Abstract
A variety of bacterial infections cause muscle necrosis in humans. Caenorhabditis elegans has epidermis and bands of muscle that resemble soft-tissue structures in mammals and humans. Here, we developed a muscle necrosis model caused by Aeromonas dhakensis infection in C. elegans. Our data showed that A. dhakensis infected and killed C. elegans rapidly. Characteristic muscle damage in C. elegans induced by A. dhakensis was demonstrated in vivo. Relative expression levels of host necrosis-associated genes, asp-3, asp-4, and crt-1 increased significantly after A. dhakensis infection. The RNAi sensitive NL2099 rrf-3 (pk1426) worms with knockdown of necrosis genes of crt-1 and asp-4 by RNAi showed prolonged survival after A. dhakensis infection. Specifically knockdown of crt-1 and asp-4 by RNAi in WM118 worms, which restricted RNAi only to the muscle cells, conferred significant resistance to A. dhakensis infection. In contrast, the severity of muscle damage and toxicity produced by the A. dhakensis hemolysin-deletion mutant is attenuated. In another example, shiga-like toxin-producing enterohemorrhagic E. coli (EHEC) known to elicit toxicity to C. elegans with concomitant enteropathogenicty, did not cause muscle necrosis as A. dhakensis did. Taken together, these results show that Aeromonas infection induces muscle necrosis and rapid death of infected C. elegans, which are similar to muscle necrosis in humans, and then validate the value of the C. elegans model with A. dhakensis infection in studying Aeromonas pathogenicity.
Keywords: Caenorhabditis elegans, Aeromonas dhakensis, disease model, muscle necrosis, infection
Introduction
Muscle necrosis is a necrotizing form of severe soft-tissue infection. These infections usually result in morbidity and mortality in humans even with early surgical intervention and antibiotic treatment. Necrotizing muscle infection is usually caused by toxin-producing bacteria, such as Streptococcus, Staphylococcus, Clostridium, Vibrio vulnificus, and Aeromonas, and characterized clinically by catastrophic progression of disease with severe tissue destruction (Anaya and Dellinger, 2007; Kuo et al., 2007; Hakkarainen et al., 2014).
Of note, the incidence of Aeromonas soft-tissue infections has been increasingly reported (Hiransuthikul et al., 2005; Chao et al., 2012; Chen et al., 2014a). Aeromonas can also cause wound infection or sepsis in burn patients (Barillo et al., 1996; Skoll et al., 1998; Kienzle et al., 2000). Cases of Aeromonas burn wound infections are often a result of water or soil exposure after the burn (Kienzle et al., 2000). Necrotizing fasciitis, myonecrosis, or both (myofascial necrosis) caused by Aeromonas usually progresses rapidly and with a fatal outcome even with antibiotic therapy and surgical intervention (Furusu et al., 1997; Tsai et al., 2007; Papadakis et al., 2012). Here, we specifically studied the pathogenesis of A. dhakensis in an animal host. The reasons for choosing A. dhakensis were: (1) A. dhakensis can cause severe soft-tissue infections in animal models and humans (Chen et al., 2014a,b); (2) A. dhakensis carries a number of virulence genes responsible for developing infectious diseases and its virulence has been recognized as the most potent among clinically important Aeromonas species (Figueras et al., 2009; Morinaga et al., 2013; Chen et al., 2014b); (3) Increasing evidence shows that A. dhakensis is widely distributed in the environment and causes a variety of infections in humans (Figueras et al., 2009; Aravena-Roman et al., 2011; Morinaga et al., 2013; Chen et al., 2014a). These findings suggest the clinical significance of this specific type of bacterial infection.
Small mammals, such as rats or mice, have been studied as experimental models of soft-tissue infection (Hidalgo-Grass et al., 2004; Cavanaugh et al., 2009; Chatterjee et al., 2016). Recently, non-mammalian models, such as Drosophila and Caenorhabditis elegans, have been increasingly used to study the pathology of soft-tissue infection (Zugasti et al., 2014; Zhang et al., 2015; Chatterjee et al., 2016). Among these models, C. elegans is attractive because of its suitability for studying host innate immunity (Kim, 2008; Marsh and May, 2012; Ermolaeva and Schumacher, 2014), convenience for gene analysis and observation, and a short life span.
Caenorhabditis elegans and Aeromonas are likely to compete in their aquatic environment and, therefore, have respective strategies to combat each other. In addition, despite their simplicity, worms have defined soft-tissues, such as epidermis and muscle bands similar to those in mammals and humans. Therefore, C. elegans can be a feasible model to study the pathogenesis of muscle necrosis. Our previous studies illustrated the applicability of virulence findings about Aeromonas species in C. elegans to mammalian cells, mice, and humans (Chen et al., 2014a,b). Clinical Aeromonas isolates that were virulent in C. elegans were also lethal to mice. The histological findings of mice with Aeromonas muscular infection, such as fragmented muscle fibers, edema of myocytes, and infiltration of inflammatory cells, resembled necrotizing myositis in humans (Chen et al., 2014b). However, the detailed histopathological characterization of muscle tissues in C. elegans with Aeromonas infection remains understudied.
In the present study, we demonstrated that C. elegans is a good surrogate model to study the pathogenesis of muscle necrosis caused by bacteria in humans. We also used this infection model to study the detrimental consequences of muscle necrosis in C. elegans after A. dhakensis infection.
Materials and Methods
C. elegans and Bacteria Strains
A wild-type Bristol N2 strain, NL2099 rrf-3(pk1426), WM118 rde-1(ne300); neIs9[myo-3p::HA::RDE-1 + rol-6(su1006)], and RW1596 myo-3(st386); stEx30[myo-3p::GFP + rol-6(su1006)] of C. elegans were provided by the Caenorhabiditis Genetics Center (CGC), which is supported by the National Institutes of Health, Office of Research Infrastructure Programs (P40 OD010440). The NL2099 strain with homozygous rrf-3 gene deletion allele was increasingly sensitive to RNAi when compared to wild-type animals (Simmer et al., 2002). The WM118 strain was used for muscle-specific RNAi (Qadota et al., 2007). The RW1596 strain with GFP-labeled sacromeres was used for analysis of muscle damage caused by A. dhakensis (Herndon et al., 2002). The animals were maintained on nematode growth (NG) plates containing Escherichia coli strain OP50 as the normal food source (Brenner, 1974).
A specific AAK1 strain of A. dhakensis (previously known as A. aquariorum; Beaz-Hidalgo et al., 2013) used in this study was a clinical isolate obtained from a patient with septicemia and necrotizing fasciitis, and its whole-genome sequences were deposited at DDBJ/EMBL/GenBank under accession nos. BAFL01000001 to BAFL01000036 and AP012343 (Wu et al., 2012).
Plate Assay of C. elegans Infected by A. dhakensis AAK1
The plate assay, as previously described (Chen et al., 2010), was conducted to measure the life span of animals infected by A. dhakensis. Briefly, eggs were prepared by treating a population of C. elegans with hypochlorite/NaOH solution and transferring the resulting eggs to NG agar plates covered with E. coli OP50, which was washed in phosphate-buffered saline, grown in LB for 18–24 h at 37°C, and standardized to an OD600 of 2.0 for tests. When these worms reached the young adult stage, 150 nematodes were transferred to fresh plates, which represented the first day of life span analysis. Animals were transferred to fresh plates of E. coli OP50 or A. dhakensis AAK1 and monitored daily for dead animals. Animals that did not respond to gentle prodding and displayed no pharyngeal pumping were scored as dead. Animals that escaped from the plate or died due to internal hatching or protrusion of the gonads through the vulva were censored. Censored animals were included in the statistical analysis until the day of the censoring event.
Life Span Assay with Heat-Killed Bacteria
Escherichia coli OP50 and A. dhakensis AAK1 were incubated with LB broth at 37°C for 18–24 h. The next day, bacterial broth was centrifuged to increase bacterial concentration to achieve an OD600 of 20.0. The concentrated bacteria were incubated at 65°C for 30 min to be inactivated. For the assays with heat-killed bacteria, 60 μl of heat-killed E. coli OP50 or heat-killed A. dhakensis AAK1 were placed on nematode growth media (NGM) and the worms were observed as described above.
Liquid-Toxic Assay of C. elegans Infected with A. dhakensis AAK1
Following washing in phosphate-buffered saline, bacteria grown in LB for 18–24 h at 37°C and standardized to an OD600 of 3.0 were prepared for tests. To obtain a synchronously growing population, eggs were prepared by treating a population of C. elegans with hypochlorite/NaOH solution and transferring the resulting eggs to NGM plates covered with E. coli OP50, as previously described (Chen et al., 2014b). The synchronized adult L4 worms on NGM plates were washed in M9 buffer. After centrifugation, the pellets of worms were re-expanded with S medium, and 5 μl of solution containing approximately 30–40 worms were placed in each lawn of 48-well plates with 5 μl fluorodeoxyuridine (Sigma–Aldrich, Saint Louis, MO, USA) to prevent reproduction. Finally, 190 μl of bacteria in LB solution were added to achieve a total 200 μl in each lawn. Assay plates were incubated at 25°C for 3–6 days. The percentages of animal death were calculated as the numbers of dead animals/total animals found each day under a dissecting microscope.
Live C. elegans Images
Synchronized late L4 to young adult stage N2 worms were plated on NGM plates seeded with A. dhakensis AAK1 or control plates seeded with E. coli OP50 at 25°C for 3 days. Stereo dissecting microscopic images were obtained using an Olympus SZX16 stereo room microscope with an Olympus DP72 cooled color digital (CCD) camera. The body length of N2 animals co-cultivated with A. dhakensis AAK1 or E. coli OP50 was measured for 4 days and compared by two-way ANOVA test.
Morphological and Physiological Analyses: Muscle Damage, Pumping Rate, Body Length, and Brood Size
Adult RW1596 animals infected with A. dhakensis and E. coli OP50 for 48 or 72 h were observed by differential interference contrast (DIC) imaging with Nomarski optics and epifluorescence imaging with corresponding filters using a Nikon Eclipse Ti inverted microscope system with a DP72 CCD camera.
The pumping rate of C. elegans was measured by counting the contractions of the terminal pharyngeal bulb of each C. elegans worm. Ten L4 stage worms were transferred to NGM with E. coli OP50, A. dhakensis AAK1, heat-killed E. coli OP50, or heat-killed A. dhakensis AAK1 at first. The pumping times for each live worm were measured everyday by counting pumps every 10 s.
The body length of ten L4 stage C. elegans worms was measured for each group every day. Images were obtained from an Olympus microscope which was connected to a digital video camera using 2.5 times magnification and captured by IC capture 2.1 software. Body length was measured from head to tail tip and analyzed by the Image-Pro Plus software. The brood size of total progeny of C. elegans was counted for 4 days after infection. Ten worms were counted for each group.
Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM)
The adult N2 worms infected with A. dhakensis AAK1 and E. coli OP50 for 48 and 72 h were fixed and embedded separately using the standard methods described in the chapter “WormMethods” of the WormBook1 (accessed March 17, 2015) for TEM and SEM study. The thin sections were collected in longitudinal aspects and observed on a Hitachi H7650 transmission electron microscope. The surface of animals was observed on a JEOL USA JSM-6390LV scanning electron microscope.
Measurement of Expression of Necrosis-Associated Genes
Expression of necrosis marker genes, asp-3, asp-4, and crt-1, was measured by quantitative real-time PCR (q-PCR) as described previously with some modifications (Wong et al., 1998). Approximately 10,000 L1 stage N2 worms were cultured on NGM plates with E. coli OP50. During the L4 to young adult stage, worms were transferred to either E. coli OP50 or A. dhakensis AAK1. After 72 h, animals were collected for RNA extraction. An RNA sample (2.0 μg) for each experimental group was converted to cDNA via reverse transcription. All q-PCRs were carried out using FastStart Universal SYBR Green Master (Rox) according to the manufacturer’s specifications and analyzed on a StepOnePlus Real-Time PCR System. Expression data were collected as Ct values, where Ct is equal to the number of PCR cycles required to amplify a given gene from a cDNA population. Changes of the expression of asp-3 (forward primer: CCA TCC AGA GAA TCA AGC TCG; reverse primer: GGA GTA ATC AGA AAG ACC CTC G), asp-4 (forward primer: CAT TTT GGC TCA ACC GTA ACC; reverse primer: CCT TGT CCA TCT TGA ATT GCC), and crt-1 (forward primer: TCC AAT ACA CCG TCA AGC AC; reverse primer: AAT CTC CCA AGT CAG CAT CAG) were initially measured as ΔCt values which subsequently normalized against a housekeeping gene: nhr-23 (forward primer: GCC GAA GAT GAT GCC GAG AT; reverse primer: GTC GCA GTG TCA AGA ATC CC). The fold-change values of the AAK1 group compared to OP50 group were estimated by following equation: Fold change = 2[-ΔCt(AAK1)]/2[-ΔCt(OP50)].
RNA Interference Assay
RNA interference (RNAi) clones to asp-3, asp-4, and crt-1 were obtained from the C. elegans RNAi library (Ashrafi et al., 2003). E. coli HT115 with L4440, an empty vector, was used as a control of RNAi. Synchronized NL2099, or WM 118 L1 larvae were cultured on plates seeding E. coli HT115 with L4440, asp-3, asp-4, or crt-1 RNAi at 20°C until the L4 stage. In the WM 118 strain, RNAi knockdowns were restricted specifically in muscle (Qadota et al., 2007). These L4 stage animals were transferred to plates together with E. coli HT115 either carrying RNAi plasmids or the L4440 plasmid together with A. dhakensis AAK1 at a ratio of 1:1. The animals were then incubated at 20°C and survival was observed.
Statistical Analysis
Statistical analysis was performed and plotted using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). The Mantel-Cox log-rank test was used to assess statistical significance of difference in survival. The two-way ANOVA test was used assess statistical significance of difference in body length, brood size, and pharyngeal pumping rate analyses. The proportions of muscle damage for A. dhakensis and the control groups were compared by Mann–Whitney U test.
Results
Aeromonas dhakensis Infects and Kills Caenorhabditis elegans
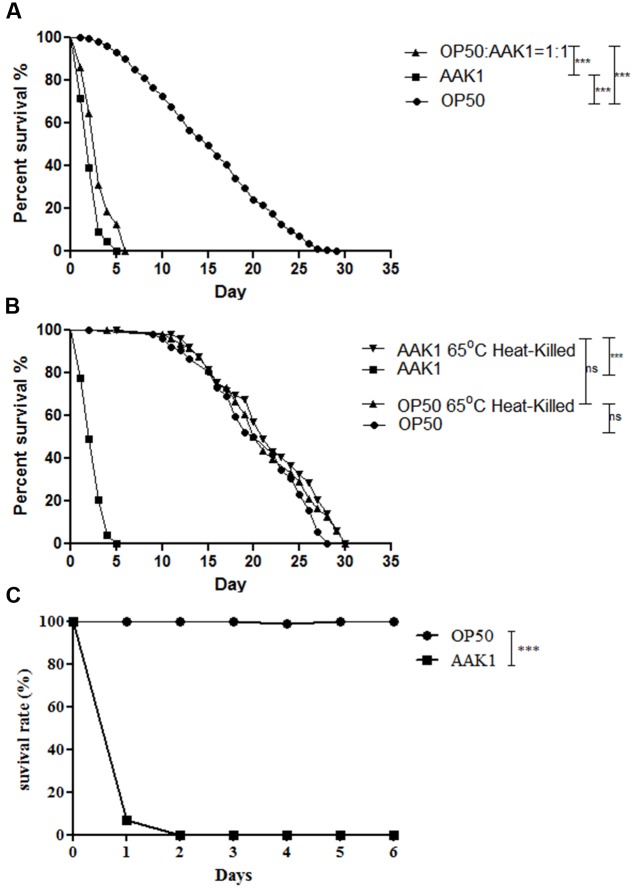
In the plate assay of C. elegans, clinical isolate A. dhakensis AAK1 significantly shortened the life span of C. elegans worms in comparison with the non-pathogenic control strain E. coli OP50, the normal laboratory food source of C. elegans (P < 0.0001; Figure 1A). A. dhakensis AAK1 mixed with E. coli OP50 in a ratio of 1:1 also decreased the life span of C. elegans worms when compared with E. coli OP50 (P < 0.0001). Moreover, heat-killed A. dhakensis AAK1 showed the toxicity to C. elegans similar to either OP50 or heat-killed bacteria (Figure 1B). Together these results suggested the short-lived phenotype of C. elegans worms feeding on A. dhakensis is not due to malnutrition but to the toxicity of A. dhakensis. In the liquid-toxic (LT) assay, the survival rate of C. elegans infected with A. dhakensis AAK1 was also significantly lower than those with E. coli OP50 (P < 0.0001, Figure 1C). Almost all animals were killed by A. dhakensis AAK1 at day 2 in the LT assay. Taken all together, our results show that A. dhakensis infects and kills C. elegans.
FIGURE 1.
Aeromonas dhakensis shortens the life span of Caenorhabditis elegans. (A) Worms infected with A. dhakensis and A. dhakensis mixed with Escherichia coli OP50 (1:1) had a significantly shorter life span when compared with control E. coli OP50. (B) A. dhakensis AAK1 showed toxicity when compared with heat-killed A. dhakensis or control E. coli OP50. (C) In liquid-toxic (LT) assay, the survival rate of C. elegans infected with A. dhakensis was significantly lower than those with control strain E. coli OP50. ∗∗∗P < 0.0001 by the Mantel-Cox log-rank test.
Morphological Changes in Caenorhabditis elegans after Aeromonas dhakensis Infection
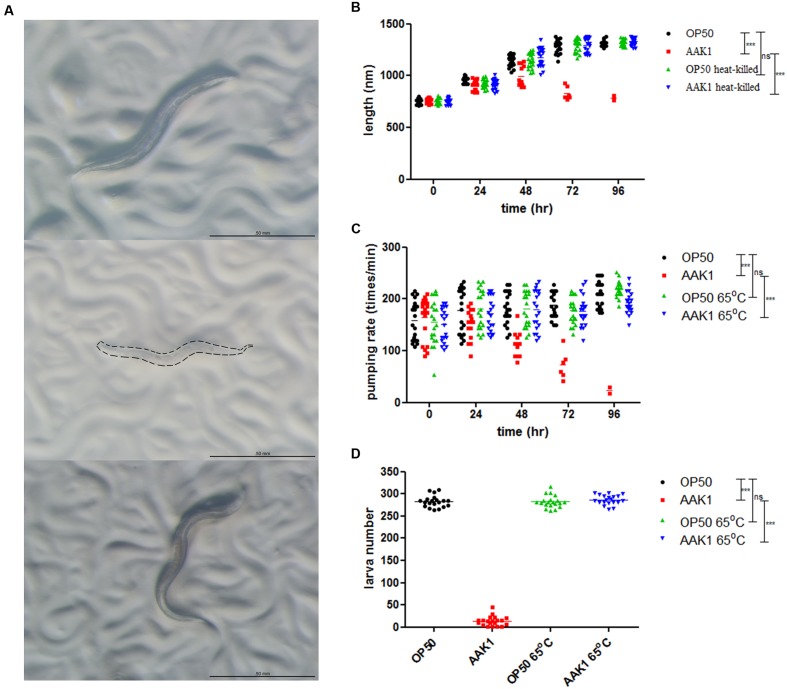
Significant morphological changes in C. elegans were observed after infection with A. dhakensis AAK1 (Figure 2). At 72 h, A. dhakensis AAK1 caused total lysis of C. elegans (middle). In contrast, C. elegans morphology was normal, if C. elegans was fed with heat-killed A. dhakensis or E. coli OP50 (top and bottom). The average body length of N2 C. elegans significantly decreased after AAK1 infection when compared to worms fed on E. coli OP50, heat-killed E. coli OP50 (OP50-65°C), and heat-killed AAK1 (AAK1-65°C; P < 0.0001, at days 3 and 4; Figure 2B). The feeding rate of AAK1 infected worms obviously diminished. Pharyngeal muscle movement (pumping times/min) of N2 worms fed with OP50, AAK1, OP50-65°C, and AAK1-65°C were monitored. The average pumping rate of N2 C. elegans significantly reduced after AAK1 infection, as compared with worms fed on OP50, OP50-65°C, or AAK1-65°C (all P < 0.0001; Figure 2C). In addition, the reproduction of AAK1 infected worms significantly decreased. Total progeny numbers of N2 worms fed with OP50, AAK1, OP50-65°C, or AAK1-65°C were counted. The average brood size of N2 C. elegans was significantly reduced after AAK1 infection when compared with worms fed on OP50, OP50-65°C, or AAK1-65°C (all P < 0.0001; Figure 2D). These findings suggest that the morphology and behavioral changes of C. elegans were caused by A. dhakensis AAK1 infection.
FIGURE 2.
Aeromonas dhakensis infection induces morphological and physiological changes in C. elegans. (A) Morphology of C. elegans treated with A. dhakensis AAK1 for 72 h (Top to bottom: E. coli OP50, A. dhakensis AAK1, 65°C heat-killed A. dhakensis AAK1). At 72 h, AAK1 infection resulted in total lysis of body. The corpse of AAK1 infected worm was marked by dashed line. The scale bar indicates 500 μm. (B) The body length of N2 C. elegans was significantly decreased after A. dhakensis AAK1 infection when compared with the control E. coli OP50 (P < 0.0001) by two-way ANOVA test. In contrast, 65°C heat-killed bacteria did not affect the size of the animals. The average feeding rate measured by pharyngeal movement (pumping times/min) (C) and brood size (D) of N2 worms infected with AAK1were significantly reduced, as compared with worms fed on OP50, OP50-65°C, or AAK1-65°C, ∗∗∗P < 0.0001.
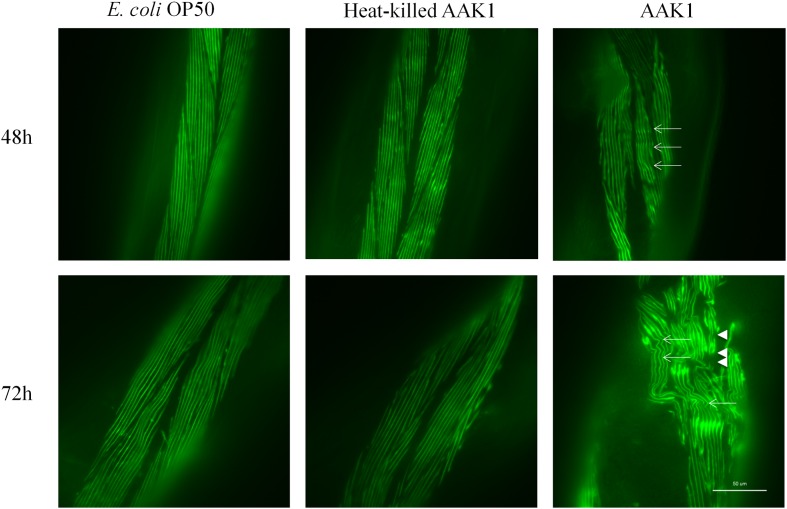
Muscle damage was observed in the transgenic C. elegans strain RW1596, in which sacromeres were labeled with MYO-3::GFP protein. Typical features of muscle damage, i.e., including bending and rupture of muscle fibers were observed at 48 and 72 h after A. dhakensis infection (Figure 3). The muscle damage caused by A. dhakensis in C. elegans became severe with time, manifesting obvious bending and rupture of muscles fiber at 72 h. In contrast, muscle fibers of C. elegans were intact either in the presence of E. coli OP50 or heat-killed A. dhakensis AAK1.
FIGURE 3.
Aeromonas dhakensis induces muscle damage in Caenorhabditis elegans. Bending of muscle fiber (arrows) was observed in worms with A. dhakensis AAK1 infection at 48 h. Rupture of muscle fibers (arrowheads) was obvious at 72 h. In contrast, E. coli OP50 or heat-killed A. dhakensis AAK1 did not cause muscle damage. Scale bar indicates 50 μm.
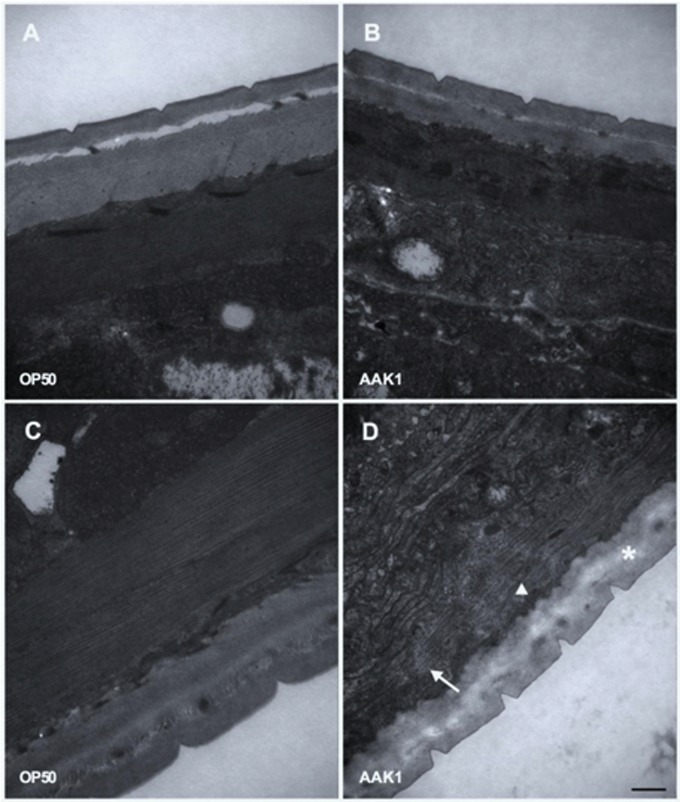
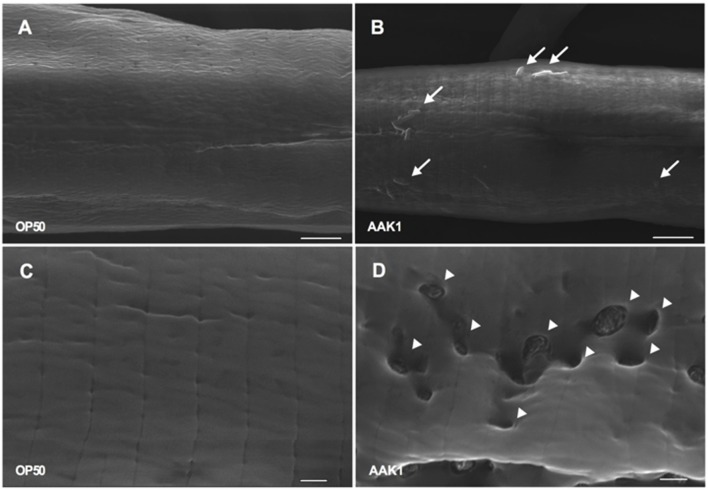
The morphological changes in C. elegans with A. dhakensis AAK1 infection were observed by TEM (Figure 4) and SEM (Figure 5). Significant muscle damage appeared at 72 h. In TEM, wavy change, patch hypo-dense lesions, and loosening of myosin filaments were evident (Figure 4D) and SEM showed that the pathogens invaded C. elegans through the cuticle. At 48 h A. dhakensis AAK1 adhered to the worm surface (Figure 5B), on which obvious erosion and pole formation were seen at 72 h (Figure 5D). Taken together, our results show that A. dhakensis AAK1 can adhere to the surfaces of C. elegans and cause cuticle invasion as well as muscle damage.
FIGURE 4.
Transmission electron microscopic analysis. The morphological changes in C. elegans after feeding with A. dhakensis AAK1 (right panels) or E. coli OP50 (left panels) for 48 h (upper panels) or 72 h (lower panels) were observed with transmission electron microscopy (A–D). Patch hypo-dense lesions (arrow), wavy change of myosin filaments (arrowhead), and decreased density of hypodermis (asterisks) were observed at 72 h after AAK1 infection (D). Scale bar indicates 500 nm.
FIGURE 5.
Scanning electron microscopic analysis. Features of N2 animals infected with A. dhakensis AAK1 (right panels, B,D) and control E. coli OP50 (left panels, A,C) for 48 h (upper panels) and 72 h (lower panels) observed by scanning electron microscopy. At 48 h, AAK1 cells that had adhered to animal surface are indicated by arrows (B). Uneven surface and punctate lesions caused by invasion of AAK1 are indicated by arrowheads at 72 h (D). Scale bars in the upper panel indicate 5 μm, and those in the lower panel indicate 1 μm.
Aeromonas dhakensis Infection-Induced Muscle Necrosis and Rapid Death in Caenorhabditis elegans
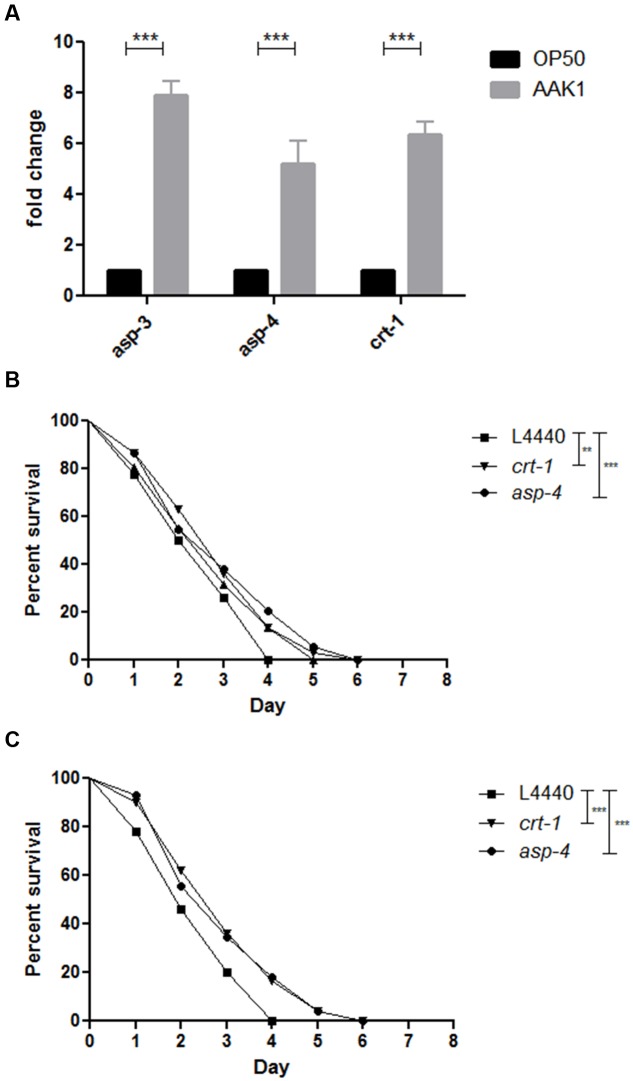
To validate whether A. dhakensis infection induces necrosis in C. elegans, we examined the expression of an array of necrosis genes, including asp-3 and asp-4 encoding aspartyl proteases, and crt-1 encoding calreticulin (Syntichaki et al., 2002) separately, by q-PCR. At 72 h, relative expression levels of these genes in C. elegans infected by A. dhakensis AAK1 were higher than those by E. coli OP50 (Figure 6A). The increased expression of necrosis-associated genes with A. dhakensis infection was compatible to the necrosis phenotype observed in the morphological analysis. To confirm whether necrosis is a part of a bystander inducible mechanism or a deleterious consequence of infection, we studied the survival of C. elegans with depressed expression of asp-4 or crt-1 upon A. dhakensis infection. The RNAi sensitive worms, NL2099 rrf-3 (pk1426), with knockdown of asp-4 or crt-1 by RNAi showed significantly enhanced survival after A. dhakensis infection, relative to the control worms with RNAi empty vector L4440 (Figure 6B). The WM118 worms with suppressed expression of asp-4 or crt-1 by RNAi restricting to the muscles were also significantly resistant to A. dhakensis infection than control worms (P < 0.0001) (Figure 6C). The RNAi used in the experiment did not influence the growth of bacteria (Supplementary Figure S1). Taken together, we demonstrated that A. dhakensis infection induces muscle necrosis, which is associated with rapid death of infected worms.
FIGURE 6.
Aeromonas dhakensis induces necrosis in C. elegans. (A) Relative expression levels of asp-3, asp-4, and crt-1 genes measured by quantitative real-time PCR (qPCR) in C. elegans infected by A. dhakensis AAK1 for 72 h were higher than C. elegans fed by E. coli OP50. (B) The RNAi-sensitive worms, NL2099 rrf-3 (pk1426), with knockdown of crt-1 or asp-4 by RNAi showed significantly enhanced survival after A. dhakensis infection, relative to the control animals with RNAi control (L4440). (C) The WM118 worms with depressed expression of crt-1 or asp-4 by RNAi restricted to the muscle were more resistant to A. dhakensis infection than RNAi control (L4440). ∗∗P < 0.01; ∗∗∗P < 0.0001.
Virulence of Hemolysin-Deletion A. dhakensis to C. elegans
The virulence of a hemolysin-deletion mutant of AAK1 (AAK1 ΔhlyA::KanR), which was produced by the methods described in the Supplementary Materials, was studied. Virulence of this mutant to C. elegans was significantly reduced when compared with the parent AAK1 strain in a survival analysis (Supplementary Figure S2). The severity of muscle damage induced by this mutant isolate in the RW1596 transgenic worms was less evident than that induced by AAK1 (Supplementary Figure S3). Similarly, the expressions of crt-1, asp-4, and asp-3 induced by AAK1 in C. elegans at 72 h were significantly abolished in those treated by the AAK1 ΔhlyA::KanR mutant (all P < 0.0001). (Supplementary Figure S4).
Discussion
Necrotizing muscle infection, a type of severe invasive infection, is usually associated with high morbidity and mortality in humans despite surgical intervention and antibiotic treatment (McHenry et al., 1995). The pathogenesis of severe muscle infection is not well understood. Here, we established a disease model of soft-tissue, namely muscle infection by A. dhakensis in C. elegans, and such a model can be used as a tool to study the pathogenesis of muscle necrosis. Traditionally, small mammal models, such as mice, rats, rabbits, or dogs, have provided valuable information to understand the pathophysiology of muscle necrosis caused by degenerative disease (Klyen et al., 2011), injury (Hargens et al., 1981; Daly et al., 2011), or infection (Hidalgo-Grass et al., 2004; Cavanaugh et al., 2009; Chatterjee et al., 2016). Among soft-tissue infection models, rats or mice were commonly adopted (Hidalgo-Grass et al., 2004; Cavanaugh et al., 2009; Chatterjee et al., 2016). However, the disadvantage of these infection models is that the results are not reproducible or sustainable due to the leakage and spread of bacteria from the inoculated tissues (Yoshioka et al., 2014). In addition, morphological analysis of the extent of muscle necrosis necessitates the sacrifice of large animals and is laborious work.
Over the past decade, increasing evidence has shown that Drosophila, zebrafish, and C. elegans are good models for studying soft-tissue pathology because they share conserved immune responses against bacterial or fungal infections in soft-tissues of mammals (Zugasti et al., 2014; Chatterjee et al., 2016). Out of these models, C. elegans is an acceptable model to explore innate defense strategies of epithelial tissues, as its epidermal cells possess cell-autonomous defense mechanisms against invading pathogens or physical injury (Ziegler et al., 2009; Zugasti et al., 2014; Zhang et al., 2015). From the temporal perspective, extensive tissue destruction usually evolves rapidly after bacterial invasion in the cases of necrotizing myonecrosis (Hakkarainen et al., 2014). Therefore, the innate immunity in muscle tissues should play an important role in the first-line host response, as it is activated after sensing pathogen-associated molecular patterns (Mogensen, 2009) or damage-associated molecular patterns at the early stage of infection (Chen and Nunez, 2010).
In the present study, A. dhakensis was chosen for testing in a disease model as it can cause a variety of forms of soft-tissue infection in humans. In some endemic areas, such as southern Taiwan, A. dhakensis is of clinical importance due to its virulence and antimicrobial resistance, and it is also the predominant species in wound infections (Chen et al., 2014a,b). In addition, the whole-genome sequence of the A. dhakensis strain AAK1 has been published (Wu et al., 2012), and genomic information of C. elegans is readily available, making further and systematic genetic studies of host-microbe interaction possible. Our study showed that A. dhakensis AAK1 can adhere to the surfaces of C. elegans and cause cuticle invasion as well as muscle damage. This phenomenon resembles necrotizing fasciitis or bacterial myositis in humans (Hiransuthikul et al., 2005; Kihiczak et al., 2006).
The pathogenicity of Aeromonas species has been considered to be multifactorial. A number of virulence factors, such as secretion systems, toxins, and the quorum sensing system (QSS), have been reported (Tomas, 2012; Chen et al., 2016). However, the pathogenesis of muscle necrosis caused by A. dhakensis infection remains unclear. Of the toxins discovered in Aeromonas species, hemolysin is one of the most important virulence factors and has been considered as a causative factor of human diarrheal disease (Burke et al., 1982). In a study of mouse infection model, intravenous injection of hemolysin can elicit lethality in mice (Asao et al., 1986). In addition, hemolysin is able to cause fluid accumulation in rabbit ligated ileal loop and infant mouse intestine in vitro (Asao et al., 1986), and hemolysin can produce inflammation of injected areas over the body surface of carps (Kanai and Takagi, 1986). In the present study, muscle necrosis caused by a hemolysin-depletion mutant of A. dhakensis was attenuated significantly when compared with parent strain. The results suggest hemolysin plays an important role in the pathogenicity of this species. Our study validate the potential of this model in studying the pathogenesis of muscle necrosis related to Aeromonas infections and provide a platform to find the potential therapeutic approaches to improve the outcome of severe Aeromonas soft-tissue infection.
Rapid death with necrosis of C. elegans is a characteristic of A. dhakensis infection. Mosser et al. (2015) found that A. dhakensis exhibited high virulence in the C. elegans infection model, with unusual presentation of rapid lysis of dead bodies by A. dhakensis BVH28b. A similar phenomenon was observed caused by our study strain, A. dhakensis AAK1 (Chen et al., 2014a). To our knowledge, these features are rarely reported in other pathogens. Other bacteria, such as Enterococcus faecalis, Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella typhimurium, and Serratia marcescens, cause intestinal infections in C. elegans (Ermolaeva and Schumacher, 2014). For example, colonization of the host intestinal tract is a key virulent mechanism of P. aeruginosa in the “slow-killing” assay (Tan et al., 1999). In another example, Shiga-like toxin-producing enterohemorrhagic E. coli (EHEC) O157:H7 has been known to infect and kill C. elegans with concomitant demonstration of bacterial colonization and induction of characteristic attaching and effacing lesions in the intestinal epithelium of worms (Chou et al., 2013). In contrast to rapid necrosis induced by A. dhakensis, no significant morphological changes in C. elegans were observed after infection with EHEC O157:H7 at 72 h (Supplementary Figure S5). Likewise, the expression of necrosis-associated genes of asp-3, asp-4, and crt-1 was not obviously changed with EHEC infection and was in accordance with morphological observation (Supplementary Figure S6). Instead, our SEM and TEM studies showed that A. dhakensis AAK1 attached to the worm skin and invaded at the initial infection site, at least during the time frame we analyzed. This evidence suggests the initiation of necrotic cell death is associated with recognition of the pathogen in the cuticle. The exact mechanism needs to be elucidated in the future.
Our data suggested that A. dhakensis infection causes necrotic cell death in muscle cells in C. elegans, and necrosis has a detrimental effect during A. dhakensis infection as necrosis-deficient worms were more resistant to bacterial infection than wild-type worms. Several mechanisms have been linked to necrotic cell death, such as autophagy, calcium, TOR-mediated nutrient sensing, and lysosomal proteases (Crook et al., 2013). Of the variety of necrotic mechanisms, homeostasis of calcium in the endoplasmic reticulum and calcium-dependent proteases may play important roles in necrotic death of muscle cells. It is well known that mitochondrial calcium overload is a general mechanism for muscle cell necrosis in various muscle diseases (Wrogemann and Pena, 1976). We discovered that host asp and crt-1 genes encoding aspartyl proteases and calreticulin (a calcium-binding protein), respectively, implicated in cell necrosis, were upregulated by A. dhakensis infection. In addition, worms with muscle deficiency of asp and crt-1 were more resistant to A. dhakensis infection. Xu et al. reported that calreticulin activation could induce calcium release from the endoplasmic reticulum (Xu et al., 2001) and downstream aspartyl proteases activity was associated with necrotic cell death and neurodegeneration in C. elegans (Syntichaki et al., 2002, 2005). Therefore, our data suggest that A. dhakensis infection may activate crt-1 expression in muscles and promotes the release of calcium from the endoplasmic reticulum, activates the cascade of aspartyl proteases, and consequently causes necrotic cell death of worms.
To sum up, our work found that gross pathology and histopathology induced by A. dhakensis infection are similar in humans and C. elegans, thus validating the value of this model. The advantages of C. elegans were exploited to visualize gross and histological soft-tissue damage. In addition, the C. elegans model can be used to study host innate immunity and bacterial factors involved in soft-tissue infection. The application of the C. elegans model provides an opportunity for high-throughput genetic screening for pathogen or host factors involved in clinically important bacterial infections.
Author Contributions
P-LC and Y-WC contributed equally to this work. P-LC, W-CK, and C-SC conceived and designed the experiments. C-CO and Y-WC performed the experiments. P-LC and C-SC analyzed the data. C-JW and T-ML contributed critical reagents and analysis tools. P-LC, W-CK, and C-SC wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge assistance from the C. elegans core facility Taiwan [funded by the Ministry of Science and Technology (MOST), Taiwan], and comments and helpful discussions from the Taiwan C. elegans research community. We also thank Miranda Loney for editing the manuscript.
Funding. This study was partially supported by grants from the Ministry of Science and Technology of Taiwan (MOST 103-2314-B-006-076-MY2, 103-2311-B-006-005-MY3, and 104-2321-B-006-019-), and National Cheng Kung University Hospital, Tainan, Taiwan (NCKUH-10408015 and 10507004).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.02058/full#supplementary-material
SUPPLEMENTARY MATERIALS | An AAK1 strain with 1.8K base-pair hemolysin gene flanked by kanamycin resistance nptII gene was generated by marker exchange utilizing a homologous recombination with suicide plasmid carrying sacB gene. We utilized the conjugation method to deliver the construct plasmid into AAK1 with the aid of E. coli S17-1 λ πstrain. After 3 h of co-culture, the colonies grew on the plates of Aeromonas Selective Medium LabM 167 (Lab M; Lab M Ltd, Lancashire, UK) with kanamycin overnight cultured at 37°C were selected. Colony PCR was performed to check the presence of sacB gene in the AAK1 strain. At the same time, we transferred those colonies to grow on the LB plate with 6% sucrose and kanamycin at 20°C in order to let sacB convert sucrose to toxic metabolite, levans, and facilitate efficient chromosome exchange between the transconjugated plasmid and the chromosome. After 2–3 days of culture, all the candidates were confirmed by genomic DNA PCR using full-length hemolysin primers to confirm an hemolysin-deficient mutant, AAK1 ΔhlyA::kanR, was obtained.
FIGURE S1 | Co-culture of Escherichia coli carrying different RNAi clones of asp-3, asp-4, and crt-1 with Aeromonas dhakensis AAK1 for 24 h did not affect the growth of A. dhakensis AAK1 by measuring colony forming units (CFUs) on kanamycin selective medium. L4440, an empty vector, was used as a negative control of RNAi.
FIGURE S2 | A hemolysin-deficient isogenic mutant of AAK1, AAK1 ΔhlyA::KanR, prolonged the survival curve of Caenorhabditis elegans (P < 0.0001).
FIGURE S3 | A hemolysin-deficient mutant of A. dhakensis, AAK1 ΔhlyA::KanRdid not cause muscle damage as parent strain. Rupture of muscle fibers (arrowheads) was obvious in worms with A. dhakensis infection at 72 h (middle). In contrast, E. coli OP50 (left) and ΔhlyA::KanR AAK1 (right) did not cause muscle damage.
FIGURE S4 | Relative expression levels of necrosis-associated genes in C. elegans infected by a hemolysin-deficient isogenic mutant of A. dhakensis AAK1. In the mutant, expression levels of crt-1, asp-3, and asp-4 at 72 h are significantly lower to those in wild-type AAK1 by quantitative real-time PCR (∗∗∗P < 0.0001).
FIGURE S5 | Enterohemorrhagic E. coli O157:H7 did not induce rapid lysis of C. elegans as A. dhakensis did.
FIGURE S6 | Expression levels of necrosis genes, asp-3, asp-4, and crt-1, in Caenorhabditis elegans infected by enterohemorrhagic E. coli O157:H7 were significantly lower than those in C. elegans infected by A. dhakensis. ∗∗∗P < 0.0001.
References
- Anaya D. A., Dellinger E. P. (2007). Necrotizing soft-tissue infection: diagnosis and management. Clin. Infect. Dis. 44 705–710. 10.1086/511638 [DOI] [PubMed] [Google Scholar]
- Aravena-Roman M., Harnett G. B., Riley T. V., Inglis T. J., Chang B. J. (2011). Aeromonas aquariorum is widely distributed in clinical and environmental specimens and can be misidentified as Aeromonas hydrophila. J. Clin. Microbiol. 49 3006–3008. 10.1128/JCM.00472-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asao T., Kinoshita Y., Kozaki S., Uemura T., Sakaguchi G. (1986). Purification and some properties of Aeromonas hydrophila hemolysin. Infect. Immun. 46 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi K., Chang F. Y., Watts J. L., Fraser A. G., Kamath R. S., Ahringer J., et al. (2003). Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421 268–272. 10.1038/nature01279 [DOI] [PubMed] [Google Scholar]
- Barillo D. J., McManus A. T., Cioffi W. G., McManus W. F., Kim S. H., Pruitt B. A., Jr. (1996). Aeromonas bacteraemia in burn patients. Burns 22 48–52. 10.1016/0305-4179(95)00075-5 [DOI] [PubMed] [Google Scholar]
- Beaz-Hidalgo R., Martinez-Murcia A., Figueras M. J. (2013). Reclassification of Aeromonas hydrophila subsp. dhakensis Huys et al. 2002 and Aeromonas aquariorum Martinez-Murcia et al. 2008 as Aeromonas dhakensis sp. nov. comb nov. and emendation of the species Aeromonas hydrophila. Syst. Appl. Microbiol. 36 171–176. 10.1016/j.syapm.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke V., Robinson J., Atkinson H. M., Gracey M. (1982). Biochemical characteristics of enterotoxigenic Aeromonas spp. J. Clin. Microbiol. 15 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh D. L., Berry J., Yarboro S. R., Dahners L. E. (2009). Better prophylaxis against surgical site infection with local as well as systemic antibiotics. An in vivo study. J. Bone Joint Surg. Am. 91 1907–1912. 10.2106/JBJS.G.01237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C. M., Lai C. C., Tang H. J., Ko W. C., Hsueh P. R. (2012). Skin and soft-tissue infections caused by Aeromonas species. Eur. J. Clin. Microbiol. Infect. Dis. 32 543–547. 10.1007/s10096-012-1771-y [DOI] [PubMed] [Google Scholar]
- Chatterjee A., Roy D., Patnaik E., Nongthomba U. (2016). Muscles provide protection during microbial infection by activating innate immune response pathways in Drosophila and zebrafish. Dis. Model Mech. 9 697–705. 10.1242/dmm.022665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. S., Bellier A., Kao C. Y., Yang Y. L., Chen H. D., Los F. C., et al. (2010). WWP-1 is a novel modulator of the DAF-2 insulin-like signaling network involved in pore-forming toxin cellular defenses in Caenorhabditis elegans. PLoS ONE 5:e9494 10.1371/journal.pone.0009494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. Y., Nunez G. (2010). Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10 826–837. 10.1038/nri2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. L., Lamy B., Ko W. C. (2016). Aeromonas dhakensis, an increasingly recognized human pathogen. Front. Microbiol. 27:793 10.3389/fmicb.2016.00793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. L., Wu C. J., Chen C. S., Tsai P. J., Tang H. J., Ko W. C. (2014a). A comparative study of clinical Aeromonas dhakensis and Aeromonas hydrophila isolates in southern Taiwan: a. dhakensis is more predominant and virulent. Clin. Microbiol. Infect. 20 O428–O434. 10.1111/1469-0691.12456 [DOI] [PubMed] [Google Scholar]
- Chen P. L., Wu C. J., Tsai P. J., Tang H. J., Chuang Y. C., Lee N. Y., et al. (2014b). Virulence diversity among bacteremic Aeromonas isolates: ex vivo, animal, and clinical evidences. PLoS ONE 9:e111213 10.1371/journal.pone.0111213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T. C., Chiu H. C., Kuo C. J., Wu C. M., Syu W. J., Chiu W. T., et al. (2013). Enterohaemorrhagic Escherichia coli O157:H7 Shiga-like toxin 1 is required for full pathogenicity and activation of the p38 mitogen-activated protein kinase pathway in Caenorhabditis elegans. Cell Microbiol. 15 82–97. 10.1111/cmi.12030 [DOI] [PubMed] [Google Scholar]
- Crook M., Upadhyay A., Hanna-Rose W. (2013). Necrosis in C. elegans. Methods Mol. Biol. 1004 171–182. 10.1007/978-1-62703-383-1_13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly K. A., Wolf M., Johnson S. A., Badylak S. F. (2011). A rabbit model of peripheral compartment syndrome with associated rhabdomyolysis and a regenerative medicine approach for treatment. Tissue Eng. Part C Methods 17 631–640. 10.1089/ten.tec.2010.0699 [DOI] [PubMed] [Google Scholar]
- Ermolaeva M. A., Schumacher B. (2014). Insights from the worm: the C. elegans model for innate immunity. Semin. Immunol. 26 303–309. 10.1016/j.smim.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueras M. J., Alperi A., Saavedra M. J., Ko W. C., Gonzalo N., Navarro M., et al. (2009). Clinical relevance of the recently described species Aeromonas aquariorum. J. Clin. Microbiol. 47 3742–3746. 10.1128/JCM.02216-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusu A., Yoshizuka N., Abe K., Sasaki O., Miyazaki K., Miyazaki M., et al. (1997). Aeromonas hydrophila necrotizing fasciitis and gas gangrene in a diabetic patient on haemodialysis. Nephrol. Dial. Transplant. 12 1730–1734. 10.1093/ndt/12.8.1730 [DOI] [PubMed] [Google Scholar]
- Hakkarainen T. W., Kopari N. M., Pham T. N., Evans H. L. (2014). Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Curr. Probl. Surg. 51 344–362. 10.1067/j.cpsurg.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargens A. R., Schmidt D. A., Evans K. L., Gonsalves M. R., Cologne J. B., Garfin S. R., et al. (1981). Quantitation of skeletal-muscle necrosis in a model compartment syndrome. J. Bone Joint Surg. Am. 63 631–636. 10.2106/00004623-198163040-00014 [DOI] [PubMed] [Google Scholar]
- Herndon L. A., Schmeissner P. J., Dudaronek J. M., Brown P. A., Listner K. M., Sakano Y., et al. (2002). Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419 808–814. 10.1038/nature01135 [DOI] [PubMed] [Google Scholar]
- Hidalgo-Grass C., Dan-Goor M., Maly A., Eran Y., Kwinn L. A., Nizet V., et al. (2004). Effect of a bacterial pheromone peptide on host chemokine degradation in group A streptococcal necrotising soft-tissue infections. Lancet 363 696–703. 10.1016/S0140-6736(04)15643-2 [DOI] [PubMed] [Google Scholar]
- Hiransuthikul N., Tantisiriwat W., Lertutsahakul K., Vibhagool A., Boonma P. (2005). Skin and soft-tissue infections among tsunami survivors in southern Thailand. Clin. Infect. Dis. 41 e93–e96. 10.1086/497372 [DOI] [PubMed] [Google Scholar]
- Kanai K., Takagi Y. (1986). Alpha-hemolytic toxin of Aeromonas hydrophila produced in vivo. Fish Pathol. 21 245–250. 10.3147/jsfp.21.245 [DOI] [Google Scholar]
- Kienzle N., Muller M., Pegg S. (2000). Aeromonas wound infection in burns. Burns 26 478–482. 10.1016/S0305-4179(99)00188-6 [DOI] [PubMed] [Google Scholar]
- Kihiczak G. G., Schwartz R. A., Kapila R. (2006). Necrotizing fasciitis: a deadly infection. J. Eur. Acad. Dermatol. Venereol. 20 365–369. 10.1111/j.1468-3083.2006.01487.x [DOI] [PubMed] [Google Scholar]
- Kim D. (2008). Studying host-pathogen interactions and innate immunity in Caenorhabditis elegans. Dis. Model Mech. 1 205–208. 10.1242/dmm.000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyen B. R., Shavlakadze T., Radley-Crabb H. G., Grounds M. D., Sampson D. D. (2011). Identification of muscle necrosis in the mdx mouse model of Duchenne muscular dystrophy using three-dimensional optical coherence tomography. J. Biomed. Opt. 16:076013 10.1117/1.3598842 [DOI] [PubMed] [Google Scholar]
- Kuo Y. L., Shieh S. J., Chiu H. Y., Lee J. W. (2007). Necrotizing fasciitis caused by Vibrio vulnificus: epidemiology, clinical findings, treatment and prevention. Eur. J. Clin. Microbiol. Infect. Dis. 26 785–792. 10.1007/s10096-007-0358-5 [DOI] [PubMed] [Google Scholar]
- Marsh E. K., May R. C. (2012). Caenorhabditis elegans, a model organism for investigating immunity. Appl. Environ. Microbiol. 78 2075–2081. 10.1128/AEM.07486-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry C. R., Piotrowski J. J., Petrinic D., Malangoni M. A. (1995). Determinants of mortality for necrotizing soft-tissue infections. Ann. Surg. 221 558–563. 10.1097/00000658-199505000-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen T. H. (2009). Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22 240–273. 10.1128/CMR.00046-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga Y., Yanagihara K., Eugenin F. L., Beaz-Hidalgo R., Kohno S., Figueras M. J. (2013). Identification error of Aeromonas aquariorum: a causative agent of septicemia. Diagn. Microbiol. Infect. Dis. 76 106–109. 10.1016/j.diagmicrobio.2013.01.019 [DOI] [PubMed] [Google Scholar]
- Mosser T., Talagrand-Reboul E., Colston S. M., Graf J., Figueras M. J., Jumas-Bilak E., et al. (2015). Exposure to pairs of Aeromonas strains enhances virulence in the Caenorhabditis elegans infection model. Front. Microbiol. 6:1218 10.3389/fmicb.2015.01218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis V., Poniros N., Katsibardi K., Charissiadou A. E., Anastasopoulos J., Polychronopoulou S. (2012). Fulminant Aeromonas hydrophila infection during acute lymphoblastic leukemia treatment. J. Microbiol. Immunol. Infect. 45 154–157. 10.1016/j.jmii.2011.09.008 [DOI] [PubMed] [Google Scholar]
- Qadota H., Inoue M., Hikita T., Koppen M., Hardin J. D., Amano M., et al. (2007). Establishment of a tissue-specific RNAi system in C. elegans. Gene 400 166–173. 10.1016/j.gene.2007.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmer F., Tijsterman M., Parrish S., Koushika S. P., Nonet M. L., Fire A., et al. (2002). Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr. Biol. 12 1317–1319. 10.1016/S0960-9822(02)01041-2 [DOI] [PubMed] [Google Scholar]
- Skoll P. J., Hudson D. A., Simpson J. A. (1998). Aeromonas hydrophila in burn patients. Burns 24 350–353. 10.1016/S0305-4179(98)00024-2 [DOI] [PubMed] [Google Scholar]
- Syntichaki P., Samara C., Tavernarakis N. (2005). The vacuolar H+ -ATPase mediates intracellular acidification required for neurodegeneration in C. elegans. Curr. Biol. 15 1249–1254. 10.1016/j.cub.2005.05.057 [DOI] [PubMed] [Google Scholar]
- Syntichaki P., Xu K., Driscoll M., Tavernarakis N. (2002). Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature 419 939–944. 10.1038/nature01108 [DOI] [PubMed] [Google Scholar]
- Tan M. W., Mahajan-Miklos S., Ausubel F. M. (1999). Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 96 715–720. 10.1073/pnas.96.5.2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas J. M. (2012). The main Aeromonas pathogenic factors. ISRN Microbiol. 2012 256261 10.5402/2012/256261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. H., Hsu R. W., Huang T. J., Hsu W. H., Huang K. C., Li Y. Y., et al. (2007). Necrotizing soft-tissue infections and sepsis caused by Vibrio vulnificus compared with those caused by Aeromonas species. J. Bone Joint Surg. Am. 89 631–636. 10.2106/00004623-200703000-00021 [DOI] [PubMed] [Google Scholar]
- Wong C. Y., Heuzenroeder M. W., Flower R. L. (1998). Inactivation of two haemolytic toxin genes in Aeromonas hydrophila attenuates virulence in a suckling mouse model. Microbiology 144(Pt 2), 291–298. 10.1099/00221287-144-2-291 [DOI] [PubMed] [Google Scholar]
- Wrogemann K., Pena S. D. (1976). Mitochondrial calcium overload: a general mechanism for cell-necrosis in muscle diseases. Lancet 1 672–674. 10.1016/S0140-6736(76)92781-1 [DOI] [PubMed] [Google Scholar]
- Wu C. J., Wang H. C., Chen C. S., Shu H. Y., Kao A. W., Chen P. L., et al. (2012). Genome sequence of a novel human pathogen, Aeromonas aquariorum. J. Bacteriol. 194 4114–4115. 10.1128/JB.00621-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Tavernarakis N., Driscoll M. (2001). Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron 31 957–971. 10.1016/S0896-6273(01)00432-9 [DOI] [PubMed] [Google Scholar]
- Yoshioka K., Ishii K., Kuramoto T., Nagai S., Funao H., Ishihama H., et al. (2014). A novel mouse model of soft-tissue infection using bioluminescence imaging allows noninvasive, real-time monitoring of bacterial growth. PLoS ONE 9:e106367 10.1371/journal.pone.0106367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li W., Li L., Li Y., Fu R., Zhu Y., et al. (2015). Structural damage in the C. elegans epidermis causes release of STA-2 and induction of an innate immune response. Immunity 42 309–320. 10.1016/j.immuni.2015.01.014 [DOI] [PubMed] [Google Scholar]
- Ziegler K., Kurz C. L., Cypowyj S., Couillault C., Pophillat M., Pujol N., et al. (2009). Antifungal innate immunity in C. elegans: PKC delta links G protein signaling and a conserved p38 MAPK cascade. Cell Host Microbe 5 341–352. 10.1016/j.chom.2009.03.006 [DOI] [PubMed] [Google Scholar]
- Zugasti O., Bose N., Squiban B., Belougne J., Kurz C. L., Schroeder F. C., et al. (2014). Activation of a G protein-coupled receptor by its endogenous ligand triggers the innate immune response of Caenorhabditis elegans. Nat. Immunol. 15 833–838. 10.1038/ni.2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 | Co-culture of Escherichia coli carrying different RNAi clones of asp-3, asp-4, and crt-1 with Aeromonas dhakensis AAK1 for 24 h did not affect the growth of A. dhakensis AAK1 by measuring colony forming units (CFUs) on kanamycin selective medium. L4440, an empty vector, was used as a negative control of RNAi.
FIGURE S2 | A hemolysin-deficient isogenic mutant of AAK1, AAK1 ΔhlyA::KanR, prolonged the survival curve of Caenorhabditis elegans (P < 0.0001).
FIGURE S3 | A hemolysin-deficient mutant of A. dhakensis, AAK1 ΔhlyA::KanRdid not cause muscle damage as parent strain. Rupture of muscle fibers (arrowheads) was obvious in worms with A. dhakensis infection at 72 h (middle). In contrast, E. coli OP50 (left) and ΔhlyA::KanR AAK1 (right) did not cause muscle damage.
FIGURE S4 | Relative expression levels of necrosis-associated genes in C. elegans infected by a hemolysin-deficient isogenic mutant of A. dhakensis AAK1. In the mutant, expression levels of crt-1, asp-3, and asp-4 at 72 h are significantly lower to those in wild-type AAK1 by quantitative real-time PCR (∗∗∗P < 0.0001).
FIGURE S5 | Enterohemorrhagic E. coli O157:H7 did not induce rapid lysis of C. elegans as A. dhakensis did.
FIGURE S6 | Expression levels of necrosis genes, asp-3, asp-4, and crt-1, in Caenorhabditis elegans infected by enterohemorrhagic E. coli O157:H7 were significantly lower than those in C. elegans infected by A. dhakensis. ∗∗∗P < 0.0001.