Abstract
Human corticosteroid binding globulin (hCBG) is a 50- to 55-kDa serum glycoprotein that binds cortisol and progesterone with high affinity. To map the steroid-binding domain and to investigate the folding pathways of hCBG, we have established an expression system based on infection of insect cells with a recombinant baculovirus encoding hCBG. Infected Spodoptera frugiperda (Sf9) cells secrete immunoreactive hCBG at high levels (16-24 pmol per 10(6) cells per 40 h), and the recombinant protein binds cortisol with an affinity and specificity equivalent to that of human serum-derived hCBG. Thus, this system has the potential to provide large amounts of wild-type and mutant hCBGs for physical-chemical analysis. Cotranslational asparagine-linked glycosylation is essential for acquisition of steroid-binding capability, as shown by the lack of cortisol-binding activity of unglycosylated hCBG secreted in the presence of tunicamycin. Golgi-associated oligosaccharide processing, however, is not required for activity, as demonstrated by the endoglycosidase H susceptibility of the fully active, secreted glycoprotein. Comparison of the steroid-binding properties of intracellular and secreted hCBG with that synthesized in vitro in the rabbit reticulocyte lysate system suggests that this protein undergoes a maturation process during transport through the secretory pathway. This system will be useful for identifying the molecular determinants of biological function in hCBG.
Full text
PDF
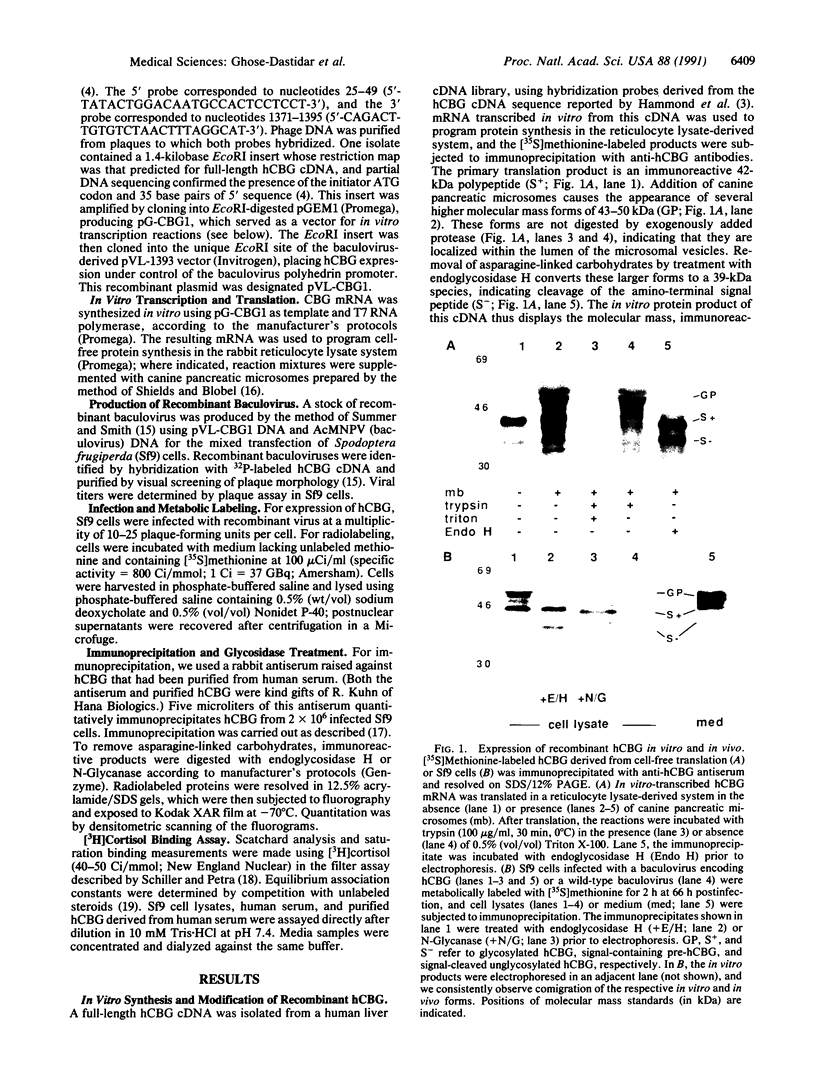
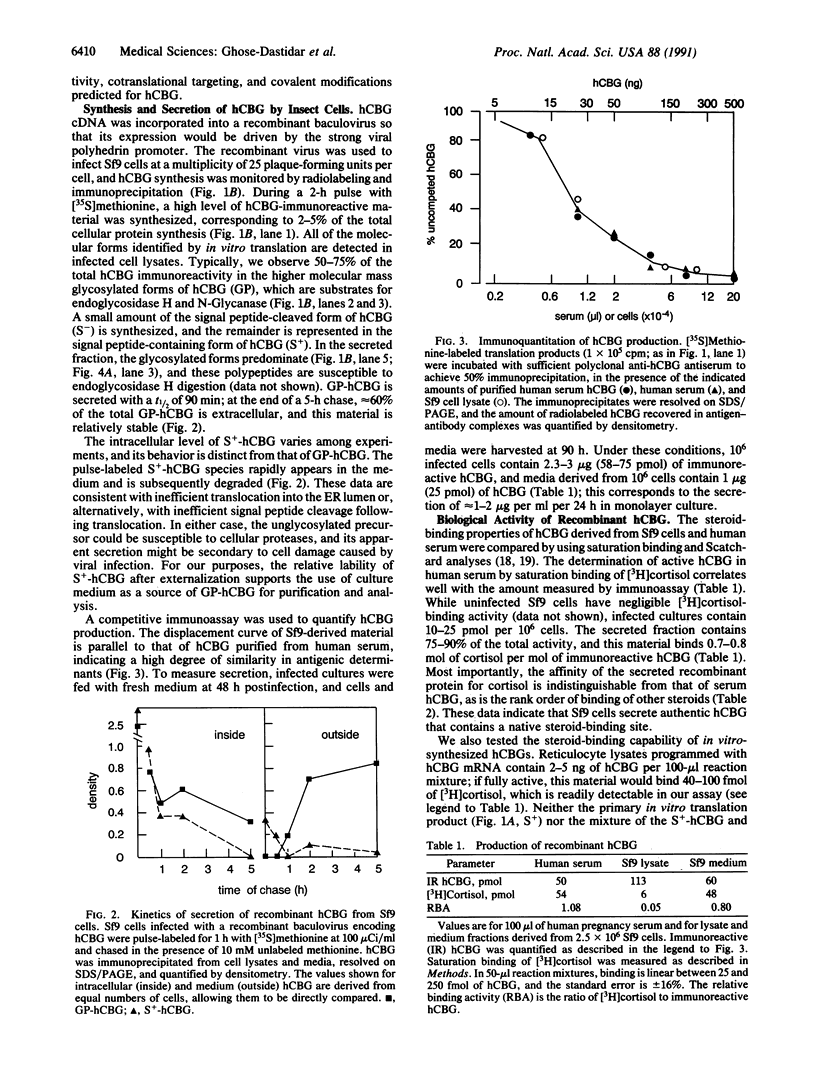
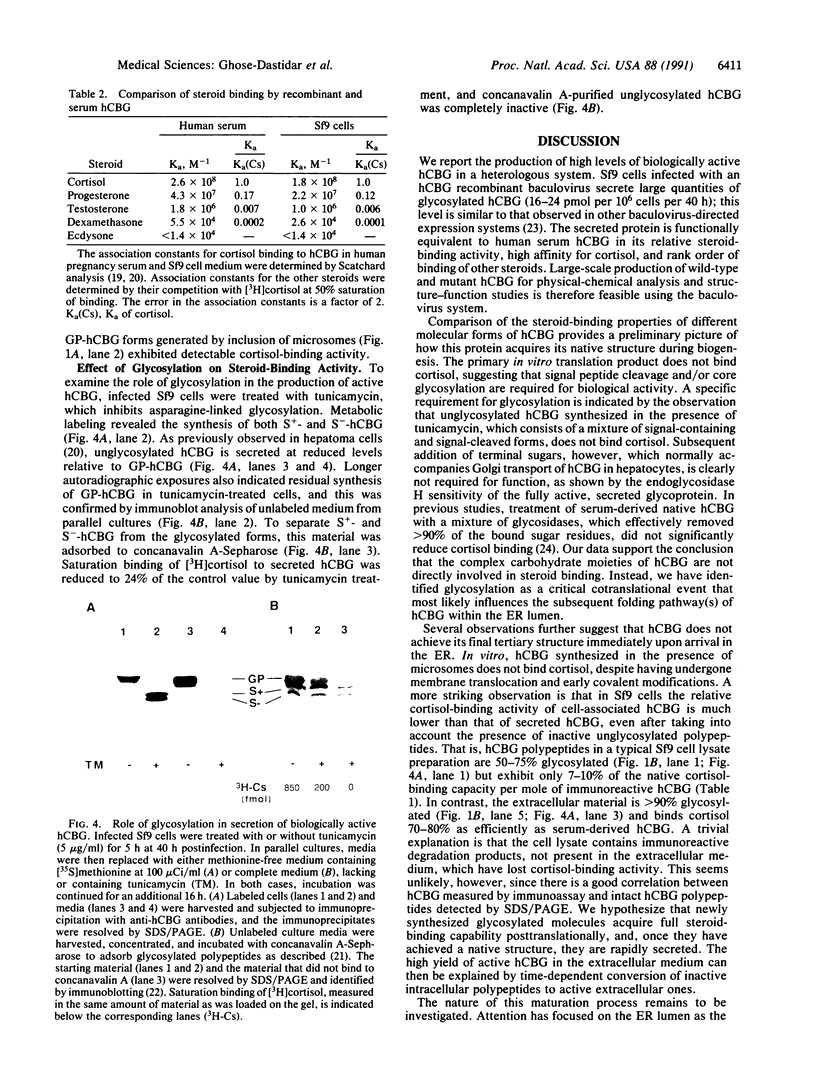

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caplan S., Green R., Rocco J., Kurjan J. Glycosylation and structure of the yeast MF alpha 1 alpha-factor precursor is important for efficient transport through the secretory pathway. J Bacteriol. 1991 Jan;173(2):627–635. doi: 10.1128/jb.173.2.627-635.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalman F. C., Bresnick E. H., Patel P. D., Perdew G. H., Watson S. J., Jr, Pratt W. B. Direct evidence that the glucocorticoid receptor binds to hsp90 at or near the termination of receptor translation in vitro. J Biol Chem. 1989 Nov 25;264(33):19815–19821. [PubMed] [Google Scholar]
- Defaye G., Basset M., Monnier N., Chambaz E. M. Electron spin resonance study of human transcortin: Thiol groups and binding site topography. Biochim Biophys Acta. 1980 Jun 26;623(2):280–294. doi: 10.1016/0005-2795(80)90256-1. [DOI] [PubMed] [Google Scholar]
- Hammond G. L., Smith C. L., Goping I. S., Underhill D. A., Harley M. J., Reventos J., Musto N. A., Gunsalus G. L., Bardin C. W. Primary structure of human corticosteroid binding globulin, deduced from hepatic and pulmonary cDNAs, exhibits homology with serine protease inhibitors. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5153–5157. doi: 10.1073/pnas.84.15.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. L., Smith C. L., Paterson N. A., Sibbald W. J. A role for corticosteroid-binding globulin in delivery of cortisol to activated neutrophils. J Clin Endocrinol Metab. 1990 Jul;71(1):34–39. doi: 10.1210/jcem-71-1-34. [DOI] [PubMed] [Google Scholar]
- Hryb D. J., Khan M. S., Romas N. A., Rosner W. Specific binding of human corticosteroid-binding globulin to cell membranes. Proc Natl Acad Sci U S A. 1986 May;83(10):3253–3256. doi: 10.1073/pnas.83.10.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Jarvis D. L., Oker-Blom C., Summers M. D. Role of glycosylation in the transport of recombinant glycoproteins through the secretory pathway of lepidopteran insect cells. J Cell Biochem. 1990 Apr;42(4):181–191. doi: 10.1002/jcb.240420402. [DOI] [PubMed] [Google Scholar]
- Kuhn R. W. Corticosteroid-binding globulin interactions with target cells and plasma membranes. Ann N Y Acad Sci. 1988;538:146–158. doi: 10.1111/j.1749-6632.1988.tb48860.x. [DOI] [PubMed] [Google Scholar]
- Lea O. A. Interaction of steroid-binding serum proteins with concanavalin A-Sepharose 4B. Horm Res. 1979;11(5):240–253. doi: 10.1159/000179060. [DOI] [PubMed] [Google Scholar]
- Mickelson K. E., Harding G. B., Forsthoefel M., Westphal U. Steroid-protein interactions. Human corticosteroid-binding globulin: characterization of dimer and electrophoretic variants. Biochemistry. 1982 Feb 16;21(4):654–660. doi: 10.1021/bi00533a010. [DOI] [PubMed] [Google Scholar]
- Murata Y., Sueda K., Seo H., Matsui N. Studies on the role of glycosylation for human corticosteroid-binding globulin: comparison with that for thyroxine-binding globulin. Endocrinology. 1989 Sep;125(3):1424–1429. doi: 10.1210/endo-125-3-1424. [DOI] [PubMed] [Google Scholar]
- Nakhla A. M., Khan M. S., Rosner W. Induction of adenylate cyclase in a mammary carcinoma cell line by human corticosteroid-binding globulin. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1012–1018. doi: 10.1016/s0006-291x(88)81329-9. [DOI] [PubMed] [Google Scholar]
- Parker E. M., Kameyama K., Higashijima T., Ross E. M. Reconstitutively active G protein-coupled receptors purified from baculovirus-infected insect cells. J Biol Chem. 1991 Jan 5;266(1):519–527. [PubMed] [Google Scholar]
- Pemberton P. A., Stein P. E., Pepys M. B., Potter J. M., Carrell R. W. Hormone binding globulins undergo serpin conformational change in inflammation. Nature. 1988 Nov 17;336(6196):257–258. doi: 10.1038/336257a0. [DOI] [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Ronne H., Ocklind C., Wiman K., Rask L., Obrink B., Peterson P. A. Ligand-dependent regulation of intracellular protein transport: effect of vitamin a on the secretion of the retinol-binding protein. J Cell Biol. 1983 Mar;96(3):907–910. doi: 10.1083/jcb.96.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner W. The functions of corticosteroid-binding globulin and sex hormone-binding globulin: recent advances. Endocr Rev. 1990 Feb;11(1):80–91. doi: 10.1210/edrv-11-1-80. [DOI] [PubMed] [Google Scholar]
- Ross J. B., Torres P., Petra P. H. Equilenin: a specific fluorescent probe for steroid-protein interactions in sex steroid-binding protein. FEBS Lett. 1982 Nov 29;149(2):240–244. doi: 10.1016/0014-5793(82)81108-3. [DOI] [PubMed] [Google Scholar]
- Schiller H. S., Pétra P. H. A filter assay for the corticosteroid binding globulin of human serum. J Steroid Biochem. 1976 Jan;7(1):55–59. doi: 10.1016/0022-4731(76)90165-5. [DOI] [PubMed] [Google Scholar]
- Seralini G. E., Smith C. L., Hammond G. L. Rabbit corticosteroid-binding globulin: primary structure and biosynthesis during pregnancy. Mol Endocrinol. 1990 Aug;4(8):1166–1172. doi: 10.1210/mend-4-8-1166. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Efficient cleavage and segregation of nascent presecretory proteins in a reticulocyte lysate supplemented with microsomal membranes. J Biol Chem. 1978 Jun 10;253(11):3753–3756. [PubMed] [Google Scholar]
- Smith C. L., Hammond G. L. Rat corticosteroid binding globulin: primary structure and messenger ribonucleic acid levels in the liver under different physiological conditions. Mol Endocrinol. 1989 Feb;3(2):420–426. doi: 10.1210/mend-3-2-420. [DOI] [PubMed] [Google Scholar]
- Stroupe S. D., Harding G. B., Forsthoefel M. W., Westphal U. Kinetic and equilibrium studies on steroid interaction with human corticosteroid-binding globulin. Biochemistry. 1978 Jan 10;17(1):177–182. doi: 10.1021/bi00594a026. [DOI] [PubMed] [Google Scholar]