Abstract
Background and Objectives
The standard of care for HIV treatment is a three-drug regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) and either a non-nucleoside reverse transcriptase inhibitor, a protease inhibitor (PI) or an integrase strand transfer inhibitor. Darunavir boosted with ritonavir (DRV/r) is the only preferred PI in the US Department of Health and Human Services (DHHS) HIV treatment guidelines for antiretroviral-naïve patients, recommended in combination with tenofovir/emtricitabine for antiretroviral-naïve patients. For treatment-experienced and certain antiretroviral-naïve patients, abacavir and lamivudine (ABC/3TC) in combination with DRV/r is considered an effective and tolerable alternative, despite limited research on the effectiveness of this particular combination. This study evaluated virologic outcomes in treatment-experienced patients taking ABC/3TC + DRV/r compared to treatment-experienced patients taking ABC/3TC with any other PI.
Methods
Treatment-experienced HIV-infected patients initiating their first regimen containing ABC/3TC in combination with any PI in the year 2005 or later were selected from the Observational Pharmaco-Epidemiology Research and Analysis (OPERA®) cohort, a prospective observational cohort reflecting routine medical care. Viral load measurements taken during follow-up were compared between patients taking ABC/3TC + DRV/r and ABC/3TC with a PI other than DRV/r. Logistic regression models were fit to assess the association between regimen exposure and viral load suppression.
Results
A total of 151 patients initiating ABC/3TC + DRV/r and 525 patients initiating ABC/3TC + a non-darunavir PI were included. Patients in both treatment groups had comparable clinical indicators (viral load, CD4) at baseline. A regimen of ABC/3TC + DRV/r was more likely to be prescribed in the later years of the study period, leading to a shorter median follow-up in the DRV/r treatment group (as-treated analysis: 14 vs. 17 months, p = 0.04; intent-to-treat analysis: 33 vs. 68 months, p < 0.001). Multivariable logistic regression models accounting for year of regimen initiation, among other factors, indicated no statistically significant differences in achieving an undetectable viral load for patients taking DRV/r with ABC/3TC compared with other PIs, both in the as-treated (odds ratio [95 % confidence interval]: 0.84 [0.53–1.34]) and intent-to-treat analyses (0.82 [0.48–1.40]). Patients in both treatment groups also showed similar reductions in viral load (median darunavir vs. non-darunavir: −23.0 vs. −23.0 copies/mL; p = 0.72) and gains in CD4 T cell counts (median darunavir vs. non-darunavir: 106 vs. 108 cells/mm3; p = 0.59] while being treated with the regimen of interest.
Conclusions
Patients receiving ABC/3TC + DRV/r appear to experience similar treatment benefit to patients taking ABC/3TC with other PIs in terms of achieving suppression, as well as absolute reductions in viral load and CD4 lymphocyte gains.
Key Points
| Darunavir boosted with ritonavir (DRV/r) paired with abacavir and lamivudine (ABC/3TC) is considered to be an effective and tolerable regimen for antiretroviral treatment-experienced HIV populations, despite little research supporting its use. |
| When assessed against ABC/3TC paired with other protease inhibitors, ABC/3TC + DRV/r is a comparably effective regimen for achieving virologic suppression in a real-world clinical setting. |
Introduction
The standard of care for HIV treatment is a three-drug regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) and either a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI), or an integrase strand transfer inhibitor (INSTI) [1]. Darunavir boosted with ritonavir (DRV/r) is the only preferred PI in the US Department of Health and Human Services (DHHS) treatment guidelines for antiretroviral-naïve patients, recommended in combination with tenofovir/emtricitabine (TDF/FTC). DRV/r has a high genetic barrier to the development of resistance mutations as compared to all NNRTIs and some INSTIs [2]. Therefore, DRV/r-containing regimens may be preferred in patients who need to begin therapy prior to resistance testing, have documented resistance to other classes, or have issues with adherence [3].
The US Food and Drug Administration (FDA) first approved DRV/r for use in antiretroviral-experienced patients in 2006 [4]. Darunavir is considered to be a second-generation PI, with a markedly better resistance profile than older comparator PIs [5–7]. Darunavir also offers superior potency and better short- and long-term tolerability, making its use more clinically practical than many first-generation PIs. Clinical trial data have indicated that treatment-experienced patients receiving DRV/r are more likely to reach and maintain treatment response when compared with antiretroviral-experienced patients receiving other ritonavir-boosted PIs [8–10].
The most common NRTI backbone prescribed with DRV/r is TDF/FTC. However, abacavir and lamivudine (ABC/3TC) is considered an acceptable alternative for certain antiretroviral-naïve patients and for treatment-experienced patients. While ABC/3TC + DRV/r is classified as an effective and tolerable regimen according to the current US antiretroviral therapy (ART) treatment guidelines [1], few studies have evaluated the effectiveness of DRV specifically in combination with ABC/3TC. In a retrospective study, Nishijima et al. [11] evaluated the effectiveness of ABC/3TC + DRV/r in 22 antiretroviral-naïve patients. Of the 18 patients who remained on the regimen through 48 weeks, 66.7 % had a viral load of <50 copies/mL and two patients experienced virologic failure (two consecutive viral loads >200 copies/mL). It is possible that the relatively low response rate observed was due to the small sample; an analysis of larger numbers of patients would add clarity to the issue. In a single-center, observational pilot study, the effectiveness of ABC/3TC + DRV/r was compared with TDF/FTC + DRV/r among 80 patients with a baseline viral load of >100,000 copies/mL [12]. No significant difference in viral suppression or time to virologic failure at 48 weeks was observed between the two arms. The SWIFT (Safety and Efficacy Study of Switching from Epzicom to Truvada) study was designed to evaluate switching to TDF/FTC from ABC/3TC in patients who were on a stable boosted PI regimen [13]. Of the 311 subjects enrolled, only 20 were on ABC/3TC + DRV/r and treatment outcomes were not reported by individual PIs. There is a need for additional data on the outcomes associated with this particular regimen.
This study sought to compare the virologic and immunologic effectiveness of ABC/3TC + DRV/r with ABC/3TC in combination with other PIs.
Methods
Study Population
The study population was selected from the Observational Pharmaco-Epidemiology Research and Analysis (OPERA®) cohort, an observational cohort including patients from 72 HIV specialty outpatient clinics in the USA. In addition to demographic and medical history information, prospectively captured details of diagnoses, medications, and laboratory results were captured through electronic medical records for all patients receiving healthcare at each of these sites. All data reflect routine medical care, with visits and testing scheduled at the discretion of the treating physicians. Information captured in the electronic medical records system at each site was retrieved, aggregated, and de-identified to maintain patient confidentiality.
Subjects for this analysis included patients in the OPERA cohort with a documented diagnosis of HIV-1 infection. The study population was restricted to antiretroviral-experienced patients starting their first antiretroviral regimen containing both abacavir and lamivudine. Patients were further restricted to those initiating abacavir and lamivudine in combination with any PI after their enrollment date in the OPERA cohort. Regimens including additional antiretroviral drugs were excluded. Patients initiating any regimen of interest prior to the year 2005 were also excluded. Patients were required to have both CD4 and viral load assessments taken during a baseline period, defined as 120 days prior to and 7 days after their ABC/3TC + PI regimen start date, as well as at least one viral load assessment taken prior to the end of follow-up on this regimen. Patients were eligible for inclusion regardless of virologic status at the time of initiating their ABC/3TC + PI regimens; both suppressed and non-suppressed patients were evaluated together in the primary analysis.
Study Design
Eligible patients were categorized as receiving either ABC/3TC and DRV/r or ABC/3TC and any other PI as a third agent. For both treatment groups, baseline was defined as the start date for the regimen. Follow-up continued until a patient’s last visit prior to the date of data extraction and aggregation for analysis (6 May 2015).
The primary objective of this analysis was to compare virologic effectiveness between the two treatment groups, defined as achieving viral suppression below detectable limits at any point during the follow-up period. The threshold for classifying a viral load as undetectable was assay dependent; viral load measurements were evaluated at different laboratories over a period of several years, leading to variable lower limits of detection ranging from <20 to <75 copies/mL. Additional outcomes associated with viral load were also assessed including lowest viral load measured during follow-up and change in viral load from baseline to the lowest copies/mL measured. The effect of treatment on CD4 cell counts was evaluated between treatment groups by comparing the highest CD4 count prior to end of follow-up, as well as change in CD4 counts between baseline and the highest count.
Each outcome of interest was assessed using an ‘intent-to-treat’ analysis, where subjects remained categorized in their initial exposure group regardless of changes to the baseline antiretroviral regimen and were followed until data extraction, as well as an ‘as-treated’ analysis, where patients were followed and contributed data to their initial exposure groups only until they changed antiretroviral regimens or stopped receiving treatment with antiretrovirals altogether.
Statistical Analysis
Baseline characteristics and certain outcome measures were compared between the two groups using Wilcoxon rank-sum tests for continuous variables and Chi-square tests for categorical variables. Results were summarized as medians with interquartile ranges (IQRs) for continuous variables and as frequencies and proportions for categorical variables.
Crude and multivariable logistic regression models were fit to assess the association between regimen exposure and viral load suppression. Separate models were run for the ‘as-treated’ follow-up data (baseline to end of regimen of interest) and the ‘intent-to-treat’ follow-up data (baseline to the end of follow-up). Multivariable models were constructed using stepwise selection methods of explanatory values. Odds ratios (ORs) and corresponding 95 % confidence intervals (CIs) were reported from the final unadjusted and adjusted logistic models.
Sensitivity Analysis
In order to limit bias created by unequal amounts of follow-up time between treatment groups, two separate sensitivity analyses were performed. First, the study population was limited to patients with at least 12 months of follow-up after baseline and with a viral load assessment taken at least 6 months after baseline. Both of these criteria were independent of the duration of the regimen of interest. Darunavir was approved more recently than other PIs taken by this cohort, and regimens containing darunavir were prescribed less frequently or not at all during the early years of this study’s initial period. In order to account for the potential for longer follow-up periods in patients taking PIs other than DRV, our second sensitivity analysis included only eligible patients initiating treatment with ABC/3TC + PI in the year 2009 or later.
Treatment effectiveness was also assessed using a more lenient threshold for the outcome of virologic control. Rather than requiring viral load to be completely undetectable, patients only had to achieve a viral load below 400 copies/mL. This outcome is referred to as suppressed rather than undetectable.
For the primary analysis, treatment-experienced patients were enrolled regardless of whether or not they switched to an ABC/3TC + PI regimen while virologically stable on a prior ART regimen (suppressed or undetectable) or due to treatment failure. In order to assess whether virologic status at baseline had an impact on regimen effectiveness, results were stratified by viral load at baseline based on a standard threshold for defining virologic failure (≤200 or >200 copies/mL).
Results
Selection of Eligible Patients
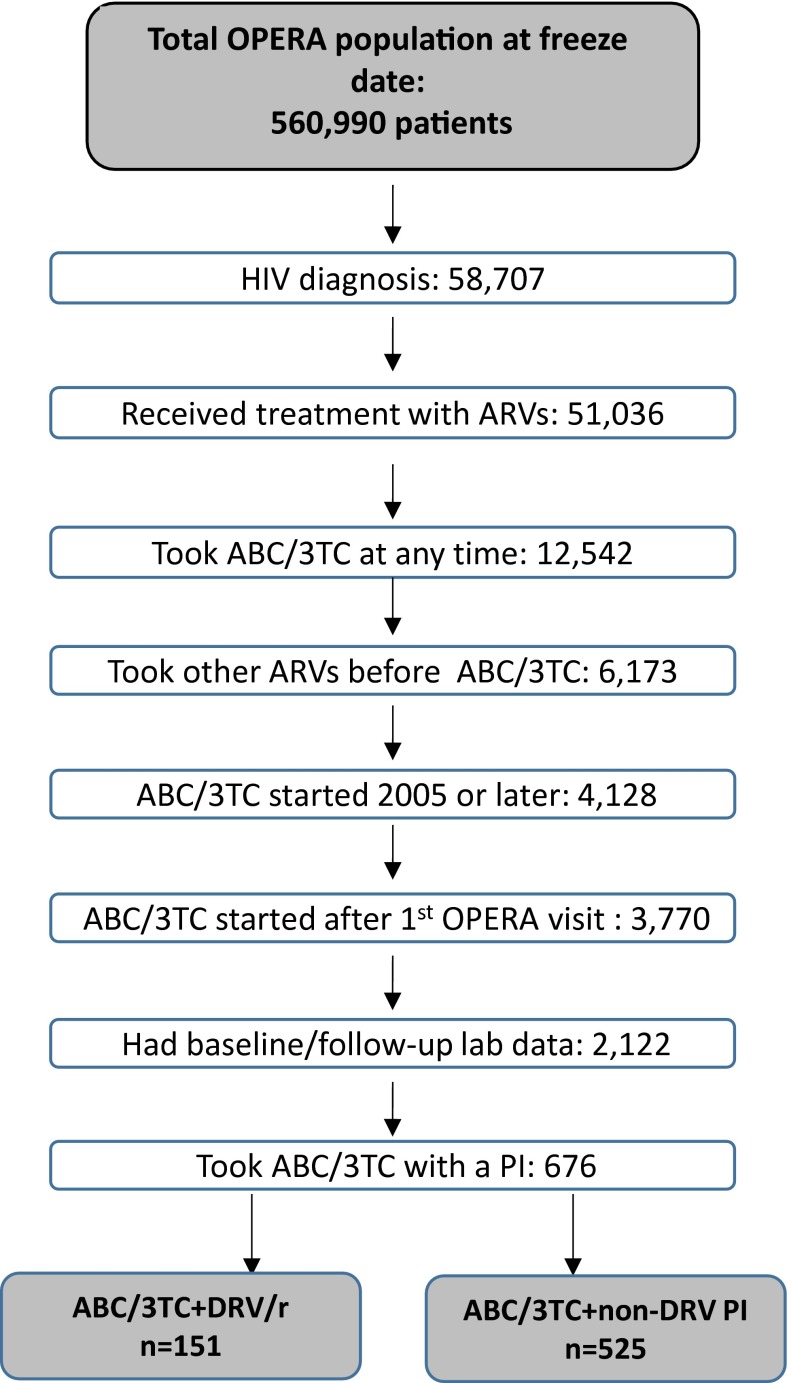
The OPERA cohort included 560,990 patients who had data from at least one clinic visit on record (Fig. 1). Of these patients, 58,707 had been diagnosed with HIV, with 87 % (n = 51,036) having any record of treatment with antiretrovirals. Of those with exposure to abacavir and lamivudine (n = 12,542), nearly half (49 %) had taken this combination of drugs as a second-line or later regimen and were considered antiretroviral-experienced prior to initiating the regimen of interest at baseline. Of these patients, 4128 (67 %) were prescribed their first ABC/3TC regimen in or after the year 2005, with 3770 (91 %) of these regimens occurring after entering care with an OPERA physician. Of the patients with both baseline and follow-up laboratory tests (n = 2122), 676 took ABC/3TC with a PI as the third regimen agent, including 151 who took a regimen contain ABC/3TC with DRV/r and 525 who took a regimen containing ABC/3TC plus a PI other than darunavir, with or without ritonavir. All patients taking darunavir (n = 151) were taking the drug in combination with ritonavir as a boosting agent (DRV/r). The majority (74 %, n = 389) of patients taking ABC/3TC with a non-darunavir PI received atazanavir (ritonavir-boosted in 67 %, n = 261). Patients also took ABC/3TC with lopinavir/r (14 %, n = 75) and fosamprenavir (8 %, n = 41). Less common (<2 %) regimens included ABC/3TC with nelfinavir, saquinavir, or indinavir.
Fig. 1.
Selection of eligible patients for primary analysis. ABC/3TC abacavir/lamivudine, ARVs antiretrovirals, DRV/r darunavir boosted with ritonavir, OPERA Observational Pharmaco-Epidemiology Research and Analysis, PI protease Inhibitor
Baseline Characteristics
Patients taking ABC/3TC + DRV/r were similar to those taking ABC/3TC with other PIs in most baseline demographic and clinical features (Table 1). In this cohort, patients did not initiate regimens containing DRV/r until 2007, with frequency of this drug combination increasing over the study period. Conversely, patients were less frequently prescribed ABC/3TC plus other PIs in the later years of the study period.
Table 1.
Baseline characteristics of antiretroviral therapy-experienced patients initiating their first regimen of either ABC/3TC + DRV/r or ABC/3TC + PI (not DRV)a
| Characteristic | ABC/3TC + DRV/rb (n = 151) [n (%)d] | ABC/3TC without DRV/rc (n = 525) [n (%)d] | p value |
|---|---|---|---|
| Male sex | 124 (82.1) | 415 (79.0) | 0.41 |
| Age, years [median (IQR)] | 46.8 (39.6–53.4) | 45.6 (39.1–52.8) | 0.61 |
| African American race | |||
| African American | 65 (43.0) | 212 (40.4) | 0.56 |
| Non-African American | 86 (57.0) | 313 (59.6) | |
| Ethnicity | |||
| Hispanic | 20 (13.2) | 67 (12.8) | 0.88 |
| Non-hispanic | 131 (86.8) | 458 (87.2) | |
| AIDS-defining event at or before baseline | 16 (11.3) | 64 (12.2) | 0.59 |
| CD4 count at baseline | |||
| <250 cells/mm3 | 51 (33.8) | 156 (29.7) | 0.34 |
| ≥250 cells/mm3 | 100 (66.2) | 369 (70.3) | |
| CD4 count, cells/mm3 [median (IQR)] | 333 (193–565) | 397 (213–621) | 0.18 |
| HIV viral load at baseline | |||
| <200 copies/mL | 89 (58.9) | 348 (66.3) | 0.10 |
| ≥200 copies/mL | 62 (41.1) | 177 (33.7) | |
| Log10 copies/mL [median (IQR)] | 1.9 (1.5–3.2) | 1.7 (1.7–3.1) | 0.93 |
| Hepatitis B or C co-infection at baseline | |||
| Yes | 4 (2.6) | 3 (0.6) | 0.03 |
| No | 147 (97.4) | 522 (99.4) | |
| Year baseline regimen started | |||
| 2005 | 0 (0.0) | 50 (9.5) | <0.0001 |
| 2006 | 0 (0.0) | 67 (12.8) | |
| 2007 | 5 (3.3) | 55 (10.5) | |
| 2008 | 11 (7.3) | 62 (11.8) | |
| 2009 | 22 (14.6) | 72 (13.7) | |
| 2010 | 18 (11.9) | 63 (12.0) | |
| 2011 | 14 (9.3) | 55 (10.5) | |
| 2012 | 18 (11.9) | 41 (7.8) | |
| 2013 | 30 (19.9) | 33 (6.3) | |
| 2014 | 30 (19.9) | 23 (4.4) | |
| 2015 | 3 (2.0) | 4 (0.8) | |
3TC lamivudine, ABC abacavir, DRV darunavir, IQR interquartile range, PI protease inhibitor, r ritonavir
aIncludes only patients that had baseline CD4, baseline viral load, and at least one viral load measurement during follow-up on the regimen of interest
bRegimen of ABC, 3TC, and DRV boosted with r
cRegimen of ABC, 3TC, and a PI other than DRV
dUnless otherwise indicated
Patients receiving DRV/r were more likely to have an active hepatitis B or C infection at the time they initiated the regimen than patients initiating regimens with other PIs (2.6 vs. 0.6 %; p = 0.03). Patients starting ABC/3TC + DRV/r have comparable CD4 counts (median [IQR] cells/mm3: 333 [193–565] vs. 397 [213–621]; p = 0.18) and viral loads (median [IQR] log10 copies/mL: 1.9 [1.5–3.2] vs. 1.7 [1.7–3.1]; p = 0.93). About half of the patients taking both DRV and non-DRV-based regimens (46 and 52 %; p = 0.2) were suppressed below 50 copies/mL at the time of regimen initiation.
Treatment and Treatment Response
As a result of DRV prescribed in the later years of the study period, patients taking ABC/3TC + DRV/r tended to have less follow-up while on DRV/r than patients taking ABC/3TC plus a non-DRV PI (median: 14 vs. 17 months; p = 0.04), as well as less follow-up time overall (median: 33 vs. 68 months; p < 0.001).
Patients receiving DRV-containing regimens were less likely to achieve an undetectable viral load both while taking the regimen (64 vs. 72 %; p = 0.04) and during their total duration of follow-up (74 vs. 86 %; p < 0.001) (Table 2). The lowest viral load achieved between baseline and the end of the regimen was similar between the two treatment groups, as was the lowest viral load achieved prior to the end of follow-up. Gains between baseline and highest CD4 measured while on the regimen of interest were similar between PI groups (median [IQR] cells/mm3: 106 [20–245] vs. 118 [19–271]; p = 0.59), but over all of follow-up, patients taking ABC/3TC with a non-darunavir PI experienced higher CD4 counts (median [IQR] cells/mm3: 696 [435–930] vs. 570 [328–842]; p = 0.02) and greater CD4 lymphocyte gains from baseline (median [IQR] cells/mm3: 217 [87–389] vs. 166 [70–312]; p = 0.04).
Table 2.
Virologic and immunologic response following treatment regimens of interest (ABC/3TC + DRV/r or ABC + 3TC + PI [not DRV/r])
| Treatment outcomes | ABC/3TC + DRV/ra (n = 151) [n (%) or median (IQR)] | ABC/3TC without DRV/rb (n = 525) [n (%) or median (IQR)] | p value |
|---|---|---|---|
| On therapy: measured between baseline and discontinuation of regimen | |||
| Viral load | |||
| Achieved undetectable viral loadc | 96 (63.6) | 380 (72.4) | 0.04 |
| Lowest viral load measured (copies/mL) | 19 (19–110) | 47 (19–50) | 0.09 |
| Change from baseline viral load to lowest viral load measured (copies/mL) | −23 (−601 to 0) | −23 (−381 to 0) | 0.72 |
| CD4 | |||
| Highest CD4 count measured (cells/mm3) | 482 (262–798) | 585 (324–823) | 0.15 |
| Change from baseline CD4 count to highest CD4 count measured (cells/mm3) | 106 (20–245) | 118 (19–271) | 0.59 |
| Intent-to-treat: measured between baseline and last date of follow-up | |||
| Viral load | |||
| Achieved undetectable viral loadc | 112 (74.2) | 452 (86.1) | 0.0005 |
| Lowest viral load measured (copies/mL) | 19 (19–40) | 19 (19–47) | 0.74 |
| Change from baseline viral load to lowest viral load measured (copies/mL) | −28 (−901 to 0) | −30 (−813 to −1) | 0.58 |
| CD4 | |||
| Highest CD4 count measured (cells/mm3) | 570 (328–842) | 696 (435–930) | 0.018 |
| Change from baseline CD4 count to highest CD4 count measured (cells/mm3) | 166 (70–312) | 217 (87–389) | 0.04 |
| On therapy | |||
| Number of CD4 count measurements | 4 (3–8) | 5 (3–9) | 0.09 |
| Number of viral load measurements | 4 (3–7) | 5 (3–9) | 0.18 |
| Months of follow-up | 13.7 (7.6–22.9) | 17.3 (6.9–34.9) | 0.04 |
| Intent-to-treat | |||
| Number of CD4 count measurements | 7 (3–14) | 12 (6–20) | <0.0001 |
| Number of viral load measurements | 7 (3–13) | 11 (6–18) | <0.0001 |
| Months of follow-up | 33.1 (17.1–63.5) | 68.1 (43.9–94.7) | <0.0001 |
3TC lamivudine, ABC abacavir, DRV darunavir, IQR interquartile range, PI protease inhibitor, r ritonavir
aRegimen of ABC, 3TC, and DRV boosted with r
bRegimen of ABC, 3TC, and a PI other than DRV
cSuppression of viral load to undetectable limit is the primary outcome of interest for this study. Undetectable measured as below assay limit (range <20 to <75 copies/mL)
In unadjusted models, patients taking ABC/3TC + DRV/r appeared to be significantly less likely to achieve an undetectable viral load, both while taking the regimen (OR [95 % CI]: 0.67 [0.45–0.98]) and for the duration of follow-up (0.46 [0.30, 0.72]) (Table 3). After adjusting for baseline viral load, CD4 count, and year of regimen initiation, there were no statistically significant differences in achieving an undetectable viral load by PI given with ABC/3TC, either while taking the regimen (darunavir compared with non-darunavir during regimen: OR [95 % CI]: 0.84 [0.53–1.34]), or during the duration of follow-up (OR [95 % CI]: 0.82 [0.48–1.40]).
Table 3.
Univariate and multivariate logistic regression analyses for primary analysis and all sensitivity analyses
| Unadjusted modela [OR (95 % CI)] | Adjusted modela [OR (95 % CI)] | |
|---|---|---|
| Primary analysis | ||
| Achieved undetectable viral loadb during baseline regimen | 0.67 (0.45–0.98) | 0.84 (0.53–1.34) |
| Achieved undetectable viral load between starting baseline regimen and last date of follow-up | 0.46 (0.30–0.72) | 0.82 (0.48–1.40) |
| Sensitivity analyses | ||
| Balancing follow-up time between treatment groups | ||
| Patients with at least 12 months of follow-up | ||
| Achieved undetectable viral load during baseline regimen | 0.77 (0.48–1.22) | 0.91 (0.54–1.51) |
| Achieved undetectable viral load between starting baseline regimen and last date of follow-up | 0.51 (0.28–0.91) | 0.55 (0.30–1.03) |
| Patients starting regimen 2009 or later | ||
| Achieved undetectable viral load during baseline regimen | 0.66 (0.42–1.03) | 0.91 (0.53–1.55) |
| Achieved undetectable viral load between starting baseline regimen and last date of follow-up | 0.53 (0.32–0.87) | 0.99 (0.53–1.87) |
| Alternative definition of effectiveness | ||
| Achieved suppressed viral load (<400 copies/mL) | ||
| Achieved suppressed viral load during baseline regimen | 0.83 (0.50–1.38) | 0.95 (0.54–1.66) |
| Achieved suppressed viral load between starting baseline regimen and last date of follow-up | 0.51 (0.26–0.97) | 0.98 (0.45–2.12) |
| Accounting for baseline viral load | ||
| Baseline viral load >200 copies/mL | ||
| Achieved undetectable viral load during baseline regimen | 0.67 (0.37–1.21) | 0.97 (0.49–1.93) |
| Achieved undetectable viral load between starting baseline regimen and last date of follow-up | 0.42 (0.23–0.75) | 0.88 (0.44–1.76) |
| Baseline viral load ≤200 copies/mL | ||
| Achieved undetectable viral load during baseline regimen | 0.78 (0.42–1.45) | 1.10 (0.54–2.23) |
| Achieved undetectable viral load between starting baseline regimen and last date of follow-up | 0.65 (0.28–1.52) | 1.34 (0.49–3.66) |
CI confidence interval, OR odds ratio
aAll models compare a regimen of abacavir/lamivudine and darunavir/ritonavir with a regimen of abacavir/lamivudine and a protease inhibitor besides darunavir/ritonavir (reference)
bSuppression of viral load to undetectable limit is the primary outcome of interest for this study. Undetectable measured as below assay limit (range <20 to <75 copies/mL)
Sensitivity Analyses
Analyses attempting to balance the follow-up time between treatment groups (by limiting the population to those with at least 12 months of follow-up after baseline and limiting the population to those initiating a regimen of interest in 2009 or later) produced similar crude effect estimates to the primary analysis (Table 3). The ORs from multivariable models were closer to null than the primary analysis, and indicated no differences in achieving undetectable viral loads between treatment groups.
Defining viral load suppression as fewer than 400 copies/mL rather than below detectable limits also resulted in adjusted effect estimates close to null, but with somewhat less precision than the results of the primary analysis. When stratified by viral load at baseline, patients with higher viral loads appear to be less likely to achieve viral suppression during follow-up on the regimen and overall than patients with a lower baseline viral loads.
Discussion
To our knowledge, this is the first study, interventional or observational, comparing the effectiveness of darunavir with other PIs when taken in combination specifically with an ABC/3TC NRTI backbone. The results of our multivariable regression models suggested no statistically significant differences in achieving viral load suppression between treatment-experienced patients receiving a regimen of ABC/3TC + DRV/r and those receiving ABC/3TC with a different PI (boosted or unboosted).
A meta-analysis conducted by Berhan and Berhan [5] looked at published evidence from randomized controlled studies of virologic response in treatment-experienced patients receiving DRV/r. Change in viral load was measured relative to regimens containing an investigator-selected boosted PI. Ten studies were identified comparing the efficacy of DRV/r with another PI. Patients taking DRV/r (OR [95 % CI]: 4.7 [2.7–7.9]) were significantly more likely to achieve viral load suppression (<50 copies/mL) than patients taking ritonavir-boosted comparator PIs. However, the backbone components of these PI-based regimens were not taken into account for this analysis. Reviewing these studies individually, none separately evaluated the different NRTI backbones, and several excluded use of abacavir altogether [8–10, 15–22].
There are few other studies of the combination of ABC/3TC + DRV/r, with most analyzing a small number of patients, limiting the ability to detect a statistical difference between treatment groups. Prior studies, however, have shown comparable efficacies with two other NRTI/PI combinations and none have indicated inferiority with regimens containing other PIs. Some of the existing data come from single-arm trials or from observational cohorts with no comparison group, making it difficult to evaluate the ABC/3TC + DRV/r regimen against the effectiveness of other drug combinations [12, 14, 15, 23].
In this cohort, observed inequalities in viral load suppression to undetectable levels, both in frequency data and in unadjusted logistic regression models, are partially attributable to differences in potential follow-up time between the two treatment groups. DRV/r trended towards wider use with ABC/3TC each year of the study period. With other PIs more frequently started in the earlier years of the study period, patients taking darunavir-containing regimens had shorter median follow-up durations, both on the regimen of interest and for the total duration of follow-up. Shorter follow-up not only results in less time for viral load to respond to treatment, but also fewer potential opportunities for testing. While the median number of viral load assessments was comparable while receiving the initial ABC/3TC + PI regimen (darunavir vs. non-darunavir median [IQR] viral load laboratory values: 4 [3–8] vs. 5 [3–9]; p = 0.09), the large disparities in total follow-up time resulted in far more viral load assessments for the intent-to-treat analysis in the non-darunavir PI treatment group (7 [3–14] vs. 12 [6–20]; p < 0.0001).
In a sensitivity analysis restricted to patients with a least 12 months of follow-up, the disparity in follow-up time while taking the regimen of interest was resolved, and the proportion of patients on the DRV/r regimen achieved an undetectable viral load was comparable with patients on other PI regimens (70 vs. 75 %; p = 0.25). Given the larger imbalance in months of follow-up for the intent-to-treat analysis, patients initiating on a non-darunavir PI regimen were still followed for a significantly longer period overall (45 vs. 69 months; p < 0.0001) and were still more likely to achieve viral load suppression than patients initiating follow-up on ABC/3TC + DRV/r. Adjusting for calendar year of regimen initiation, among other patient characteristics, in the multivariable logistic regression models for the primary analysis also partially accounted for the trend towards greater DRV/r use in the later study years, with adjusted models showing no statistical differences in achieving an undetectable viral load.
In addition to differences in potential time to accumulate more viral load laboratory values, the more frequent use of DRV/r in later years of the study period could have impacted testing in other ways, with trends towards less frequent viral load monitoring, particularly among patients who seem to be responding well to treatment. Furthermore, darunavir is a potent PI with a superior resistance profile to first-generation PIs. If clinicians perceived patients on DRV/r as less likely to experience treatment failure, this could be reflected in less frequent viral load evaluations. When given equal time and opportunity to show treatment success, DRV/r appears to be comparable with other PIs in their ability to suppress viral loads to undetectable levels when combined with ABC/3TC.
Patients with a wide variety of prior treatment experiences and clinical characteristics at baseline were eligible to enter the analysis cohort, and for the primary aim of this study were analyzed as a single group, compared only by the PI taken with ABC/3TC. The variability in clinical characteristics was mostly comparable between the two treatment groups. Patients receiving ABC/3TC with DRV/r or other PIs had similar prior experience on ART before starting their initial ABC/3TC regimen with a PI (DRV/r vs. non-darunavir PI, time on ART at baseline [IQR]: 1.4 years [0.4–3.8] vs. 1.5 years [0.6–3.9]; p = 0.4). Baseline viral load was highly variable among all patients, regardless of treatment group. About half of patients, whether starting ABC/3TC with DRV/r or another PI, had a viral load of <50 copies/mL at baseline, while another 14 and 17 %, respectively (p = 0.4), had a viral load >20,000 copies/mL at baseline.
Not accounting for the initial viral load limits interpretation of the results from the primary analysis. To account for baseline viral load, sensitivity analyses were performed that were restricted to either patients with a viral load (1) at or below or (2) above a threshold indicating virologic control (200 copies/mL) at the time of switch. For patients with a baseline viral load ≤200 copies/mL at baseline, crude and adjusted models both indicated no difference in ability to achieve an undetectable viral load between patients taking ABC/3TC with DRV/r and those taking ABC/3TC with another PI. For patients with a baseline viral load >200 copies/mL, the crude model for all of follow-up indicated reduced odds of suppression among those taking DRV/r. The multivariable model for this subgroup, which included an adjustment for time (year of initiation), indicated no significant differences in odds of achieving an undetectable viral load. This suggests that for patients starting out with higher viral loads, the amount of time available to reach undetectable levels of viremia is more critical than for those starting with a low viral load.
While not covered in this analysis, the variability in baseline viral loads suggest that treatment-experienced patients often switched to a backbone of ABC/3TC with a PI for reasons beyond better virologic control, including issues of tolerability and adherence. While darunavir is considered to have favorable tolerability compared with other PIs, patients receiving DRV were not significantly more likely to switch while virologically controlled. This suggests that the perceived benefit, whether for better treatment tolerance or simplified regimen, may be in the change of NRTI backbone, as all patients were taking ABC/3TC for the first time in their treatment history. This analysis could be enhanced by examining adverse event data, to assess both differences in indicators for switch to an ABC/3TC + PI regimen as well as to compare tolerability as a factor in treatment success between patients taking ABC/3TC + DRV/r and ABC/3TC plus a non-darunavir PI.
Conclusion
The results of this study suggest that in clinical practice in the USA, no difference was observed in multivariable logistic regression analysis comparing the use of ABC/3TC + DRV/r versus ABC/3TC plus another PI (non-DRV/r). Additionally, patients receiving DRV/r with ABC/3TC were able to achieve equivalent gains in raising the CD4 cell count and lowering viral load compared with those taking other PIs with ABC/3TC.
Acknowledgments
The authors extend special thanks to Robin Beckerman for SAS® programming and quality assurance, Emily Brouwer for contributions to the study design and analysis, Ted Ising and Bernie Stooks for database creation and management, and Rodney Mood for site integration and management. This research would not be possible without the collaboration of physicians, personal assistants, nurses, other allied healthcare providers, and patients throughout the USA participating in the OPERA database. We are indebted to them.
Compliance with Ethical Standards
Funding
This work was support by a project grant from ViiV Healthcare, which was performed using Epividian’s OPERA® database. ViiV Healthcare had no editorial control in the content of this article or involvement in the design, conduct, analysis, and interpretation of this study.
Conflicts of interest
Philip Lackey is a member of the OPERA® Clinical and Epidemiological Advisory Board. Anthony Mills has received research support and served as a consultant for Gilead Sciences, Merck, ViiV Healthcare, EMD Serono, and Bristol-Myers Squibb, and has received lecture fees from Gilead Sciences, ViiV Healthcare, and Merck. He is also a member of the OPERA® Clinical and Epidemiological Advisory Board. Felix Carpio is a member of the OPERA® Clinical and Epidemiological Advisory Board. Ricky Hsu has received research support from Gilead Sciences, and has served as a consultant and received lecture honoraria from Gilead Sciences, ViiV Healthcare, Janssen, Bristol-Myers Squibb, and AbbVie. Edwin DeJesus is a member of the OPERA® Clinical and Epidemiological Advisory Board. Gerald Pierone is a member of the OPERA® Clinical and Epidemiological Advisory Board. Cassidy Henegar is an employee of Epividian. Jennifer Fusco is an employee of Epividian and a member of the OPERA® Clinical and Epidemiological Advisory Board. Gregory Fusco is chairperson for the OPERA® Clinical and Epidemiological Advisory Board. Michael Wohlfeiler has served as a consultant for ViiV Healthcare, and has received lecture honoraria from EMD Serono and Gilead Sciences. He is also a member of the OPERA® Clinical and Epidemiological Advisory Board.
Ethical approval
All procedures in this study were in accordance with the 1964 Helsinki Declaration (and its amendments). No approval by ethical committee or institutional review board was needed. Analysis was performed on de-identified patient data collected for routine clinical purposes.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 1 Oct 2015.
- 2.Blanco JL, Varghese V, Rhee S, et al. HIV-1 integrase inhibitor resistance and its clinical implications. J Infect Dis. 2011;203:1204–1214. doi: 10.1093/infdis/jir025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naggie S, Hicks C. Protease inhibitor-based antiretroviral therapy in treatment-naïve HIV-1-infected patients: the evidence behind the options. J Antimicrob Chemother. 2010;65(6):1094–1099. doi: 10.1093/jac/dkq130. [DOI] [PubMed] [Google Scholar]
- 4.Wilson LE, Gallant JE. The management of treatment-experienced HIV-infected patients: new drugs and drug combinations. Clin Infect Dis. 2009;48:214–221. doi: 10.1086/595701. [DOI] [PubMed] [Google Scholar]
- 5.Berhan A, Berhan Y. Virologic response to tipranavir-ritonavir or darunavir-ritonavir based regimens in antiretroviral therapy experienced HIV-1 patients: a meta-analysis and meta-regression of randomized controlled clinical trials. PLoS One. 2013;8(4):e60814. doi: 10.1371/journal.pone.0060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elgadi MM, Piliero PJ. Boosted tipranavir versus darunavir in treatment-experienced patients: observational data from the randomized POTENT trial. Drugs R&D. 2011;11(4):295–302. doi: 10.2165/11596340-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lascar RM, Benn P. Role of darunavir in the management of HIV infection. HIV AIDS (Auckl) 2009;1:31–39. doi: 10.2147/hiv.s5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willig JH, Aban I, Nevin CR, et al. Darunavir outcomes study: comparative effectiveness of virologic suppression, regimen durability, and discontinuation reasons for three-class experienced patients at 48 weeks. AIDS Res Hum Retrov. 2010;26(12):1279–1285. doi: 10.1089/aid.2010.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKinnell JA, Lin HY, Nevin CN, et al. Early virologic suppression with three-class experienced patients: 24-week effectiveness in the darunavir outcomes study. AIDS. 2009;23:1539–1546. doi: 10.1097/QAD.0b013e32832c7b5c. [DOI] [PubMed] [Google Scholar]
- 10.Pozniak A, Opravil M, Beatty G, et al. Effect of baseline viral susceptibility on response to darunavir/ritonavir versus control protease inhibitors in treatment-experienced HIV type 1-infected patients: pOWER 1 and 2. AIDS Res Hum Retrov. 2008;24(10):1275–1280. doi: 10.1089/aid.2007.0275. [DOI] [PubMed] [Google Scholar]
- 11.Nishijima T, Tsukada K, Teruya K, et al. Efficacy and safety of once-daily ritonavir-boosted darunavir and abacavir/lamivudine for treatment-naïve patients: a pilot study. AIDS. 2012;26(5):649–651. doi: 10.1097/QAD.0b013e328350fb85. [DOI] [PubMed] [Google Scholar]
- 12.Nishijima T, Komatsu H, Teruya K, et al. Once-daily darunavir/ritonavir and abacavir/lamivudine versus tenofovir/emtricitabine for treatment-naïve patients with a baseline viral load of more than 100000 copies/ml. AIDS. 2013;27(5):839–842. doi: 10.1097/QAD.0b013e32835cadb7. [DOI] [PubMed] [Google Scholar]
- 13.Mills AM, Nelson M, Jayaweera D, et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV1-infected patients: 96-week analysis. AIDS. 2009;23:1679–1688. doi: 10.1097/QAD.0b013e32832d7350. [DOI] [PubMed] [Google Scholar]
- 14.Podzamczer D, Imaz A, Perez I, et al. Abacavir/lamivudine plus darunavir/ritonavir in routine clinical practice: a multicenter experience in antiretroviral therapy-naïve and -experienced patients. J Antimicrob Chemother. 2014;69:2536–2540. doi: 10.1093/jac/dku157. [DOI] [PubMed] [Google Scholar]
- 15.Trottier B, Machouf N, Thomas R, et al. Abacavir/lamivudine fixed-dose combination with ritonavir-boosted darunavir: a safe and efficacious regimen for HIV therapy. HIV Clin Trials. 2012;13(6):335–342. doi: 10.1310/hct1306-335. [DOI] [PubMed] [Google Scholar]
- 16.Madruga JV, Berger D, McMurchie M, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomized controlled phase III trial. Lancet. 2007;370:49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 17.Katlama C, Esposito R, Gatell JM, et al. Efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients: 24-week results of POWER 1. AIDS. 2007;21:395–402. doi: 10.1097/QAD.0b013e328013d9d7. [DOI] [PubMed] [Google Scholar]
- 18.Arasteh K, Yeni P, Pozniak A, et al. Efficacy and safety of darunavir/ritonavir in treatment experienced HIV type 1 trials at week 96. Antivir Ther. 2009;14:859–864. doi: 10.3851/IMP1301. [DOI] [PubMed] [Google Scholar]
- 19.Haubrich R, Berger D, Chiliade P, et al. Week 24 efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients. AIDS. 2007;21:F11–F18. doi: 10.1097/QAD.0b013e3280b07b47. [DOI] [PubMed] [Google Scholar]
- 20.Clotet B, Bellos N, Molina JM, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169–1178. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- 21.De Meyer SMJ, Spinosa-Guzman S, Vangeneugden TJ, et al. Efficacy of once-daily darunavir/ritonavir 800/100 mg in HIV-infected, treatment-experienced patients with no baseline resistance-associated mutations to darunavir. J Acquir Immune Defic Syndr. 2008;49:179–182. doi: 10.1097/QAI.0b013e318183a959. [DOI] [PubMed] [Google Scholar]
- 22.Hill A, Moyle G. Relative antiviral efficacy of ritonavir-boosted darunavir and ritonavir-boosted tipranavir vs. control protease inhibitor in the POWER and RESIST trials. HIV Med. 2007;8:259–264. doi: 10.1111/j.1468-1293.2007.00465.x. [DOI] [PubMed] [Google Scholar]
- 23.de los Santos I, Gomex-Berroccal A, Valencia E, et al. Efficacy and tolerability of darunavir/ritonavir in combination with abacavir/lamivudine: an option in selected HIV-infected patients. HIV Clin Trials. 2013;14(5):254–259. doi: 10.1310/hct1405-254. [DOI] [PubMed] [Google Scholar]