Abstract
Background
Anastomotic leakage is a serious complication associated with anterior resection for rectal cancer, the long-term effects of which are unclear. Therefore, a systematic review and meta-analysis were conducted to evaluate the impact of anastomotic leakage on disease recurrence and survival.
Methods
We searched PubMed, Embase, and the Cochrane Library databases from their inception to January 2016. Studies evaluating the oncologic impact of anastomotic leakage were included in the meta-analysis. Outcome measures were local recurrence, overall survival, cancer-specific survival, and distant recurrence. Pooled hazard ratio (HR) with 95 % confidence interval (CI) was calculated using random effects models.
Results
Fourteen studies containing 11,353 patients met inclusion criteria. Anastomotic leakage was associated with a greater local recurrence (HR 1.71; 95 % CI 1.22–2.38) and decreased in both overall survival (HR 1.67; 95 % CI 1.19–2.35) and cancer-specific survival (HR 1.30; 95 % CI 1.08–1.56); anastomotic leakage did not increase distant recurrence (HR 1.03; 95 % CI 0.76–1.40).
Conclusions
Anastomotic leakage was associated with high local recurrence and poor survival (both overall and cancer-specific), but not with distant recurrence.
Introduction
Because of advances in operative techniques and our knowledge of the biology of rectal cancer, an increasing number of patients with rectal cancer have undergone sphincter preserving surgery. Anastomotic leakage, however, is a serious surgical complication with an incidence that varies from 4 to 29.5 % [1, 2] and is associated with short-term mortality, high reoperation rate, and increased healthcare costs [3–5].
The long-term outcome of curative rectal cancer resections is affected by many factors, such as lower differentiation, later stage, and older age [6, 7]. Though some studies found that anastomotic leakage was associated with a poorer long-term outcome [8, 9], others did not [10, 11]. Thus, we performed a systematic review and meta-analysis to determine the evidence-based impact of anastomotic leakage on long-term outcomes after curative anterior resection.
Materials and methods
Literature search and inclusion criteria
Two authors (S.W. and J.L.) searched independently the electronic databases (inception to January 2016) of Pubmed, Embase, and the Cochrane Library. Search terms included the following keywords in various combinations: “rectal neoplasms,” “anastomotic leak,” “recurrence,” “neoplasm metastasis,” “survival,” and “mortality.” Searches of subject headings (MeSH) and text words were performed with no language restrictions applied. We followed the Cochrane approach of PICO (population intervention, comparison, outcome, and context). We also searched for references included in the articles to identify related studies.
For this meta-analysis, we followed the PRISMA guidelines [12]. The inclusion criteria were as follows: (1) patients who underwent a curative anterior resection for rectal cancer; (2) studies that analyzed the impact of anastomotic leakage on long-term outcomes, including local recurrence, overall survival, cancer-specific survival, or distant recurrence; and (3) patients with and without anastomotic leakage who were compared using a multivariate Cox proportional hazards model.
Conversely, the exclusion criteria were as follows: (1) patients who underwent an emergency operation; and (2) studies including all anatomic locations of colorectal cancer, unless the data were presented separately.
Anastomotic leakage was defined as a communication between the intra- and extraluminal compartments, determined by either clinical or radiologic evidence [13]. Local recurrence was defined as a mass in the lesser pelvis documented by clinical, radiologic, or pathologic examination. Distant recurrence was defined as tumor growth in any lymph node outside the pelvis, or in any other organ documented by clinical, radiologic, or pathologic examination.
Data extraction, outcome measures, and quality assessment
Disagreements regarding the independently extracted data (by authors S.W. and S.G.) were settled through discussion, when no consensus could be reached, a third specialist was consulted (W.W.). For this study, outcome measures evaluated included local recurrence, overall survival, cancer-specific survival, and distant recurrence.
The quality of the included studies was assessed by two independent authors (S.W. and H.Z.) using the criteria shown in File S1. The guideline for appraising the studies was adopted from a quality assessment framework for systematic reviews of prognostic studies [14]. This framework includes the following six areas of potential bias: study participation, study attrition, measurement of prognostic factors, measurement of and controlling for confounding variables, measurement of outcomes, and approaches in analysis.
Statistical analysis
Pooled HR and 95 % CI were estimated for each outcome. Statistical heterogeneity was assessed with I 2 and χ2 statistics. Heterogeneity was considered significant if the p value (χ2) was <0.1 and I 2 was >50 %. A random effects model was used regardless of heterogeneity [15]. Whenever significant heterogeneity was present, potential sources of heterogeneity were assessed. For example, a sensitivity analysis was performed, and the study was excluded if the results were outside the range established by others. Potential publication bias was assessed through visual inspection of Begg’s funnel plots where the log HR was plotted against their standard errors. The presence of publication bias was then evaluated using the Begg’s test [16]. Statistical analysis was performed using Stata 12.0 (Stata Corporation, College Station, TX, USA) and RevMan 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark).
Results
Search results and study descriptions
The predefined search strategy identified 2140 studies, and after removal of 371 duplicate studies, 1769 articles remained. A total of 1718 studies were excluded after reading the titles and abstracts, mainly because they were not pertinent to the topic, leaving 51 studies for full-text review. After further review, 37 studies were excluded for the following reasons: 16 studies had unavailable data, 10 studies included patients who underwent palliative operations, 7 studies included colorectal cancer with data unable to be sorted specifically to anterior resection for rectal cancer, 3 studies included patients who underwent emergency operations, and 1 study contained duplicate data. Finally, 14 studies were included in this meta-analysis [17–30] as seen in the PRISMA flow diagram (Fig. 1).
Fig. 1.
Prisma flow diagram of the literature screening and selection
All included studies were published between 2001 and 2015. Sample size ranged from 108 to 2480 patients, giving a total of 11,353 patients available for inclusion. Seven studies were prospective cohort studies, and seven were retrospective cohort studies. Furthermore, nine studies provided data on local recurrence, ten provided data on overall survival, seven examined cancer-specific survival, and three assessed distant recurrence. The multivariate Cox proportional hazards model was applied to all 14 studies to adjust for potential confounding data. Further characteristics of these studies are presented in Table 1.
Table 1.
Characteristics of included articles
| References | Year | Country | Period | Journal | Sample size | Definition of AL | Follow-up in yearsa |
|---|---|---|---|---|---|---|---|
| Bell et al. [17] | 2003 | Australia | 1971–1991 | Br J Surg | 403 | Clinical or radiological | 10.80 (5–23) |
| Bertelsen et al. [18] | 2010 | Denmark | 2001–2004 | Colorectal Dis | 1494 | Clinical | 3.80 (0.09–6.18) |
| den Dulk et al. [19] | 2009 | Multination | 1987–2003 | Br J Surg | 2480 | Clinical | 5.90 (0.20–14.90) |
| Ebinger et al. [20] | 2015 | Switzerland | 1991–2010 | Int J Colorectal Dis | 584 | Clinical or radiological | 5.20 (0.20–21.20) |
| Espin et al. [21] | 2015 | Spain | 2006–2008 | Br J Surg | 1153 | Clinical | At least 5 |
| Gong et al. [22] | 2014 | China | 2003–2007 | Asian Pac J Cancer Prev | 460 | Clinical | 3.50 |
| Gunkova et al. [23] | 2013 | Czech | 2001–2009 | Rozhl Chir | 174 | Clinical or radiological | NA |
| Jager et al. [24] | 2015 | Osterreich | 2003–2010 | Chirurg | 108 | Clinical or radiological | 5.80 (2–10.30) |
| Jorgren et al. [25] | 2011 | Sweden | 1995–1997 | Colorectal Dis | 250 | Clinical | 5 |
| Kang et al. [26] | 2015 | Korea | 2006–2009 | Medicine (Baltimore) | 1083 | Clinical | 4.50 (0.08–7.70) |
| Ke et al. [27] | 2015 | China | 2007–2011 | Zhonghua Wei Chang Wai Ke Za Zhi | 653 | Clinical or radiological | 3.90 (0.10–7.58) |
| Kulu et al. [28] | 2015 | Multination | 2002–2011 | Ann Surg Oncol | 570 | Clinical or radiological | 4.70 ± 2.90 |
| Merkel et al. [29] | 2001 | Germany | 1978–1996 | Colorectal Dis | 814 | Clinical | 7.50 (0.30–20.80) |
| Smith et al. [30] | 2012 | USA | 1991–2010 | Ann Surg | 1127 | Clinical | 6.20 (IQR 1.60–8.90) |
NA not available, AL anastomotic leakage, IQR interquartile range
aMedian (range)
Study quality
We used 14 quality domains reflecting 6 main quality aspects in the framework established by Hayden et al. [14]. Each domain was evaluated using a quality score of 0, 0.5, or 1. The total score was obtained through the addition of each domain, thereby making the maximum quality score 14. The median (interquartile range) of the total quality score was 12 (10.6, 12.5). The results of the quality assessment of the included studies are shown in Table 2.
Table 2.
Quality assessment of included articles
| References | Study participation max 3 pts | Study attrition max 2 pts | Prognostic factor measurement max 2 pts | Outcome measurement max 2 pts | Confounding measurement and account max 2 pts | Analysis max 3 pts | Total score max 14 pts |
|---|---|---|---|---|---|---|---|
| Bell et al. [17] | 3 | 2 | 2 | 2 | 1.5 | 2.5 | 13 |
| Bertelsen et al. [18] | 3 | 2 | 2 | 1 | 1.5 | 3 | 12.5 |
| den Dulk et al. [19] | 3 | 2 | 2 | 2 | 1 | 2.5 | 12.5 |
| Ebinger et al. [20] | 3 | 2 | 2 | 2 | 1.5 | 3 | 13.5 |
| Espin et al. [21] | 3 | 1 | 2 | 2 | 1 | 3 | 12 |
| Gong et al. [22] | 3 | 2 | 2 | 2 | 1 | 2 | 12 |
| Gunkova et al. [23] | NA | NA | NA | NA | NA | NA | NA |
| Jager et al. [24] | NA | NA | NA | NA | NA | NA | NA |
| Jorgren et al. [25] | 3 | 1 | 2 | 2 | 1 | 2 | 11 |
| Kang et al. [26] | 1.5 | 1 | 2 | 2 | 1 | 3 | 10.5 |
| Ke et al. [27] | 1.5 | 1 | 1 | 1 | 1 | 1.5 | 7 |
| Kulu et al. [28] | 3 | 2 | 2 | 1.5 | 1 | 3 | 12.5 |
| Merkel et al. [29] | 3 | 1 | 2 | 2 | 1 | 2.5 | 11.5 |
| Smith et al. [30] | 1.5 | 2 | 2 | 1 | 1 | 3 | 10.5 |
Max maximum, pts points, NA not available
Anastomotic leakage and local recurrence
Nine studies reported local recurrence after anastomotic leakage, with no significant heterogeneity among them (P = 0.25, I 2 = 22 %). In the random effects model, anastomotic leakage was significantly associated with greater local recurrence rate (HR 1.71; 95 % CI 1.22–2.38; P = 0.002), as shown in Fig. 2.
Fig. 2.
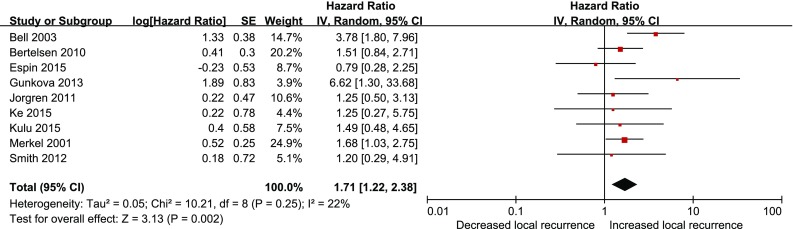
Effect of anastomotic leakage on the risk of local recurrence of rectal cancer after anterior resection
Anastomotic leakage and overall survival
Ten studies assessed overall survival after anastomotic leakage. Our meta-analysis found that anastomotic leakage decreased overall survival (HR 1.67; 95 % CI 1.19–2.35; P = 0.003, Fig. 3), but with significant heterogeneity among studies (P < 0.00001, I 2 = 82 %). As shown in Fig. 3, the results of the study conducted by Gong et al. were notably outside of the range established by others, probably contributing to this heterogeneity. After excluding this study, results indicated that anastomotic leakage was associated with poor overall survival (HR 1.38; 95 % CI 1.14–1.66; P = 0.001) and no significant heterogeneity was observed among the remaining studies (P = 0.13, I 2 = 37 %).
Fig. 3.
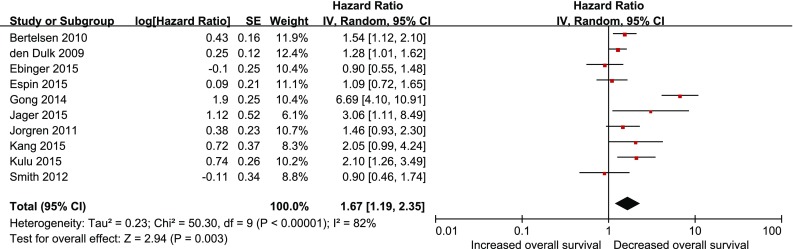
Effect of anastomotic leakage on overall survival
Anastomotic leakage and cancer-specific survival
In the random effects model, anastomotic leakage was associated with lesser cancer-specific survival (HR 1.30; 95 % CI 1.08–1.56; P = 0.005), as shown in Fig. 4. There was no heterogeneity among the seven studies that assessed cancer-specific survival after anastomotic leakage (P = 0.50, I 2 = 0 %).
Fig. 4.
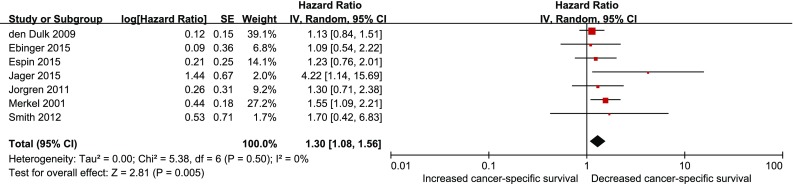
Effect of anastomotic leakage on cancer-specific survival
Anastomotic leakage and distant recurrence
Three studies provided data on distant recurrence after anastomotic leakage. Results showed that anastomotic leakage had no significant effect on distant recurrence (1.03; 95 % CI 0.76–1.40, P = 0.86, Fig. 5), and there was no heterogeneity among the studies (P = 0.71, I 2 = 0 %).
Fig. 5.

Effect of anastomotic leakage on distant recurrence
Publication bias
Assessment of publication bias revealed no potential publication bias among the included studies (Begg’s test, P = 0.18).
Discussion
Our study evaluated the oncologic impact of anastomotic leakage after a “curative” anterior resection for rectal cancer. The results of the present meta-analysis revealed that anastomotic leakage was associated with worse overall survival, as well as cancer-specific survival and greater risk of local recurrence; anastomotic leakage had no significant difference in terms of distant recurrence.
There is only one meta-analysis on a similar topic, which was done by Mirnezami et al. [31] and published in 2011. There are many dissimilar characteristics between the two studies. First, the prior study included patients with both colon and rectal cancer. Because the incidence of anastomotic leakage after resection of colon cancer is very low (2.4 %) compared to resections for rectal cancer [32], our study specifically only included those patients with rectal cancers. Furthermore, the prior study included patients with stage IV colorectal cancer and palliative operations which confound the analysis. These factors were very likely to affect the long-term outcomes of patients [30, 33, 34], which is the reason for exclusion of colon cancer, emergency operations, patients with stage IV disease, and palliative operations in the present study. Second, the prior study analyzed time-to-event data as dichotomous and expressed effect size as an odds ratio. Our meta-analysis used methods of survival analysis and expressed effect size as an HR, which is considered the most appropriate means of summarizing time-to-event data [35]. Lastly, the present meta-analysis included more recently published articles than the analysis conducted by Mirnezami et al.
Some factors may explain our findings of anastomotic leakage being associated with high local recurrence, poorer overall survival, and poorer cancer-specific survival. Colorectal cancer exfoliates cancer cells remaining in the lumen of the bowel and from the large intestinal mucosa, potentially seeding the local environment after resection [36]. Although the rectal stump was routinely washed out, free malignant cells can be found in the anastomosis of the anterior resection [37, 38]. When anastomotic leakage occurs, these cells may lead to extraluminal tumor implantation and pelvic recurrence. Moreover, anastomotic leakage causes postoperative peritoneal and pelvic infection, which may enhance proliferation, migration, and invasion capacities of cancer cells as shown in cancer cell lines in vitro [39]. Furthermore, some studies found that peritoneal infection increased serum interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), and C-reactive protein (CRP) concentrations, which are associated with poor overall and cancer-specific survival [40–42]. Postoperative adjuvant chemotherapy for patients undergoing oncologic resections for rectal cancer has provided significantly positive effects on overall survival and distant metastasis [43], but anastomotic leakage may prevent or delay the receipt of adjuvant chemotherapy. This may also explain poorer survival in patients with anastomotic leakage [44].
Even though heterogeneity was present in our study, we detected its major source through sensitivity analysis. Quality appraisal is incomplete in most reviews of prognosis studies [14], but our meta-analysis incorporated adequate quality assessment and included studies that achieved a high median score. In addition, there was no publication bias, suggesting that our conclusions are not an artifact of unpublished studies. As more evidence becomes available, the test power to provide reliable estimates of risk increases.
There are a number of limitations of our study that must be considered. First, different definitions of anastomotic leakage have been applied throughout the studies included in this meta-analysis, regardless of a definition and grading of anastomotic leakage being proposed by the International Study Group of Rectal Cancer in 2010 [13]. Moreover, some included studies only contained patients with clinical anastomotic leakage, while others contained patients with clinical and radiologic anastomotic leakage. Second, though the relationship between anastomotic leakage and outcome measures was analyzed using multivariate the Cox proportional hazards model, each study had different confounder variables. These two concerns may have influenced the association of anastomotic leakage with recurrence or survival. Finally, although we concluded that anastomotic leakage did not increase distant recurrence, only three studies addressed this topic, and patient sample size was relatively small (n = 2397), indicating lesser statistical power.
In conclusion, based on available evidence, anastomotic leakage is associated with a greater risk of local recurrence and poorer overall and cancer-specific survival. These findings suggest that all attempts to decrease the incidence of anastomotic leakage should be employed when performing anterior resection of the rectum. Furthermore, close follow-up of patients with anastomotic leakage should be conducted. Whether local adjuvant radiation is beneficial in patients with anastomotic leakage is not known, but this special use of radiation therapy in this clinical situation might be a topic of interest in future studies.
Acknowledgments
The authors gratefully acknowledge LetPub (www.letpub.com) for its linguistic assistance in the preparation of this manuscript.
Compliance with ethical standards
Conflict of interest
The authors have declared that no competing interests exist.
Contributor Information
Shuanhu Wang, Phone: +86-552-3086151, Email: knight01030103@126.com.
Wenbin Wang, Email: wbwangay@163.com.
References
- 1.Asoglu O, Kunduz E, Serin KR, et al. Standardized laparoscopic sphincter-preserving total mesorectal excision for rectal cancer: long-term oncologic outcome in 217 unselected patients European Surgery. Acta Chirurgica Austriaca. 2012;44:6. doi: 10.1007/s10353-011-0067-2. [DOI] [Google Scholar]
- 2.Di Mauro D, Uthayanan M, Austin R. Outcomes of laparoscopic true anterior resection of the rectum. Colorectal Dis. 2014;16:100. [Google Scholar]
- 3.Kang CY, Halabi WJ, Chaudhry OO, et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg. 2013;148:65–71. doi: 10.1001/2013.jamasurg.2. [DOI] [PubMed] [Google Scholar]
- 4.Ashraf SQ, Burns EM, Jani A, et al. The economic impact of anastomotic leakage after anterior resections in English NHS hospitals: Are we adequately remunerating them? Colorectal Dis. 2013;15:e190–e198. doi: 10.1111/codi.12125. [DOI] [PubMed] [Google Scholar]
- 5.Eriksen MT, Wibe A, Norstein J, et al. Anastomotic leakage following routine mesorectal excision for rectal cancer in a national cohort of patients. Colorectal Dis. 2005;7:51–57. doi: 10.1111/j.1463-1318.2004.00700.x. [DOI] [PubMed] [Google Scholar]
- 6.Manas MJ, Espin E, Lopez-Cano M, et al. Multivisceral resection for locally advanced rectal cancer: prognostic factors influencing outcome. Scand J Surg. 2015;104:154–160. doi: 10.1177/1457496914552341. [DOI] [PubMed] [Google Scholar]
- 7.Reshef A, Lavery I, Kiran RP. Factors associated with oncologic outcomes after abdominoperineal resection compared with restorative resection for low rectal cancer: patient- and tumor-related or technical factors only? Dis Colon Rectum. 2012;55:51–58. doi: 10.1097/DCR.0b013e3182351c1f. [DOI] [PubMed] [Google Scholar]
- 8.Lin JK, Yueh TC, Chang SC, et al. The influence of fecal diversion and anastomotic leakage on survival after resection of rectal cancer. J Gastrointest Surg. 2011;15:2251–2261. doi: 10.1007/s11605-011-1721-5. [DOI] [PubMed] [Google Scholar]
- 9.Manley K, Elkins B, Gillam M, et al. Oncological outcomes following anastomotic leaks in rectal cancer surgery. Colorectal Dis. 2013;15:41. [Google Scholar]
- 10.Adelsdorfer C, Delgado S, Adelsdorfer W, et al. Influence of anastomotic leakage in the longterm results of laparoscopic treatment of curative rectal cancer Surgical Endoscopy and Other Interventional. Techniques. 2012;26:S276. [Google Scholar]
- 11.Hai-Lin K, Pan C, Hui-Ming L, et al. The influence of anastomotic leakage on longterm survival after resection for rectal cancer European Surgery. Acta Chirurgica Austriaca. 2015;47:S61. [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269 [DOI] [PubMed]
- 13.Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- 15.Sedgwick P. How to read a forest plot in a meta-analysis. BMJ. 2015;351:h4028. doi: 10.1136/bmj.h4028. [DOI] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 17.Bell SW, Walker KG, Rickard MJ, et al. Anastomotic leakage after curative anterior resection results in a higher prevalence of local recurrence. Br J Surg. 2003;90:1261–1266. doi: 10.1002/bjs.4219. [DOI] [PubMed] [Google Scholar]
- 18.Bertelsen CA, Andreasen AH, Jorgensen T, et al. Anastomotic leakage after curative anterior resection for rectal cancer: short and long-term outcome. Colorectal Dis. 2010;12:e76–e81. doi: 10.1111/j.1463-1318.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 19.den Dulk M, Marijnen CA, Collette L, et al. Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg. 2009;96:1066–1075. doi: 10.1002/bjs.6694. [DOI] [PubMed] [Google Scholar]
- 20.Ebinger SM, Warschkow R, Tarantino I, et al. Anastomotic leakage after curative rectal cancer resection has no impact on long-term survival: a propensity score analysis. Int J Colorectal Dis. 2015;30:1667–1675. doi: 10.1007/s00384-015-2331-6. [DOI] [PubMed] [Google Scholar]
- 21.Espin E, Ciga MA, Pera M, et al. Oncological outcome following anastomotic leak in rectal surgery. Br J Surg. 2015;102:416–422. doi: 10.1002/bjs.9748. [DOI] [PubMed] [Google Scholar]
- 22.Gong JP, Yang L, Huang XE, et al. Outcomes based on risk assessment of anastomotic leakage after rectal cancer surgery. Asian Pac J Cancer Prev. 2014;15:707–712. doi: 10.7314/APJCP.2014.15.2.707. [DOI] [PubMed] [Google Scholar]
- 23.Gunkova P, Gunka I, Martinek L, et al. Impact of anastomotic leakage on oncological outcomes after rectal cancer resection. Rozhl Chir. 2013;92:244–249. [PubMed] [Google Scholar]
- 24.Jager T, Nawara C, Neureiter D, et al. Impact of anastomotic leakage on long-term survival in mid-to-low rectal cancer. Chirurg. 2015;86:1072–1082. doi: 10.1007/s00104-015-0090-0. [DOI] [PubMed] [Google Scholar]
- 25.Jorgren F, Johansson R, Damber L, et al. Anastomotic leakage after surgery for rectal cancer: a risk factor for local recurrence, distant metastasis and reduced cancer-specific survival? Colorectal Dis. 2011;13:272–283. doi: 10.1111/j.1463-1318.2009.02136.x. [DOI] [PubMed] [Google Scholar]
- 26.Kang J, Choi GS, Oh JH, et al. Multicenter analysis of long-term oncologic impact of anastomotic leakage after laparoscopic total mesorectal excision: the Korean Laparoscopic Colorectal Surgery Study Group. Medicine. 2015;94:e1202. doi: 10.1097/MD.0000000000001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ke H, Chi P, Lin H, et al. Influence of anastomotic leakage on long-term survival after resection for rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2015;18:920–924. [PubMed] [Google Scholar]
- 28.Kulu Y, Tarantio I, Warschkow R, et al. Anastomotic leakage is associated with impaired overall and disease-free survival after curative rectal cancer resection: a propensity score analysis. Ann Surg Oncol. 2015;22:2059–2067. doi: 10.1245/s10434-014-4187-3. [DOI] [PubMed] [Google Scholar]
- 29.Merkel S, Wang WY, Schmidt O, et al. Locoregional recurrence in patients with anastomotic leakage after anterior resection for rectal carcinoma. Colorectal Dis. 2001;3:154–160. doi: 10.1046/j.1463-1318.2001.00232.x. [DOI] [PubMed] [Google Scholar]
- 30.Smith JD, Paty PB, Guillem JG, et al. Anastomotic leak is not associated with oncologic outcome in patients undergoing low anterior resection for rectal cancer. Ann Surg. 2012;256:1034–1038. doi: 10.1097/SLA.0b013e318257d2c1. [DOI] [PubMed] [Google Scholar]
- 31.Mirnezami A, Mirnezami R, Chandrakumaran K, et al. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253:890–899. doi: 10.1097/SLA.0b013e3182128929. [DOI] [PubMed] [Google Scholar]
- 32.Kanellos I, Blouhos K, Demetriades H, et al. The failed intraperitoneal colon anastomosis after colon resection. Tech Coloproctol. 2004;8(Suppl 1):s53–s55. doi: 10.1007/s10151-004-0111-3. [DOI] [PubMed] [Google Scholar]
- 33.Yun HR, Lee WY, Lee WS, et al. The prognostic factors of stage IV colorectal cancer and assessment of proper treatment according to the patient’s status. Int J Colorectal Dis. 2007;22:1301–1310. doi: 10.1007/s00384-007-0315-x. [DOI] [PubMed] [Google Scholar]
- 34.Smith JD, Butte JM, Weiser MR, et al. Anastomotic leak following low anterior resection in stage IV rectal cancer is associated with poor survival. Ann Surg Oncol. 2013;20:2641–2646. doi: 10.1245/s10434-012-2854-9. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The cochrane collaboration, 2011. www.cochrane-handbook.org
- 36.Kouraklis G, Glinavou A, Kouvaraki M, et al. Anal lesion resulting from implantation of viable tumour cells in a pre-existing anal fistula. A case report. Acta Chir Belg. 2002;102:212–213. doi: 10.1080/00015458.2002.11679299. [DOI] [PubMed] [Google Scholar]
- 37.Gertsch P, Baer HU, Kraft R, et al. Malignant cells are collected on circular staplers. Dis Colon Rectum. 1992;35:238–241. doi: 10.1007/BF02051014. [DOI] [PubMed] [Google Scholar]
- 38.Sayfan J, Averbuch F, Koltun L, et al. Effect of rectal stump washout on the presence of free malignant cells in the rectum during anterior resection for rectal cancer. Dis Colon Rectum. 2000;43:1710–1712. doi: 10.1007/BF02236855. [DOI] [PubMed] [Google Scholar]
- 39.Salvans S, Mayol X, Alonso S, et al. Postoperative peritoneal infection enhances migration and invasion capacities of tumor cells in vitro: an insight into the association between anastomotic leak and recurrence after surgery for colorectal cancer. Ann Surg. 2014;260:939–943. doi: 10.1097/SLA.0000000000000958. [DOI] [PubMed] [Google Scholar]
- 40.Alonso S, Pascual M, Salvans S, et al. Postoperative intra-abdominal infection and colorectal cancer recurrence: a prospective matched cohort study of inflammatory and angiogenic responses as mechanisms involved in this association. Eur J Surg Oncol. 2015;41:208–214. doi: 10.1016/j.ejso.2014.10.052. [DOI] [PubMed] [Google Scholar]
- 41.McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003;90:215–219. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen HJ, Christensen IJ, Sorensen S, et al. Preoperative plasma plasminogen activator inhibitor type-1 and serum C-reactive protein levels in patients with colorectal cancer. The RANX05 Colorectal Cancer Study Group. Ann Surg Oncol. 2000;7:617–623. doi: 10.1007/BF02725342. [DOI] [PubMed] [Google Scholar]
- 43.Petersen SH, Harling H, Kirkeby LT, et al. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev. 2012;3:CD004078. doi: 10.1002/14651858.CD004078.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Des Guetz G, Nicolas P, Perret GY, et al. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010;46:1049–1055. doi: 10.1016/j.ejca.2010.01.020. [DOI] [PubMed] [Google Scholar]