Abstract
Prostate cancer (PCa) is the second lethal disease for men in western countries. Although androgen receptor (AR) signaling has been widely investigated, noncoding RNAs (ncRNAs), deficient of open reading frame, have also received considerable attention. Growing studies showed that the aberrant ncRNAs expression contributed to cell proliferation, metastasis and drug resistance in PCa. Therefore, therapeutically targeting ncRNAs may synergize androgen deprivation therapy (ADT) to have a better effect to fight against PCa, especially castration-resistant prostate cancer (CRPC). This review would systematically summarize the multicellular events controlled by ncRNAs and give a snapshot of future scientific activities and clinical applications.
Keywords: Prostate cancer, lncRNA, miRNA, diagnosis, therapeutic target
Introduction
PCa, the second lethal disease among male in western countries [1], is responsible for approximate 300,000 deaths annually worldwide [2]. ADT shows promising effect, castration resistance invariably occurs due to multiple reasons. Therefore, it is a need to find novel therapeutic targets for advanced PCa.
NcRNAs, originally viewed as “junk RNAs”, bear no translational potential and account for approximate 90% RNAs in human [3]. Accumulating evidences suggest that ncRNAs should be implicated into numerous biological processes: alternative RNA splicing, epigenetic modification, mRNA titration, regulation of protein stability and so on [4-7]. Of note, the spatial and temporal deregulation of ncRNA in different types of cancer strongly highlights its crucial role in cancer pathogenesis. Indeed, a variety of ncRNAs have been experimentally and clinically reported to participate into cancer progression including PCa. Overall, the importance of ncRNAs has been recognized even though they cannot be translated into protein. This mini review would summarize the roles of several well-known ncRNAs in the pathogenesis of PCa and also give some insights for further studies or clinical applications.
Long non-coding RNAs in PCa
Long non-coding RNAs (lncRNAs) are RNA species with a length of more than 200 nucleotides. After being transcribed by Polymerase II, lncRNAs are 5’ capped and 3’ adenylated [8]. Generally speaking, lncRNA functions to regulate gene expression at the transcriptional level via recruiting chromatin modifying complexes or other transcription factors (Figure 1). LncRNA can also stabilize protein via direct binding (Figure 1). Recent reports demonstrated that some lncRNAs act as sponge to titrate miRNAs, leading their targeting proteins to be post-transcriptionally altered (Figure 1). Additionally, lncRNAs have been well acknowledged as prognostic factor in many cancers. Here, we would review some cellular consequences triggered by the deregulation of several well-known lncRNAs in PCa.
Figure 1.
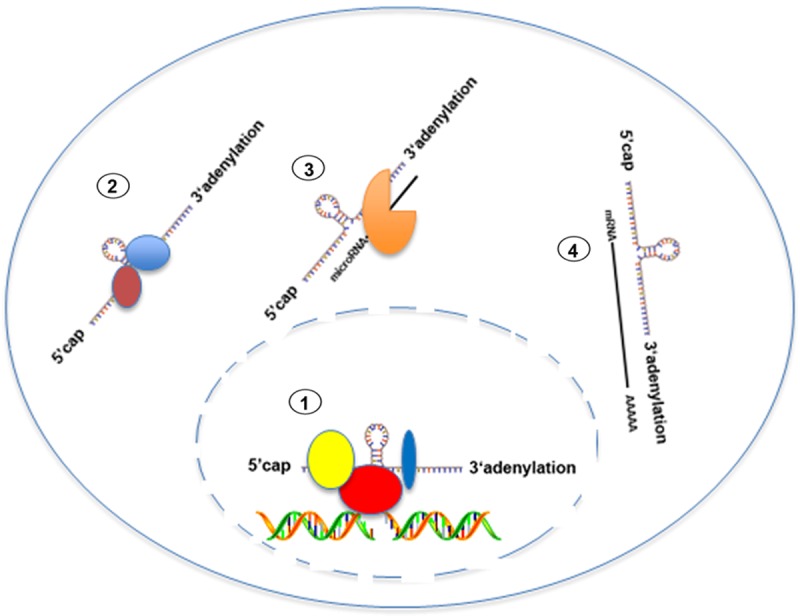
Schematic map of the functions of lncRNAs. LncRNAs regulate gene expression via recruiting chromatin modifying complex, stabilize protein and mRNA via direct binding. LncRNAs still can titrate miRNAs levels.
PCAT-1
Prostate Cancer Associated Transcript 1 (PCAT-1), located on chromosome 8, encodes an lncRNA that is PCa specific. Through high throughout RNA-seq, Prensner found that about 112 lncRNAs were differentially expressed in benign, localized and metastatic PCa [9]. PCAT-1 was further investigated because it was highly upregulated in subset of metastatic and high-grade localized PCa. Indeed, forced expression of PCAT-1 in normal prostate cell line RWPE triggered the cells to transform into cancerous cells. In parallel, knockdown of PCAT-1 in LNCaP cells with independent small interfering RNAs (siRNAs) could cause a decreased cell proliferation, supporting the clinical relevance of PCAT-1 with PCa malignant status.
Unexpectedly, the expression of PCAT-1 is mutually exclusive to that of enhancer of zeste homolog 2 (EZH2), another oncogene that is also enriched in advanced PCa, indicating that PCAT-1 and EZH2 may define two different subsets of advanced PCa. In fact, inhibition of EZH2 by short hairpin RNA (shRNA) or pharmacologic inhibitor (3-deazaneplanocin) in VCaP cells induced PCAT-1 expression level dramatically with a release expression of its repressed genes (i.e. breast cancer 1, BRCA1, centromere protein E, CENP E and centromere protein F, CENP F).
As EZH2 is a component of polycomb repressive complex 2 (PRC2), there is a rational to hypothesize that PCAT-1 may act as one of the downstream genes repressed by PRC2. Indeed, ChIP assay with SUZ12 (another component of PRC2) antibody showed that PRC2 was recruited to the promoter of PCAT-1. Meanwhile, PCAT-1 was also reported to impair double-stranded DNA repair (DSBs) via down-regulating the expression of breast cancer 2 (BRCA2) that was considered as a tumor suppressor by repairing damaged DNA [10,11]. Importantly, loss of BRCA2 or BRCA2 mutation frequently occurs in a variety of cancers including PCa [12]. PCAT-1 mediated reduction of BRCA2 expectedly involved in PCa progression. Intriguingly, PCAT-1 suppressed BRCA2 expression via binding to the 3’UTR of its messenger RNA, which extends our knowledge of lncRNAs and sets an example for future lncRNA exploration.
Given the expression profile of PCAT-1 and EZH2 in metastatic PCa, we thought that anti-EHZ2 drug would ignore another subset of PCa patients who have higher expression levels of PCAT-1. Therefore, a combinational therapy with PCAT-1 and EZH2 would be highly recommended for clinical trials in the future. Furthermore, the importance of PCAT-1 in advanced PCa prompts our expectation of the cross talk between PCAT-1 and androgen receptor signaling.
PCGEM1
As one of the earliest identified lncRNAs, prostate cancer gene expression marker 1 (PCGEM1) located on chromosome 2q32, is over-expressed in 84% PCa patients by in situ hybridization. This might imply that PCGEM1 should act as an onco-lncRNA which is involved in the progression of PCa [13,14]. In a previous study, PCGEM1 was reported to show no protein coding capacity based on the in vitro translation assay and online analysis of TESTCODE program [13]. Further analysis of PCGEM1 level in various PCa cell lines found that such a lncRNA was undetectable in AR-null cell lines such as DU145 and PC3 [13,15]. Accordantly, it could be substantially induced by 10nM synthetic androgen R1881 [15]. All these datas suggest that PCGEM1 should be androgen/androgen receptor dependent. The function of PCGEM1 has not been determined before Petrovics’s group shared their data in oncogene. According to their report [16], forced overexpression of PCGEM1 into LNCaP and NIH3T3 evidently enhanced cell proliferation and their colony-forming capacity. By detecting cell cycle related genes, they demonstrated that the biological function of PCGEM1 was resulted from its ability to stimulate Rb phosphorylation at serine 801 residue, which prevented Rb from binding to E2F and allowed cell to enter into S phase. Nevertheless, the detailed mechanism by which PCGEM1 impairs the inhibitory effect of Rb on cell cycle progression still remains unknown. It is possible that PCGEM1 functions as a scaffold lncRNA which might bring Rb together to let the phosphorylation reaction happen.
Furthermore, Rosenfeld and colleagues in 2013 found that both of PCGEM1 and prostate cancer associated non-coding RNA 1 (PRNCR1) (also named PCAT8) are responsible for AR activation [17]. The result from native RNA immunoprecipitation revealed that both PCGEM1 and PRNCR1 bind to AR, and such an interaction could be further enhanced by dihydrotestosterone propionate (DHT) treatment. Knockdown of PCGEM or PRNCR1 abolished DHT-induced gene expressions and inhibited cell proliferation in vitro. In xenograft model, silenced expression of PCGEM1 or PRNCR1 by doxycycline-induced shRNA also diminished tumor size compared to the control cohorts (scramble shRNA) [17]. The PRNCR1-AR interaction mediated the methylation of AR at K349 by disruptor of telomeric silencing 1-like (DOT1L) at the molecular levels, which was indispensable for the binding of PCGEM1 to the N terminal of AR [17]. ChIP-3C assay confirmed that the recruitment of AR to the enhancer region of its downstream genes required the binding of PRNCR1 and PCGEM1, supporting the idea that PRNCR1 and PCGEM1 make the distant regulation of AR possible by exerting as scaffold lncRNA to connect chromatin structure together. Interestingly, PCGEM1 and PRNCR1 were highly induced in castration resistant PCa. Thereby, whether PCGEM1/PRNCR1 participates in the development of castration resistance especially enzalutamide resistance deserves more efforts in the future scientific exploration. In addition, it is also prompting to block the interaction of PCGEM1/PRNCR1 with AR by synthesis of oligoes in clinical trials.
HOTAIR
HOX transcript antisense RNA (HOTAIR), located within HOX gene cluster, encodes 2.2 kb RNA transcript to influence chromatin state via binding PRC2 at its 5’ termini [18,19]. More importantly, the function of Lysine Specific Demethylase (LSD1) on the epigenetic modification was also coordinated with PRC2 with the assistance of HOTAIR. In clinical practice, HOTAIR has been recognized to be strongly associated with metastasis in various cancers, including breast cancer, renal cell carcinoma, colorectal cancer and PCa [19-25].
Particularly in PCa, Zhang found that HOTAIR expression was indistinguishable between benign prostate and localized PCa, which was attributable to the transcriptional suppression of HOTAIR expression by androgen/AR in androgen dependent PCa [25]. However, upon castration resistance, the dominance of androgen independent pathway in PCa allows cells to release HOTAIR expression from androgen/AR inhibition. Based on these previous clinical findings, Zhang implemented a series of experiments to uncover HOTAIR’s function in PCa. Targeted expression of HOTAIR in androgen-dependent cell line LNCaP could promote cell growth and cell invasion even in the absence of androgen stimulation. Meanwhile, knockdown of HOTAIR in androgen independent cell line C4-2B dramatically suppressed cell proliferation and cell invasion, indicating HOTAIR was tumor-progression driver in castration resistant PCa [25]. Mechanistically, HOTAIR binds to the N terminal of AR with its 5’ end, preventing its interaction with E3 ubiquitination ligase MDM2 [26]. As a result, AR is more stable and more active even without androgen stimulation.
Given the interaction between the HOTAIR and the N terminal of AR, it is possible that HOTAIR may also bind to another AR isoform AR-v7, which has been widely accepted as one of essential driving forces to confer enzalutamide resistance to PCa patients, to mediate castration resistance. Consistently, Zhang found that enzalutamide resistant cells were noticed by their higher induction of HOTAIR level compared to parental cells. In the future, much attention should be paid on how HOTAIR synergizes AR-v7 to control enzalutamide resistance.
MALAT1
MALAT1, also known as metastasis associated lung adenocarcinoma transcript 1, was documented as critical regulator in metastasis, alternative RNA splicing, nuclear organization and epigenetic modification [27,28]. It is worth noting that MALAT1 has been highlighted for its crucial role in the progression of multiple cancers such as lung cancer, PCa, bladder cancer and breast cancer [29-32].
In 2013, Sun and colleagues demonstrated that MALAT1 was significantly elevated in human prostate cancer compared to the adjacent normal prostate tissues, and its expression was highly associated with Gleason stage, tumor stage, prostate specific antigen (PSA) level and castration resistance. All these strongly imply that MALAT1 may function as an onco-lncRNA in PCa [29]. To test MALAT1 function in PCa, they knocked down MALAT1 expression by using siRNA oligoes and observed a reduced cell growth and metastasis in MALAT1 deficient PCa cells. In vivo xenograft animal model, delivery of siRNA against MALAT1 contributed to the delay of tumor growth and inhibition of metastasis. However, the detailed mechanism by which MALAT1 controls cell growth and cell invasion is not clear at that moment.
Recently, Wang discovered that MALAT1 could bind the N terminal of zeste homolog 2 (EZH2), a component of PRC2 complex that is frequently over-expressed in castration resistant prostate cancer, to regulate its methylating activity. SiRNA against MALAT1 could impair EZH2 recruitment to its favorable chromatin locus, leading to the transcriptional suppression of EZH2 downstream genes [33]. Now it is considerably clear that MALAT1 promotes PCa progression by partially modulating EZH2 activity.
Since MALAT1 is up-regulated in castration resistant PCa, scientists have compelling rational to explore MALAT1 status/MALAT1 function in enzalutamide resistant PCa. The fact that MALAT1 is documented as a splicing regulator and AR splice variant 7 (AR-v7) is produced by alternative splicing gives us much scientific imagination. Therefore, more efforts are suggested to put into the exploration of the relationship between MALAT1 and enzalutamide resistance or AR-v7 production.
PCAT3
Development of diagnostic and prognostic biomarkers for PCa is important to reduce overtreatment and prolong overall survival. Serum PSA has been used to predicate the prognosis of PCa since 1994 [34]. Nevertheless, such a marker is organ specific rather than cancer specific, of which the indiscriminate utilization would result in over-diagnosis and over-treatment in many patients. Therefore, it is necessary to develop an efficient marker for the diagnosis of PCa. Of note, prostate cancer antigen 3 PCA3 (also referred as DD3), a prostate tissue specific lncRNA, is developed as an advanced PCa biomarker [35-37]. Compared to PSA, PCA3 is of a lower sensitivity but a higher specificity. Nowadays, urinary detection of PCA3 by Progensa PCA3 has been proved by Food and Drug Administration (FDA) to predict the malignancy of PCa.
However, there is a conflicting evidence when PCAT3 is used to correlate with prognostic factors such as Gleason score and tumor stage, indicating only PCAT3 alone is not adequate and enough to predict PCa status. To improve its prognostic value, the combination of PCAT3 with one fused gene TMPRSS22-ERG, which is induced in advanced PCa, is highly recommended in clinical practice [38]. This combined detection had surprisingly increased the predictive sensitivity by about 20% and significantly reduced the rate of unnecessary biopsies.
Small ncRNAs in PCa
Given the evidence that miRNAs play regulatory roles in the development of various cancers and deregulation of miRNAs could cause adverse consequences, we therefore only focus on the discussion of miRNAs in this mini review. MiRNAs were 20-22 nucleotides RNA molecules derived from so-called “pri-miRNA”. After being transcribed by Pol II, pri-miRNA is cleaved by nuclear RNase III endonuclease Drosha to produce pre-miRNA, which is exported from the nucleus and further processed by another endonuclease Dicer to generate mature miRNA (Figure 2) [39,40]. Functionally, miRNA directly binds to the 3’ UTR of mRNA via incomplete base pairing and inhibits its translation. It is estimated that 60% mRNAs can be regulated by miRNAs [41]. Extensive studies have demonstrated that aberrant miRNA expression could contribute to cancer carcinogenesis including PCa [42,43]. Meanwhile, several miRNAs have been highlighted for their crucial roles in determining PCa survival and metastasis.
Figure 2.
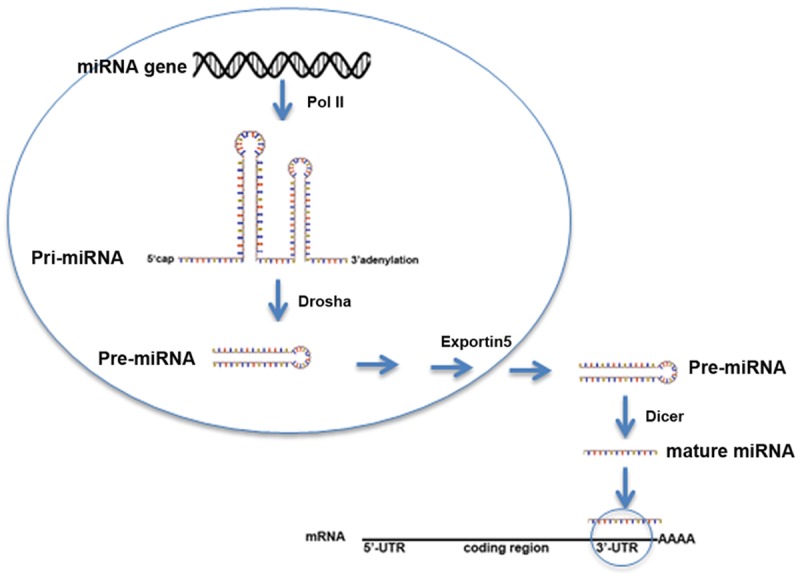
Schematic model of biogenesis of miRNA. After being transcribed by Pol II, pri-miRNA is further digested by Drosha to generate pre-miRNA. Pre-miRNA is exported to cytosol by exportin5, where it is processed by Dicer to form mature miRNA.
MIR-205
One of the well-documented miRNAs in PCa is miR-205, which is substantially decreased in prostate cancer compared to the matched benign PCa. Its level was further decreased in advanced or metastatic PCa [44]. In cancerous cells, the folded chromosomal structure led by hyper-methylation on miR-205 locus contributed to its silenced expression. Indeed, addition of 5-Aza, one de-methylating reagent, into LNCaP caused dramatic induction of miR-205, which is associated with a reduced cell proliferation. Therefore, the methylation status in miR-205 promoter has been considered as a predictive biomarker for advanced or recurrent PCa.
Based on online software prediction and experimental results, miR-205 targeting genes are involved in signaling pathways which are related to MAPK (mitogen activated protein kinase), androgen receptor, EMT (epithelial to mesenchymal transition), migration and invasion [45]. For instance, zinc finger E-box binding homeobox 1 (ZEB1) and zinc finger E-box binding homeobox 2 (ZEB2), two crucial molecules in governing EMT transition, were directly suppressed by miR-205 [46]. Androgen receptor, one of the most importantly regulatory factors in PCa, was also post-transcriptionally inhibited by miR-205 [47]. Since up-regulation of miR-205 provides beneficial effects to PCa cells, the study of its upstream pathways looks attractive for scientists. Recent findings suggested that P63 showed anti-tumor activity via transcriptional activation of miR-205 in PC3 cells [48]. More efforts should be taken to dissect how miR-205 is regulated in PCa.
On this basis, development of new drugs that could induce miR-205 expression is appealing for the treatment of PCa. The 5-Aza may be one candidate but is not the perfect one due to its off-targeting effect. Therefore, identification of the specific methylating enzyme responsible for the hyper-methylation of miR-205 promoter is of necessity to design matched drug to alter miR-205 status without attendant toxicity.
MIR-34a
Just like the other cancers, PCa is also heterogeneous, composing of various types of cells including epithelial cells, fibroblasts, immune cells and cancer stem cells (CSCs) [49]. Prostate cancer stem cells (PCSCs) are potently tumorigenic because of their strong self-renewal capability and potential differentiation ability [49,50]. More importantly, PCSCs are relatively resistant to ADT and chemotherapy treatment due to their AR-negative characteristics [51].
To explore the function of miRNAs in PCSCs, Liu found that miR-34a was under-expressed in isolated CD44 molecule (CD44+) cancer stem cells derived from either xenografts or primary tumors compared to their relative CD44- parental cells [52]. Enforced expression of miR-34a in CD44+ cells could suppress their sphere-forming ability, tumor regeneration and cell invasion. Consistently, introduction of miR-34a inhibitor into CD44- cells could redirect cells to be stem cell like [53]. In xenograft model, systematic delivery of miR-34a delayed tumor growth and inhibited metastasis. The mechanistic dissection found that CD44 was a direct target of miR-34a. A decreased level of miR-34a in CD44+ cells guaranteed CD44 expression level and maintained their tumorigenic status. All these data suggest that miR-34a could be served as a therapeutic target for PCa, PCSCs in particular.
MiR-34a is one of the downstream targets mediated by p53 [54,55]. Therefore, researchers should have compelling rational to investigate the role of p53 in balancing the homeostasis of PCSCs. Also, prostate cancer stem cells were also considered as AR-negative cells. Is there any cross-talk between p53 and AR signaling in PCSCs to regulate miR-34a expression level? All these questions deserve further studies.
AR/AR-v7 associated miRNAs
Enormous efforts have been paid to develop methods for inhibiting AR/AR signaling given the roles of AR/AR signaling in determining the pathology of PCa. ADT, anti-androgen and anti-AR are all well-defined methods to block AR/AR signaling in PCa. However, further studies are still needed to develop miRNA-based therapeutic approaches to target AR in order to fight against PCa.
To systematically analyze AR-based miRNAs, Ostling applied LMA screening using 2 miRNA libraries with 1129 miRNAs to identify the miRNA candidates which have potential ability to target AR via binding to its 3’ UTR [56]. Data showed that about 77 miRNAs were reported to negatively affect AR protein level in 5 cell lines. After validation by 3’UTR based assay, 14 out 77 miRNAs (miR-135b, miR-185, miR-297, miR-299-3p, miR-34a, miR-9, miR-34c, miR-654-5p, miR-634, miR-449b, miR-371-3p, miR-421, miR-449a, miR-449b) were reported to bind 3’UTR of AR directly and inhibited cell proliferation of LNCaP and CWR22Rv1. Since AR-v7 had its unique 3’UTR, its regulatory miRNAs were supposed to be different from these against full length AR. In fact, miR-124 was the only reported one that directly targeted AR-v7 [57]. In vitro study showed that miR-124 evidently suppressed cell proliferation of CWR22Rv1 cells via degrading AR-v7 protein level. As AR-v7 is a critical molecule that confers enzalutamide resistance to PCa, miR-124 could re-sensitize enzalutamide resistance by probably altering AR-v7 level. In vivo intravenous delivery of miR-124 could increase tumor apoptosis in combination with enzalutamide administration. Taken together, these findings would build up strong rational to develop miRNAs as therapeutic approach to fight against PCa via reducing AR/AR-v7 level.
MIR-15A-MIR-16-1
Allelic loss of miR-15a-miR-16-1 on chromosome 13 was viewed as predictive signature of metastatic PCa. Previous investigation showed that introduction of miR-15a-miR-16-1 inhibitor into non transformed prostate cells promoted cell carcinogenesis [58]. Delivery of mR-15a-miR-16-1 antagomirs into normal mouse prostate could induce hyperplasia. Conversely, over-expression of miR-15a-miR-16-1 in prostate cancer cells resulted in remarkable growth arrest, cell apoptosis and tumor shrinkage of tumor bearing xenografts. MiR-15a-miR-16-1 cluster could reduce CCND1, WNT3A and BCL2 via directly binding to their 3’UTRs, as well as preventing their translation. Furthermore, miR-15a level was considered to be highly associated with cMYB function in PCa. MiR-15a reconstitution induced a significant down-regulation of cMYB expression and an evident up-regulation of AR in androgen dependent LNCaP cell lines, suggesting that the deficiency of miR-15a in PCa cells contributed to cMYB-induced cell progression. However, two questions are raised from this report and deserves further investigation: does miR-15a directly regulate the cMYB via base pairing binding; what’s the underlying mechanism by which miR-15a up-regulates AR.
Summary
Novel target-based drugs are urgently needed for better improving the treatment of PCa. NcRNAs, including long noncoding RNAs and miRNAs have been extensively investigated in recent years. The aberrant expression of ncRNA caused by either genomic modification or transcriptional regulation was highly related to PCa progression. Even though targeting ncRNAs are effective in both the cell line experiment and animal model study, the application of ncRNAs into clinical trial is still far away from satisfaction. The off-target effect and the low efficiency of small RNA delivery are currently two major obstacles to prevent ncRNAs from further clinical application.
The exploration on ncRNAs is still in its infancy. On one hand, the biological processes ncRNAs involved are largely uncovered. Earlier reports have mentioned that some ncRNAs could be translocated to certain organelle such as mitochondria or endoplasmic reticulum [59,60], whereas they possibly control certain biological events that requires our exploration. On the other hand, for those lncRNAs functioning to control gene regulation, their regulatory codes remain largely unknown. The map of lncRNA-regulated downstream genes and the DNA binding code are still mysterious to us. More investigations are required to solve these problems if we want to translate lncRNA functions into clinical use.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81271692), the Ocean Antithrombotic Fibrinolytic Enzyme Gene Bank of Taiwan Strait (No. 2014FJPT08), the Science and Technology Innovation Public Technology Service Platform of Function of Drugs and Food (No. 3502Z20141015), Huaqiao University (14BS111) and Education Department of Fujian Province (JA14020).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermuller J, Hofacker IL, Bell I, Cheung E, Drenkow J, Dumais E, Patel S, Helt G, Ganesh M, Ghosh S, Piccolboni A, Sementchenko V, Tammana H, Gingeras TR. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 4.Heo JB, Lee YS, Sung S. Epigenetic regulation by long noncoding RNAs in plants. Chromosome Res. 2013;21:685–693. doi: 10.1007/s10577-013-9392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012;338:1435–1439. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez I, Munita R, Agirre E, Dittmer TA, Gysling K, Misteli T, Luco RF. A lncRNA regulates alternative splicing via establishment of a splicing-specific chromatin signature. Nat Struct Mol Biol. 2015;22:370–376. doi: 10.1038/nsmb.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li JH, Liu S, Zheng LL, Wu J, Sun WJ, Wang ZL, Zhou H, Qu LH, Yang JH. Discovery of Protein-lncRNA Interactions by Integrating Large-Scale CLIP-Seq and RNA-Seq Datasets. Front Bioeng Biotechnol. 2014;2:88. doi: 10.3389/fbioe.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolton EM, Tuzova AV, Walsh AL, Lynch T, Perry AS. Noncoding RNAs in prostate cancer: the long and the short of it. Clin Cancer Res. 2014;20:35–43. doi: 10.1158/1078-0432.CCR-13-1989. [DOI] [PubMed] [Google Scholar]
- 9.Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso CS, Kominsky HD, Cao X, Jing X, Wang X, Siddiqui J, Wei JT, Robinson D, Iyer HK, Palanisamy N, Maher CA, Chinnaiyan AM. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29:742–749. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prensner JR, Chen W, Iyer MK, Cao Q, Ma T, Han S, Sahu A, Malik R, Wilder-Romans K, Navone N, Logothetis CJ, Araujo JC, Pisters LL, Tewari AK, Canman CE, Knudsen KE, Kitabayashi N, Rubin MA, Demichelis F, Lawrence TS, Chinnaiyan AM, Feng FY. PCAT-1, a long noncoding RNA, regulates BRCA2 and controls homologous recombination in cancer. Cancer Res. 2014;74:1651–1660. doi: 10.1158/0008-5472.CAN-13-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004;95:866–871. doi: 10.1111/j.1349-7006.2004.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorne H, Willems AJ, Niedermayr E, Hoh IM, Li J, Clouston D, Mitchell G, Fox S, Hopper JL Kathleen Cunningham Consortium for Research in Familial Breast Cancer Consortium. Bolton D. Decreased prostate cancer-specific survival of men with BRCA2 mutations from multiple breast cancer families. Cancer Prev Res (Phila) 2011;4:1002–1010. doi: 10.1158/1940-6207.CAPR-10-0397. [DOI] [PubMed] [Google Scholar]
- 13.Srikantan V, Zou Z, Petrovics G, Xu L, Augustus M, Davis L, Livezey JR, Connell T, Sesterhenn IA, Yoshino K, Buzard GS, Mostofi FK, McLeod DG, Moul JW, Srivastava S. PCGEM1, a prostate-specific gene, is overexpressed in prostate cancer. Proc Natl Acad Sci U S A. 2000;97:12216–12221. doi: 10.1073/pnas.97.22.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ifere GO, Ananaba GA. Prostate cancer gene expression marker 1 (PCGEM1): a patented prostate-specific non-coding gene and regulator of prostate cancer progression. Recent Pat DNA Gene Seq. 2009;3:151–163. doi: 10.2174/187221509789318360. [DOI] [PubMed] [Google Scholar]
- 15.Parolia A, Crea F, Xue H, Wang Y, Mo F, Ramnarine VR, Liu HH, Lin D, Saidy NR, Clermont PL, Cheng H, Collins C, Wang Y, Helgason CD. The long non-coding RNA PCGEM1 is regulated by androgen receptor activity in vivo. Mol Cancer. 2015;14:46. doi: 10.1186/s12943-015-0314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrovics G, Zhang W, Makarem M, Street JP, Connelly R, Sun L, Sesterhenn IA, Srikantan V, Moul JW, Srivastava S. Elevated expression of PCGEM1, a prostate-specific gene with cell growth-promoting function, is associated with high-risk prostate cancer patients. Oncogene. 2004;23:605–611. doi: 10.1038/sj.onc.1207069. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Lin C, Jin C, Yang JC, Tanasa B, Li W, Merkurjev D, Ohgi KA, Meng D, Zhang J, Evans CP, Rosenfeld MG. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature. 2013;500:598–602. doi: 10.1038/nature12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorensen KP, Thomassen M, Tan Q, Bak M, Cold S, Burton M, Larsen MJ, Kruse TA. Long non-coding RNA HOTAIR is an independent prognostic marker of metastasis in estrogen receptor-positive primary breast cancer. Breast Cancer Res Treat. 2013;142:529–536. doi: 10.1007/s10549-013-2776-7. [DOI] [PubMed] [Google Scholar]
- 20.Pei CS, Wu HY, Fan FT, Wu Y, Shen CS, Pan LQ. Influence of curcumin on HOTAIR-mediated migration of human renal cell carcinoma cells. Asian Pac J Cancer Prev. 2014;15:4239–4243. doi: 10.7314/apjcp.2014.15.10.4239. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Liu J, Zheng Y, You L, Kuang D, Liu T. Suppressed expression of long non-coding RNA HOTAIR inhibits proliferation and tumourigenicity of renal carcinoma cells. Tumour Biol. 2014;35:11887–11894. doi: 10.1007/s13277-014-2453-4. [DOI] [PubMed] [Google Scholar]
- 22.Chiyomaru T, Fukuhara S, Saini S, Majid S, Deng G, Shahryari V, Chang I, Tanaka Y, Enokida H, Nakagawa M, Dahiya R, Yamamura S. Long non-coding RNA HOTAIR is targeted and regulated by miR-141 in human cancer cells. J Biol Chem. 2014;289:12550–12565. doi: 10.1074/jbc.M113.488593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, Miyano S, Mori M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 24.Xue Y, Gu D, Ma G, Zhu L, Hua Q, Chu H, Tong N, Chen J, Zhang Z, Wang M. Genetic variants in lncRNA HOTAIR are associated with risk of colorectal cancer. Mutagenesis. 2015;30:303–310. doi: 10.1093/mutage/geu076. [DOI] [PubMed] [Google Scholar]
- 25.Zhang A, Zhao JC, Kim J, Fong KW, Yang YA, Chakravarti D, Mo YY, Yu J. LncRNA HOTAIR Enhances the Androgen-Receptor-Mediated Transcriptional Program and Drives Castration-Resistant Prostate Cancer. Cell Rep. 2015;13:209–221. doi: 10.1016/j.celrep.2015.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong L, Yuan Q, Zhu H, Li Y, Guo Q, Wang Q, Bi X, Gao X. The suppression of prostate LNCaP cancer cells growth by Selenium nanoparticles through Akt/Mdm2/AR controlled apoptosis. Biomaterials. 2011;32:6515–6522. doi: 10.1016/j.biomaterials.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, Blencowe BJ, Prasanth SG, Prasanth KV. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripathi V, Shen Z, Chakraborty A, Giri S, Freier SM, Wu X, Zhang Y, Gorospe M, Prasanth SG, Lal A, Prasanth KV. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet. 2013;9:e1003368. doi: 10.1371/journal.pgen.1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren S, Liu Y, Xu W, Sun Y, Lu J, Wang F, Wei M, Shen J, Hou J, Gao X, Xu C, Huang J, Zhao Y, Sun Y. Long noncoding RNA MALAT-1 is a new potential therapeutic target for castration resistant prostate cancer. J Urol. 2013;190:2278–2287. doi: 10.1016/j.juro.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Xu S, Sui S, Zhang J, Bai N, Shi Q, Zhang G, Gao S, You Z, Zhan C, Liu F, Pang D. Downregulation of long noncoding RNA MALAT1 induces epithelial-to-mesenchymal transition via the PI3K-AKT pathway in breast cancer. Int J Clin Exp Pathol. 2015;8:4881–4891. [PMC free article] [PubMed] [Google Scholar]
- 31.Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, Liu Y. TGF-beta-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res. 2014;20:1531–1541. doi: 10.1158/1078-0432.CCR-13-1455. [DOI] [PubMed] [Google Scholar]
- 32.Guo F, Guo L, Li Y, Zhou Q, Li Z. MALAT1 is an oncogenic long non-coding RNA associated with tumor invasion in non-small cell lung cancer regulated by DNA methylation. Int J Clin Exp Pathol. 2015;8:15903–15910. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D, Ding L, Wang L, Zhao Y, Sun Z, Karnes RJ, Zhang J, Huang H. LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget. 2015;6:41045–41055. doi: 10.18632/oncotarget.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O’Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD PLCO Project Team. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bussemakers MJ, van Bokhoven A, Verhaegh GW, Smit FP, Karthaus HF, Schalken JA, Debruyne FM, Ru N, Isaacs WB. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999;59:5975–5979. [PubMed] [Google Scholar]
- 36.Auprich M, Chun FK, Ward JF, Pummer K, Babaian R, Augustin H, Luger F, Gutschi S, Budaus L, Fisch M, Huland H, Graefen M, Haese A. Critical assessment of preoperative urinary prostate cancer antigen 3 on the accuracy of prostate cancer staging. Eur Urol. 2011;59:96–105. doi: 10.1016/j.eururo.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145:907–923. doi: 10.1016/s0022-5347(17)38491-4. [DOI] [PubMed] [Google Scholar]
- 38.Lin DW, Newcomb LF, Brown EC, Brooks JD, Carroll PR, Feng Z, Gleave ME, Lance RS, Sanda MG, Thompson IM, Wei JT, Nelson PS Canary Prostate Active Surveillance Study Investigators. Urinary TMPRSS2:ERG and PCA3 in an active surveillance cohort: results from a baseline analysis in the Canary Prostate Active Surveillance Study. Clin Cancer Res. 2013;19:2442–2450. doi: 10.1158/1078-0432.CCR-12-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Duarte RJ, Cazares-Ordonez V, Avila-Chavez E. The microRNA biogenesis machinery: regulation by steroid hormones and alterations in cancer. Rev Invest Clin. 2014;66:460–464. [PubMed] [Google Scholar]
- 41.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33:1126–1133. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khanmi K, Ignacimuthu S, Paulraj MG. MicroRNA in prostate cancer. Clin Chim Acta. 2015;451:154–160. doi: 10.1016/j.cca.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 44.Hulf T, Sibbritt T, Wiklund ED, Patterson K, Song JZ, Stirzaker C, Qu W, Nair S, Horvath LG, Armstrong NJ, Kench JG, Sutherland RL, Clark SJ. Epigenetic-induced repression of microRNA-205 is associated with MED1 activation and a poorer prognosis in localized prostate cancer. Oncogene. 2013;32:2891–2899. doi: 10.1038/onc.2012.300. [DOI] [PubMed] [Google Scholar]
- 45.Boll K, Reiche K, Kasack K, Morbt N, Kretzschmar AK, Tomm JM, Verhaegh G, Schalken J, von Bergen M, Horn F, Hackermuller J. MiR-130a, miR-203 and miR-205 jointly repress key oncogenic pathways and are downregulated in prostate carcinoma. Oncogene. 2013;32:277–285. doi: 10.1038/onc.2012.55. [DOI] [PubMed] [Google Scholar]
- 46.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 47.Hagman Z, Haflidadottir BS, Ceder JA, Larne O, Bjartell A, Lilja H, Edsjo A, Ceder Y. miR-205 negatively regulates the androgen receptor and is associated with adverse outcome of prostate cancer patients. Br J Cancer. 2013;108:1668–1676. doi: 10.1038/bjc.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tucci P, Agostini M, Grespi F, Markert EK, Terrinoni A, Vousden KH, Muller PA, Dotsch V, Kehrloesser S, Sayan BS, Giaccone G, Lowe SW, Takahashi N, Vandenabeele P, Knight RA, Levine AJ, Melino G. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci U S A. 2012;109:15312–15317. doi: 10.1073/pnas.1110977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith BA, Sokolov A, Uzunangelov V, Baertsch R, Newton Y, Graim K, Mathis C, Cheng D, Stuart JM, Witte ON. A basal stem cell signature identifies aggressive prostate cancer phenotypes. Proc Natl Acad Sci U S A. 2015;112:E6544–6552. doi: 10.1073/pnas.1518007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo J, Lee SO, Cui Y, Yang R, Li L, Chang C. Infiltrating bone marrow mesenchymal stem cells (BM-MSCs) increase prostate cancer cell invasion via altering the CCL5/HIF2alpha/androgen receptor signals. Oncotarget. 2015;6:27555–27565. doi: 10.18632/oncotarget.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharifi N, Kawasaki BT, Hurt EM, Farrar WL. Stem cells in prostate cancer: resolving the castrate-resistant conundrum and implications for hormonal therapy. Cancer Biol Ther. 2006;5:901–906. doi: 10.4161/cbt.5.8.2949. [DOI] [PubMed] [Google Scholar]
- 52.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han L, Shi S, Gong T, Zhang Z, Sun X. Cancer stem cells: therapeutic implications and perspectives in cancer therapy. Acta Pharmaceutica Sinica B. 2013;3:65–75. [Google Scholar]
- 54.Tan J, Fan L, Mao JJ, Chen B, Zheng L, Zhang T, Li T, Duan J, Duan Y, Jin Z, Kuang W. Restoration of miR-34a in p53 deficient cells unexpectedly promotes the cell survival by increasing NFkappaB activity. J Cell Biochem. 2012;113:2903–2908. doi: 10.1002/jcb.24167. [DOI] [PubMed] [Google Scholar]
- 55.Xiong H, Pang J, Yang H, Dai M, Liu Y, Ou Y, Huang Q, Chen S, Zhang Z, Xu Y, Lai L, Zheng Y. Activation of miR-34a/SIRT1/p53 signaling contributes to cochlear hair cell apoptosis: implications for age-related hearing loss. Neurobiol Aging. 2015;36:1692–1701. doi: 10.1016/j.neurobiolaging.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 56.Ostling P, Leivonen SK, Aakula A, Kohonen P, Makela R, Hagman Z, Edsjo A, Kangaspeska S, Edgren H, Nicorici D, Bjartell A, Ceder Y, Perala M, Kallioniemi O. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res. 2011;71:1956–1967. doi: 10.1158/0008-5472.CAN-10-2421. [DOI] [PubMed] [Google Scholar]
- 57.Shi XB, Ma AH, Xue L, Li M, Nguyen HG, Yang JC, Tepper CG, Gandour-Edwards R, Evans CP, Kung HJ, deVere White RW. miR-124 and Androgen Receptor Signaling Inhibitors Repress Prostate Cancer Growth by Downregulating Androgen Receptor Splice Variants, EZH2, and Src. Cancer Res. 2015;75:5309–5317. doi: 10.1158/0008-5472.CAN-14-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L, D’Urso L, Pagliuca A, Biffoni M, Labbaye C, Bartucci M, Muto G, Peschle C, De Maria R. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14:1271–1277. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 59.Bianchessi V, Badi I, Bertolotti M, Nigro P, D’Alessandra Y, Capogrossi MC, Zanobini M, Pompilio G, Raucci A, Lauri A. The mitochondrial lncRNA ASncmtRNA-2 is induced in aging and replicative senescence in Endothelial Cells. J Mol Cell Cardiol. 2015;81:62–70. doi: 10.1016/j.yjmcc.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 60.van Heesch S, van Iterson M, Jacobi J, Boymans S, Essers PB, de Bruijn E, Hao W, MacInnes AW, Cuppen E, Simonis M. Extensive localization of long noncoding RNAs to the cytosol and mono- and polyribosomal complexes. Genome Biol. 2014;15:R6. doi: 10.1186/gb-2014-15-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]


