Abstract
Neuroglioma is the most common primary malignant tumor in neurosurgery. Due to unfavorable life quality of patients, the treatment of glioma is a major challenge in clinics. The search for effect treatment drugs thus benefits patient prognosis. As one derivative of resveratrol, pterostilbene has a wide spectrum of pharmaceutical functions, especially with the anti-tumor effects. This study thus investigated the effect of pterostilbene on neuroglioma and related mechanisms. U87 glioma cell line was divided into control, normal culture and different dosages of pterostilbene groups, which received 5 mM or 10 mM pterostilbene for 48 h. MTT assay was used to detect U87 cell proliferation, while invasion assay was employed to test the effect of pterostilbene on cell invasion, followed by flow cytometry assay for analyzing U87 cell apoptosis. Real-time PCR was used to test mRNA expression of Bcl-2 and Bax in glioma cells under the effect of pterostilbene, while Western blotting was used to detect alternation of Bcl-2 and Bax protein levels. Pterostilbene significantly inhibited proliferation and invasion abilities of glioma cells compared to those in control group (P<0.05). It can also enhance cell apoptosis, decrease mRNA and protein of Bcl-2 expression, and increase mRNA and protein expressions of Bax (P<0.05 compared to control group) in a dose-dependent manner. Pterostilbene can facilitate apoptosis of glioma cells, and inhibit their proliferation and invasion via mediating apoptotic/anti-apoptotic homeostasis.
Keywords: Glioma, pterostilbene, Bcl-2, Bax, cell proliferation, cell invasion
Introduction
Neuroglioma is the most common malignant tumor in neurosurgery, and has a high incidence among all intracranial tumors, with elevated occurrence rate and younger age of onset [1]. Among all primary brain tumors, neuroglioma has high invasiveness and unfavorable prognosis, leading to short lifespan and worse life quality [2,3]. Neuroglioma has now become the most popular tumor in central nervous system in China [4]. Glioma has a rapid disease progression, and can cause different clinical symptoms due to the size and location of intracranial tumors [5]. Due to the space occupying effect, intracranial pressure (ICP) may be elevated, accompanied with neural symptoms [6]. Although treatment for brain glioma has been developed with major progress, radical surgery is still a major challenge due to complicated mechanism [7,8]. Residual tumor may again proliferation, leading to its invasive growth, severely compromising treatment efficacy and patient life quality, thus making the treatment of glioma as one major challenge worldwide [9]. Therefore, the development of effective treatment medicine could help to improve patient’s prognosis.
Previous studies have found the pluripotent role of resveratrol, including anti-oxidation, anti-bacterial, anti-tumor, modulating vascular dilation, inhibiting platelet coagulation, mediating lipoprotein metabolism and enhancing body immune defense [10,11]. As one derivative of resveratrol, pterostilbene is one non-flavonoid polyphenol compound that is enriched in grapes, nuts, strawberries, Guangxi Xuexi and propolis. As one poly-hydroxyl-diphenyl ethylene compound of resveratrol [12,13], pterostilbene has similar pharmaceutical role as those of resveratrol. In a wide spectrum of functions, pterostilbene could exert certain roles against fungal infection, bacteria, mediating cell proliferation and growth, modulating lipid metabolism and participating in oxidative/reductive reaction or anti-inflammation. Current study has confirmed its effective roles in treating hypoxia-ischemia brain disease, Alzheimer’s disease and tumors. With superior biological activity and selectivity over resveratrol, pterostilbene mainly exerts anti-inflammation, anti-oxidation and anti-tumors [14,15]. This study thus investigated the role of pterostilbene on glioma cells and related mechanisms.
Materials and methods
Reagent and equipment
Human glioma U87 cell line was purchased from ATCC cell bank (US). Pterostilbene was purchased from Fujistu (Japan). DMEM medium, fetal bovine serum (FBS), and streptomycin-penicillin were purchased from Hyclone (US). DMSO and MTT powders were purchased from Gibco (US). Trypsin-EDTA lysis buffer was purchased from Sigma (US). Caspase 3 activity assay kit and PVDF membrane were purchased from Pall Life Sciences (US). EDTA was purchased from Hyclone (US). Western blotting reagent was purchased from Beyotime (China). ECL reagent was purchased from Amersham Biosciences (US). Rabbit anti-human Bcl-2 monoclonal antibody, rabbit anti-human Bax monoclonal antibody, and mouse anti-rabbit horseradish peroxidase (HRP)-conjugated IgG secondary antibody were all purchased from Cell Signaling (US). Transwell chamber was purchased from Corning (US). RNA extraction kit and reverse transcription kit were purchased from Axygen (US). Annexin V-FITC apoptotic assay kit was purchased from BD (US). FACA Calibur flow cytometry apparatus was purchased from BD (US). Labsystem Version 1.3.1 microplate reader was purchased from Bio-rad (US).
Glioma U87 cell culture and grouping
U87 cells kept in liquid nitrogen were resuscitated in 37°C water-bath until fully thawing. Cells were centrifuged at 1000 rpm for 3 min, and were re-suspended in 1 ml fresh medium and were removed to 5 ml culture flask which contained 3 ml fresh culture medium. Cells were kept in a humidified chamber with 5% CO2 at 37°C for 24~48 h. U87 cells were seeded in 6-well plate at 1×105 per cm2. Cells at log-phase with 2nd to 8th generation were randomly divided into control, normal culture and high/low dosage of pterostilbene (5 mM or 10 mM) groups in 48 h continuous culture.
MTT assay for cell proliferation
U87 cells at log-phase were seeded into 96-well plate which contained DMEM medium with 10% FBS at 5×103 density. After 24 h incubation, the supernatant was removed. Cells were randomly divided into control or high/low pterostilbene groups, which were treated as abovementioned. In brief, 20 μl sterile MTT was added into each test well in triplicates after 48 h cell culture. After 4 h continuous cultivation, the supernatant was removed, with the addition of 150 μl DMSO for 10 min vortex until the complete resolving of crystal violet. Absorbance (A) values was measured at 570 nm in a microplate reader. The proliferation rate was calculated in each group. Each experiment was repeated for more than three times.
Transwell assay for cell invasion
Following the manual instruction, serum-free culture medium was used for 24 h cell culture. Transwell chamber was pre-coated using 1:5 50 mg/L Matrigel dilutions on the bottom and upper layer of the membrane, followed by 4°C air-dry. 500 μl DMEM culture medium containing 10% FBS was then added into inner and outer surface of the chamber, which contained 100 μl tumor cell suspensions prepared by serum-free culture medium in triplicates. The chamber was placed in a 24-well plate. Control cells were cultured in Transwell chamber without Matrigel. After 48 h, PBS was used to rinse Transwell chamber, with the removal of membrane-fixed cells, which were then fixed in cold ethanol and stained by crystal violet. The number of cells at the lower surface of the micro-pore membrane was then counted in triplicates (N=3).
Flow cytometry measuring U87 cell apoptosis
Tumor cells were digested, counted and inoculated into 50 ml culture flask at 5×105/mL concentration, and were randomly divided into three groups as abovementioned (N=3 each). After 48 h transfection, cells were counted, and collected at 2×106 per ml. After centrifugation in 1× PBS at 1000 rpm for 5 min, cells were rinsed and fixed in 75% cold ethanol for 4°C overnight incubation. After discarding 75% ethanol, cells were-centrifuged in 1× PBS at 1000 rpm for 1 min, followed by rinsing and re-suspension in 800 μl 1× PBS containing 1% BSA, with the addition of 100 μg/ml PI dye (in 3.8% Na-citrate, pH 7) and 100 RNAase A (10 mg/ml) for 37°C dark incubation (30 min). Flow cytometry apparatus was used to test cell apoptosis, with the data analysis by FCSExpress3.0 software.
Real-time PCR for measuring Bcl-2 and Bax mRNA expression
Trizol reagent was used to extract RNA from all groups for cells. Reverse transcription was performed according to the manual instruction, using primers designed by Primer6.0 and synthesized by Invitrogen, Shanghai (China) as shown in Table 1. Real-time PCR was performed on target genes under the following conditions: 55°C for 1 min, followed by 35 cycles each containing 92°C for 30 s, 58°C for 45 s, and 72°C for 35 s. Data were collected and calculated for CT values of all samples and standards based on fluorescent quantification using GAPDH as the baseline. Standard curve was firstly plotted using CT values of standards, followed by semi-quantitative analysis by 2-ΔCt method.
Table 1.
Primer sequence
| Target gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| GAPDH | ACCAGGTATCTGCTGGTTG | TAACCATGATGTCAGCGTGGT |
| Bcl-2 | ATCATGGTATGATGGACCCCGCAACTTC | ATTCGAAGTCGCACAGCAGCTGGTC |
| Bax | CTTAGTGGTCTCTGTATGACGT | TCACCCTCTCACAGCTTGAGTCGA |
Western blotting
U87 cell proteins were firstly extracted. In brief, RIPA lysis buffer containing proteinase inhibitor was used to lyse cells on ice for 15~30 min, followed by ultrasound rupture (5 s×4) and centrifugation (4°C, 10000 g, 15 min). Supernatants were saved and quantified for protein contents, and were stored at -20°C for further Western blotting. Proteins were then separated using 10% SDS-PAGE gel, and were transferred to PVDF membrane using semi-dry method. Non-specific background was removed by 5% defatted milk powder at room temperature for 2 h, followed by the addition of anti-Bcl-2 monoclonal antibody (1:1000 dilution) in 4°C overnight incubation. On the next day, the membrane was rinsed in PBST, and incubated with 1:2000 goat anti-rabbit secondary antibody for 30 min incubation. After PBST rinsing, ECL reagent was used to develop the membrane, which was exposed under X-ray for observing results. Protein imaging analysis system and Quantity One software were used to scan X-ray films for observing band density. Each experiment was repeated for four times (N=4) for further analysis.
Statistical analysis
All data were presented as mean ± standard deviation (SD). Student t-test was used to compare means between two groups. SPSS11.5 software was used in statistical analysis. Analysis of variance (ANOVA) was used for between-group analysis. A statistical significance was identified when P<0.05.
Results
Effect of pterostilbene on glioma cell proliferation
We employed MTT assay to check the mediation of pterostilbene on glioma U87 cell proliferation. Results showed the significant inhibition of pterostilbene on cell proliferation (P<0.05) in a positive dose-dependent manner, as higher pterostilbene enhanced the inhibitory effect on tumor cell proliferation (Figure 1). These results suggested that pterostilbene could inhibit proliferation and survival of glioma cells.
Figure 1.
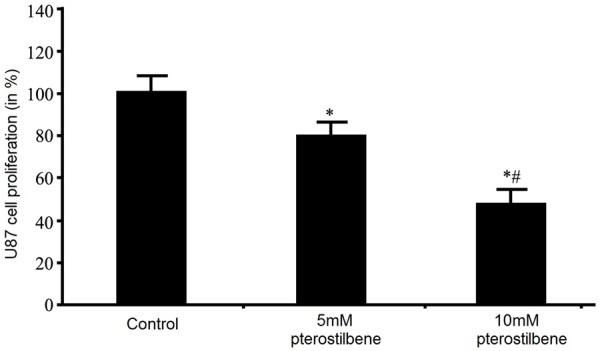
Effect of pterostilbene on glioma cell proliferation. *P<0.05 compared to control group; #P<0.05 compared to 5 mM pterostilbene group.
Effects of pterostilbene on glioma cell invasion
We performed cell invasion assay using Transwell chamber under the influence of pterostilbene. Results showed that, after 48 h of pterostilbene treatment on glioma U87 cells, the invasion potency of tumor cells was remarkably suppressed (P<0.05 compared to control group). With elevated pterostilbene dosage, such inhibition was further potentiated (Figures 2 and 3), suggesting that pterostilbene could inhibit tumor growth via suppressing glioma cell invasion.
Figure 2.
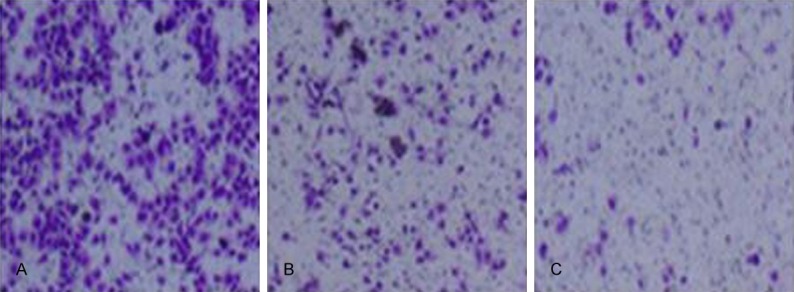
Effect of pterostilbene on glioma cell invasion. A. Control group; B. 5 mM pterostilbene group; C. 10 mM pterostilbene group.
Figure 3.
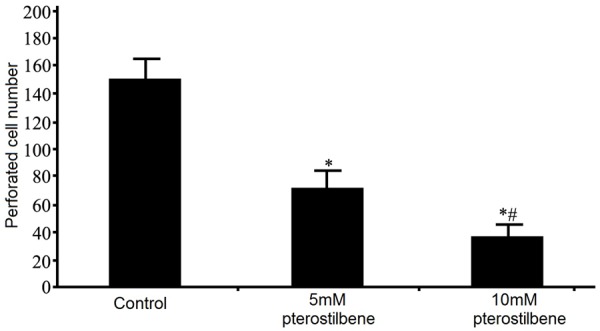
Analysis of pterostilbene effect on invasion of glioma cells. *P<0.05 compared to control group; #P<0.05 compared to 5 mM pterostilbene group.
Effect of pterostilbene on glioma cell apoptosis
Flow cytometry was used to analyze the effect of pterostilbene on apoptosis of U87 cells. Results showed that after 48 h of pterostilbene treatment, the apoptosis of glioma cells was significantly inhibited (P<0.05 compared to control group). With elevated pterostilbene concentration, such potentiation effect on tumor cell apoptosis was further enhanced (Figures 4 and 5). These results thus indicated the induction of cell apoptosis by pterostilbene.
Figure 4.
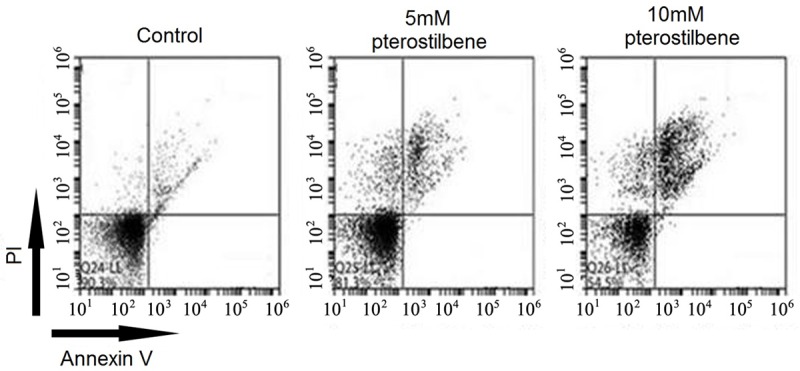
Effect of pterostilbene on glioma cell apoptosis.
Figure 5.

Analysis of pterostilbene on glioma cell apoptosis. *P<0.05 compared to control group; #P<0.05 compared to 5 mM pterostilbene group.
Pterostilbene and Bcl-2 mRNA expression in glioma cells
Real-time PCR was further replenished to test the effect of pterostilbene on mRNA expression of Bcl-2 in U87 glioma cells. Results showed that after 48 h of pterostilbene treatment, mRNA expression of Bcl-2 gene was remarkably depressed (P<0.05 compared to control group). With further higher concentration, the inhibitory effect on Bcl-2 mRNA level in tumor cells was potentiated (Figure 6).
Figure 6.
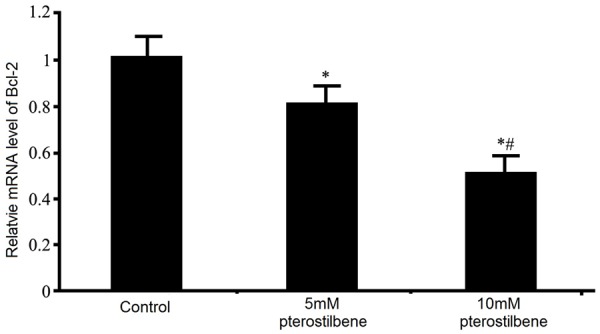
Effects of pterostilbene of Bcl-2 mRNA expression in glioma cells. *P<0.05 compared to control group; #P<0.05 compared to 5 mM pterostilbene group.
The effect of pterostilbene on Bax mRNA expression in glioma cells
Further real-time PCR assay was employed to test the effect of pterostilbene on Bax mRNA expression of glioma U87 cells under the treatment of pterostilbene. Results showed that after 48 h of pterostilbene treatment, Bax mRNA level in U87 cells was significantly elevated (P<0.05 compared to control group). With higher concentration of pterostilbene, such enhancing effect was further potentiated (Figure 7).
Figure 7.
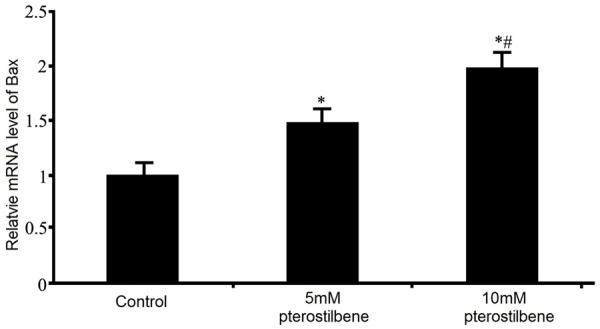
Effects of pterostilbene on Bax mRNA expression in glioma cells. *P<0.05 compared to control group; #P<0.05 compared to 5 mM pterostilbene group.
Regulatory effects of pterostilbene on protein levels of Bcl-2 and Bax in glioma cells
Western blotting was used to test the effect of pterostilbene treatment on Bcl-2 and Bax protein expression in U87 glioma cells. Results were consistent with those in mRNA analysis by real-time PCR, as 48 h pterostilbene treatment significantly suppressed anti-apoptotic Bcl-2 protein expression and facilitated pro-apoptotic Bax protein expression. The ratio of Bcl-2/Bax protein was significantly lowered (P<0.05 compared to that in control group). With higher dosage of pterostilbene, such Bcl-2 inhibition and Bax potentiation effects were further enhanced (Figures 8 and 9).
Figure 8.
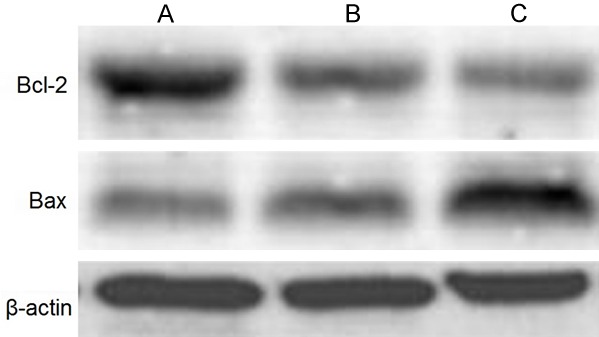
Western blotting bands showing Bcl-2 and Bax proteins in glioma cells. A. Control group; B. 5 mM pterostilbene group; C. 10 mM pterostilbene group.
Figure 9.
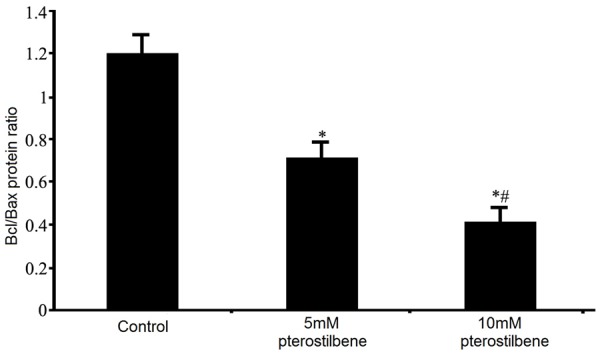
Effects of pterostilbene on Bcl-2 and Bax protein expression. *P<0.05 compared to control group; #P<0.05 compared to 5 mM pterostilbene group.
Discussion
Glioma has complicated pathogenesis mechanisms among malignant tumors of nervous system, causing its unfavorable prognosis and the major challenge for neurosurgeon. As its increasing incidence worldwide, glioma has become a major concern threating the public health and leads to heavy economic burden and mental stress [16]. Although the advancement of medical sciences has improved the treatment of neuroglioma, the composited application of various treatment strategies did improve patient’s life quality to certain extents. The clinical treatment efficacy is still unfavorable, repressing the overall 5-year survival rate. In addition, higher recurrence rate also existed for glioma even after surgery, making it one refractory tumor [17,18]. The invasion and migration property of glioma has a close relationship with tumor recurrence and refractory nature. Glioma cells has intrinsic features of migration and invasion, both of which are correlated with anti-apoptosis and enhanced cell adhesion of tumor cells, composing on complicated pathological process [19]. Therefore, the establishment of effective anti-glioma drugs can improve the treatment efficacy of glioma.
Pterostilbene can effectively clear reactive oxygen, and regulate oxidation/reduction homeostasis via anti-oxidation pathway, and may exert functions for various diseases including ischemia-reperfusion injury, inflammation and tumors. As one of the most effective natural compound for anti-tumor treatment currently used in clinics [20], pterostilbene can induce the apoptosis of human acute promyelocytic leukemia cells, and inhibit dimethylbenzanthracene-induced skin tumor cells, with chemical anti-cancer effects. Pterostilbene has more potent pharmaceutical functions over resveratrol, due to its advantage of medicine effects and highly selective specificity [21,22]. However, the role of pterostilbene has not been illustrated yet. The over-expression of Bcl-2 and inhibition of Bax expression are closely correlated with anti-apoptosis/apoptosis imbalance of glioma cells. As one regulatory mechanism for body homeostasis, apoptosis can retard the occurrence of tumors via inhibiting its over-growth. When Bcl-2 is over-expressed, those injured cells resist apoptosis, induce the interaction between downstream proliferation or growth related genes, developing into tumors. When apoptotic protein Bax is over-expressed, the apoptosis signal is initiated for facilitating cell apoptosis, which may antagonize or inhibit Bcl-2 protein expression [23]. This study demonstrated that after pterostilbene treatment, the proliferation or invasion of glioma cells was remarkably inhibited, enhancing tumor cell apoptosis, decreasing mRNA/protein expression of Bcl-2 while increasing Bax mRNA or protein expression.
In summary, pterostilbene could facilitate the apoptosis of glioma cells via regulating apoptotic/anti-apoptotic balance for inhibiting glioma cell proliferation or invasion. This study provided evidence for analyzing the pathogenesis mechanism of glioma in clinics, and suggested novel strategies for treatment.
Disclosure of conflict of interest
None.
References
- 1.Young JS, Morshed RA, Kim JW, Balyasnikova IV, Ahmed AU, Lesniak MS. Advances in stem cells, induced pluripotent stem cells, and engineered cells: delivery vehicles for anti-glioma therapy. Expert Opin Drug Deliv. 2014;11:1733–1746. doi: 10.1517/17425247.2014.937420. [DOI] [PubMed] [Google Scholar]
- 2.Chung DS, Shin HJ, Hong YK. A new hope in immunotherapy for malignant gliomas: adoptive T cell transfer therapy. J Immunol Res. 2014;2014:326545. doi: 10.1155/2014/326545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiao JT, Jiang C, Huang J, Dai MC, Wang C, Cheng C, Shao JF. Metabolic syndrome factors and risk of postoperative depression in high-grade glioma patients in a 1.5-year prospective study. Med Oncol. 2014;31:234. doi: 10.1007/s12032-014-0234-y. [DOI] [PubMed] [Google Scholar]
- 4.Avdieiev S, Gera L, Havrylyuk D, Hodges RS, Lesyk R, Ribrag V, Vassetzky Y, Kavsan V. Bradykinin antagonists and thiazolidinone derivatives as new potential anti-cancer compounds. Bioorg Med Chem. 2014;22:3815–3823. doi: 10.1016/j.bmc.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Sai K, Gong F, Yang Q, Chen F, Lin J. Mutation of isocitrate dehydrogenase 1 induces glioma cell proliferation via nuclear factor-kappaB activation in a hypoxia-inducible factor 1-alpha dependent manner. Mol Med Rep. 2014;9:1799–1805. doi: 10.3892/mmr.2014.2052. [DOI] [PubMed] [Google Scholar]
- 6.Anderson G, Maes M. Local melatonin regulates inflammation resolution: a common factor in neurodegenerative, psychiatric and systemic inflammatory disorders. CNS Neurol Disord Drug Targets. 2014;13:817–827. doi: 10.2174/1871527313666140711091400. [DOI] [PubMed] [Google Scholar]
- 7.Lim YC, Roberts TL, Day BW, Stringer BW, Kozlov S, Fazry S, Bruce ZC, Ensbey KS, Walker DG, Boyd AW, Lavin MF. Increased sensitivity to ionizing radiation by targeting the homologous recombination pathway in glioma initiating cells. Mol Oncol. 2014;8:1603–1615. doi: 10.1016/j.molonc.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramachandran R, Malarvizhi GL, Chandran P, Gupta N, Menon D, Panikar D, Nair S, Koyakutty M. A polymer-protein core-shell nanomedicine for inhibiting cancer migration followed by photo-triggered killing. J Biomed Nanotechnol. 2014;10:1401–1415. doi: 10.1166/jbn.2014.1847. [DOI] [PubMed] [Google Scholar]
- 9.Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C, Nicholas S, Kellett M, Ruzevick J, Jackson C, Albesiano E, Durham NM, Ye X, Tran PT, Tyler B, Wong JW, Brem H, Pardoll DM, Drake CG, Lim M. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One. 2014;9:e101764. doi: 10.1371/journal.pone.0101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Ding XQ, Gu TT, Song L, Li JM, Xue QC, Kong LD. Pterostilbene and allopurinol reduce fructose-induced podocyte oxidative stress and inflammation via microRNA-377. Free Radic Biol Med. 2015;83:214–226. doi: 10.1016/j.freeradbiomed.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Hsiao PC, Chou YE, Tan P, Lee WJ, Yang SF, Chow JM, Chen HY, Lin CH, Lee LM, Chien MH. Pterostilbene simultaneously induced G0/G1-phase arrest and MAPK-mediated mitochondrial-derived apoptosis in human acute myeloid leukemia cell lines. PLoS One. 2014;9:e105342. doi: 10.1371/journal.pone.0105342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan C, Hu Y, Li J, Wang Z, Huang J, Zhang S, Ding L. Estrogen receptor-alpha36 is involved in pterostilbene-induced apoptosis and anti-proliferation in in vitro and in vivo breast cancer. PLoS One. 2014;9:e104459. doi: 10.1371/journal.pone.0104459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saw CL, Guo Y, Yang AY, Paredes-Gonzalez X, Ramirez C, Pung D, Kong AN. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: involvement of the Nrf2-ARE signaling pathway. Food Chem Toxicol. 2014;72:303–311. doi: 10.1016/j.fct.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 14.Jagadeb M, Konkimalla VB, Rath SN, Das RP. Elucidation of the Inhibitory Effect of Phytochemicals with Kir6.2 Wild-Type and Mutant Models Associated in Type-1 Diabetes through Molecular Docking Approach. Genomics Inform. 2014;12:283–288. doi: 10.5808/GI.2014.12.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato D, Shimizu N, Shimizu Y, Akagi M, Eshita Y, Ozaki S, Nakajima N, Ishihara K, Masuoka N, Hamada H, Shimoda K, Kubota N. Synthesis of glycosides of resveratrol, pterostilbene, and piceatannol, and their anti-oxidant, anti-allergic, and neuroprotective activities. Biosci Biotechnol Biochem. 2014;78:1123–1128. doi: 10.1080/09168451.2014.921551. [DOI] [PubMed] [Google Scholar]
- 16.Waghmare I, Roebke A, Minata M, Kango-Singh M, Nakano I. Intercellular cooperation and competition in brain cancers: lessons from Drosophila and human studies. Stem Cells Transl Med. 2014;3:1262–1268. doi: 10.5966/sctm.2014-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rathod SS, Rani SB, Khan M, Muzumdar D, Shiras A. Tumor suppressive miRNA-34a suppresses cell proliferation and tumor growth of glioma stem cells by targeting Akt and Wnt signaling pathways. FEBS Open Bio. 2014;4:485–495. doi: 10.1016/j.fob.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Shi ZM, Jiang CF, Liu X, Chen QD, Qian X, Li DM, Ge X, Wang XF, Liu LZ, You YP, Liu N, Jiang BH. MiR-143 acts as a tumor suppressor by targeting N-RAS and enhances temozolomide-induced apoptosis in glioma. Oncotarget. 2014;5:5416–5427. doi: 10.18632/oncotarget.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brower JV, Clark PA, Lyon W, Kuo JS. MicroRNAs in cancer: glioblastoma and glioblastoma cancer stem cells. Neurochem Int. 2014;77:68–77. doi: 10.1016/j.neuint.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey SC, Amedei A, Aquilano K, Azmi AS, Benencia F, Bhakta D, Bilsland AE, Boosani CS, Chen S, Ciriolo MR, Crawford S, Fujii H, Georgakilas AG, Guha G, Halicka D, Helferich WG, Heneberg P, Honoki K, Keith WN, Kerkar SP, Mohammed SI, Niccolai E, Nowsheen S, Vasantha Rupasinghe HP, Samadi A, Singh N, Talib WH, Venkateswaran V, Whelan RL, Yang X, Felsher DW. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin Cancer Biol. 2015;35(Suppl):S199–223. doi: 10.1016/j.semcancer.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Androutsopoulos VP, Fragiadaki I, Tosca A. Activation of ERK1/2 is required for the antimitotic activity of the resveratrol analogue 3,4,5,4’-tetramethoxystilbene (DMU-212) in human melanoma cells. Exp Dermatol. 2015;24:632–634. doi: 10.1111/exd.12721. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, He C, Ran R, Zhang D, Li D, Xiao PG, Altman E. The resveratrol oligomers, cis- and trans-gnetin H, from Paeonia suffruticosa seeds inhibit the growth of several human cancer cell lines. J Ethnopharmacol. 2015;169:24–33. doi: 10.1016/j.jep.2015.03.074. [DOI] [PubMed] [Google Scholar]
- 23.Bi D, Yang M, Zhao X, Huang S. Effect of Cnidium Lactone on Serum Mutant P53 and BCL-2/BAX Expression in Human Prostate Cancer Cells PC-3 Tumor-Bearing BALB/C Nude Mouse Model. Med Sci Monit. 2015;21:2421–2427. doi: 10.12659/MSM.893745. [DOI] [PMC free article] [PubMed] [Google Scholar]


