Abstract
To examine if transplantation of human umbilical cord mesenchymal stem cells (UMSC) into cochlea can be used to repair sensorineural hearing. Here we transplanted the fifth and sixth generations of UMSCs through the subarachnoid cavity of congenital deaf albino pigs. Auditory brainstem responses (ABR) were measured before and after UMSC transplantation. Cochlear samples were collected at 2 hrs, 3 days, 1, 2, 3, 4 and 8 weeks after transplantation. Immunohistochemistry was used to detect the proliferated cell nuclear antigen (PCNA). The UMSCs were found in different regions of the cochlea, including the stria vascularis, the basal membrane and the spiral ganglions, 3 days to 4 weeks after the transplantation. UMSCs and their DNA were found also in the areas of the brain, the heart, the liver, the kidney and the lung. ABR tests displayed a new waveform in the congenital deaf albino pigs after the UMSCs transplantation. We conclude that human UMSCs injected into the subarachnoid space can migrate into the inner ear, the central nervous system and the periphery organs. The presence of UMSCs in the cochlea maybe associated with changes of ABR waveforms in the congenital deaf albino pigs.
Keywords: Human umbilical cord mesenchymal stem cells (UMSC), pigs, subarachnoid cavity, auditory brainstem response
Introduction
Hearing loss or deafness is a severe disorder affects people’s communication and quality of life. Sensorineural hearing loss, commonly caused by genetic dysfunction, ototoxic drugs, noise exposure and aging, is induced by hair cell loss [1]. The current option to restore profound sensorineural hearing loss is cochlear implant. Cochlear implant transfers mechanical signals into electric signals and delivers electrical pulse into auditory nerves to replace the function of hair cells. The success of cochlear implant depends on the auditory nerves that receive and carry the electrical signals to the auditory brain for further speech processing and recognition. However, cochlear implant has limited benefit to the patients with impaired auditory nerves.
Using stem cell transportation to replace the damaged SGN and auditory nerves has potential to improve cochlear implant performance. Given the fact that the auditory nerve and auditory centre floats in the cerebrospinal fluid (CSF) compartment, which communicates with perilymph, we hypothesized that stem cell transplanted into the CSF could enter into perilymph, and subsequently come in contact with and repair inner ear damage. CSF is important in the central nervous system to maintain the metabolism, protect brain tissue and adjust its pressure. 80% of the CSF is produced by the choroid plexuses in the brain ventricles. After formation, CSF flows down the channel from the lateral ventricles to third ventricles, and continue to flow through the cerebral aqueduct to the fourth ventricle, then it empties into the subarachnoid space (SAS). CSF is absorbed from the SAS into the blood via the arachnoid villi which protrudes into the venus sinuses. In addition, CSF drains along the cranial nerves and spinal roots, and enters into the lymphatics [2]. We hypothesize that stem cells transplanted in the CSF may flow into the SAS and ultimately get into the inner ear.
We had previously described a congenital deaf albino pig (Mitf-M -/-) found in Rongchang, Chongqing, southwest of China. Unlike the wild-type (Mitf-M +/+) counterparts with black hair, the congenital deaf pigs have white hairs. The genetically mutant pigs show profound sensorineural hearing loss, which comparable to human Waardenburg syndrome type II [3-5]. The structural damage seen in these Rongchang albino pigs includes missing of cochlear hair cells, damage of stria vascularis, loss of the spiral ganglion neurons (SGN) and impairment of auditory nerves. Functional assessments in these pigs revealed no recordable auditory brainstem response (ABR). Given the similarity of its inner ear anatomy and physiology to human inner ear structure, this is an excellent animal model for study cochlear implant [6-8]. This study was designed to test the hypothesis that transplanted human UMSCs may reach to the inner ear. The success of the experiment may help to find a useful routine for stem cell transplantation in order to repair permanent hearing loss.
Materials and methods
Human UMSC preparation
Umbilical cords were obtained from human between 12 to 24 hours after baby delivery. UMSCs were later isolated from human umbilical cord after twenty days of tissue adherence. These UMSCs were repeatedly cultured till the fifth or sixth generation of cultured cells which showed uniformed swirl appearance (Figure 1). Immunophenotyping by flow cytometry showed that these UMSCs expressed CD73 and CD105, but not CD31 and CD34 markers (Figure 2).
Figure 1.
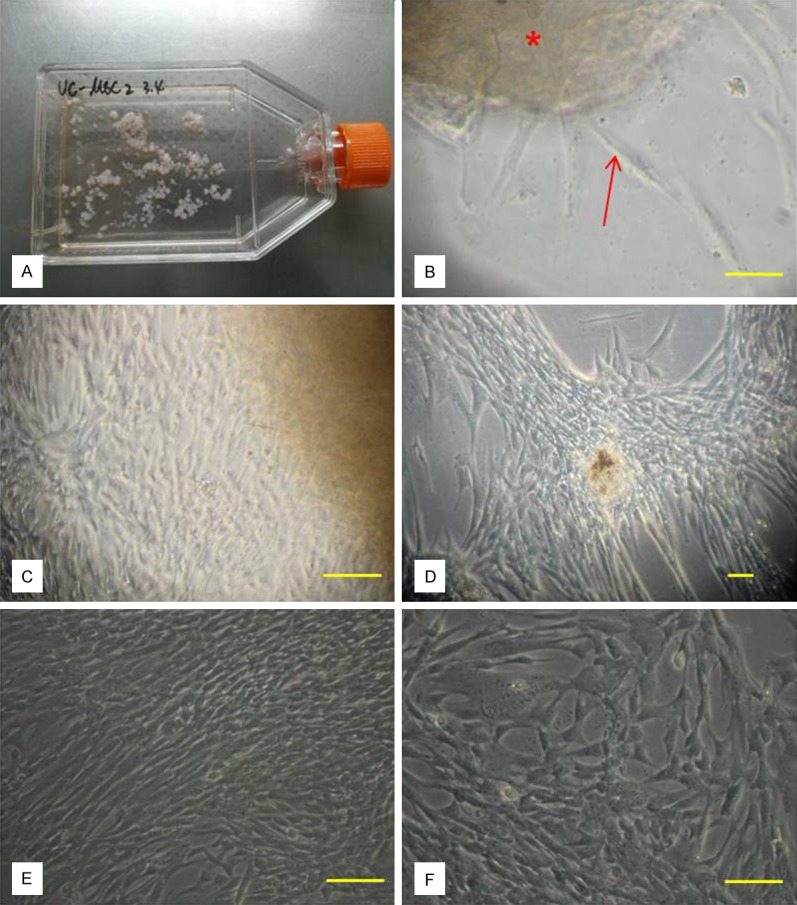
UMSCs isolated from human umbilical cord. A: The first day of tissue adherence. B: Twenty days of tissue adherence (red star). C: Original UMSC of P0 generation (20×); D: Original UMSC of P0 generation (10×); E: UMSC of P0 generation ready to be passage; F: Fusiform shaped UMSCs of P1 generation (bar = 100 μm).
Figure 2.
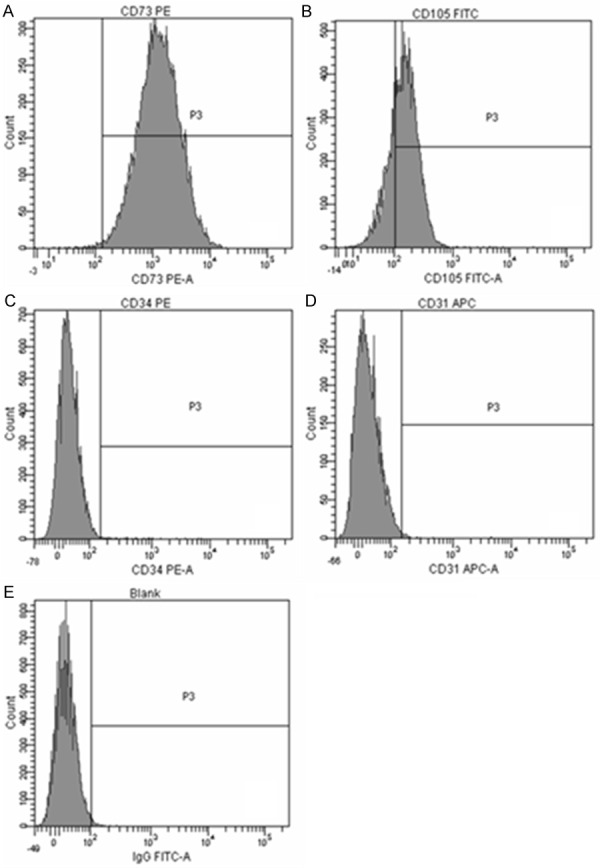
Immunophenotyping of P6 UMSCS by flow cytometry. A: CD73 is positive; B: CD105 is positive; C: CD45 is negative; D: CD31 is negative; E: Blank control.
Animals
Fifteen healthy Rongchang swine (3 months old, 6-10 kg) have been used for this study: ten congenital deaf albino swine (Mitf-M -/-) and five wild-type swine (Mitf-M +/+). The ABR waves of the wild-type Rongchang swine could be evoked by clicks and tone-burst at 0.5, 1, 4, 8, 16 and 32 kHz. The albino Rongchang pigs could not be evoked by clicks or tone-bursts (0.5 to 32 kHz, 100 dB SPL). The gene type (Mitf-M) of all the albino swine were tested by PCR (Figure 3). This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Minnesota (Permit Number: 27-2956). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Figure 3.
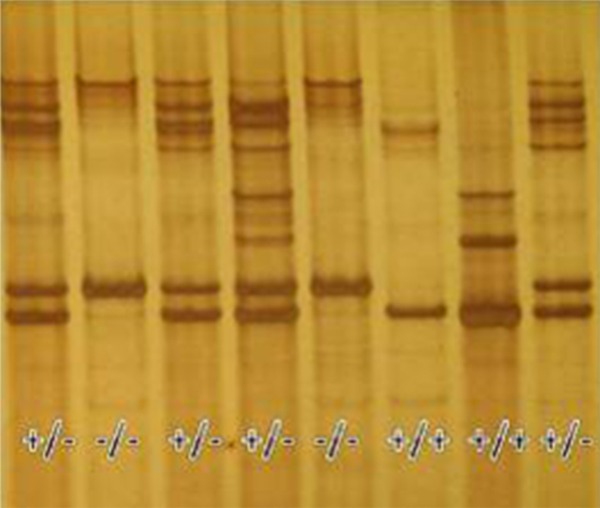
The gene type (Mitf-M) tested by PCR (+/+, wild-type swine; -/-, albino swine; +/-, heterozygous swine).
Xenotransplantation
As listed in Table 1, eleven pigs (both albino and wild-type pigs) were transplanted with fifth or sixth generations of cultured UMSCs (conc. of 3×10^5 to 1×10^7) by injections into the subarachnoid cavities. Two swine were injected with D-hanks solution and the other two were surgically treated without injection of any cells. Swine with normal hearing were served as controls. The animals were sacrificed after deep anesthetized with pentobarbital at different time intervals after UMSCs transplantation at 2 hrs, 3 days, 1, 2, 3, 4 or 8 weeks. Cochlea, blood, bone marrow cells and other organs/tissues including heart, liver, spleen, kidney and lungs were collected. Half of samples were frozen in liquid nitrogen and the other half samples were fixed overnight in 4% formaldehyde. The cochlear samples were assessed by immunohistochemistry and polymerase chain reaction (PCR) for detection and distribution of human proliferated cell nuclear antigen (PCNA) which helped to identify human UMSC. ABR was tested before animals were scarified.
Table 1.
Human UMSCs transplantation
| Group 1 | Sacrificed time | Cells or treatment | Group 2 | Sacrificed time | Cells or treatment |
|---|---|---|---|---|---|
| MZ1 | 2 weeks | 1×10^6 | WZ1 | 2 hours | 6.5×10^6 |
| MZ2 | 4 weeks | 1×10^7 | WZ2 | 1 week | 6×10^6 |
| MZ3 | 2 weeks | 5×10^6 | WZ3 | 3 weeks | 1×10^7 |
| MZ4 | 1 week | 3×10^5 | WZ4 | 4 weeks | D-hanks |
| MZ5 | 8 weeks | 5×10^5 | WZ5 | 4 weeks | only surgery |
| MZ6 | 3 weeks | 5×10^6 | |||
| MZ7 | 3 days | 6.6×10^6 | |||
| MZ8 | 2 hours | 1×10^6 | |||
| MZ9 | 4 weeks | D-hanks | |||
| MZ10 | 4 weeks | only surgery |
Cochlea and organs sample preparation and immunohistochemistry
The cochlea was opened at the apex and 5 ml 4% formaldehyde were injected through the perforated round window. The fixation was carried out overnight at 4°C in formaldehyde. Fixed cochleas were washed in 0.1 M PBS (pH = 7.4) and then decalcified with EDTA solution for 30 days at room temperate. After removed from the temporal bones, the cochlea was dehydrated with 30% and 10% sucrose. The cochlea was then embedded in OCT gum and frozen at -20°C for 30 minutes. Sections (10 µm) were cut and permeabilized with Triton 0.1% in PBS, for 30 minutes at room temperature, blocked with 5% goat serum for 30 minutes and then incubated with primary antibody; mice monoclonal IgG anti-human proliferated cell nuclear antigen (PCNA) which is a specific marker of human cell, (Millipore, Germany) for 12 hours at 4°C. The sections were washed with 0.1 M PBS for 15 minutes and incubated with secondary antibody; goat 488-conjugated anti-mouse IgG (Invitrogen, USA) for 2 hours at room temperature, for visualization of anti-PCNA. DAPI was employed for visualization of nuclear materials in these sections.
Organ samples were fixed with formaldehyde overnight at 4°C, and then dehydrated with 30% and 10% sucrose, followed by the immunohistochemistry techniques as described for the cochlea.
PCR analysis
Genomic DNA was extracted from frozen tissues according to the manufacturer’s protocol (QIAamp DNA, Mini Kit, Qiagen, Germany). This was further analyzed by PCR for the presence of human CETP (cholesterol ester transport protein) as a marker for human DNA sequence, using the forward (5’-CACCCTCAAAGGCTGGAAAC-3’) and reverse (5’-AGCTGAGAACACGG AGCCTC-3’) primers. Single positive 725-bp PCR bands were sequenced to confirm the presence of human CETP DNA sequence in analyzed samples thus confirming the presence of human UMSCs in swine tissues.
Confocal scan analysis
Slides were observed under a confocal microscope system (LSM780, Zeiss, Germany) and Light Edition software (Zen 2009).
Results
Engraftment in body tissues of swine after transplantation
PCR analysis showed that DNA isolated from tissues taken at specific intervals from swine treated with UMSCs, had CETP sequence. Such engraftments appeared more frequently in the central neural tissues and body organs within three weeks of transplantation (Table 2 and Figure 4), and more frequently in the brain, spleen, liver and lung. There was no difference in human UMSCs distribution in tissues of wild-type and albino swine. We found that positive detection of human UMSCs in tissue samples varied with time as there was more cell engraftment by three days and one week than 3 weeks post UMSCs transplantation.
Table 2.
Engraftment in body tissues of swine after transplantation
| Tissue | 3 days | 1 week | 3 weeks |
|---|---|---|---|
| Cochlea | +/- | + | +/- |
| Heart | . | + | . |
| Liver | + | + | . |
| Spleen | + | + | . |
| Lung | + | . | . |
| Kindey | . | . | + |
| Cerebra | + | . | . |
| Cerebella | +/- | . | . |
| Cerestem | . | + | . |
| Spinal cord | +/- | + | + |
| Vein blood | . | . | . |
| Bone marrow | . | . | . |
Figure 4.
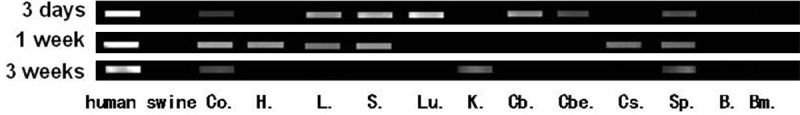
The human UMSCs detected by CETP DNA sequence (Co = cochlea, H = heart, L = liver, S = spleen, Lu = lung, K = kindey, Cb = cerebra, Cbe = cerebella, Cs = cerestem, Sp = spinal cord, B = blood, Bm = Bone morrow).
Engraftment in cochlea of swine after transplantation
To demonstrate the presence of human cell within transplanted swine, we employed the combination of immunohistochemistry confocal microscopy. Using this method, we detected human PCNA in SGN, Corti’s organ, stria vascularis and spiral ligament in the cochlea of swine infused with UMSCs. There was no difference in the distribution pattern of UMSCs in tissues from the wild type or deaf albino swine. Similar to PCR findings, positive detection of human PCNA varied with time. We detected PCNA at 3 days, 1, 2, 3 and 4 weeks, but not at very early (2 hrs) or late (8 weeks) time points (Table 3 and Figures 5, 6, 7).
Table 3.
engraftment in cochlea of swine after transplantation
| Time interval | Stria vascularis | Corti’s organ | SGN |
|---|---|---|---|
| 2 hours | - | - | - |
| 3 days | + | + | + |
| 1 week | + | + | + |
| 2 weeks | + | + | + |
| 3 weeks | + | + | + |
| 4 weeks | + | + | + |
| 8 weeks | - | - | - |
Figure 5.
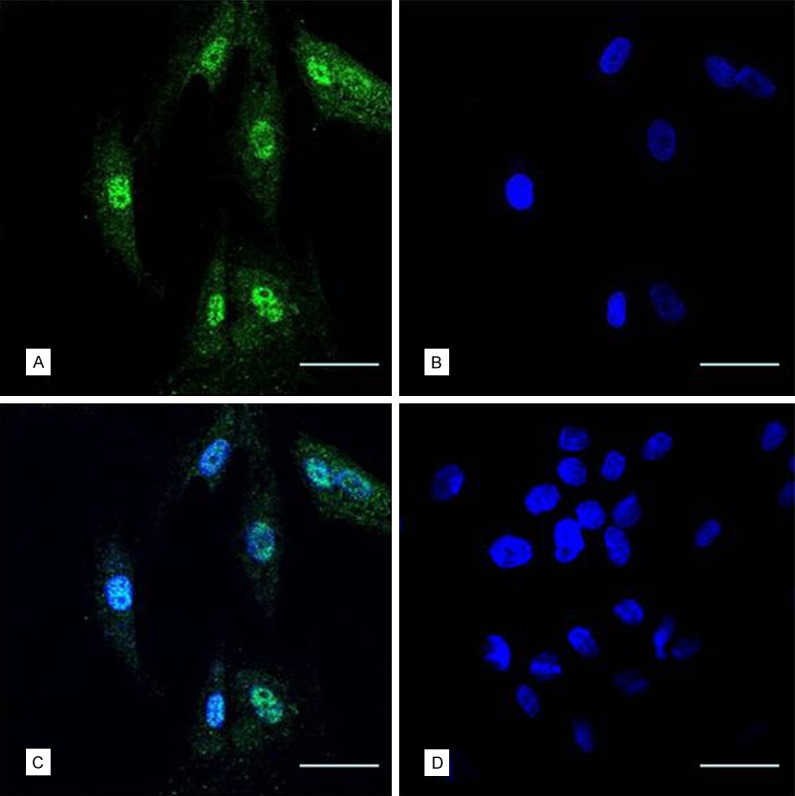
Human UMSCs cultured in vitro showed Anti-PCNA positive. (A) Green, second antibody was goat 488-conjugated anti-mouse IgG with 488, blue nucleolus (B) dyeing with DAPI) and merge image (C). The swine cells cultured in vitro showed Anti-PCNA negative (bar = 50 μm) (D).
Figure 6.
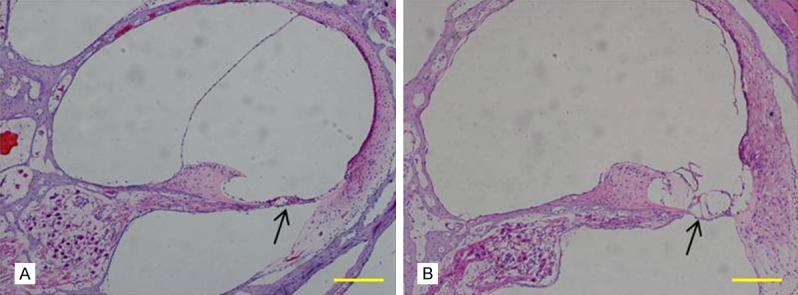
Cochlear frozen slice under HE staining. A: Wild-type swine have normal cochlear structure; B: Cochlea of albino Rongchang swine showed degeneration of inner ear hair cells of the organ of Corti, damaged stria vascularis and loss of spiral ganglion neurons (SGNs) (bar = 200 μm).
Figure 7.
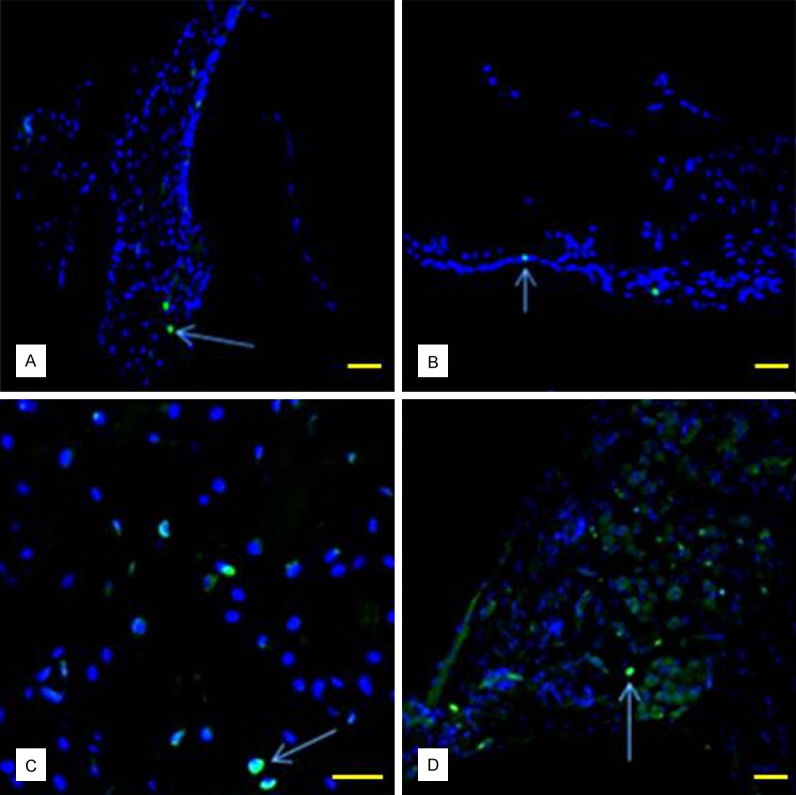
PCNAs expressed in the cochlea (showed by arrow) were detected in spiral ligament (A), Organ of Corti (B), stria vascularis (C) and SGNs (D) (bar = 50 μm).
Change of ABR waveforms
Functional performance was determined by measuring auditory brainstem evoked response (ABR). Wave III to V of ABR responses was generated by the cochlear nucleus and the superior olivary complex cells, and they reflected neural connections with the central auditory pathway. We tested and recorded ABR thresholds of wild-type and albino swine at before, 2 hrs, 3 days, 1, 2, 3, and 4 weeks after transplantation. The auditory function of albino swine was severely impaired, with thresholds were above 120 dB SPL. Control animals showed no sign of functional recovery (for deaf albino swine). However, in three out of eight albino animals (37.5%) injected with UMSCs, there was a detectable wave at the position expected between wave III and IV. This wave detection started about day 3 post transplantation (Table 4; Figure 8), with its threshold lowered (sign of improvement) to 95 dB by the 3rd week. The most obvious maximum wave point appeared after one week of transplantation, and this disappeared after 3 weeks. This represents an overall functional improvement of approximately 33%.
Table 4.
New ABR wave after transplantation
| Swine | 2 hours | 3 days | 1 week | 2 weeks |
|---|---|---|---|---|
| MZ1 | . | . | . | . |
| MZ2 | . | . | . | . |
| MZ3 | +/- | + | - | - |
| MZ4 | +/- | + | . | . |
| MZ5 | - | - | - | - |
| MZ6 | - | +/- | + | +/- |
| MZ7 | - | - | . | . |
| MZ8 | - | . | . | . |
| MZ9 | - | - | - | - |
| MZ10 | - | - | - | - |
| WZ1 | N | . | . | . |
| WZ2 | N | N | N | . |
| WZ3 | N | N | N | - |
| WZ4 | N | N | N | N |
| WZ5 | N | N | N | N |
“+” Means a new detectable wave shape at the position expected for wave III, “-” Means no wave, “+/-” Means hard to judge, “N” Means normal ABR and “.” Means swine had been executed or did not test.
Figure 8.
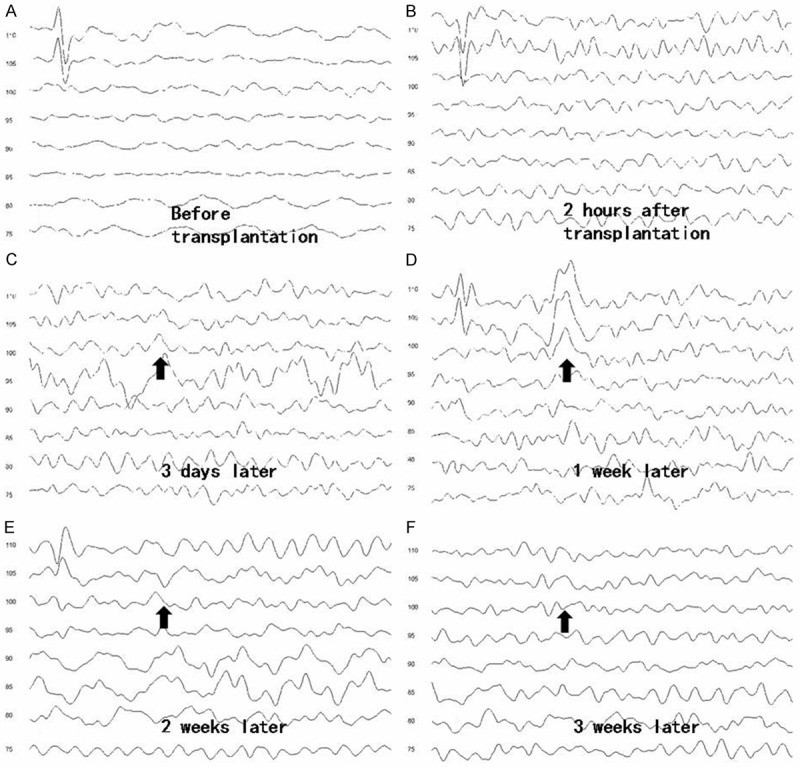
The ABR wave of albino swine (WZ6) before and 3 weeks after the UMSC transplantation. A detectable wave was found at wave III and IV (arrow) at post-one week after transplantation. A detectable wave shape was found at the position expected between wave III and IV (arrow) most obviously at the point of one week later.
Discussion
Determination of stem cells for study
In previous studies, embryonic stem cells (ESC) and inducible pluripotent stem cells (IPS) have been used in clinical treatment [9]. Recently, mesenchymal stem cells (MSC), which have partial differentiation potentials and are less immunogenic than ESC and easier to be isolated and cultured [7,10], has been used in transplantation. In this study, we chose UMSCs for transplantation, rather than auto-transplantation or homo-transplantation, as it was a more convenient inoculation method. In addition, it was relative easy to isolate enough UMSCs for the study and to check their specific properties. Considering about the long-term affection and safety of patient receiving transplantation, autotransplantation would be better choice in clinical treatment in the future.
Determination of pathway for stem cell transplantation
Stem cell transplantations can either be done by intravenous administration [11] or by local transplantation. For local administration of stem cells, cells can be injected in the round window, scala tympani or scala media, or direct injection into the modiolus. All of these various routes of administration have shown positive effects on hearing loss in previous studies [9,11,12]. However, possible damage to the cochlea can occur by direct or local inoculation of stem cells, we did not use direct delivery. Because the intravenous route of delivery requires larger amount of stem cells as the cells can barely bypass the blood-brain barrier, we chose transplantation of the stems into the CSF through subarachnoid. The auditory nerve and auditory centre float in the CSF environment, which communicated with perilymph. Therefore, we assume that the transplantation via subarachnoid cavity offers advantages similar to the direct inner ear inoculation. It needs fewer cells than the intravenous inoculation and still has access to the blood compartment.
Mehta and colleagues used subarachnoid placement of stem cells in neurological disorders [13], and carried out a prospective analysis to assess the technical difficulties and short- and long-term effects of SC infusion. In Mehta’s study, 31.67% patients had functional improvement. Although more than half of patients have side effects including headache, low-grad fever and meningism after the transplantation, none of these syndrome lasted more than 24 hours. This means the subarachnoid stem cell transplantation is safe, less invasive, and a convenient procedure. During the experiment, we found that after the cells have been injected, the needle tip should be left in the same position for at least 2 minutes before it was withdrawn to prevent any backflow.
Impact of stem cells transplantation on inner ear and ABR
This study didn’t reveal any structural damage of the inner ear by the 4th week after the transplantation. The reason for this lack of anatomical improvement in the inner ear maybe due to short observation time or inadequate transplanted stem cells. This is the area needs further studies in the future as transplanted cells were detected in the spiral ligament, stria vascularis, organ of Corti and SGN.
ABR response cannot be recorded from Rongchang albino swine at up to click sound stimuli at 120 dB SPL. After UMSCs transplantation, there was still no evidence of ABR response in these pigs. However, we found III~IV waves were recorded in 3 transplanted animals between 3-7 days post transplantation elicited by clicks at 95 dB SPL. These new response in these 3 animals may be induced by stem cells adherent to the auditory nerves. Another possible explanation for this new wave is that stem cells may alter ion concentration of the CSF and endolymph, thus causing changes in neural conduction and ABR wave. We suspected that this new wave found in the 3 swine by 3-7 days has to do with the subarachnoid inoculation of UMSCs into the CSF, but the mechanism underlying this is not clear yet.
Impact of blood circulation on stem cells distribution
Once the stem cells were inoculated into the body from irrespective routes of administration, they will reach varies organs of the body. The rate of arrival, extent of distribution and the organ that was first reached depends on the direct types of inoculation. The first station of stem cells is CNS, where have most stem cells settle.
As the delay in immune system mobilization due to paucity of leucocyes in the CNS, increases the half life of stem cells in that compartment, we think that xenotransplanted stem cells may have a high survival chance in the CNS if the cells entered these organs. The cochlea is thought to be able to harbour stem cells for a considerable time as immune reaction is poor in that tissue. The non-detection of human cells after 3 weeks of inoculation in this study is thought to be due to immune clearance of stem cells which ultimately set in.
As mentioned earlier, due to the transportation role of the blood and the connection of CSF to perilymph to blood, we found human cells in the heart, liver and lung, with more cells located in the lung. The large amount of stem cells in the lung is thought to be as result of the relative large amount of blood that gets to the lungs. This is similar to what is found in intravenous administration of stem cells. As may be expected of an immune organ, we did not detect any human cells in spleen samples, after stem cells transplantation. This result suggests that entrance of stem cells into the blood stream will lead to its clearance by leucocytes and other immune cells. This may be true for allogenic cell transplantation found a previous study [14]. This is not likely the same with MSCs which have low immunogenicity [7], immune tolerance and anti-inflammatory cytokine response [15-17]. Therefore, MSCs, including human UMSCs, are expected to have increased survival rate at least for a short time.
In our experiment, we also noted that swine were stable and healthy for at least 8 weeks after transplantation. There was no loss of weight, fever, hair loss or movement disability. We think the transplantation of human stem cells at a concentration of 10^7 is safe for swine. The possibility of tumor development [18] or other long term adverse effects [19,20] should be evaluated before clinical translation.
In conclusion we found that the human UMSCs, which have been inoculated into the CSF of young deaf albino Rongchang swine, are able to migrate into the inner ears, the central nervous system and peripheral organs. Importantly, UMSCs appeared to cause some changes on ABR response. The procedure is safe as the animals remained healthy up to 8 weeks after the transplantation.
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (973 Program) (#2012CB967900), the Major State Basic Research Development Program of China (973 Program) (#2011CBA01000), the National Natural Science Foundation of China (81400472), the National Natural Science Foundation of China (81271082).
Disclosure of conflict of interest
None.
References
- 1.Guo W, Yuan Y, Hou Z, Wang X, Yang S. Profiles of the auditory epithelia related microRNA expression in neonatal and adult rats. Eur J Med Res. 2014;19:48. doi: 10.1186/s40001-014-0048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chodobski A, Szmydynger-Chodobska J. Choroid plexus: target for polypeptides and site of their synthesis. Microsc Res Tech. 2001;52:65–82. doi: 10.1002/1097-0029(20010101)52:1<65::AID-JEMT9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Graw J, Pretsch W, Loster J. Mutation in intron 6 of the hamster Mitf gene leads to skipping of the subsequent exon and creates a novel animal model for the human Waardenburg syndrome type II. Genetics. 2003;164:1035–1041. doi: 10.1093/genetics/164.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opdecamp K, Vanvooren P, Riviere M, Arnheiter H, Motta R, Szpirer J, Szpirer C. The rat microphthalmia-associated transcription factor gene (Mitf) maps at 4q34-q41 and is mutated in the mib rats. Mamm Genome. 1998;9:617–621. doi: 10.1007/s003359900832. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Guo W, Ren L, Yang M, Zhao Y, Guo Z, Yi H, Li M, Hu Y, Long X, Sun B, Li J, Zhai S, Zhang T, Tian S, Meng Q, Yu N, Zhu D, Tang G, Tang Q, Liu K, Zhang S, Che T, Yu Z, Wu N, Jing L, Zhang R, Cong T, Chen S, Zhang Y, Bai X, Guo Y, Zhao L, Zhang F, Zhao H, Zhang L, Hou Z, Zhao J, Sun W, Zou X, Wang T, Ge L, Liu Z, Hu X, Wang J, Yang S, Li N. A de novo silencer causes elimination of MITF-M expression and profound hearing loss in pigs. BMC Biol. 2016;14:52. doi: 10.1186/s12915-016-0273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo W, Yi H, Ren L, Chen L, Zhao L, Sun W, Yang SM. The morphology and electrophysiology of the cochlea of the miniature pig. Anat Rec (Hoboken) 2015;298:494–500. doi: 10.1002/ar.23095. [DOI] [PubMed] [Google Scholar]
- 7.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Xiang LX, Shao JZ, Pan RL, Wang YX, Dong XJ, Zhang GR. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J Cell Mol Med. 2010;14:1494–1508. doi: 10.1111/j.1582-4934.2009.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, Kuhn S, Milo M, Thurlow JK, Andrews PW, Marcotti W, Moore HD, Rivolta MN. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012;490:278–282. doi: 10.1038/nature11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boer JC, Carney KE, van der Zee S. Differentiation of mouse embryonic stem cells into spiral ganglion neurons: a therapeutic approach to deafness. J Neurosci. 2009;29:5711–5712. doi: 10.1523/JNEUROSCI.0433-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho YB, Cho HH, Jang S, Jeong HS, Park JS. Transplantation of neural differentiated human mesenchymal stem cells into the cochlea of an auditory-neuropathy guinea pig model. J Korean Med Sci. 2015;26:492–498. doi: 10.3346/jkms.2011.26.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamiya K, Fujinami Y, Hoya N, Okamoto Y, Kouike H, Komatsuzaki R, Kusano R, Nakagawa S, Satoh H, Fujii M, Matsunaga T. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol. 2007;171:214–226. doi: 10.2353/ajpath.2007.060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta T, Feroz A, Thakkar U, Vanikar A, Shah V, Trivedi H. Subarachnoid placement of stem cells in neurological disorders. Transplant Proc. 2008;40:1145–1147. doi: 10.1016/j.transproceed.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Akpek G, Pasquini MC, Logan B, Agovi MA, Lazarus HM, Marks DI, Bornhaeuser M, Ringden O, Maziarz RT, Gupta V, Popat U, Maharaj D, Bolwell BJ, Rizzo JD, Ballen KK, Cooke KR, McCarthy PL, Ho VT. Effects of spleen status on early outcomes after hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48:825–831. doi: 10.1038/bmt.2012.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 19.Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG, Simmons PJ, Sensebe L, Keating A. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010;12:576–578. doi: 10.3109/14653249.2010.507330. [DOI] [PubMed] [Google Scholar]
- 20.Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, Kim DW, Yoon YS. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340–1347. doi: 10.1161/CIRCRESAHA.110.239848. [DOI] [PMC free article] [PubMed] [Google Scholar]


