Abstract
Menadione (vitamin K3) has been reported to induce apoptotic cell death and growth inhibition in various types of cancer cells. However, involvement of menadione in cell cycle control has not been considered in gastric cancer cells yet. In the current study, we have investigated whether menadione is involved in the cell cycle regulation and suppression of growth in gastric cancer cells. In the cell cycle analysis, we found that menadione induced G2/M cell cycle arrest in AGS cells. To elucidate the underlying mechanism, we investigated the cell cycle regulatory molecules involved in the G2/M cell cycle transition. After 24 h of menadione treatment, the protein level of CDK1, CDC25C and cyclin B1 in AGS cells was decreased in a menadione dose-dependent manner. In the time course experiment, the protein level of CDC25C decreased in 6 h, and CDK1and cyclin B1 protein levels began to decrease after 18 h of menadione treatment. We found that mRNA level of CDC25C decreased by menadione treatment in 6 h. Menadione did not have an influence on mRNA level of CDK1 and cyclin B1 though the protein levels were decreased. However, the decreased protein levels of CDK1 and cyclin B1 were recovered by inhibition of proteasome. Collectively, these results suggest that menadione inhibits growth of gastric cancer cells by reducing expression of CDC25C and promoting proteasome mediated degradation of CDK1 and cyclin B1 thereby blocking transition of the cell cycle from G2 phase to M phase.
Keywords: Menadione, AGS, G2/M arrest, CDK1, Cyclin B1, CDC25C
Introduction
Menadione (vitamin K3) is a synthetic form of vitamin K which is generally known to be necessary for blood clotting, bone formation and carbohydrate storage [1,2]. In the recent studies, however, menadione has been reported to inhibit various types of cancer cells [1,3-9]. These reports described menadione as an apoptosis inducing factor that is working in gastric cancer, lung cancer, breast cancer, oral cancer, prostate cancer, bladder cancer, hepatocellular carcinoma, pancreatic cancer and ovarian cancers [1,3-9].
The cell cycle is a tightly regulated process to monitor and regulate cell cycle progression and it is crucial to the growth of a cell [10]. The cell cycle consists of two main cell cycle checkpoints which are the G1/S checkpoint and the G2/M checkpoint and they are regulated by various molecules. Among the various molecules, the cyclin dependent kinase 1 (CDK1) is a key regulatory molecule in the G2/M cell cycle transition. CDK1 is involved in the reorganization of the nucleus, chromosome condensation, and formation of the mitotic spindle via the phosphorylation of various mitotic substrates [11]. Therefore, activation of CDK1 is indispensible step for entry into mitosis. The activation of CDK1 is controlled by cyclin binding and CDK1 phosphorylation [12]. The cell division cycle 25 C (CDC25C) protein is a phosphatase responsible for dephosphorylation and activation of CDK1, and cyclin B is a G2 phase-associated cyclin which binds to and activates CDK1 to promote transition of cells to mitosis [11].
In the previous study, we reported menadione induces XIAP-mediated apoptotic cell death in gastric cancer cell line [13]. However, the role of menadione in cell cycle regulation was not examined in gastric cancer cells. Many reports regarding anti-cancer effect of menadione has been focused on apoptotic cell death. Here, we investigated the involvement of menadione in cell cycle arrest and inhibition of cancer proliferation in human gastric cancer cell line.
Materials and methods
Materials
RPMI1640 medium, fetal bovine serum (FBS), streptomycin-penicillin, trypsin-EDTA and trypan blue stain solution were obtained from BRL Life Technologies (Grand Island, NY, USA). Propidium iodide (PI) staining solution was purchased from BD Biosciences (Sparks, MD, USA). Trizol reagent, random hexamer, and Moloney Murine Leukemia Virus Reverse Transcriptase (MMLV-RT) were purchased from Invitrogen (Grand Island, NY, USA). Protease inhibitor cocktail were obtained from Sigma-Aldrich (Saint Louis, MO, USA). Antibodies to detect CDK1, CDC25C and cyclin B1 were purchased from Cell Signaling Technology (Danvers, MA, USA) and β-actin antibody was purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
Proteosome inhibitor assay
MG132, a proteasome inhibitor, (Calbiochem, Darmstadt, Germany) was dissolved in dimethylsulfoxide (DMSO). After 24 h incubation, cells were treated with 15 μM of menadione and 0.5 μM of MG132 for 24 h. Cell lysates were subjected to Western blot analysis.
Cell culture
AGS cells (American Type Culture Collection CRL-1739, Manassas, VA, USA) were cultured in RPMI1640 medium supplemented with 10% fetal bovine serum and streptomycin-penicillin. The cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
Trypan blue exclusion assay
AGS cells (3×105 per well) were plated in 6-well plates. After 24 h, cells were treated with 15 μM of menadione. The cells were then incubated for indicated time periods and subjected to trypan blue exclusion assay as described previously [14]. Briefly, the incubated cells were trypsinized and trypan blue stain solution (10 µl of 0.4%) was mixed with 10 µl of the trypsinized cells. Non-stained cells were counted from this mixture using a hematocytometer.
Cell cycle analysis
Cell cycle analysis was performed as previously described. Briefly, trypsinized cells were washed twice with PBS, fixed with 70% ethanol in PBS and incubated for 2 h at 4°C. Fixed cells were stained with a solution containing RNase A (0.1 mg/ml) and propidium iodide (5 mg/ml) in PBS. After incubation for 40 min at 37°C, the cell suspension was analyzed using FACS Calibur (BD Biosciences, Sparks, MD, USA). Percentage of cells in each phase of the cell cycle was analyzed by ModFit LT software (Verity Software House, Topsham, ME, USA).
RT-PCR (reverse transcription-polymerase chain reaction)
Cultured cells were washed with PBS and total RNA was extracted using Trizol reagent as described in the manufacturer’s instructions. cDNA was synthesized and subjected to PCR as described previously [15]. The PCR primer sequences used in this study are listed in Table 1. Densities of the PCR bands were measured by ImageLab software (Bio-Rad, Hercules, CA, USA) and illustrated as a graph.
Table 1.
List of primers used in this study
| Primers | Sequences (5’-3’) | Product(bp) | Annealingtemp. (°C) | Cycles | |
|---|---|---|---|---|---|
|
| |||||
| Forward | Reverse | ||||
| CDK1 | GGTTCCTAGTACTGCAATTCG | TTTGCCAGAAATTCGTTTGG | 709 | 62 | 26 |
| Cyclin B1 | TGGGTCGGCCTCTACCTTTGCACTTC | CGATGTGGCATACTTGTTCTTGACAGTCA | 332 | 60 | 24 |
| CDC25C | TTTATGTCATTGATTGTCGC | AAGAAGTCTCTGTAGCCGCC | 286 | 55 | 24 |
| GAPDH | CGGGAAGCTTGTCATCAATGG | GGCAGTGATGGCATGGACTG | 349 | 55 | 20 |
Western blotting
Cells were washed with PBS and then lysed at 4°C with lysis buffer containing 1% Triton X-100, protease inhibitor cocktail, and PBS. Lysates were centrifuged and the supernatants were subjected to Westernblot as described previously [16]. Densities of the Western bands were measured by Bio1D software (Vilber Lourmat, Marne la Vallée, France) and illustrated as graphs.
Statistical analysis
Data in the bar graphs are presented as mean ± standard error of mean (SEM). All the statistical analyses were performed using GraphPad Prism 5.02 software (GraphPad Software, San Diego, CA, USA). All the data were analyzed by unpaired Student’s t-test and P < 0.05 was considered to be statistically significant (*P < 0.05, **P < 0.01 and ***P < 0.001).
Results
Menadione reduces growth of gastric cancer cells by inducing G2/M arrest
We previously reported menadione induces apoptosis in a gastric cancer cell line, however, inhibitory effect of menadione on growth of the gastric cancer cells was not examined [13]. In our previous report, menadione induced apoptosis in AGS cells when treated with 20 μM or higher doses [13]. To avoid apoptotic cell death, therefore, we treated 15 μM of menadione on AGS cells and observed growth of the cells in a time dependent manner. The number of cells was enumerated by trypan blue exclusion assay. In the results, we found that 15 μM of menadione reduced growth of AGS gastric cancer cells in a time dependent manner (Figure 1A). Furthermore, we investigated whether 15 μM of menadione induces apoptosis in AGS cells. However, annexin-V staining result showed that 15 μM of menadione does not lead cells to the apoptosis (Figure 1B). These results suggest that menadione can inhibit growth of gastric cancer cells apart from apoptosis-mediated cell death.
Figure 1.
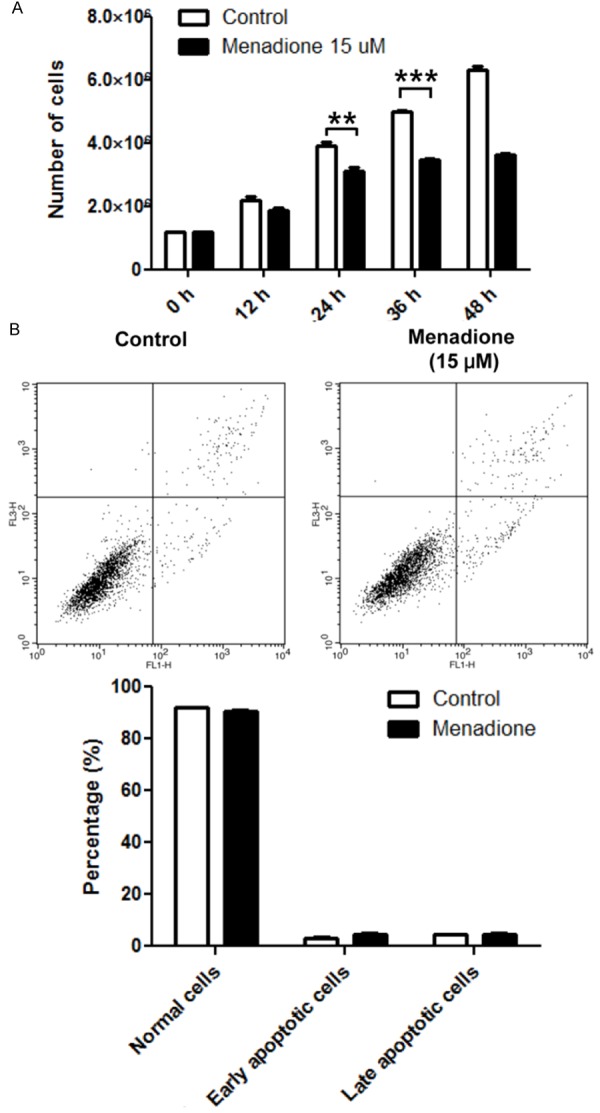
Effect of menadione on cell viability of gastric cancer cell line. A: AGS gastric cancer cells were treated with 15 μM of menadione for indicated time periods and numbers of cells were counted by trypan blue exclusion assay. B: AGS cells were treated with 15 μM of menadione for 24 h. Cells were stained with annexin V-FITC and PI then subjected to flowcytometry. Stained cells were analyzed and illustrated on the quadrant by CellQuestPro software. Percentage of cells in apoptosis was illustrated as a graph. Data were from triplicate experiments and analyzed by Student’s t-test (*P < 0.05, **P < 0.01 and ***P < 0.001).
To elucidate whether menadione interferes the cell cycle transition for growth inhibition of AGS cells, we investigated cell cycle status of AGS cells after exposure to menadione. After 24 h of menadione (15 μM) treatment, cells were stained with PI and analyzed by flowcytometry. In the results, percentage of cells in G2/M phase was increased and G0/G1 phase was correspondingly decreased (Figure 2). Moreover, percentage of cells in S phase remained constant (Figure 2). Therefore, these results indicate menadione delayed cell cycle progression by inducing G2/M arrest in AGS cells.
Figure 2.
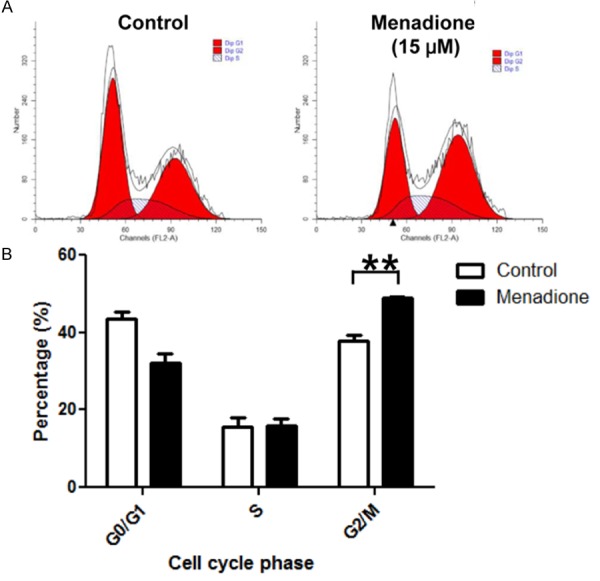
Menadione induces G2/M cell cycle arrest in gastric cancer cell line. AGS cells were treated with 15 μM of menadione for 24 h. Cells were stained with PI and subjected to cell cycle analysis by flowcytometry. Data were analyzed by ModFit LT software and percentage of cells in each phase of the cell cycle was graphically presented (upper panel) and illustrated as a graph (lower panel). Data were from triplicate experiments and analyzed by Student’s t-test (*P < 0.05, **P < 0.01 and ***P < 0.001).
Menadione decreases protein level of CDK1, cyclin B1 and CDC25C in AGS cells
Various molecules are involv-ed in the regulation of cell cycle transition from G2 to M phase. Among those molecules, CDK1, CyclinB and CDC25C are the key regulatory molecules indispensible for G2/M transition. CDK1CyclinB complex conducts reorganization of the nucleus, chromosome condensation, and formation of the mitotic spindle via the phosphorylation of various mitotic substrates, and CDC25C is a phosphatase responsible for activation of CDK1 [11]. Therefore, we investigated CDK1, Cyclin B1 and CDC25C to elucidate how menadione induced G2/M arrest in AGS cells. AGS cells were treated with various concentrations of menadione (0, 5, 10, 15 μM) for 24 h and subjected to Westernblot. In the result, CDK1, Cyclin B1 and CDC25C protein levels were decreased by menadione treatment (Figure 3A and 3B). The results were confirmed in the time course experiment. Protein levels of CDK1, Cyclin B1 and CDC25C were decreased by menadione treatment (15 μM) in a time dependent manner (Figure 4A and 4B).
Figure 3.
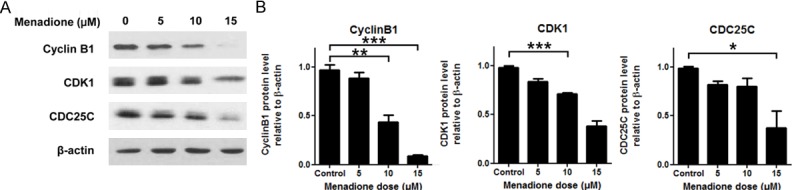
Cell cycle regulatory molecules associated with the menadione induced G2/M arrest of AGS cells. AGS cells were treated with indicated dose of menadione (0, 5, 10, 15 μM) for 24 h. A: The cell lysates were subjected to Westernblot for CyclinB1, CDK1, and CDC25C. B: Densities of the Western bands were measured by Bio1D software and illustrated as graphs. Data were from triplicate experiments and analyzed by Student’s t-test (*P < 0.05, **P < 0.01 and ***P < 0.001).
Figure 4.
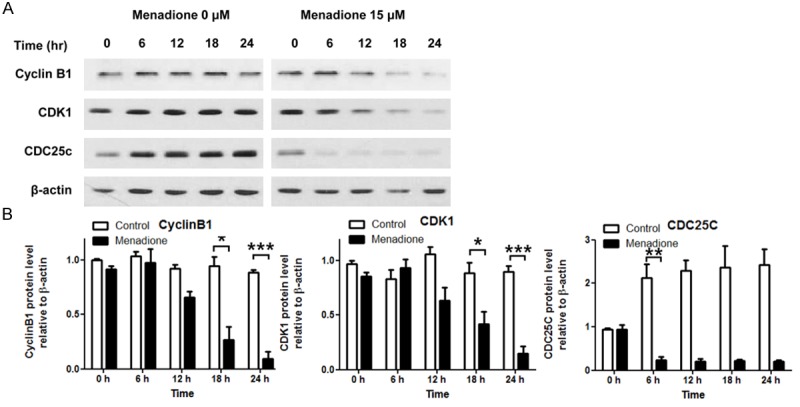
Time course experiments to monitor protein levels of cell cycle regulatory molecules after menadione treatment. AGS cells were treated with 15 μM of menadione for indicated time periods (0, 6, 12, 18, 24 h). A: The cell lysates were subjected to Westernblot for CyclinB1, CDK1, and CDC25C. B: Densities of the Western bands were measured by Bio1D software and illustrated as graphs. Data were from triplicate experiments and analyzed by Student’s t-test (*P < 0.05, **P < 0.01 and ***P < 0.001).
Menadione reduces mRNA expression of CDC25C in AGS cells
To investigate whether reduced protein levels of CDK1, Cyclin B1 and CDC25C by menadione treatment in AGS cells were due to the down-regulation of the mRNA expression, we extracted RNAs from menadione treated AGS cells and performed RT-PCR. AGS cells were treated with menadione for 6 h, because CDC25C protein level was decreased in 6 h in our time course experiment (Figure 4A and 4B). The results showed that CDC25C mRNA expression was decreased by menadione treatment in 6 h, though CDK1 and Cyclin B1 expression was unchanged (Figure 5A and 5B). CDK1 and Cyclin B1 mRNA levels were also unchanged in 24 h (data not shown). These results suggest that menadione treatment reduces CDC25C level in AGS cells by down-regulation of mRNA expression.
Figure 5.
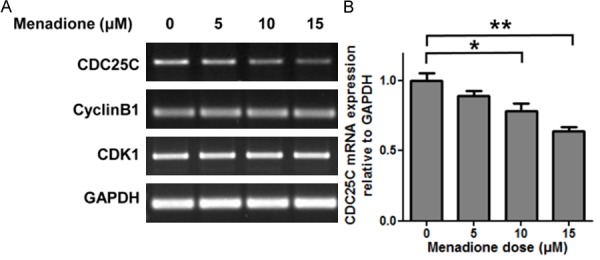
Menadione reduces expression of CDC25C by inhibiting mRNA expression in AGS cells. AGS cells were treated with indicated dose of menadione (0, 5, 10, 15 μM) for 24 h. A: Total RNA was extracted from the cell lysates and subjected to RT-PCR to detect CDC25C, CyclinB1, and CDK1. B: Densities of the PCR bands were measured by ImageLab software and illustrated as a graph. Data were from triplicate experiments and analyzed by Student’s t-test (*P < 0.05, **P < 0.01 and ***P < 0.001).
Menadione induces proteasome mediated degradation of CDK1 and cyclin B1 in AGS cells
Menadione treatment did not affect CDK1 and Cyclin B1 mRNA expression in AGS cells. Therefore, we investigated other possible mechanism for reduction of CDK1 and Cyclin B1 apart from the transcriptional regulation. Levels of CDKs and cyclins are tightly regulated during cell cycle. In particular, CDK1 and cyclin B1 are rapidly degraded by ubiquitin-proteaosome ma-chinery after a cell cycle phase reaches the M phase [17]. Thus we presumed that menadione decreased CDK1 and Cyclin B1 protein levels by proteasome mediated mechanism. To investigate whether the proteins were dec-reased by proteasome mediated degradation, AGS cells were treated with proteasome inhibitor (MG132) before exposure to menadione. If the proteins were reduced by proteasome mediated degradation, inhibition of proteasome will rescue the proteins from degradation. As expected, the decreased protein levels of CDK1 and Cyclin B1 by menadione treatment were recovered by inhibition of proteasome which suggest menadione facilitated proteasome mediated degradation of CDK1 and Cyclin B1 in AGS cells (Figure 6A and 6B). However, CDC25C protein level remained decreased although proteasome inhibitor was treated (Figure 6A and 6B). Collectively, menadione reduced CDK1, Cyclin B1 and CDC25C levels by two distinct mechanisms (proteasome mediated degradation and down-regulation of mRNA expression) and subsequently led AGS cells to G2/M cell cycle arrest.
Figure 6.
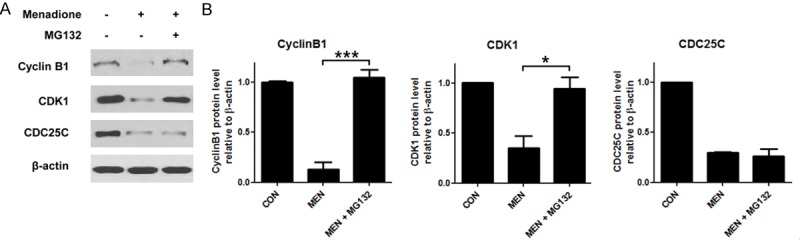
Menadione reduces CyclinB1 and CDK1 protein levels by promoting proteasome mediated degradation. AGS cells were treated with 15 μM of menadione and 0.5 μM of MG132 for 24 h. A: The cell lysates were subjected to Westernblot for CyclinB1, CDK1, and CDC25C. B: Densities of the Western bands were measured by Bio1D software and illustrated as graphs. Data were from triplicate experiments and analyzed by Student’s t-test (*P < 0.05, **P < 0.01 and ***P < 0.001).
Menadione induces G2/M arrest in MKN45 cells
To confirm inhibitory effect of menadione on the growth of gastric cancer cells, we conducted further experiments in another gastric cancer cells line MKN45. Menadione treatment reduced confluency of the cells on the microscopic examination (Figure 7A). In the cell cycle analysis, menadione also induced G2/M arrest in MKN45 cells and we also observed decrease of CDK1 protein in a menadione dose dependent manner (Figure 7B-D). These results confirmed menadione induces growth arrest in gastric cancer cells.
Figure 7.
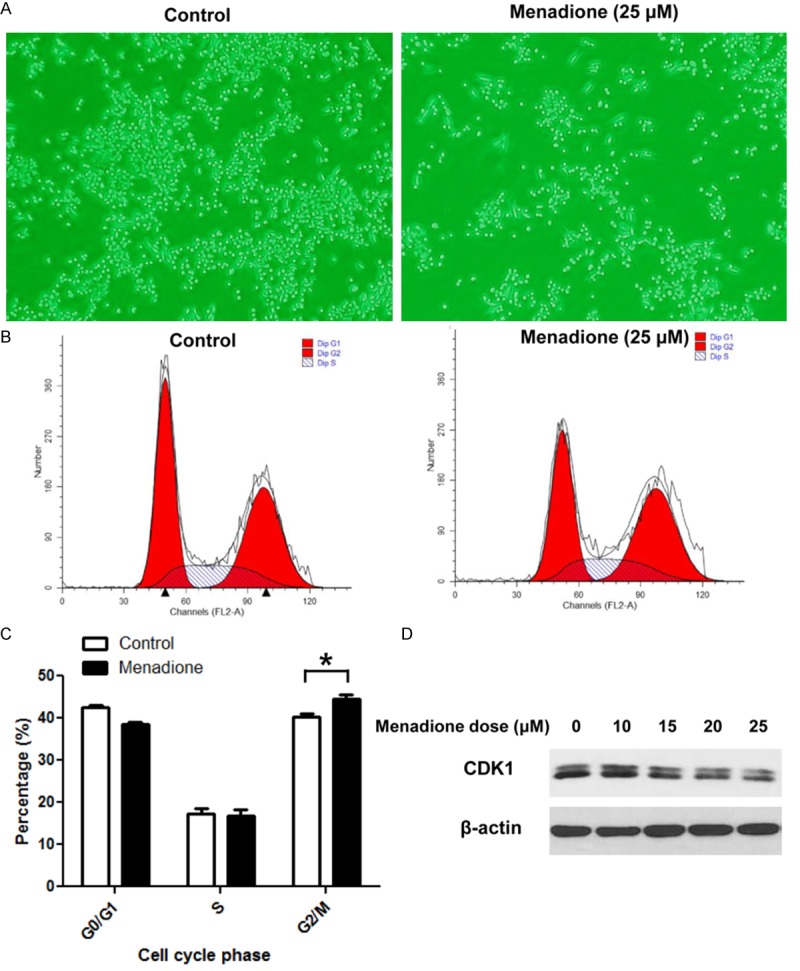
Confirmation of growth arrest of gastric cancer cells by menadione in MKN45 cells. (A) : AGS cells were treated with 25 μM of menadione for 24 h and images were captured using an inverted microscope (X 200). (B) : AGS cells were treated with 25 μM of menadione for 24 h. Cells were stained with PI and subjected to cell cycle analysis by flowcytometry. (C) : Data in (B) were analyzed by ModFit LT software and percentage of cells in each phase of the cell cycle was illustrated as a graph (lower panel). Data were from triplicate experiments and analyzed by Student’s t-test (*P < 0.05, **P < 0.01 and ***P < 0.001). D: AGS cells were treated with indicated concentrations of menadione for 24 h and subjected to Westernblot for CDK1.
Discussion
In this study, we investigated effect of menadione on gastric cancer growth and its growth-inhibitory mechanism. Here we have found that menadione inhibited growth of gastric cancer cells by inducing G2/M cell cycle arrest. We also have elucidated that G2/M arrest of gastric cancer cells by menadione was due to the decrease of CDK1, cyclin B1 and CDC25C. Furthermore, decrease of CDK1 and cyclin B1 was due to the proteasome mediated degradation of the proteins, and CDC25C was down-regulated in its mRNA expression level. Menadione induced G2/M arrest and the mechanism has been summarized in a schematic diagram (Figure 8).
Figure 8.
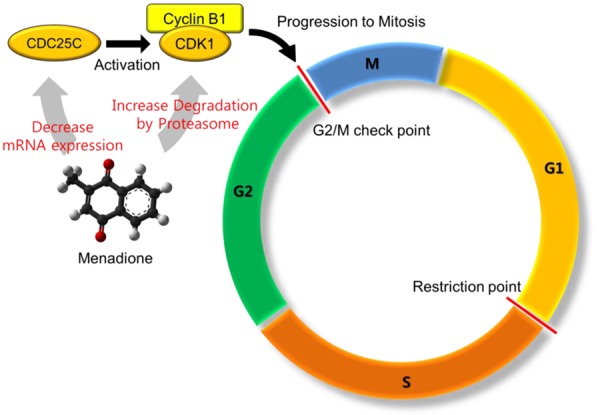
A schematic diagram illustrating the mechanism of menadione induced G2/M arrest in AGS cells.
Menadione was implicated in the cell cycle arrest of several cancer cells. In particular, involvement of menadione with cell cycle regulatory molecules was reported during G2/M cell cycle arrest [18,19]. It was reported that menadione inhibits function of CDK1, which is a key molecule necessary for G2/M transition, by inducing hyperphosphorylation on the protein [19]. It was also reported that CDC25, a protein phosphatase involved in CDK1 activation, function was attenuated by interaction of menadione with active site of the enzyme [20]. Although, these reports were from different type of cancer cells (hepatocellular carcinoma) and the mechanism can be different dependent on the cancer types, the previous reports and our results concordantly indicate menadione induces G2/M arrest in cancer cells.
In our study, menadione induced G2/M arrest by two distinct mechanisms. Menadione re-duced mRNA expression of CDC25C, and at the same time it promoted proteasome mediated degradation of CDK1 and Cyclin B1 proteins. According to the previous reports, menadione inhibited CDC25C function and subsequently inactivated CDK1-CyclinB complex in hepatocellular carcinoma [20]. Dysfunction of CDC25C results in hyperphosphorylation of CDK1 [11,20]. Therefore, we investigated phospho-CDK1 (Tyr15) level by Westernblot, but hyperphosphorylation was unobservable presumably because protein level of CDK1 was decreased in this study (data not shown). Although the mechanism is different from the previous reports in the different cancer types, reduced protein levels of CDK1, Cyclin B1 and CDC25C by menadione in AGS cells are sufficient cause for G2/M arrest of the cancer cells. The levels of CDK1 and cyclin B1 are closely associated with G2/M cell cycle arrest. Thus reduced level of CDK1 or cyclin B1 was reported to induce G2/M cell cycle arrest [21]. Therefore, our results indicate that menadione induces G2/M arrest by reducing protein level of CDK1, Cyclin B1 and CDC25C in AGS cells. Furthermore, we confirmed menadione induced G2/M arrest in gastric cancer cells by using another gastric cancer cell line MKN45, although growth arrest and decrease of CDK1 was observed in the higher dose of menadione in MKN45 cells.
Gastric cancer was the fourth prevalent (8.5%) type of cancer worldwide [22]. In particular, 35.4% of gastric cancer has occurred in Eastern Asia [22,23]. Therefore, discovery of the possible medications for gastric cancer seem to be necessary especially for Eastern Asian countries as well as for worldwide. Furthermore, several studies reported that naphthoquinones including menadione inhibits H. pylori growth although the mechanism is yet to be elucidated [24,25]. H. pylori is a gram-negative curved bacterium associated with the carcinogenesis on the stomach, thus World Health Organization classified H. pylori as a class I carcinogen [26]. Chronic infection of H. pylori on the stomach induces various gastric diseases such as gastritis, gastric ulcer and gastric cancer in severe case [26]. Therefore, it is expected that administration of menadione has advantages that works for treament of gastric cancer as well as for prevention and elimintion of H. pylori infection. Moreover, Tariq et al. reported menadione has gastroprotective effect by reducing gastric ulcer that is another advantageous effect of menadione treatment [27]. These reports illuminate the potential availability of menadione for H. pylori eradication as well as for prevention of gastric disease. Moreover, the inhibitory effect of menadione on gastric cancer cells and the inhibitory mechanism demonstrated in this study suggests the potential availability of menadione for treatment of gastric cancer. However, further investigations still seem to be necessary to completely understand the mechanisms by which menadione inhibits gastric cancer cells, and application in vivo should be accompanied to evaluate the physiological availability and cytotoxicity.
Disclosure of conflict of interest
None.
References
- 1.Al-Suhaimi E. Molecular mechanisms of leptin and pro-apoptotic signals induced by menadione in HepG2 cells. Saudi J Biol Sci. 2014;21:582–588. doi: 10.1016/j.sjbs.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy PK, Lall SP. Vitamin K deficiency inhibits mineralization and enhances deformity in vertebrae of haddock (Melanogrammus aeglefinus L. ) Comp Biochem Physiol B Biochem Mol Biol. 2007;148:174–183. doi: 10.1016/j.cbpb.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Gilloteaux J, Jamison JM, Arnold D, Taper HS, Summers JL. Ultrastructural aspects of autoschizis: a new cancer cell death induced by the synergistic action of ascorbate/menadione on human bladder carcinoma cells. Ultrastruct Pathol. 2001;25:183–192. doi: 10.1080/019131201300343810. [DOI] [PubMed] [Google Scholar]
- 4.Gilloteaux J, Jamison JM, Neal D, Summers JL. Synergistic antitumor cytotoxic actions of ascorbate and menadione on human prostate (DU145) cancer cells in vitro: nucleus and other injuries preceding cell death by autoschizis. Ultrastruct Pathol. 2014;38:116–140. doi: 10.3109/01913123.2013.852645. [DOI] [PubMed] [Google Scholar]
- 5.Marchionatti AM, Picotto G, Narvaez CJ, Welsh J, Tolosa de Talamoni NG. Antiproliferative action of menadione and 1,25 (OH) 2D3 on breast cancer cells. J Steroid Biochem Mol Biol. 2009;113:227–232. doi: 10.1016/j.jsbmb.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Osada S, Tomita H, Tanaka Y, Tokuyama Y, Tanaka H, Sakashita F, Takahashi T. The utility of vitamin K3 (menadione) against pancreatic cancer. Anticancer Res. 2008;28:45–50. [PubMed] [Google Scholar]
- 7.Tetef M, Margolin K, Ahn C, Akman S, Chow W, Leong L, Morgan RJ Jr, Raschko J, Somlo G, Doroshow JH. Mitomycin C and menadione for the treatment of lung cancer: a phase II trial. Invest New Drugs. 1995;13:157–162. doi: 10.1007/BF00872865. [DOI] [PubMed] [Google Scholar]
- 8.Kim YJ, Shin YK, Sohn DS, Lee CS. Menadione induces the formation of reactive oxygen species and depletion of GSH-mediated apoptosis and inhibits the FAK-mediated cell invasion. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:799–809. doi: 10.1007/s00210-014-0997-x. [DOI] [PubMed] [Google Scholar]
- 9.Oztopcu-Vatan P, Sayitoglu M, Gunindi M, Inan E. Cytotoxic and apoptotic effects of menadione on rat hepatocellular carcinoma cells. Cytotechnology. 2015;67:1003–9. doi: 10.1007/s10616-014-9739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 11.Rhind N, Russell P. Signaling pathways that regulate cell division. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atherton-Fessler S, Liu F, Gabrielli B, Lee MS, Peng CY, Piwnica-Worms H. Cell cycle regulation of the p34cdc2 inhibitory kinases. Mol Biol Cell. 1994;5:989–1001. doi: 10.1091/mbc.5.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MH, Yang JY, Cho Y, Park M, Woo HJ, Kim HW, Kwon HJ, Kim SH, Moon C, Kim TW, Kim JB. Menadione induces apoptosis in a gastric cancer cell line mediated by down-regulation of X-linked inhibitor of apoptosis. Int J Clin Exp Med. 2016;9:2437–2443. [Google Scholar]
- 14.Kim SH, Kang YW, Lee J, Kim HK, Jung BC, Kim B, Kim DJ, Kim YS. Ultraviolet B (UVB) Induces Down-regulation of Parkin Gene Expression. Biomed Sci Lett. 2016;22:18–23. [Google Scholar]
- 15.Lim J, Kim SH, Kang YW, Jung BC, Kim HK, Lee D, Rhee KJ, Kim YS. Triglyceride up-regulates expression of ABCG1 in PMA-induced THP-1 macrophages through activation of JNK and p38 MAPK pathways. Biomed Sci Lett. 2014;20:237–243. [Google Scholar]
- 16.Lee K, Lee MH, Kang YW, Rhee KJ, Kim TU, Kim YS. Parkin induces apoptotic cell death in TNF-alpha-treated cervical cancer cells. BMB Rep. 2012;45:526–531. doi: 10.5483/bmbrep.2012.45.9.104. [DOI] [PubMed] [Google Scholar]
- 17.Hershko A. Mechanisms and regulation of the degradation of cyclin B. Philos Trans R Soc Lond B Biol Sci. 1999;354:1571–1575. doi: 10.1098/rstb.1999.0500. discussion 1575-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu FY, Chang NT, Chen WJ, Juan CC. Vitamin K3-induced cell cycle arrest and apoptotic cell death are accompanied by altered expression of c-fos and c-myc in nasopharyngeal carcinoma cells. Oncogene. 1993;8:2237–2244. [PubMed] [Google Scholar]
- 19.Juan CC, Wu FY. Vitamin K3 inhibits growth of human hepatoma HepG2 cells by decreasing activities of both p34cdc2 kinase and phosphatase. Biochem Biophys Res Commun. 1993;190:907–913. doi: 10.1006/bbrc.1993.1135. [DOI] [PubMed] [Google Scholar]
- 20.Wu FY, Sun TP. Vitamin K3 induces cell cycle arrest and cell death by inhibiting Cdc25 phosphatase. Eur J Cancer. 1999;35:1388–1393. doi: 10.1016/s0959-8049(99)00156-2. [DOI] [PubMed] [Google Scholar]
- 21.Badie C, Bourhis J, Sobczak-Thepot J, Haddada H, Chiron M, Janicot M, Janot F, Tursz T, Vassal G. p53-dependent G2 arrest associated with a decrease in cyclins A2 and B1 levels in a human carcinoma cell line. Br J Cancer. 2000;82:642–650. doi: 10.1054/bjoc.1999.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 23.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 24.Park BS, Lee HK, Lee SE, Piao XL, Takeoka GR, Wong RY, Ahn YJ, Kim JH. Antibacterial activity of Tabebuia impetiginosa Martius ex DC (Taheebo) against Helicobacter pylori. J Ethnopharmacol. 2006;105:255–262. doi: 10.1016/j.jep.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Inatsu S, Ohsaki A, Nagata K. Idebenone acts against growth of Helicobacter pylori by inhibiting its respiration. Antimicrob Agents Chemother. 2006;50:2237–2239. doi: 10.1128/AAC.01118-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero-Gallo J, Harris EJ, Krishna U, Washington MK, Perez-Perez GI, Peek RM Jr. Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab Invest. 2008;88:328–336. doi: 10.1038/labinvest.3700719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tariq M, Al Moutaery A. Menadione protects gastric mucosa against ethanol-induced ulcers. Exp Toxicol Pathol. 2005;56:393–399. doi: 10.1016/j.etp.2004.12.003. [DOI] [PubMed] [Google Scholar]


