Abstract
Immune regulatory system dysfunction plays a role in the pathogenesis of asthma. The therapeutic effect of allergic asthma is to be improved. The immune regulatory function of probiotics has been recognized. This study tests a hypothesis that Clostridium butyricum (CB) enhances the effect of allergen specific immunotherapy (AIT) on asthma. In this study patients with allergic asthma were treated with AIT or/and CB for six months. The therapeutic effect and IgE production of the patients were observed. The results showed that administration with AIT alone alleviated the asthma symptoms; but the serum levels of interleukin (IL)-4, IL-5, IL-13 and specific IgE were not altered, which was markedly improved by the administration with CB plus AIT. Such effects were maintained only for two months in the patients treated with AIT alone; but maintained more than 12 months in those patients treated with both AIT and CB. CB facilitated AIT to induce IL-10+ B cells (B10 cells) in asthma patients. AIT/CB therapy converted antigen specific B cells to antigen specific regulatory B cells. Butyrate modulated the gene transcription of IgE and IL-10 in the allergen specific B cells. In conclusion, administration of CB can enhance the therapeutic effect of AIT in the treatment of allergic asthma via facilitating generation of B10 cells.
Keywords: Allergy, asthma, allergen specific immunotherapy, IgE, probiotics
Introduction
The allergen specific immunotherapy (AIT) is regarded as a specific therapeutic remedy for allergic diseases, such as allergic asthma, allergic rhinitis, allergic dermatitis, etc [1]. By administrating with small doses of specific allergens to patients, AIT intends to induce allergen specific regulatory immune cells, such as regulatory T cells (Treg) and regulatory B cells (Breg), and specific IgG4 production [2]. Yet, the therapeutic effect of AIT is not satisfactory currently and needs to be further improved. The therapeutic period of AIT is too long [3].
It is well understood that Immunoglobulin (Ig) E is the major mediator in the pathogenesis of allergic diseases [4]. Via binding the high affinity receptors of IgE on mast cells, which makes mast cells sensitized. Upon re-exposure to specific allergens, the sensitized mast cells are activated to release allergic mediators to initiate the allergy attack [5]. Although the mechanism of IgE production has been extensively investigated, the over-production of the allergen specific IgE in patients with allergic diseases is still difficult to be regulated to the normal levels currently [6].
The allergen-specific IgE is produced by plasma cells. Allergen-specific B cells differentiate into plasma cells or B10 cells depending on the microenvironment. The thrombospondin-1-producing B cells may develop into the transforming growth factor (TGF)-β-producing B10 cells [7]. Some B cells produce IL-10 and have been described as potent suppressive cells to inhibit immune inflammation [8]; this fraction of B cells is termed as B10 cells. B10 cells also induce T-cell apoptosis via the Fas-Fas ligand pathway [9] or the granzyme-B pathway [10]. B10 cells are capable of producing the inhibitory IgG4 to block mast cell activation [11]. Thus, to induce allergen-specific B10 cells may be an efficient strategy to induce allergen specific immune tolerance.
Probiotics are extensively used as a supplement to improve immunity [12]. The mechanism by which probiotics improve immunity is not fully elucidated yet. Some probiotics, such as Clostridium butyricum (CB), produce butyrate [13]. The butyrate is an inhibitor of histone deacetylase (HDAC) [14]. HDAC1 is associated with the pathogenesis of airway allergy [15]. Butyrate can regulate B cell’s activity [16]. Based on the information above, we hypothesize that the CB may enhance the effect of AIT by promoting the development of allergen-specific B10 cells. The results showed that the concurrent administration of AIT and CB significantly enhanced the therapeutic effect on asthma.
Materials and methods
Ethic statement
The using human specimen in the present study was approved by the Human Ethic Committees at Shenzhen University, Shanxi Medical University and Shanghai Jiaotong University. A written informed consent was obtained from each subject. The study was carried out in accordance with the approved guidelines.
Human subjects
Sixty patients (Table 1) with mild/moderate perennial asthma (with or without allergic rhinitis) sensitized to HDM alone were selected from 288 asthma patients (the rest 228 asthma patients were sensitized more than one allergen) and recruited into this study. The mild asthma was defined as no asthma controller medications, forced expired volume in 1 s (FEV1)>80% predicted. The moderate asthma was defined as low-moderate dose inhaled corticosteroid, FEV1 between 60 to <80% predicted. The diagnosis of asthma was recorded based on skin prick test (Alutard SQ, ALK-Abelló, Denmark), specific IgE and IgG4 (UniCAP®, Phadia, Sweden) and the history of asthma as described in the international consensus on AIT [17,18]. The exclusion criteria include: 1, severe asthma; 2, had AIT history; 3, asthma symptom control was relied on steroids or other immune suppressors; 4, had the history using theophylline, or other bronchodialators; 5, suffered from other severe organ diseases. Thirty-four healthy subjects were also recruited to this study based on no allergic history, the negative allergen skin test and the serum IgE levels were less than 0.35 kU/L. If specific treatment was required for alleviating asthma clinical symptoms, the patients were excluded from the study and switched to proper treatments. In total 56 patients completed the study (Figure 1).
Table 1.
Basic demographic data
| Items | AIT | AIT/CB | CB | Placebo |
|---|---|---|---|---|
| Gender (F/M) | 8/7 | 6/8 | 6/7 | 6/8 |
| Age (year) | 23.5 ± 5.6 | 19.8 ± 4.5 | 22.8 ± 3.9 | 21.6 ± 5.1 |
| Asthma history | 2-5 years | 2-5 years | 2-5 years | 2-5 years |
| Previous SIT | No | No | No | No |
| Previous CB | No | No | No | No |
| Smoking | No | No | No | No |
Data are presented as mean ± SD. FEV1, forced expiratory volume in first second. FVC, forced vital capacity. L, litre. % pred, percentage of predicted value. *, P <0.01, compared with the placebo group. #, P <0.01, compared with the SIT group. $, P <0.01, compared with the time point of “Before” in the same group. Serum samples from individual patients were processed separately. SIgE: Specific IgE (KU/L).
Figure 1.
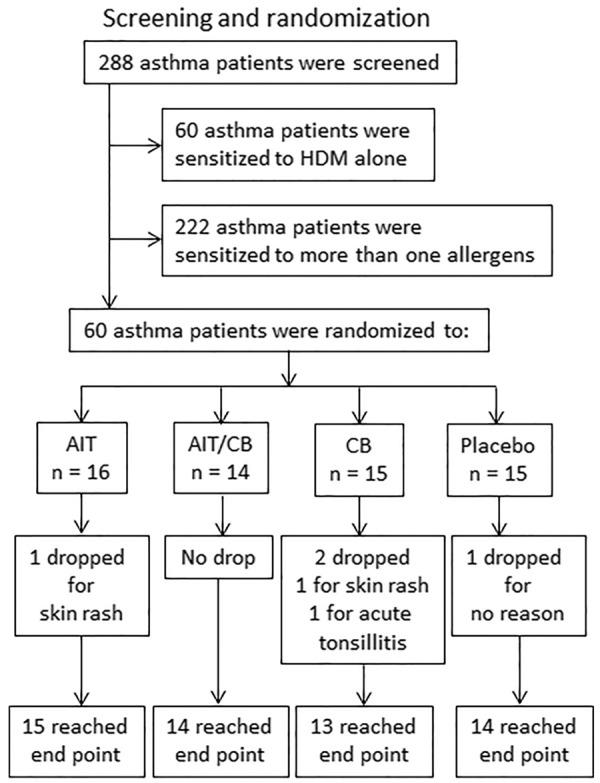
Screening and randomization. We screened 288 asthma patients, among which 222 patients were sensitized to more than one allergen; 60 patients were sensitized to HDM (house dust mite) alone. The 60 patients were randomly divided into 4 groups: AIT group: Patients were treated with AIT alone. AIT/CB group: Patients were treated with AIT and CB (C. butyricum). CB group: Patients were treated with CB alone. Placebo group: Patients were treated with placebo. In total 4 patients dropped from the study because of skin rash (n = 2) and tonsillitis (n = 1). One patient dropped for no reason. In total 56 patients completed the study.
Treating asthma patients with AIT or/and CB
Patients were randomized to 4 groups treated with AIT, AIT/CB, CB and placebo, respectively (The CB is an over-the-counter medicine produced by Shandong Kexing Biotech, Shandong, China). The AIT was carried out with an extract of Dermatophagoides pteronyssinus (Der p) absorbed to aluminium hydroxide (Alutard SQ, ALK-Abelló, Denmark) with our established protocol (using 1 μg/ml of the Der p vaccine; wk1: 0.1 ml; wk2: 0.2 ml; wk3, 0.3 ml; wk4: 0.4 ml; and 0.5 ml biweekly from wk5 to month 6) via subcutaneous injection. The patients remained in the clinic under observation for 1 h after the injection. A group of patients was treated with both AIT and oral CB. In addition to AIT, the patients took two capsules of CB (strain CGMCC 0313-2; 420 mg/capsule; Kexing Biotech, Shandong, China) twice daily from day one to month 6. The CB group patients took two capsules of CB twice daily without AIT. The placebo group patients were treated with saline (to replace Der p vaccine) and capsules containing vehicle. The patients were coded. The physicians recorded the therapeutic results were not aware of the code to avoid the observer bias.
Diary card
The patients were required to record asthmatic scores, medication consumption, and peak expiratory flow rate (PEFR; it was measured three times in the morning, the best one was recorded) throughout the observation periods on diary cards. The asthma symptoms were recorded on a four-point scale (0 = no symptom, 1 = mild, 2 = moderate, 3 = severe symptoms). During the trial, patients were allowed to take the following rescue medications if necessary: inhaled corticosteroids (mometasone for nasal allergy; budesonide turbuhaler for asthma), inhaled brochodialator (albuterol inhaler), and oral antihistiamine (levocetirizine, 5 mg). The number of puffs and/or tablets was scored as: 1 point: used one nasal spray per day; 2 points: used one tablet per day; 3 points, used one spray and one tablet per day. The results are presented as the averages per week.
Asthma control questionnaire, 5-item version (ACQ5)
The ACQ5 consists of nocturnal waking, morning symptoms, activity limitation, shortness of breath, and wheeze, each scored on a scale of 0-6, where 0 represents good control and 6 represents poor control [19]. The total asthma symptom score of the ACQ5 is the mean of the five responses [20].
Assessment of pulmonary functions
FVC and FEV1 were recorded for each patient using a spirometry and expressed as a percentage of predicted values. The use of additive short-acting β2 agonists was prohibited for at least 6 hours prior to the spirometry evaluation.
Assessment of serum Der p-specific IgE antibody
The blood (20 ml/subject) was obtained from each patient by the ulnar vein puncture before treatment and 6, 7 and 12 months after. The serum Der p-specific IgE level was measured by an in-house enzyme-linked immunosorbent assay (ELISA). The micro-plates were coated with mite crude extracts (1 mg/ml) and incubated at 4°C overnight. The plates were blocked with 5% fetal bovine serum (FBS) in PBS for 30 min at room temperature. After washing with PBS containing 0.05% Tween 20 (PBST) for 3 times, the serum samples (1/20 dilution; 0.1 ml/well) were added to the micro-plates and incubated overnight at 4°C. After washing with PBST, the plates were incubated with anti-human IgE biotinylated mAb for 1 h at room temperature. After washing with PBST for 3 times, the plates were incubated with horseradish-peroxidase-conjugated streptavidin at room temperature for 30 min. The wells were washed and then incubated with TMB substrate (Sigma Aldrich) for 30 minutes at room temperature. The enzyme reaction was stopped by adding 2N H2SO4. The plates were read with a microplate reader at 450 nm.
Assessment of fractional exhaled nitric oxide (FeNO)
FeNO was measured with a Niox Mino device (Aerocrine, Solna, Sweden) by trained technicians.
Reagents
The antibodies of CD3, CD28, STAT3, STAT6, HDAC1 and the shRNA kit for STAT6 were purchased from Santa Cruz Biotech (Santa Cruz, CA). The fluorochrome-labeled antibodies of CD4, CD25, Foxp3, IL-10, CD19 and IgE were purchased from eBioscience (San Diego, CA). The immune cell isolation kits were purchased from Miltenyi Biotech (San Diego, CA). The ELISA kits of IL-4, IL-5, IL-13 and IFN-γ were purchased from R&D Systems (Minneapolis, MN). The Derp1 was synthesized by us via molecular cloning approach. The luciferase kit and ChIP kit were purchased from Sigma Aldrich (St. Louis., MO). The reagents for real time RT-PCR, Western blotting and gene transcription were purchased from Invitrogen (Carlsbad, CA).
Peripheral blood sample collection and immune cell isolation
Blood samples were collected from each subject (20 ml/person/time) via ulnar vein puncture. The peripheral blood mononuclear cells (PBMC) were isolated by the gradient density centrifugation. The immune cells, including CD11c+ dendritic cells (DC), CD4+ CD25+ CD- 127- T cells, CD19+ CD138- B cells were isolated from the PBMCs by the magnetic cell sorting (MACS) with commercial reagent kits following the manufacturer’s instructions. The cell purity was checked by flow cytometry. If the purity did not reach 95%, the MACS was performed again.
Cell culture
The isolated immune cells were cultured in RPMI1640 medium supplemented with 10% fetal bovine albumin (BSA), 100 U/ml penicillin, 0.1 mg/ml streptomycin and 2 mM L-glutamine. The medium was changed in 2-3 days. The cell viability was assessed by the Trypan blue exclusion assay. In the case of B cell culture, anti-CD40 Ab (20 ng/ml) was added to the culture. The cell viability was greater than 99% as checked by Trypan blue exclusion assay.
Construction of a Der p-specific tetramer
To isolate the Derp1 specific B cells (DerBC), a tetramer was constructed following reported procedures [21,22] with a minor modification. The biotinylated Derp1 was incubated with magnetic particle-conjugated streptavidin for 30 min at room temperature. Unconjugated reagents less than 10 kDa were filtered through a filter tube by centrifugation. The Derp1 tetramers were collected for Derp1-specific B cell isolation.
Derp1-specific B cell Isolation
Following our established procedures [22], PBMCs were isolated from the peripheral blood samples. The Derp1 tetramer was added to the PBMCs at 2 μg/ml and incubated for 30 min at room temperature. The cells were passed through a column in the magnetic apparatus (Miltenyi Biotech). Cells were collected, washed with acidic phosphate-buffered saline (PBS) (pH = 3) to remove the bound Derp1 on the cell surface, and transferred to RPMI 1640 medium for further experiments.
Enzyme-linked immunosorbent assay (ELISA)
The levels of cytokines in the serum were determined by ELISA with commercial reagent kits following the manufacturer’s instructions.
Flow cytometry
In the surface staining, the cells were stained with fluorochrome-labeled primary antibodies (0.5 mg/ml, or isotype IgG using as a control) for 30 min on ice. If intracellular staining was required, the cells were fixed by 2% paraformaldehyde and permeabilized with 0.5% saponin for 2 h, and followed by incubating with fluorochrome-labeled antibodies (0.5 µg/ml, or isotype IgG using as a control) for 1 h on ice. In the case of assessing the antigen specific B cell proliferation, the isolated antigen specific B cells were isolated by the Der p specific tetramer and labeled with CFSE, and cultured in the presence of specific antigens for 3 days. After washing with PBS, the cells were analyzed by flow cytometry. The data were analyzed by software Flowjo. The data of isotype IgG staining were used as a gating reference.
Western blotting
The total proteins were extracted from cells and quantified with the BCA method (Bio-Rad). The proteins were fractioned by SDS-PAGE and transferred onto a PVDF membrane. The membrane was blocked by incubating with 5% skim milk for 30 min, incubated with the primary antibodies or isotype IgG (200 ng/ml) overnight at 4°C, and followed by incubating with the second antibodies (conjugated with peroxidase) for 1 h at room temperature. Washing with TBST (Tris buffered saline Tween 20) was performed after each incubation. The membrane was developed by the enhanced chemiluminescence. The results were photographed with an Image Station (UVI Image System; Cambridge, UK).
Real time RT-PCR (RT-qPCR)
The total RNA was extracted from the cells with the TRIzol reagent. The cDNA was synthesized with the extracted RNA using a reverse transcription kit following the manufacturer’s instructions. The cDNA was amplified by qPCR in a real time PCR device (MiniOpticon Real-Time PCR Detection System; Bio Rad) with the SYBR Green Master Mix. The primers using in the present study include: IgE, tagtgactctgatgccaccc and ccccagaggtccaagtaaca; IL-10, gttctttggggagccaacag and gctccctggtttctcttcct. The results were calculated with the 2-∆∆Ct method and presented as folds of change against controls.
Chromatin IP (ChIP)
The cells were fixed with 1% formaldehyde for 15 min to cross link the DNA binding proteins. The samples were sonicated to shear DNA along with bound proteins into small fragments. The lysates were precleared by incubating with protein G agarose beads for 4 h at 4°C. After removing the beads by centrifugation, the samples were incubated with antibodies of STAT3, or STAT6, or HDAC1, or isotype IgG, and protein G agarose beads overnight at 4°C to bind antibodies specific to the DNA-binding proteins. The beads were harvested by centrifugation. The immune complex on the beads was eluted with an eluting buffer. After cross-link-reversal and DNA purification, qPCR was performed with the samples and inputs. The primers of the promoter regions include: Ig heavy chain germline Igϵ (tgggcctgagagagaagaga and agctctgcctcagtgctttc) and IL-10 (tcaagacaacactactaaggctt and agatggggtggaagaagttga). The results are presented as a relevant value against the input.
RNA interference (RNAi)
The gene of STAT6 was knockdown by RNAi with a commercial shRNA kit following the manufacturer’s instructions. The knockdown effect was assessed by Western blotting.
STAT6 overexpression
The STAT6 expressing plasmid was constructed by Genescript (Nanjing, China) and transfected to B cells with the lipofectamine reagent kit following the manufacturer’s instructions.
Luciferase reporter assay
HEK293 cells were seeded in 24-well plates and transfected with either a control vector or a STAT3 vector using Lipofectamine 2000 following the manufacturer’s instructions. The cells were lysed 24-h after the transfection with lysis buffer. Luciferase assays were performed with a commercial reagent kit following the manufacturer’s instructions. The results were read on a luminometer.
Statistics
The data are presented as mean ± SD. Two-way analysis of variance (ANOVA) along with Bonferroni correction was used in for continuous variable between treatment groups. The paired t-test was used to determine changes from baseline within each treatment group. A P<0.05 was set as a significant criterion.
Results
Administration of CB enhances the therapeutic efficacy of AIT in asthma patients
To elucidate if probiotics facilitate the effect of AIT, we treated asthma patients with AIT, or AIT/CB, or CB alone, or placebo for 6 months. The major asthma parameters (Figure 2; Tables 2, 3), including ACQ5, serum specific IgE and Th2 cytokines, were markedly down regulated, serum IgG4 was markedly up regulated, in the AIT group, which were further improved in the AIT/CB group, while no apparent improvement was observed in the CB group and the placebo group. These parameters were re-examined in the patients on the 7th month and 12th month (one and 6 months after the termination of the therapy) respectively. The results showed that the asthma parameters returned to the levels before the therapy in the AIT group within one month after the termination of AIT. In the AIT/CB group, however, the asthma parameters were further improved at the time points of month 7 and 12 respectively (Figure 2; Tables 2, 3). The results indicate that AIT does alleviate asthma, which can be enhanced by the concurrent administration with CB. The therapeutic effect of AIT on asthma symptoms does not last long after the termination of the therapy; the therapeutic effect can be well maintained by the concurrent administration of CB and AIT.
Figure 2.
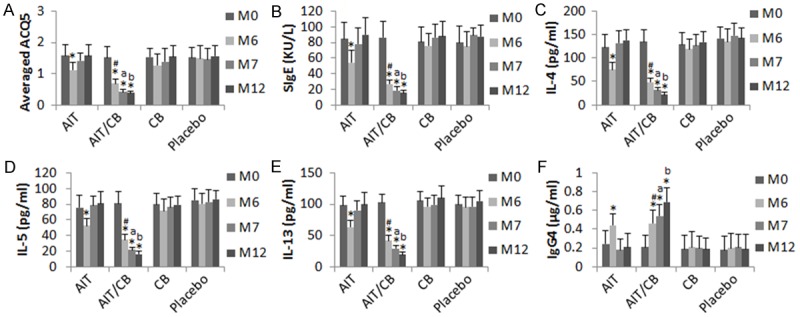
CB enhances AIT in asthmatic patients. Asthma patients were treated with AIT (n = 15), or AIT/CB (n = 14), or CB (n = 13), or placebo (n = 14) for 6 months. The TAS was recorded and the peripheral blood samples were collected from the patients in month 0, 6, 7 and 12 respectively. The bars indicate the TAS (A), specific (S) IgE (B), IL-4 (C), IL-5 (D), IL-13 (E) and IgG4 (F). Samples from individual subjects were analyzed separately. The data of bars are presented as mean ± SD. The #, *, a and b, P<0.01, compared with the AIT group (#), or month (M) 0 (*), or M6 (a), or M7 (b).
Table 2.
Medication scores$
| AIT | AIT/CB | CB | Placebo | |
|---|---|---|---|---|
| Month 0 | 14.1 ± 3.2 | 15.1 ± 4.9 | 15.6 ± 4.5 | 14.5 ± 4.3 |
| Month 6 | 9.1 ± 4.2* | 3.5 ± 2.1* | 14.7 ± 3.5 | 15.2 ± 3.6 |
| Month 7 | 16.1 ± 3.4 | 2.6 ± 1.3* | 14.9 ± 3.2 | 14.6 ± 3.4 |
| Month 12 | 15.3 ± 3.1 | 2.1 ± 0.5* | 15.3 ± 3.4 | 15.5 ± 2.7 |
Data are presented as mean ± SD.
The data are an average of medication score in one week prior to the indicated time points.
P<0.01.
Table 3.
Asthma related parameters
| Parameters | AIT | AIT/CB | CB | Placebo |
|---|---|---|---|---|
| FEV1 (% pred) | ||||
| 0 month | 78 ± 11 | 77 ± 11 | 79 ± 12 | 80 ± 12 |
| 6 months | 88 ± 12 | 97 ± 13* | 80 ± 15 | 81 ± 18 |
| 7 months | 81 ± 15 | 99 ± 14*,# | 78 ± 16 | 79 ± 19 |
| 12 months | 77 ± 18 | 101 ± 15*,# | 81 ± 18 | 78 ± 20 |
| PEFR (% pred) | ||||
| 0 month | 78 ± 15 | 76 ± 18 | 74 ± 19 | 73 ± 19 |
| 6 months | 78 ± 15 | 98 ± 18* | 74 ± 18 | 73 ± 10 |
| 7 months | 75 ± 11 | 103 ± 18*,# | 72 ± 16 | 72 ± 17 |
| 12 months | 72 ± 19 | 108 ± 19*,# | 72 ± 13 | 72 ± 15 |
| FeNO | ||||
| 0 month | 31.8 ± 14.6 | 33.5 ± 18.8 | 34.2 ± 12.7 | 32.5 ± 17.6 |
| 6 months | 24.9 ± 12.9 | 14.8 ± 9.9*,# | 33.9 ± 14.8 | 33.6 ± 14.5 |
| 7 months | 32.3 ± 18.5 | 12.7 ± 11.4*,# | 31.7 ± 14.2 | 31.8 ± 18.5 |
| 12 months | 33.5 ± 13.5 | 12.9 ± 9.7*,# | 33.6 ± 15.2 | 33.7 ± 15.9 |
| Eosinophils | ||||
| 0 month | 9.56 ± 3.61 | 9.82 ± 2.68 | 9.47 ± 3.19 | 9.66 ± 3.51 |
| 6 months | 7.63 ± 2.55 | 3.67 ± 1.54*,# | 8.96 ± 3.32 | 9.58 ± 2.88 |
| 7 months | 9.17 ± 2.35 | 3.48 ± 1.28*,# | 9.18 ± 3.61 | 9.28 ± 3.41 |
| 12 months | 9.63 ± 3.11 | 3.22 ± 1.31*,# | 9.69 ± 3.67 | 9.47 ± 3.52 |
Data are presented as mean ± SD. FEV1: Forced expiratory volume in one second. Pred: Predicted. PEFR: Peak expiratory flow rate. Eosinophils: Percentage of eosinophils in total white blood cells.
P<0.01, compared with the data of 0 month in the same group.
P<0.01, compared to the AIT group.
CB enhances AIT to induce IL-10+ B cells in asthma patients
To elucidate if CB enhances the development of regulatory immune cells, we assessed the frequency of the peripheral CD4+ CD25+ Foxp3+ Treg and IL-10+ Breg in the asthma patients before and after the therapy. As shown by Figure 3, however, before the AIT/CB therapy, the frequency of Treg in the asthma patients did not show any significant difference from healthy subjects. We also isolated CD4+ CD25+ CD127- Tregs from the PBMCs and analyzed by flow cytometry. An interesting finding was that the Derp1 specific Tregs were detected in healthy subjects. The frequency of Derp1 specific Tregs was a little less in the asthma patients than in healthy subjects, but the difference did not reach the significant levels. The treatment with AIT/CB for 6 months only slightly increased the Derp1 specific Tregs in the asthma patients (Figure 3).
Figure 3.
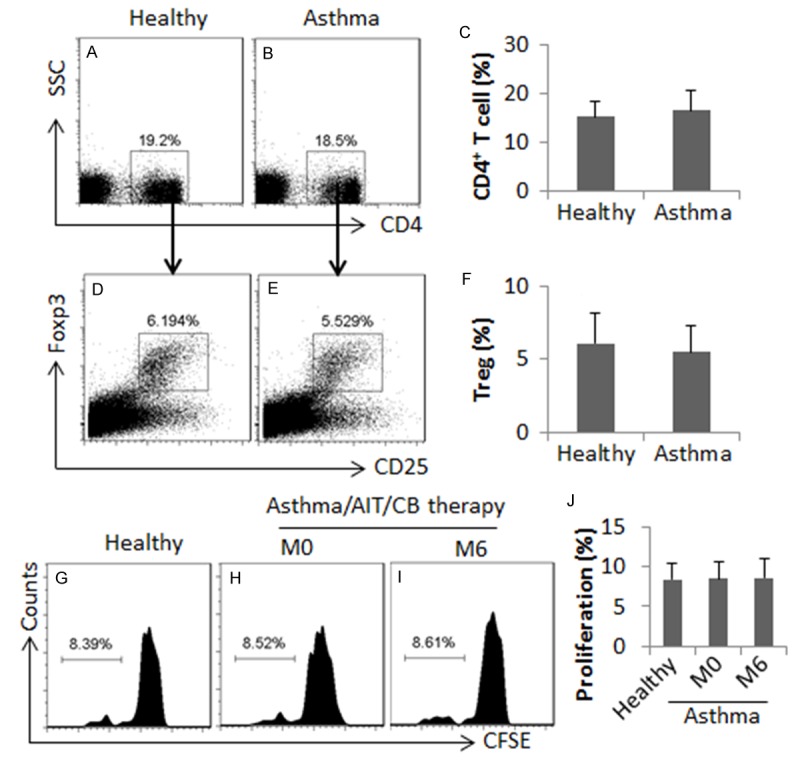
Assessment of peripheral Tregs and B10 cells. PBMCs were isolated from blood samples collected from healthy subjects (n = 10) and asthma patients (n = 10) and analyzed by flow cytometry. (A, B) The gated plots indicate the frequency of CD4+ T cells. (D, E) The gated plots indicate the frequency of Tregs. (G-I) The gated histograms indicate the proliferating CD4+ CD25+ CD127- T cells, which were isolated from the PBMCs, labeled with CFSE and cultured in the presence of DCs (T cell: DC = 104: 2×103/well) and Derp1 (1 µg/ml) for 3 days. The bars are summarized data (mean ± SD) of (A-F) and (G-J). M0: Month 0. M6: Month 6. Samples from individual subjects were analyzed separately.
On the other hand, we observed that the frequency of the peripheral B10 cells in asthma patients was not significantly different from that of healthy subjects (Figure 4A). Considering asthma may only affect the allergen specific B10 cells, we assessed the frequency of the Derp1 specific B10 cells using a specific tetramer [22]. The results showed that even though in healthy subjects, the Derp1 specific B10 cells were detected in the PBMCs, which were about 5 folds less (P<0.01) in asthma patients (Figure 4B). The AIT/CB therapy markedly enhanced the frequency of Derp1 specific B10 cells in asthma patients, which lasted throughout the observation period. Treating with either AIT or CB alone did not induce appreciable increases in the Derp1 specific B10 cells in the asthma patients (Figure 4B). The results demonstrate that the AIT/CB therapy is capable of increasing the Derp1 specific B10 cells, but not Tregs, in asthma patients sensitized to HDM. Neither the AIT therapy, nor the CB therapy does show the effect on altering the Derp1 specific B10 cells or Tregs in HDM asthma patients.
Figure 4.
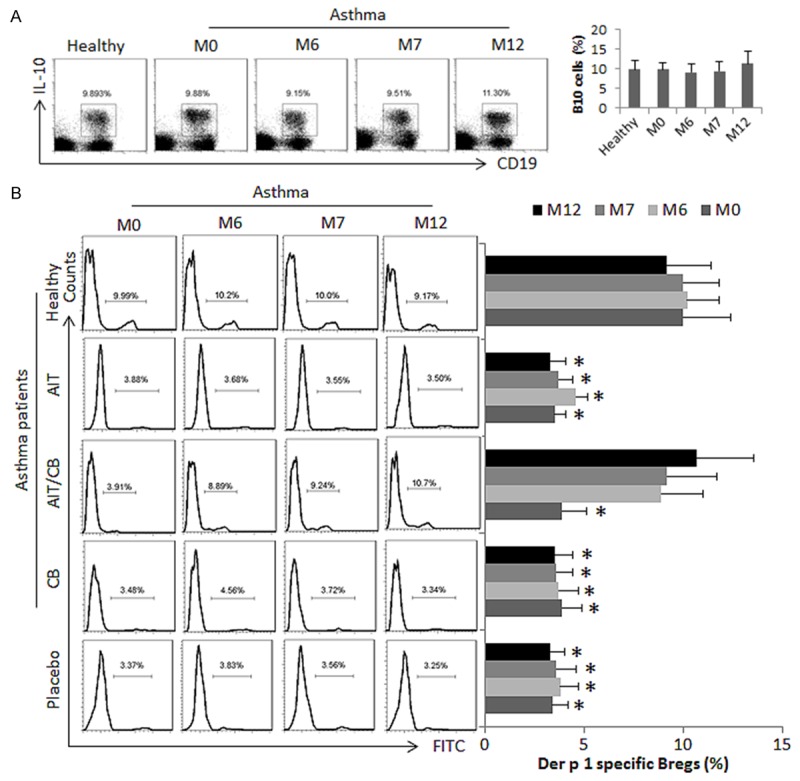
The AIT/CB therapy induces allergen specific B10 cells. Asthma patients were treated with AIT or/and CB, or placebo as denoted in the figure. PBMCs were isolated from healthy subjects and asthma patients. The cells were analyzed by flow cytometry. A: The gated dot plost show the frequency of IL-10+ B10 cells; the bar graphs show the summarized data of the dot plots. B: The gated histograms show the frequency of the Derp1 specific B10 cells (stained by FITC-labeled Derp1 tetramer). The bar graphs show the summarized data of the histograms. The data of bars are presented as mean ± SD. *, P<0.01, compared with the healthy group. The human subject numbers are the same as Figure 1. Samples from individual subjects were processed separately.
AIT/CB therapy converts antigen specific B cells to antigen specific B10 cells
The results reported above implicate that the AIT/CB therapy converted the Derp1 specific B cells to Derp1 specific B10 cells in asthma patients. To corroborate the results, we isolated the Derp1 specific B cells from asthma patients with our established procedures [22] before receiving any treatment. The cells were treated with Derp1 or/and butyrate (a product of CB) in the culture for 4 days. The results indicate that the treatment with Derp1/butyrate converted about 48.6% of the Derp1 specific B cells to IL-10+ B10 cells, which did not occur in the cells treated with either Derp1 alone or butyrate alone. On the other hand, B cells stimulated by Derp1 alone showed about 42.5% IgE+ B cells. Treating with butyrate alone did not induce either IL-10+ B10 cells or IgE+ B cells (Figure 5). The results indicate that exposure to the specific allergens alone converts allergen specific B cells to the IgE-producing plasma cells, while exposure to both specific allergens and butyrate converts the allergen specific B cells to IL-10+ B10 cells.
Figure 5.
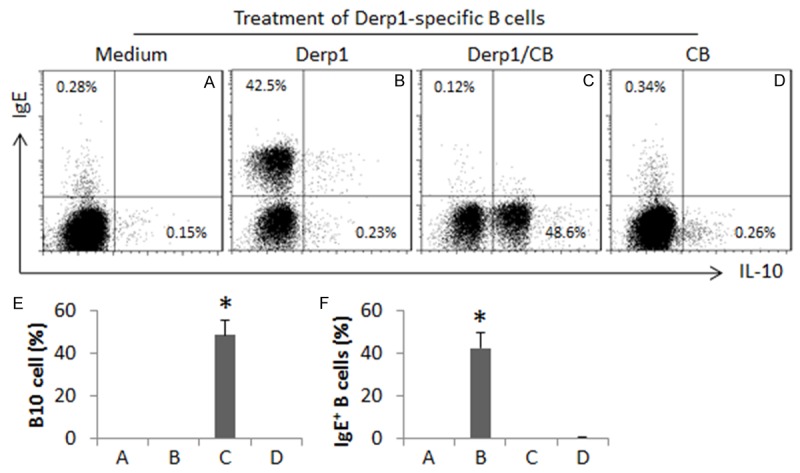
Butyrate modulates allergen specific B cell differentiation. The Derp1 specific CD138- B cells were isolated from the peripheral blood samples of asthma patients (n = 6) with a specific tetramer. The cells were cultured in the presence of Derp1 (1 µg/ml) or/and butyrate (1 mM), and anti-CD40 (20 ng/ml) for 4 days. The cells were analyzed by flow cytometry. (A-D) The gated dot plots show the IgE positive or IL-10 positive cells. The bars graphs (E, F: Mean ± SD. *, P<0.01, compared to group A) show the summarized data of IL-10 positive (E) or IgE positive B cells. The Derp1 specific B10 cells obtained from individual subjects were processed separately.
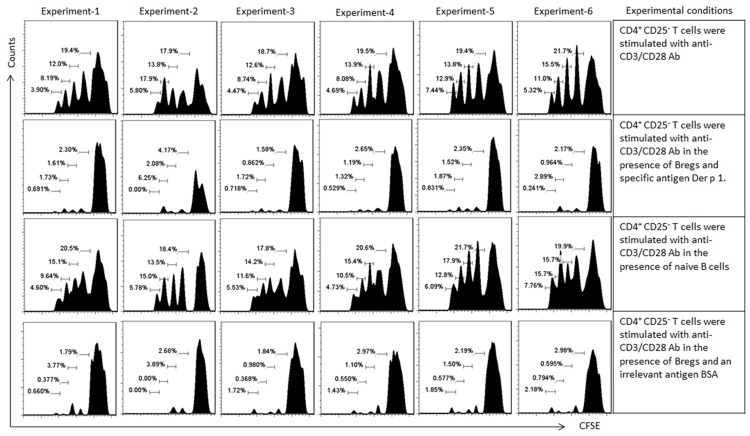
To test the immune suppressor effect of the generated B10 cells, after exposure to Derp1/butyrate in the culture for 4 days, the cells were washed with fresh medium and cocultured with CD4+ CD25- effector T cells (labeled with CFSE) in the presence of anti-CD3/CD28 for 3 days. As shown by the flow cytometry data, the effector T cells proliferated markedly in response to the TCR stimulation, which was suppressed by the presence of the Derp1/butyrate-primed B10 cells (Figure 6), indicating the B10 cells have the immune suppressor function.
Figure 6.
Activation of Derp1-specific B10 cells suppress T cell proliferation. CD4+ CD25- T cells and naïve B cells were isolated from PBMCs of healthy subjects (by MACS; n = 24). Derp1-specific B cells were isolated from 24 asthma patients sensitized to HDM (before any specific treatment); the cells were primed by Derp1/butyrate for 4 days. The cultural condition is denoted in the table on the right side column of the histograms. B cell: T cell = 104: 5×104/well. The gated histograms indicate the proliferating T cells. Samples from individual subjects were processed separately.
Butyrate modulates the gene transcription of IgE and IL-10 in the allergen specific B cells
We next took a further insight into the mechanism. After exposure to Derp1 or/and butyrate in the culture for 4 days, the Derp1 specific B cells were harvested and analyzed by ChIP. The results showed that exposure to Derp1 significantly increased the levels of STAT6 at the Iε promoter locus (Figure 7A), and the expression of IgE was also detected in the B cells (Figure 7B), which was abolished by the co-presence of butyrate, indicating that HDAC might be involved in the Derp1-induced IgE expression in the B cells. Since HDAC1 plays an important role in the pathogenesis of allergy [14,23], we assessed the levels of HDAC1 at the Iε promoter locus of the B cells after exposure to Derp1. Indeed, the levels of HDAC1 were significantly increased after exposure to the specific allergens (Figure 7C).
Figure 7.
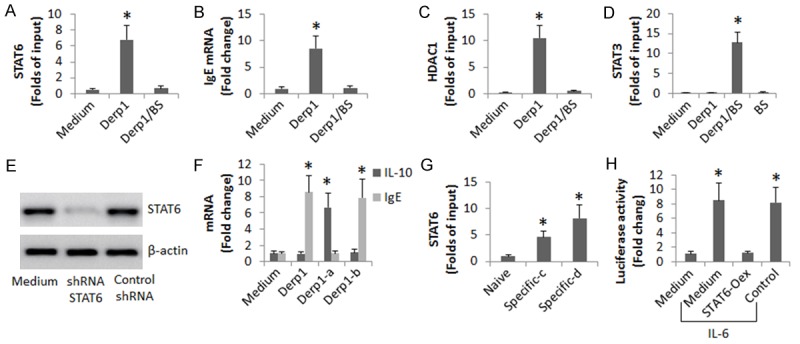
Butyrate modulates Derp1-specific B cell activity. Derp1-specific B cells were isolated from the peripheral blood of asthma patients (n = 12; before treatment). The cells were stimulated with Derp1 with or without the presence of butyrate sodium (BS; 5 mM) for 48 h. A: The cells were analyzed by ChIP. The bars indicate the STAT6 levels at the Iε promoter locus. B: The B cell extracts were analyzed by RT-qPCR. The bars indicate the mRNA levels of IgE. C: The bars indicate the HDAC1 levels at the Iε promoter locus (by ChIP). D: The bars indicate the STAT3 levels at the IL-10 promoter locus (by ChIP). E: The immune blots indicate the results of STAT6 gene knockdown. F: The bars indicate the mRNA levels of IgE and IL-10 in the B cells. G: The bars indicate the STAT6 levels at the IL-10 gene locus (by ChIP). Naive: Naive B cells. Specific: Derp1-specific B cells; the cells were treated with saline (c) or exposed to Derp1 (1 µg/ml) in the culture (d). H: The bars indicate the STAT3 reporter gene activity (by ChIP). STAT6-Oex: The cells with STAT6-overexpression. Control: The cells were transfected with an empty plasmid. The concentration of IL-6 was 20 ng/ml. The data are representatives of 3 independent experiments. *, P<0.01, compared to the medium group (compared to the naive group in panel G).
Since exposure to Derp1/butyrate increased the expression of IL-10 in the Derp1-specific B cells, published data indicate that the STAT3 is the gene transcription factor of IL-10 [24], we inferred that the levels of STAT3 might be also up regulated. To test this, we performed ChIP assay with the B cells. The results showed that the levels of STAT3 at the IL-10 promoter locus were indeed up regulated in the B cells by exposure to both Derp1 and butyrate, but not to either one alone (Figure 7D). The results suggest that the Derp1-increased STAT6 interferes with the expression of IL-10 in B cells via repressing STAT3. To test this, we treated the STAT6-knockdown Derp1-specific cells (Figure 7E) with Derp1 in the culture. Indeed, the cells expressed high levels of IL-10, but not the IgE (Figure 7F). On the other hand, we detected that STAT6 bound to the STAT3 gene locus in the Derp1-specific B cells after exposure to the specific allergens (Figure 7G). The results implicate that the STAT6 binds the STAT3 gene to repress its transcription. To test this, we overexpressed STAT6 in HEK293 cells; the cells were transfected with a STAT3 luciferase reporter; the cells were then activated by IL-6 [25]. The results showed that the presence of IL-6 induced high levels of luciferase activity, which was significantly suppressed by the overexpression of STAT6.
Discussion
The incidence of allergic diseases increased rapidly in the last several decades. Current therapies of allergic diseases are not satisfactory. Thus, to find more efficient novel therapies for allergic diseases is of great significance. The present data show that administration of C. butyricum (CB) significantly increased the therapeutic effect of AIT on asthma. Moreover, in the AIT alone group, one month after stopping the treatment, the asthma clinical symptoms and serum specific IgE returned to the levels before the therapy, while in the AIT/CB group, the asthma symptoms, serum specific IgE and Th2 cytokines were satisfactorily suppressed throughout the entire observation period. Thus, from the present study, we have found a novel, more efficient therapy for the treatment of asthma.
Probiotics have been extensively used to improve immunity in the body. Arrieta et al inoculated germ-free mice with probiotics ameliorated airway inflammation in their adult progeny, and found that probiotics prevent the development of asthma [26]. Simpson et al treated pregnant women with probiotics and found that maternal probiotic ingestion may reduce the incidence of allergic dermatitis [27]. CB is one of the probiotics. It is an over-the-counter medicine. Our previous work showed that oral administration of CB improved the intestinal epithelial barrier function and prevented the development of food allergy in a mouse model [28]. Yet, the efficacy of the probiotic therapy on allergic diseases is still limited [29]. The present results are in line with the published data by showing that treating with CB alone did not show apparent therapeutic effect on asthma patients. However, administration of CB significantly enhanced AIT to ameliorate asthma symptoms as shown by the present data.
AIT has been employed to treat allergic diseases in clinic for a long time. Regarding its therapeutic efficacy, however, diverse results have been reported. Roger et al reported that no significant changes were observed in concentrations of total IgE, specific IgE or Th2 cytokines in patients with allergic rhinitis after AIT although satisfactory relief of allergic rhinitis symptoms was declared by most patients in the period of the treatment [30]. Glover et al indicated that the efficacy of AIT on asthma is “uncertain” [31] while Galli even observed no difference between AIT group and control group [32]. Our data are in line with reported data by showing that AIT alleviated asthma clinical symptoms somehow, but relapsed one month after stopping AIT. The results may be explained as that AIT introduces small doses of specific allergens to subjects with allergic diseases; the allergens inevitably bind the sensitized mast cells and slowly exhaust the allergic mediators in the mast cells. The inference is supported by the present data, in which the specific IgE and Th2 cytokines in asthma patients were not satisfactorily suppressed by AIT and the asthma clinical symptoms relapsed quickly (within one month) after stopping AIT.
The combination of AIT and CB got the expected results. After treating with AIT/CB for 6 months, the asthma clinical symptoms were markedly alleviated; serum specific IgE and Th2 cytokines were significantly down regulated in the asthma patients. The therapeutic effect lasted throughout the entire observation period (12 months). The subsequent data provide supporting evidence. After the AIT/CB therapy, the frequency of the allergen-specific B10 cells was increased in asthma patients. Such a phenomenon was also reported by other investigators [11]. The data from in vitro experiments provide foundational evidence, in which exposure to specific allergens and butyrate converted allergen-specific B cells to Breg, while exposure to specific allergens alone converted allergen-specific B cells to IgE-producing plasma cells.
It is suggested that the generation of Tregs to restore the tolerance is one of the mechanisms of AIT [11]. In our study, however, after the therapy of AIT/CB, although the frequency of Tregs was slightly up regulated, it did not reach the significant criterion. As Dr. Berin MC comments on, tolerance has been notoriously difficult to restore in animal disease models, but limited data from human trials suggest that tolerance can be re-established in a subset of patients [33]. In fact, the rationale in the AIT-generating Tregs is less clear currently. It seems that the major functional part in the AIT/CB therapy is to convert the allergen-specific B cells to the allergen-specific B10 cells. The data demonstrate that AIT and CB have a synergistic effect on regulating the allergen-specific B cell activity because either AIT alone or CB alone could not induce the allergen-specific B10 cells. It is clear that to activate allergen-specific B cells, at least two activators are required. In the present in vitro experiments, the specific allergen, Derp1, is one activator; the anti-CD40 antibody can be another. These two activators did activate the allergen-specific B cells to develop into the IgE-producing plasma cells. The addition of the third activator, butyrate, made a critical change to induce the allergen-specific B cells to differentiate into B10 cells, in which the chromatin remolding was induced at the IL-10 gene locus in the B cells.
In summary, the present data show that concurrent administration of AIT and C. butyricum significantly enhanced the therapeutic effect on asthma, in which the allergen-specific B10 cells were generated via inducing the chromatin remolding at the IL-10 gene locus in the B cells.
Acknowledgements
This study was supported by grants from the innovation of science and Technology Commission of Shenzhen Municipality (JCYJ20150402090413008, JCYJ20160422101725667, JCYJ20140418095735611, JCYJ20160429091935720 and ZDSYS201506050935272), the Natural Science Foundation of China (81373176, 31570932, 31400856, 81571790 and 81501573).
Disclosure of conflict of interest
None.
References
- 1.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J. 2015;8:17. doi: 10.1186/s40413-015-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soyka MB, van de Veen W, Holzmann D, Akdis M, Akdis CA. Scientific foundations of allergen-specific immunotherapy for allergic disease. Chest. 2014;146:1347–1357. doi: 10.1378/chest.14-0049. [DOI] [PubMed] [Google Scholar]
- 3.Ridolo E, Montagni M, Bonzano L, Senna G, Incorvaia C. Arguing the misconceptions in allergen-specific immunotherapy. Immunotherapy. 2014;6:587–595. doi: 10.2217/imt.14.23. [DOI] [PubMed] [Google Scholar]
- 4.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 5.Kawakami T, Kashiwakura J, Kawakami Y. Histamine-releasing factor and immunoglobulins in asthma and allergy. Allergy Asthma Immunol Res. 2014;6:6–12. doi: 10.4168/aair.2014.6.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol. 2003;3:721–732. doi: 10.1038/nri1181. [DOI] [PubMed] [Google Scholar]
- 7.Zhang HP, Wu Y, Liu J, Jiang J, Geng XR, Yang G, Mo L, Liu ZQ, Liu ZG, Yang PC. TSP1-producing B cells show immune regulatory property and suppress allergy-related mucosal inflammation. Sci Rep. 2013;3:3345. doi: 10.1038/srep03345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braza F, Chesne J, Castagnet S, Magnan A, Brouard S. Regulatory functions of B cells in allergic diseases. Allergy. 2014;69:1454–1463. doi: 10.1111/all.12490. [DOI] [PubMed] [Google Scholar]
- 9.Lundy SK, Klinker MW. Characterization and activity of Fas ligand producing CD5(+) B cells. Methods Mol Biol. 2014;1190:81–102. doi: 10.1007/978-1-4939-1161-5_7. [DOI] [PubMed] [Google Scholar]
- 10.Kaltenmeier C, Gawanbacht A, Beyer T, Lindner S, Trzaska T, van der Merwe JA, Harter G, Gruner B, Fabricius D, Lotfi R, Schwarz K, Schutz C, Honig M, Schulz A, Kern P, Bommer M, Schrezenmeier H, Kirchhoff F, Jahrsdorfer B. CD4+ T cell-derived IL-21 and deprivation of CD40 signaling favor the in vivo development of granzyme B-expressing regulatory B cells in HIV patients. J Immunol. 2015;194:3768–3777. doi: 10.4049/jimmunol.1402568. [DOI] [PubMed] [Google Scholar]
- 11.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133:621–631. doi: 10.1016/j.jaci.2013.12.1088. [DOI] [PubMed] [Google Scholar]
- 12.Bubnov RV, Spivak MY, Lazarenko LM, Bomba A, Boyko NV. Probiotics and immunity: provisional role for personalized diets and disease prevention. EPMA J. 2015;6:14. doi: 10.1186/s13167-015-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Guo Y, Liu H, Gao J, Nie W. Clostridium butyricum reduce lipogenesis through bacterial wall components and butyrate. Appl Microbiol Biotechnol. 2014;98:7549–7557. doi: 10.1007/s00253-014-5829-x. [DOI] [PubMed] [Google Scholar]
- 14.Jiang J, Liu JQ, Li J, Li M, Chen HB, Yan H, Mo LH, Qiu SQ, Liu ZG, Yang PC. Trek1 contributes to maintaining nasal epithelial barrier integrity. Sci Rep. 2015;5:9191. doi: 10.1038/srep09191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler CA, McQuaid S, Taggart CC, Weldon S, Carter R, Skibinski G, Warke TJ, Choy DF, McGarvey LP, Bradding P, Arron JR, Heaney LG. Glucocorticoid receptor beta and histone deacetylase 1 and 2 expression in the airways of severe asthma. Thorax. 2012;67:392–398. doi: 10.1136/thoraxjnl-2011-200760. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto I, Matsunaga T, Sakata K, Nakamura Y, Doi S, Hanmyou F. Histone hyperacetylation plays a role in augmentation of IL-4-induced IgE production in LPS-stimulated murine B-lymphocytes by sodium butyrate. J Biochem. 1996;119:1056–1061. doi: 10.1093/oxfordjournals.jbchem.a021347. [DOI] [PubMed] [Google Scholar]
- 17.Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, Cox L, Demoly P, Frew AJ, O’Hehir R, Kleine-Tebbe J, Muraro A, Lack G, Larenas D, Levin M, Nelson H, Pawankar R, Pfaar O, van Ree R, Sampson H, Santos AF, Du Toit G, Werfel T, Gerth van Wijk R, Zhang L, Akdis CA. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–568. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Grzelewski T, Witkowski K, Makandjou-Ola E, Grzelewska A, Majak P, Jerzynska J, Janas A, Stelmach R, Stelmach W, Stelmach I. Diagnostic value of lung function parameters and FeNO for asthma in schoolchildren in large, real-life population. Pediatr Pulmonol. 2014;49:632–640. doi: 10.1002/ppul.22888. [DOI] [PubMed] [Google Scholar]
- 19.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99:553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the asthma control test. J Allergy Clin Immunol. 2009;124:719–723. e711. doi: 10.1016/j.jaci.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 21.Taylor JJ, Martinez RJ, Titcombe PJ, Barsness LO, Thomas SR, Zhang N, Katzman SD, Jenkins MK, Mueller DL. Deletion and anergy of polyclonal B cells specific for ubiquitous membrane-bound self-antigen. J Exp Med. 2012;209:2065–2077. doi: 10.1084/jem.20112272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geng XR, Yang G, Li M, Song JP, Liu ZQ, Qiu S, Liu Z, Yang PC. Insulin-like growth factor-2 enhances functions of antigen (Ag)-specific regulatory B cells. J Biol Chem. 2014;289:17941–17950. doi: 10.1074/jbc.M113.515262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grausenburger R, Bilic I, Boucheron N, Zupkovitz G, El-Housseiny L, Tschismarov R, Zhang Y, Rembold M, Gaisberger M, Hartl A, Epstein MM, Matthias P, Seiser C, Ellmeier W. Conditional deletion of histone deacetylase 1 in T cells leads to enhanced airway inflammation and increased Th2 cytokine production. J Immunol. 2010;185:3489–3497. doi: 10.4049/jimmunol.0903610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedrich CM, Rauen T, Apostolidis SA, Grammatikos AP, Rodriguez Rodriguez N, Ioannidis C, Kyttaris VC, Crispin JC, Tsokos GC. Stat3 promotes IL-10 expression in lupus T cells through trans-activation and chromatin remodeling. Proc Natl Acad Sci U S A. 2014;111:13457–13462. doi: 10.1073/pnas.1408023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuo R, Morihara H, Mohri T, Murasawa S, Takewaki K, Nakayama H, Maeda M, Fujio Y. The inhibition of N-glycosylation of glycoprotein 130 molecule abolishes STAT3 activation by IL-6 family cytokines in cultured cardiac myocytes. PLoS One. 2014;9:e111097. doi: 10.1371/journal.pone.0111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, Subbarao P, Mandhane P, Becker A, McNagny KM, Sears MR, Kollmann T, Mohn WW, Turvey SE, Brett Finlay B. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 27.Simpson MR, Dotterud CK, Storro O, Johnsen R, Oien T. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol. 2015;15:13. doi: 10.1186/s12895-015-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H, Liu JQ, Yu Y, Mo LH, Ge RT, Zhang HP, Liu ZG, Zheng PY, Yang PC. Regulation of TWIK-related potassium channel-1 (Trek1) restitutes intestinal epithelial barrier function. Cell Mol Immunol. 2016;13:110–8. doi: 10.1038/cmi.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang RB, Chang JK, Chen HL. Can probiotics be used to treat allergic diseases? J Chin Med Assoc. 2015;78:154–157. doi: 10.1016/j.jcma.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Roger A, Depreux N, Jurgens Y, Heath MD, Garcia G, Skinner MA. A novel and well tolerated mite allergoid subcutaneous immunotherapy: evidence of clinical and immunologic efficacy. Immun Inflamm Dis. 2014;2:92–98. doi: 10.1002/iid3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glover MT, Atherton DJ. A double-blind controlled trial of hyposensitization to Dermatophagoides pteronyssinus in children with atopic eczema. Clin Exp Allergy. 1992;22:440–446. doi: 10.1111/j.1365-2222.1992.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 32.Galli E, Chini L, Nardi S, Benincori N, Panei P, Fraioli G, Moschese V, Rossi P. Use of a specific oral hyposensitization therapy to Dermatophagoides pteronyssinus in children with atopic dermatitis. Allergol Immunopathol (Madr) 1994;22:18–22. [PubMed] [Google Scholar]
- 33.Berin MC, Mayer L. Can we produce true tolerance in patients with food allergy? J Allergy Clin Immunol. 2013;131:14–22. doi: 10.1016/j.jaci.2012.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]