Abstract
The LOX-1/p38 mitogen-activated protein kinase (MAPK) pathway has been proved to participate in the endothelial dysfunction in atherosclerosis. Trichosanatineis is an active compound isolated from the peel of Trichosanthes kirilowii. This study aims to determine whether trichosanatine prevents the oxidized low-density lipoprotein (ox-LDL)-induced insult through inhibition of the LOX-1/p38 MAPK pathway in HUVECs. HUVECs were treated with 150 mg/ml ox-LDL for 24 h to establish an ox-LDL-induced endothelial injury model. Cell viability, mitochondrial membrane potential (MMP), apoptosis, reactive oxygen species (ROS) level, LOX-1 and p38 MAPK expression level were measured. The results indicated that HUVECs were pretreated with either 100 mM trichosanatine or LOX-1 shRNA prior to exposure to ox-LDL for 24 h. Exposure of HUVECs to 150 mg/ml ox-LDL for 24 h significantly up-regulated the expression levels of LOX-1. The increased expression levels of LOX-1 were markedly attenuated by pretreatment with 100 mM trichosanatine. In addition, the ox-LDL-induced increase in phosphorylated (p) p38 MAPK expression was ameliorated by pretreatment with LOX-1 shRNA. Pretreatment of HUVECs with either trichosanatine or LOX-1 shRNA before exposure to ox-LDL significantly inhibited the ox-LDL-induced injuries, as evidenced by an increase in cell viability, a decrease in apoptotic cells, a ROS generation and a loss of MMP. In conclusion, we have demonstrated for the first time that the LOX-1/p38 MAPK pathway contributes to the ox-LDL-induced injury in HUVECs. Meanwhile, the trichosanatine protects the HUVECs against ox-LDL-induced injury at least in part by inhibiting the activated of LOX-1/p38 MAPK pathway.
Keywords: Trichosanatine, oxidized low-density lipoprotein, LOX-1, p38 mitogen-activated protein kinase
Introduction
Atherosclerosis is a chronic multi-factorial disease, which has become the major cause for morbidity and mortality worldwide [1]. Endothelial dysfunction is a precursor factor for the atherosclerosis, during which the oxidized low-density lipoprotein (ox-LDL) is now considered to play a critical role [2]. Ox-LDL leads to endothelial dysfunction through up-regulating the expression of adhesion molecules, and recruiting the monocytes to the sub endothelial space, which also impairs the secretory activities of endothelium and reducing the antioxidant capability [2]. All these effects of ox-LDL are tightly related to a novel receptor for ox-LDL, lectin-like low density lipoproteinreceptor-1(LOX-1) [3,4].
LOX-1 is a 50 kDa type II membrane glycoprotein, which belongs to the C-type lectin family [5]. LOX-1 was highly expressed in vascular wall in patients or animals with atherosclerosis, diabetes, hypertension, and other diseases [5]. Many reports have demonstrated that LOX-1 play an important role in the mediation of the effects of ox-LDL on endothelial biology [3,4,6,7]. Activation of LOX-1 by ox-LDL initiates the intracellular signaling pathways. LOX-1 promotes the generation of ROS. The ROS over-production leads to the sequential phosphorylation of a series of protein kinases, tyrosine kinases, and mitogen-activated protein kinases (MAPK). A recent report showed that LOX-1 signaling and its down-stream p38 MAPK pathways were participated in the lipopolysaccharide insult in Human umbilical vein endothelial cells (HUVECs) [8-10]. However, whether the LOX-1/p38MAPK pathway is involved in ox-LDL -induced endothelial cells injury is also unknown.
Trichosanthes kirilowii is a traditional medicine used in East Asian countries, which exhibits various medicinal effects and has been long used clinically in China to treat trophoblastic carcinomas and ectopic pregnancies, and to interrupt early and mid-trimester pregnancies [11]. Of note, trichosanthes species are reported to have beneficial effects on inflammation and oxidative stress [12,13]. Trichosanatine is an active compound isolated from the peel of Trichosanthes kirilowii [14]. However, it is still unclear whether or not trichosanatine preventsox-LDL induced endothelial cellular injury and with which intra-cellular factors trichosanatine interferes.
Since the ox-LDL induced endothelial cell injury and the important role of LOX-1 in the progress of atherosclerosis, we investigated whether trichosanatine protects endothelial cells from ox-LDL induced injury via modulating the expression of LOX-1, interfering with the ox-LDL mediated intra-cellular signaling pathway (ROS over-production, p38MAPK phosphorylation). In this report, to our knowledge, we showed for the first time that exogenous trichosanatine protects against ox-LDL induced endothelial cells injury via inhibition of LOX-1/p38 MAPK pathway in HUVECs.
Materials and methods
Materials
Ox-LDL was supplied by Institute of Biochemistry, Peking Union Medical College. Hoechst 33258, 2-7-dichlorodihydrofluorecein diacetate (DCFH-DA) was purchased from Sigma-Aldrich Corporation (St Louis, MO, USA). Cell counter kit-8 (CCK-8) and rhodamine 123 (Rh123) were purchased from Dojindo (Kumamoto, Kyushu, Japan). Dulbecco’s modified eagle medium (DMEM)-F12 and fetal bovine serums (FBS) were supplied by Gibico BRL (Calsbad, CA, USA). Rabbit polyclonal antibody to LOX-1 was provided by Abcam (New Territories, HK, ab60178), mouse anti-p38 MAPK monoclonal antibody (sc-81621), mouse anti-p-p38MAPK monoclonal antibody (sc-7973), second antibody were provided by Santa Cruz Biotechnology Inc. BCA protein assay kit was purchased from Kangchen Biotech (Shanghai, China). Western Blot Detection Kit (ECL solution) was purchased from KeyGen Biotech (Nanjing, China).
HUVECs culture and treatment
HUVEC cell line was originally supplied by the American Type Culture Collection (Manassas, VA, USA). Endothelial cell line HUVECs was cultured according to standard procedure as described previously [15]. Cells were seeded in DMEM supplemented with 10% fetal bovine serum, and maintained in a humidified atmosphere containing 5% CO2 at 37°C. The cells between passages 2 and 15 were used in this study. For the treatment, HUVECs were pre-treated for 20 min with different concentrations of trichosanatine (100 mM, according to our pilot study), and then exposed to ox-LDL (150 mg/ml) for the indicated time periods. Since trichosanatine was dissolved in DMSO, we therefore used 0.1% DMSO as vehicle control. In our pilot study, we found that this concentration of DMSO had no effect on the cells in the presence or absence of ox-LDL. To inhibit LOX-1 expression, we designed a shRNA targeting theLOX-1 (NM_002543) transcript.
Cell viability assay
The CCK-8 assay was used to investigate the cell viability of HUVECs cells cultured in 96-well plates. In brief, after different treatments, 100 μl CCK-8 solution at a 1:10 dilution was added into each well of the plate followed by a further 2~4 h incubation in the incubator. Absorbance was measured at 450 nm with a micro-plate reader (Molecular Devices, Sunnyvale, CA, USA). The mean optical density (OD) of 5 wells in the indicated groups was used to calculate the cell viability percentage. Experiments were performed in triplicate.
Apoptosis assessment
Morphological changes of apoptosis, such as chromosomal condensation and fragmentation in the nuclei of HUVECs cells, were observed by Hoechst 33258 staining followed by photofluorography. In brief, after different treatments, HUVECs cells were fixed with 4% paraformaldehyde in phosphate buffered saline for 10 min. After stained with 5 mg/l Hoechst 33258 for 30 min, the cells were visualized under a fluorescent microscope (BX50-FLA; Olympus, Tokyo, Japan). Viable cells displayed normal nuclear size and uniform fluorescence, whereas apoptotic cells showed condensed, fractured or distorted nuclei.
Measurement of intracellular reactive oxygen species (ROS)
Intercellular ROS detection was performed as described previously. Intracellular ROS levels were determined by oxidative conversion of cell-permeable DCFH-DA to fluorescent DCF. After different treatments, DCFH-DA (10 μM) in FBS-free medium was added to the slides and the cells were incubated for a further 30 min at 37°C. The slides were washed three times with FBS-free DEME medium. The DCF fluorescence was measured over the entire field of vision using a fluorescent microscope connected to an imaging system (BX50-FLA; Olympus, Tokyo, Japan). Mean fluorescence intensity (MFI) from three random fields was analyzed using Image J 1.41 software (National Institutes of Health, Bethesda, MD, USA), and MFI was used to represent the amount of ROS.
Measurement of mitochondrial membrane potential (MMP)
MMP was measured using the fluorescent cell-permeable dye Rh123 that preferentially enters mitochondria based on the highly negative MMP. Depolarization of MMP results in the loss of Rh123 from the mitochondria and a decrease in intracellular fluorescence. In the present study, Rh123 (100 mg/L) was added to medium for 45 min at 37°C. Rh123 fluorescence was measured over the entire field of vision using a fluorescent microscope connected to an imaging system (BX50-FLA; Olympus Tokyo, Japan). The MFI of Rh123 from five random fields was analyzed by using the Image J 1.41 software, with MFI taken as an index of MMP.
Western blot analysis
The protein expression of LOX-1, t-p38MAPK and p-p38MAPK was measured by western blotting analysis as described previously. After the indicated treatments, HUVECs cells were harvested and splited with ice-cold lysis solution. After quantitated with BCA protein assay kit, total proteins in the cell lysate were fractionated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Then the proteins were transferred into poly vinyli denedi fluoride (PVDF) membranes. The membranes were blocked in 5% fat-free milk (in TBST) for 1.5 h at room temperature and then incubated with LOX-1, p38 and p-p38 antibody with gentle agitation at 4°C. Following 3 time washes with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. The immune reactive signals were visualized by the ECL detection system. For quantifying the protein expression, the X-ray films were scanned and analyzed with Image J 1.41 software.
Quantitative real-time PCR for LOX-1 mRNA expression
HUVECs were incubated in 6-well plates at the density of 2×105 cells per well and pre-treated with trichosanatine (100 mM) for 20 min, and subsequently treated with ox-LDL (150 mg/ml) for 24 h. After incubation, the cells were washed twice with PBS and the total mRNA was extracted by Trizol reagent according to the manufacturer’s instructions. The concentration of RNA was determined by measuring the absorbance at 260 nm. The total RNA was then reverse-transcribed to cDNA as the condition of follows: 25°C, 5 min, 42°C 60 min, and 70°C, 5 min. The cDNA was used for quantitative real-time PCR. The primer pair sequences specific to human LOX-1 was listed as the follows: Forward primer 5’-GAGAGTAGCAAATTGTTCAGCTCCTT-3’, Reverse primer 5’-GCCCGAGGAAAATAGGTAACAGT-3’. The primer pair sequences specific to human GAPDH was listed as the follows: Forward primer, 5’-TGGCCTCCAAGGAGTAAGAAAC-3’, Reverse primer, 5’-GGCCTCTCTCTTGCTCTCAGTATC-3’. The real-time PCR was performed by using the ABIPRISM® 7300Fast Real-Time PCR System (Applied Biosystems). There action products were detected by measuring the binding of SYBR Green to DNA using the SYBR Green PCR Master Mix. The optimization of the amplification reaction was assured by a dissociation curve analysis. The basic protocol for real-time PCR was an initial incubation at 95°C for 1 min, followed by 40 cycles of 95°C 15 s, 60°C 30 s, and 72°C 30 s. All samples were run in triplicate, and the gene expression values were normalized to the expression value of GAPDH and then analyzed using the 2(ΔΔCt) methods as previously described.
Data analysis
All data were presented as the mean ± SD from at least three different experiments. Differences between groups were analyzed by one-way analyses of variance (ANOVA) with SPSS 16.0 software (Chicago, IL, USA), and followed by LSD post hoc comparison test. Statistical significance was defined as P<0.05.
Results
Trichosanatine inhibits ox-LDL-induced expression of LOX-1 in HUVECs
To explore the effect of ox-LDL on expression of LOX-1, the HUVECs were treated with 150 mg/ml ox-LDL for 24 h. The results of western blot analysis showed that treatment of HUVECs with ox-LDL significantly up-regulated the expression of LOX-1 (Figure 1). Prior to the HUVECs exposure to 150 mg/ml ox-LDL for 24 h, the HUVECs were pretreated with 100 mM Trichosanatine for 30 min. It markedly attenuated ox-LDL-induced over-expression of LOX-1, while trichosanatine alone had no effect on the basal expression of LOX-1. These results indicated that ox-LDL induces activation of LOX-1, which can be suppressed by the trichosanatine in HUVECs.
Figure 1.
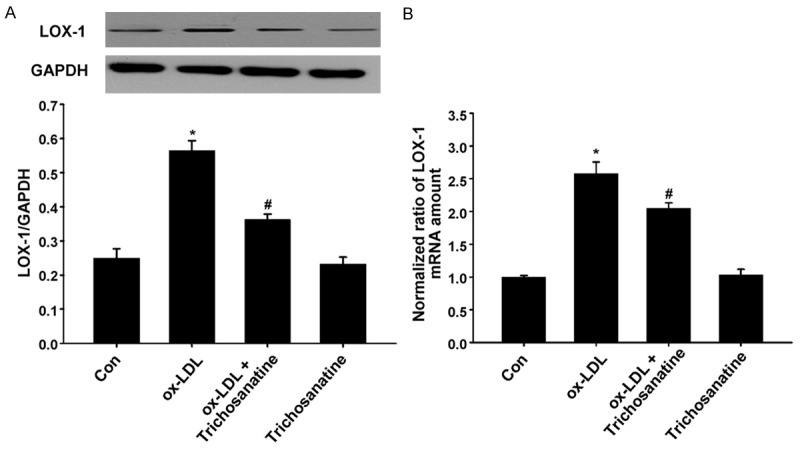
Effect of trichosanatine on ox-LDL-induced overexpression of LOX-1 (A) HUVECs were treated with 150 mg/ml ox-LDL for 24 h in the absence or presence of pretreatment with 100 mM trichosanatine for 30 min. The western blot assay was used to detect expressions of LOX-1. (B) Effect of trichosanatine on ox-LDL-induced over-expression of LOX-1 by quantitative real-time PCR. Data are shown as mean ± SD, n = 3. *P<0.01 compared with control group, #P<0.01 compared with ox-LDL treatment group.
Ox-LDL-induced phosphorylation of p38 MAPK and can be attenuated by inhibition of LOX-1
To clarify the role of LOX-1 in the phosphorylation of p38 MAPK induced by ox-LDL in HUVECs, LOX-1 shRNA was used to elucidate the role on ox-LDL induced p38 phosphorylation. The results of Figure 2 showed that exposure of HUVECs to ox-LDL for 24 h significantly enhanced the phosphorylation of p38 MAPK. In order to further confirm whether leptin mediated ox-LDL-induced activation of p38 MAPK, HUVECs were transfected with LOX-1 shRNA or non-targeting shRNA. As shown in Figure 1A, LOX-1 shRNA significantly inhibited p38 phosphorylation. Non-targeting shRNA had no effect on ox-LDL-induced p38 phosphorylation. These results indicated that LOX-1 mediated ox-LDL-induced activation of p38 MAPK.
Figure 2.
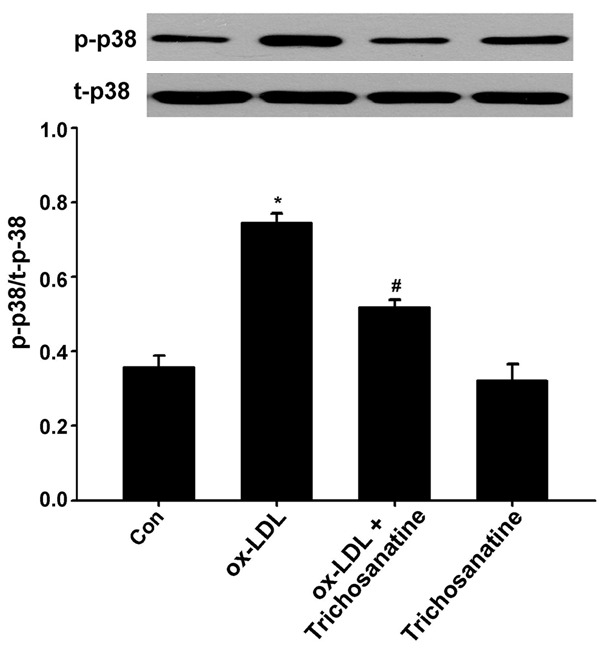
Effects of trichosanatine and LOX-1 shRNA on ox-LDL-induced phosphorylation of p38 MAPK. HUVECs were treated with 150 mg/ml ox-LDL for 24 h in the absence or presence of pretreatment with 100 mM trichosanatine for 30 min or LOX-1 shRNA. The western blot assay was used to detect phosphorylation of p38 MAPK. Data are shown as mean ± SD, n = 3. *P<0.01 compared with control group, #P<0.01 compared with ox-LDL treatment group.
Inhibition of LOX-1 contributed to protection of trichosanatine against ox-LDL-induced cytotoxicity
We firstly examined whether LOX-1 activation was involved in ox-LDL-induced cytotoxicity in HUVECs. As presented in Figure 3, exposure of HUVECs to 150 mg/ml ox-LDL for 24 h obviously led to a decrease in cell viability, compared with control group (P<0.01). However, HUVECs transfected with LOX-1 shRNA dramatically attenuated ox-LDL-induced cell viability decreasing by approximately 20%, compared with non-targeting shRNA group (P<0.01). The data revealed that LOX-1 activation mediated ox-LDL-induced cytotoxicity in HUVECs. Similarly, pretreatment with 100 mM trichosanatine for 30 min prior to 150 mg/ml ox-LDL exposure protected HUVECs against viability decreasing induced by ox-LDL. Alone, trichosanatine (100 mM) didn’t affected cell viability. The findings suggest that trichosanatine blocks ox-LDL-induced cytotoxicity partially by inhibition of LOX-1 activation.
Figure 3.
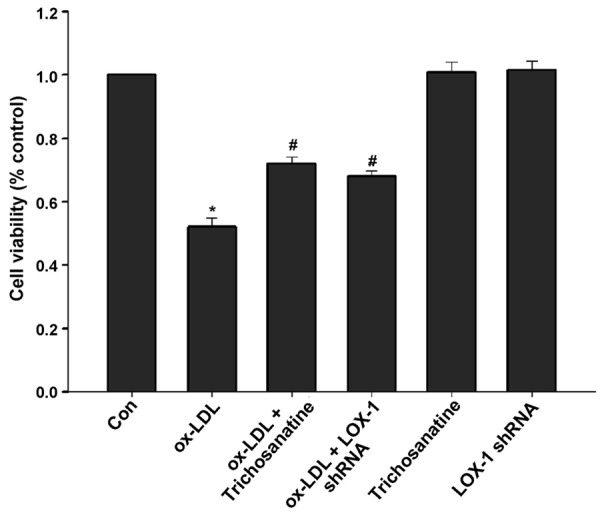
Effects of trichosanatine and LOX-1 shRNA on ox-LDL-induced decrease in cell viability HUVECs were treated with 150 mg/ml ox-LDL for 24 h in the absence or presence of pretreatment with 100 mM trichosanatine for 30 min or LOX-1 shRNA. Cell viability was measured by CCK-8 assay. Mean ± SD, n = 3. *P<0.01 compared with control group, #P<0.01 compared with ox-LDL treatment group.
Inhibition of LOX-1 contributed to protection of trichosanatine against ox-LDL-induced apoptosis
We secondary tested whether LOX-1 activation was associated with ox-LDL-induced apoptosis in HUVECs. The results showed that exposure of HUVECs to 150 mg/ml ox-LDL for 24 h caused a significant increase in apoptotic cells (Figure 4). Ox-LDL-induced apoptosis was considerably decreased in HUVECs transfected with LOX-1 shRNA. Pretreatment with 100 mM trichosanatine for 30 min prior to exposure of cells to ox-LDL had the similar protective effect against apoptosis. Alone, neither non-targeting shRNA nor trichosanatine caused apoptosis in HUVECs. These results suggested that LOX-1 activation mediated ox-LDL-induced apoptosis and that the protective effect of trichosanatine against ox-LDL-induced apoptosis was associated with inhibition of LOX-1 activation.
Figure 4.

Effects of trichosanatine and LOX-1 shRNA on ox-LDL-induced apoptosis in HUVECs. After the indicated treatments, cellular apoptosis was assessed by Hoechst 33258 staining followed by photofluorography. Mean ± SD, n = 3. *P<0.01 compared with control group, #P<0.01 compared with ox-LDL treatment group.
Inhibition of LOX-1 was implicated in protection of trichosanatine against ox-LDL-induced mitochondrial damage
As shown in Figure 5, after HUVECs were treated with 150 mg/ml ox-LDL for 24 h, mitochondria were considerably damaged, characterized by a loss of MMP (P<0.01). The dissipation of MMP was ameliorated in LOX-1 shRNA group. Also, pretreatment with 100 mM trichosanatine also plays the role in mitochondrial protection. These results suggest that trichosanatine can cause a preservation of mitochondrial function by inhibiting LOX-1 activation.
Figure 5.
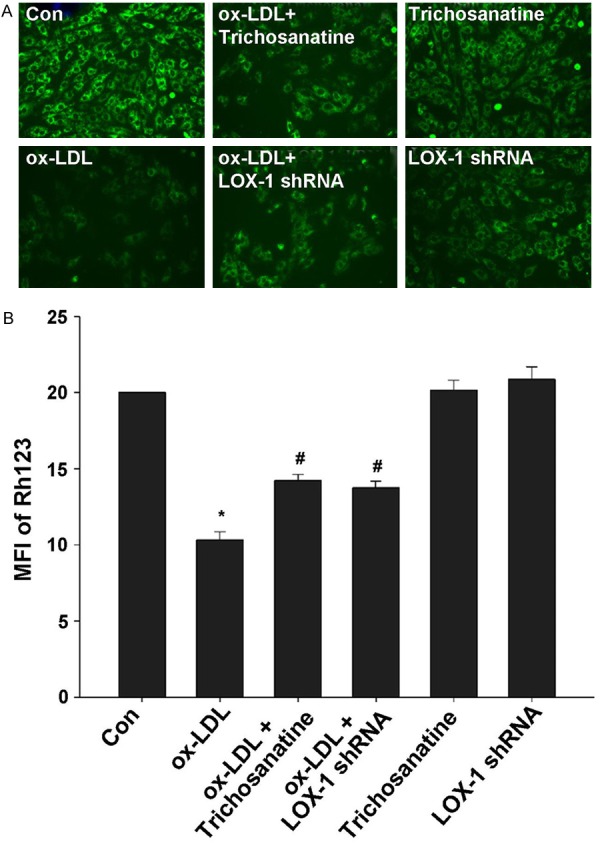
Effects of trichosanatine and LOX-1 shRNA on ox-LDL-induced MMP loss in HUVECs. After the indicated treatments, cellular MMP was measured by Rh123 staining followed by photofluorography. Quantitative analysis of the MFI of Rh123 in A-F with Image J 1.41 software. Mean ± SD, n = 3. *P<0.01 compared with control group, #P<0.01 compared with ox-LDL treatment group.
Inhibition of LOX-1 activation was involved in protective effect of trichosanatine against ox-LDL-induced ROS generation
Finally, we examined whether activation of LOX-1 contributes to ROS production induced by ox-LDL. As shown in Figure 6, treatment with 150 mg/ml ox-LDL for 24 h led to an obvious increase accumulation of intracellular ROS. Inhibition of LOX-1 activation by LOX-1 shRNA significantly reduced the intracellular ROS levels. Similarly, pretreatment with 100 mM trichosanatine attenuated ox-LDL-induced ROS generation. Non-targeting shRNA or trichosanatine alone did not elicit intracellular ROS generation. These data revealed that LOX-1 activation is implicated in ox-LDL-induced ROS generation and that antioxidant effect of trichosanatine is partly associated with the inhibition of LOX-1 activation in HUVECs.
Figure 6.
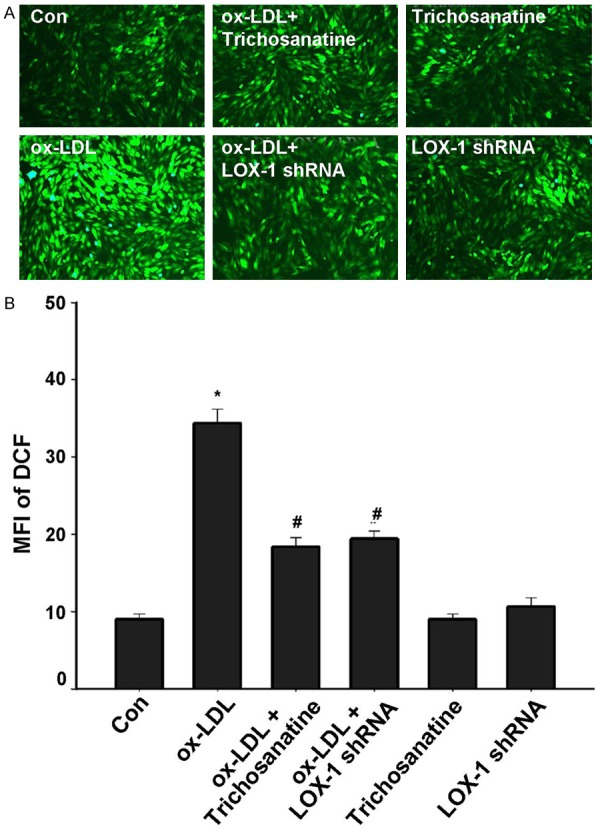
Effects of trichosanatine and LOX-1 shRNA on ox-LDL-induced ROS accumulation in HUVECs. After the indicated treatments, intracellular ROS generation was measured by DCFH-DA staining followed by photofluorography. Mean ± SD, n = 3. *P<0.01 compared with control group, #P<0.01 compared with ox-LDL treatment group.
Discussion
In this study, we provide a new insight into the mechanisms responsible for ox-LDL-induced injury in HUVECs. The results demonstrated that LOX-1/p38 MAPK pathway is an important regulatory mechanism for ox-LDL-induced endothelial cells injury. Importantly, by inhibiting this pathway, trichosanatine protects HUVECs against ox-LDL-induced HUVECs injury, leads to an increase in cell viability, and decreases in apoptotic cells and ROS generation, as well as dissipation of MMP. To our best knowledge, this is the first study to demonstrate that trichosanatine can protect against ox-LDL-induced HUVECs damage by inhibiting the LOX-1/p38 MAPK pathway.
The endothelium dysfunction is now regarded as an early event in the pathogenesis of atherosclerosis [16,17]. It is reported that the injury of endothelial cells contributes to destabilization of atherosclerotic plaques and thrombosis, which leads to the acute symptoms in patients with CAD [17-19]. Ox-LDL elicited endothelial dysfunction plays a critical role in atherogenesis. Ox-LDL changes the secretory activities of endothelium, induces endothelial cell apoptosis, and reduces the antioxidant capability and nitric oxide synthesis in endothelium.
Many studies implicates that these effects of ox-LDL can be mediated predominantly by an endothelial receptor, LOX-1 [3,4,6]. The identified presence of LOX-1 expression in endothelial cells also indicates that LOX-1 signaling may serve as an important biological regulator in cardiac disorders. However, whether the LOX-1 signaling is involved in ox-LDL-induced endothelial cells injury remains unclear. Ox-LDL induced over-expression of LOX-1 can cause endothelial activation and/or injuries, and this was confirmed by our present work. To further understand the mechanism of LOX-1 signaling in ox-LDL-treated cells, we evaluated the role of LOX-1 during ox-LDL-induced endothelial cells injury. Here, we provide a clear evident that ox-LDL induces expression of LOX-1, leading to HUVECs injury characterized by decrease in cell viability, and increases in apoptotic cells and ROS generation as well as dissipation of MMP. Moreover, these effects are attenuated by the specific inhibition of LOX-1.
MAPKs are a group of related serine-threonine protein kinases, which participate in the mediation of cell growth, apoptosis, proliferation and function [20]. It has been reported that various intracellular signal pathways are involved in the ox-LDL induced endothelial cells apoptosis, including phosphorylation and activation of ERK1/2, JNK, and p38MAPKs [21,22]. The blocking of p38 MAPK activation protects several cells against apoptosis [23,24]. Therefore, we investigated the inhibitory effects of trichosanatine on the phosphorylation of p38MAPK. The findings showed that trichosanatine suppresses ox-LDL-induced p38 MAPK activation in HUVEC, suggesting that p38MAPK involves in the protective effect of trichosanatine.
Importantly, in this study, we demonstrated the protective effects of trichosanatine in ox-LDL-treated HUVECs. Trichosanatine is an active compound isolated from the peel of Trichosanthes kirilowii [11,14]. However, it is still unclear whether or not trichosanatine prevents ox-LDL induced endothelial cellular injury and with which intracellular factors trichosanatine interferes. Increasing evidence shows that the active compound of Trichosanthes exerts protective effects against several models of myocardial injury in the setting of diseases by preventing apoptosis and oxidative stress [25,26]. Similar to these studies, we found that pretreatment with trichosanatine before exposure to ox-LDL reduced the ox-LDL-induced endothelial dysfunction in HUVECs, which was characterized by increased cell viability, decreased apoptosis and ROS generation, as well as MMP improvement. To further explore the mechanisms of the protective effect of trichosanatine on endothelial dysfunction, we observed the effect of trichosanatine on the LOX-1/p38 MAPK pathway induced by ox-LDL. The findings of this study showed that pretreatment of HUVECs with trichosanatine significantly down-regulated ox-LDL-induced expressions of LOX-1 and p38 MAPK, which also accompanied by increased cell viability, decreased in apoptotic cells and ROS generation as well as dissipation of MMP. Additionally, LOX-1 shRNA mimics the protective effects of trichosanatine in ox-LDL-induced injury, suggesting that inhibition of LOX-1/p38 MAPK pathway may be associated with the protection of trichosanatine from ox-LDL-induced endothelial dysfunction.
In conclusion, our study demonstrated for the first time that the LOX-1/p38 MAPK pathway is involved in ox-LDL-induced endothelial cells injury and that trichosanatine supplementation can protect against endothelial cells injury at least partly by inhibiting the above signaling pathway in HUVECs. The findings provide a novel insight to elucidate the mechanisms underlying the pathogenesis of atherosclerosis. Moreover, exogenous administration of trichosanatine may be a new therapeutic strategy for the protective effect against ox-LDL-induced injury in atherosclerosis.
Acknowledgements
This study was granted by the National Nature Science Fund of China (Grant No. 81373574).
Disclosure of conflict of interest
None.
References
- 1.Wang W, Lee Y, Lee CH. Review: the physiological and computational approaches for atherosclerosis treatment. Int J Cardiol. 2013;167:1664–1676. doi: 10.1016/j.ijcard.2012.09.195. [DOI] [PubMed] [Google Scholar]
- 2.Niu N, Sun SZ, Han B, Wang Y. Relationship of ox-LDL/LOX-1 and vascular endothelial dysfunction of diet-induced obese immature rats and nicotinic acid’s intervention outcomes. Zhonghua Yi Xue Za Zhi. 2013;93:3388–3393. [PubMed] [Google Scholar]
- 3.Dong Q, Xiang R, Zhang ZY, Qin S. Ox-LDL increases OX40L in endothelial cells through a LOX-1-dependent mechanism. Braz J Med Biol Res. 2013;46:765–70. doi: 10.1590/1414-431X20132733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong D, Bai YP, Gao HC, Wang X, Li LF, Zhang GG, Hu CP. Ox-LDL induces endothelial cell apoptosis via the LOX-1-dependent endoplasmic reticulum stress pathway. Atherosclerosis. 2014;235:310–317. doi: 10.1016/j.atherosclerosis.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Christ A, Latz E. LOX-1 and mitochondria: an inflammatory relationship. Cardiovasc Res. 2014;103:435–437. doi: 10.1093/cvr/cvu187. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Guo Y, Zhu P, Yang T. Role of Ox-LDL/LOX-1/NF-kappaB signaling pathway in regulation of atherosclerotic plaque growth by testosterone in male rabbits. Vascul Pharmacol. 2013;59:131–137. doi: 10.1016/j.vph.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Bai YP, Zhang GG, Shi RZ, Li YJ, Tan GS, Chen J. Inhibitory effect of reinioside C on LOX-1 expression induced by ox-LDL. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31:659–662. [PubMed] [Google Scholar]
- 8.Chen J, Li D, Schaefer R, Mehta JL. Cross-talk between dyslipidemia and renin-angiotensin system and the role of LOX-1 and MAPK in atherogenesis studies with the combined use of rosuvastatin and candesartan. Atherosclerosis. 2006;184:295–301. doi: 10.1016/j.atherosclerosis.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi N, Hara K, Tojo A, Onozato ML, Honda T, Yoshida K, Mita S, Nakano S, Tsubokou S, Matsuoka H. Eplerenone shows renoprotective effect by reducing LOX-1-mediated adhesion molecule, PKCepsilon-MAPK-p90RSK, and Rho-kinase pathway. Hypertension. 2005;45:538–544. doi: 10.1161/01.HYP.0000157408.43807.5a. [DOI] [PubMed] [Google Scholar]
- 10.Guo R, Su Y, Liu B, Li S, Zhou S, Xu Y. Resveratrol suppresses oxidised low-density lipoprotein-induced macrophage apoptosis through inhibition of intracellular reactive oxygen species generation, LOX-1, and the p38 MAPK pathway. Cell Physiol Biochem. 2014;34:603–616. doi: 10.1159/000363026. [DOI] [PubMed] [Google Scholar]
- 11.Li AF, Sun AL, Liu RM, Zhang YQ. Chemical constituents of Trichosanthes kirilowii peels. Zhong Yao Cai. 2014;37:428–431. [PubMed] [Google Scholar]
- 12.Seo CS, Kim TW, Kim YJ, Park SR, Ha H, Shin HK, Jung JY. Trichosanthes kirilowii ameliorates cisplatin-induced nephrotoxicity in both in vitro and in vivo. Nat Prod Res. 2015;29:554–7. doi: 10.1080/14786419.2014.952229. [DOI] [PubMed] [Google Scholar]
- 13.Mondal A. A novel extraction of trichosanthin from Trichosanthes kirilowii roots using three-phase partitioning and its in vitro anticancer activity. Pharm Biol. 2014;52:677–680. doi: 10.3109/13880209.2013.864684. [DOI] [PubMed] [Google Scholar]
- 14.Chen KC, Chen HY, Chen CY. Potential protein phosphatase 2A agents from traditional Chinese medicine against cancer. Evid Based Complement Alternat Med. 2014;2014:436863. doi: 10.1155/2014/436863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Zheng J, Xu Y, Zhang Y, Gu L, Dong J, Zhu Y, Zhou R, Zheng L, Zhang X, Du J. Melatonin suppresses hypoxia-induced migration of HUVECs via inhibition of ERK/Rac1 activation. Int J Mol Sci. 2014;15:14102–14121. doi: 10.3390/ijms150814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y. Transcriptional regulation of endothelial dysfunction in atherosclerosis: an epigenetic perspective. J Biomed Res. 2014;28:47–52. doi: 10.7555/JBR.27.20130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mudau M, Genis A, Lochner A, Strijdom H. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr. 2012;23:222–231. doi: 10.5830/CVJA-2011-068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyseni A, Roest M, Braun SL, Barendrecht AD, de Groot PG, Ndrepepa G, Kastrati A. Chronic dysfunction of the endothelium is associated with mortality in acute coronary syndrome patients. Thromb Res. 2013;131:198–203. doi: 10.1016/j.thromres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Andreeva EO, Kuril’skaia TE, Pivovarov IuI, Eniseeva ES, Koriakina LB, Belokhvostikova TS. Dysfunction of vascular endothelium in patients with coronary heart disease. Klin Med (Mosk) 2009;87:23–27. [PubMed] [Google Scholar]
- 20.Peter AT, Dhanasekaran N. Apoptosis of granulosa cells: a review on the role of MAPK-signalling modules. Reprod Domest Anim. 2003;38:209–213. doi: 10.1046/j.1439-0531.2003.00438.x. [DOI] [PubMed] [Google Scholar]
- 21.Dai Y, Palade P, Wang X, Mercanti F, Ding Z, Dai D, Mehta JL. High fat diet causes renal fibrosis in LDLr-null mice through MAPK-NF-kappaB pathway mediated by Ox-LDL. J Cardiovasc Pharmacol. 2014;63:158–166. doi: 10.1097/FJC.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 22.Zhang K, Meng X, Kong J, Liu FF, Yang JM, Gao F, Zhang Y, Zhang C. Simvastatin increases Prolyl-4-Hydroxylase alpha1 expression in atherosclerotic plaque and ox-LDL-stimulated human aortic smooth muscle cells via p38 MAPK and ERK1/2 signaling. J Mol Cell Cardiol. 2013;65:43–50. doi: 10.1016/j.yjmcc.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Lee JH, Park HS, Lee GS, Kim HW, Ha KT, Kim BJ. Schizandra chinensis extracts induce apoptosis in human gastric cancer cells via JNK/p38 MAPK activation and the ROS-mediated/mitochondria-dependent pathway. Pharm Biol. 2015;53:212–219. doi: 10.3109/13880209.2014.913297. [DOI] [PubMed] [Google Scholar]
- 24.Guo R, Su Y, Liu B, Li S, Zhou S, Xu Y. Resveratrol suppresses oxidised low-density lipoprotein-induced macrophage apoptosis through inhibition of intracellular reactive oxygen species generation, LOX-1, and the p38 MAPK pathway. Cell Physiol Biochem. 2014;34:603–616. doi: 10.1159/000363026. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharya S, Das SK, Haldar PK. Arsenic induced myocardial toxicity in rats: alleviative effect of Trichosanthes dioica fruit. J Diet Suppl. 2014;11:248–261. doi: 10.3109/19390211.2014.937044. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharya S, Haldar PK. Trichosanthes dioica root alleviates arsenic induced myocardial toxicity in rats. J Environ Pathol Toxicol Oncol. 2013;32:251–261. doi: 10.1615/jenvironpatholtoxicoloncol.2013008541. [DOI] [PubMed] [Google Scholar]


