Abstract
Runx2, a member of the Runt domain family, is a well-known master transcription factor for osteoblast differentiation. Runx2 has also been shown to play essential roles during chondrocyte hypertrophy, an important late stage of endochondral ossification linking both bone and cartilage development. To identify the co-factors that may interact with Runx2 together to regulate this critical process, we have performed yeast two-hybrid (Y2H) screening using Runx2 as a bait to screen a cDNA library of hypertrophic chondrocytes. The bait expressing cassette was constructed by fusing Runx2 with the pGBKT7 vector containing the Gal4 DNA binding domain (BD). The Mate & Plate libraries were constructed using pGADT7-Rec and cDNAs derived from hypertrophic chondrocytes enriched limb tissues or hypertrophic MCT cells. After co-transformation of pGBKT7-Runx2 and the cDNA libraries, colonies that grew in nutrition deficient medium were selected and subjected to PCR and sequencing analysis. We successfully identified more than 30 candidate genes, including Lectin-1 (Lgals1), Col1a2, Edf1 and Timp-2. We have performed literature review and bioinformatics analysis of these genes using GenePaint. Most of them show ubiquitous expression with Lgals1 show enhanced expression in hypertrophic chondrocytes. We further performed preliminary expression analysis by quantitative PCR and detected differential expression of these candidate genes in proliferative and hypertrophic MCT cells, with Timp-2 significantly (around 3-fold) and Lgals1 moderately (around 1.5 fold) upregulated in hypertrophic MCT cells. Our results suggest that, candidate gene Timp-2 is very likely to interact with Runx2 and together to play essential function during cartilage development, and possibly its homeostasis.
Keywords: Runx2, yeast two-hybrid screening, hypertrophic chondrocytes, Timp-2, GenePaint
Introduction
Endochondral ossification is known as the major skeletal developmental pathway. During this process, the resting chondrocytes proliferative and develop into large and round hypertrophic chondrocytes. It was generally accepted that chondrocyte hypertrophy represents a stage of chondrocyte terminal differentiation, which subsequently undergoes apoptosis and allows for blood vessel invasion and replaced by osteoblasts [1]. However, recent studies have reported survival of hypertrophic chondrocytes at the chondro-osseous junction and continual differentiation into osteoblasts and osteocytes [2,3]. These findings make chondrocyte hypertrophy not only a critical process linking both bone and cartilage development, but also an important process affecting bone homeostasis.
There are multiple factors, including transcription factors (TFs), growth factors, and matrix proteins that have been shown to control the process of chondrocyte hypertrophy. Runx2 (or Cbfa1, core binding factor a1), one of the Runt domain containing TFs, has been shown by multiple studies as a master transcription factor both for osteoblast differentiation and for chondrocyte hypertrophy. Runx2 null mice (Runx2(-/-)) completely lack mineralized tissue and bone formation, whereas Runx2 heterozygotes (Runx2(+/-)) showed similar skeletal defects that have been observed in human cleidocranial dysplasia (CCD) [4,5]. CCD, which is caused by haploinsufficiency of human RUNX2, is characterized by delayed closure of the fontanel, hypoplastic clavicles, as well as multiple dental abnormalities, indicating its defective intramembranous bone formation. CCD also has typical phenotype of short stature, while Runx2 heterozygotes show growth plate abnormalities, indicating that the endochondral bone formation is injured as well. Indeed, we have previously detected significantly shortened hypertrophic zone along with decreased expression of hypertrophic chondrocyte-specific markers COL10A1 and MMP13, in a fetal case of CCD [6]. There are also multiple in vitro and in vivo studies that support a central regulatory function of Runx2 during chondrocyte hypertrophy and its associated marker genes as we and others previously reported [7-11]. However, chondrocyte hypertrophy is a very complicated biological process. Many other TFs and signaling molecules may serve as independent factors or work with Runx2 together to control chondrocyte hypertrophic differentiation.
In this study, we aimed to identify the candidate factors that may associate with Runx2 and together to control chondrocyte hypertrophy. We have performed yeast two-hybrid screening using Runx2 as a bait and the specific cDNA libraries generated from hypertrophic chondrocytes-enriched limb tissue and MCT cells. We successfully identified multiple candidate Runx2-interacting genes, including Timp-2, Lgals1, Edf1, and Col1a2. Based on expression and bioinformatics analysis, these candidate genes are expected to play essential function during bone and cartilage development.
Materials and methods
Generation of pGBKT7-Runx2 bait plasmid
The murine Runx2/Cbfa1 cDNA fragment covering the whole coding region was cloned in frame with the GAL4 DNA binding domain of the bait plasmid pGBKT7. Briefly, the Runx2 cDNA cloned in a pBluescript plasmid was a gift from Dr. Geoffroy. This Runx2 cDNA contains two major translation start sites and may encode both the longest (MASNSL) and shorter Runx2 isoform [12,13]. The pGBKT7 bait vector was purchased from Clontech which was described in the Matchmaker Gold Yeast Two-Hybrid System User Manual (PT4084-1, see www.clontech.com). The Runx2 cDNA released from the pBluescript plasmid was cloned into the EcoR I and BamH I sites of pGBKT7 bait vector and subjected to sequencing confirmation.
Yeast transformation with bait and test experiments
The pGBKT7-Runx2 BD-Bait construct was transformed into the yeast strain Y2HGold using the Matchmaker Gold Yeast Two-Hybrid System (Cat. No. 630489) according to the protocol described in the Yeastmaker Yeast Transformation System 2 User Manual (PT1172-1, Cat No. 630439). This bait integrated yeast strain was subjected to expression test and check for autoactivation and toxicity, which can be determined by observing the color and growth status of the diluent yeast transformants spread on a series of nutrient deficient selective agar plates. The bait strain did not grow in SD/-Trp/X-a-Gal/AbA plate and was considered no autoactivation, while the bait protein was considered not toxic when it grew well after 3-5 days’ culture in SD/-Trp plate.
Cell culture and collection of hypertrophic chondrocytes-enriched tissue
The mouse chondrocytes transformed by large T antigen (MCT) were originally obtained from Dr. de Crombrugghe’s laboratory at MD Anderson Cancer Center (Houston, TX, USA). These cells were cultured at 32°C for proliferation and may or may not subjected to temperature switch from 32°C to 37°C to acquire hypertrophy-like properties, which are characterized by significant upregulation of Col10a1 and other relevant markers of chondrocyte maturation and mineralization [14]. To collect hypertrophic chondrocytes-enriched tissue, new-born mice were euthanized by isoflurane inhalation and chilled on ice for 30 minutes before being skinned and eviscerated. Then, the mouse limbs and ribs were dissected and tissues from the chondro-osseous junctions were collected for subsequent RNA extraction, cDNA synthesis and library construction were conducted as described below.
Construction and screening of a Mate & Plate yeast two-hybrid library
Total RNAs were extracted from dissected mouse limbs and ribs and from MCT cells cultured at 32°C to 37°C respectively using TRIzol Reagents (Invitrogen). The first-strand cDNA synthesis was obtained using 1-2 μg of total RNA as template, the random primer (CDSIII/6), and reversely transcribed using the SMART MMLV Reverse Transcriptase, which will generate cDNA ending with known sequence homology to pGADT7-Rec prey vector. This first strand cDNA was then used as template for low cycle (~20) long distance PCR (LD-PCR) amplification to generate 3-6 μg double strand cDNA (dscDNA). These dscDNAs (PCR product) were purified using the CHROMA SPIN TE-400 Column to obtain DNA molecules larger than 200 bp. The column purified cDNA was further undergone ethanol purification to obtain high quality of cDNA for following library construction. Co-transformation of the library cDNA (prey) and the purified pGBKT7-Runx2 (bait) into Y187 yeast strain allowed interaction between prey and bait. Yeast colonies grown on selective agar medium (Double dropout media containing 40 µg/ml X-a-Gal and 200 ng/ml Aureobasidin A) was then harvested and subjected to further analysis.
Confirmation of positive interactions and rescue of the prey plasmid
Yeast colonies grown in DDO/X/A agar plate are colonies with potential positive interaction between bait and prey proteins and were subjected to colony PCR and rescue of the plasmid for subsequent analysis. Briefly, yeast colony PCR using the Matchmaker insert check PCR mix 2 (Cat. No. 630497) was performed to eliminate duplicate clones. Additional co-transformation of pGBKT7/Bait or Empty pGBKT7 with candidate prey isolated was performed to distinguish genuine or false positive interaction. Then, the genuine prey plasmid was rescued from yeast, after amplification and plasmid DNA extraction, the prey insert can be identified by sequencing and the sequence compared to the NCBI/GenBank databases.
Bioinformatics analysis of candidate genes using GenePaint
GenePaint is a web-based tool and database that has been developed over a decade ago (http://www.genepaint.org). This database, which is freely available to the scientific community, collects expression patterns of genes that are derived from non-radioactive in situ hybridization (ISH) majorly on series of mouse embryo sections at E14.5 [15]. The digital atlas of gene expression patterns can be retrieved by searching the database using gene name, site of expression, GenBank accession number or sequence homology as described online (http://www.genepaint.org). Candidate genes identified by above mentioned yeast two-hybrid screening were queried against this database and compared with the expression pattern of Col10a1, the hypertrophic chondrocyte-specific marker gene.
Expression analysis of candidate genes by (q)RT-PCR
The above total RNAs (~1 μg) from dissected mouse limbs and ribs and from MCT cells were reversely transcribed in a 20 μl volume using superscript II (Invitrogen) according to the manufacturer’s instructions. 2 μl of diluted cDNA samples were used as templates for semi-quantitative RT-PCR or quantitative real-time RT-PCR (qPCR) analysis of following candidate genes: Col10a1, Runx2, Col1a2, Timp-2, Lgals1, and Gapdh as an internal control to quantify the mRNA levels. The specific primers for these genes were listed in Table 1. The RT-PCR product was subjected to gel electrophoresis and qPCR was performed on the real-time PCR detection system from Bio-Rad using SYBR Premix Ex Taq™; II. Comparative 2-ΔΔCt method was used to calculate the expression levels of above genes [16].
Table 1.
Primers designed for real-time RT-PCR
| Name | RefSeqID | Sense Primer (5’-3’) | Antisense Primer (5’-3’) | Amplicon (bp) |
|---|---|---|---|---|
| Gapdh | NM_008084 | ACCCAGAAGACTGTGGATGG | CACATTGGGGGTAGGAACAC | 171 |
| Col10α1 | NM_009925 | GCAGCATTACGACCCAAGATC | TCTGTGAGCTCCATGATTGC | 201 |
| Col1α2 | NM_007743 | AGAGAAGGGAACCAAAGG | GGAAGCCAGTCATACCAG | 149 |
| Timp-2 | NM_011594 | AGGAAAGGCATACCAG | GAGGAGATGTAGGCAAGGGA | 165 |
| Lgals1 | NM_008495 | TCTCAAACCTGGGGAATGTC | GCGAGGATTGAAGTGTAGGC | 115 |
Statistical analysis
Relative mRNA expression levels of marker genes and Gapdh were quantified by qRT-PCR and the comparative 2-ΔΔCt method and analyzed using the GraphPad prism 5 software [45]. Date were collected from three repeated runs with duplicated templates. P<0.05 indicates significant fold changes of genes of interest in hypertrophic vs proliferative MCT cells.
Results
pGBKT7-Runx2 bait construct
Based on the National Center for Biotechnology Information (NCBI) database, the mouse Runx2 gene is consisted of 9 exons with the coding region locates in exons 2-9 (Figure 1, top). The Runt domain that is encoded by exons 3-6 contains about 120 (116-237) amino acids. The Runx2 open reading frame (ORF, ATG to TGA) is around 1.6-kb, which was previously cloned into a pBluescript plasmid [12,13]. This 1.6-kb ORF fragment was cloned in-frame into the pGBKT7 bait vector with the sequence coding for amino acids 1-147 of the GAL4 DNA binding domain (PT3248-5, www.clontech.com, Figure 1, bottom). The property of Kanamycin resistance is for selection in E. coli and the TRP1 nutritional marker is for selection in yeast as described in the manual.
Figure 1.
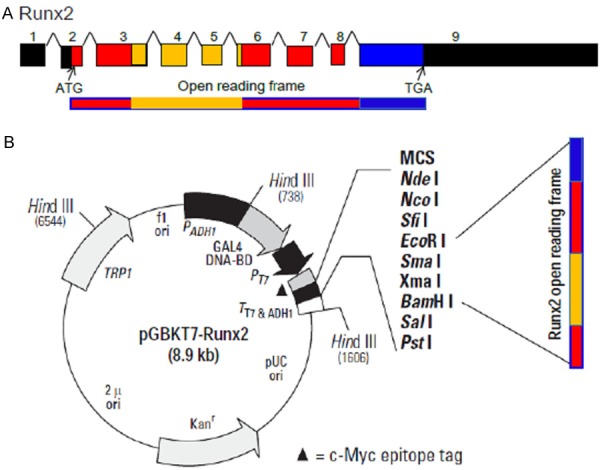
Cloning of pGBKT7-Runx2 bait construct. A. The mouse Runx2 gene is consisted of 9 exons with a 1.6 kb open reading frame covering exons 2-9. ATG: start codon, TGA: stop codon. Yellow bar represents sequence coding the Runt domain which is about 120 amino acids. B. The pGBKT7 bait is a 7.3 kb plasmid containing sequence coding for the GAL4 DNA binding domain. This vector has both antibiotic (Kanamycin) and nutritional (TRP1) selection markers in Ecoli and yeast respectively (PT4084-1)_092413 (1) (see www.clontech.com). The Runx2 cDNA fragment covering the ORF was cloned into the EcoR I and BamH I sites of pGBKT7 bait vector and confirmed by sequencing.
Generation of BD-Bait-expressing yeast strain (Y2HGold)
The pGBKT7-Runx2 bait construct was transformed into yeast competent cells prepared from Y2HGold yeast strain according to the protocol of the Yeast Transformation System 2 User Manual (PT1172-1, Cat No. 630439). After expression confirmation and exclusion of autoactivation (data not shown), the obtained bait-integrated yeast strain was tested for toxicity. The results showed that there are colonies from bait-containing yeast diluent that grew well after 3-5 days’ culture in SD/-Trp plate, demonstrating its in-toxicity to yeast growth (Figure 2).
Figure 2.
Generation of BD-Bait-expressing Y2HGold yeast strain. Illustrated were colonies derived from transformants of pGBKT7-Runx2 bait integrated Y2HGold yeast strain and grew well in SD/-Trp plate, showing its in-toxicity of pGBKT7-Runx2 bait to yeast cell growth.
Mate & Plate library construction and screening
The first strand cDNAs derived from total RNAs of the tissues from hypertrophic zone (HZ) and MCT cells were PCR amplified using specific random primer (CDSIII/6). The results showed that most of the PCR products are around 500-bp (Figure 3A). After co-transformation of these library cDNA and the PGBKT7-Runx2 bait plasmid, multiple colonies grew on above selective media (SD/-Leu-His-Trp-Ade/ABA/X-αgal) indicating a potential interaction (two-hybrid) between prey and bait (Figure 3B). These colonies were further picked and re-streaked on the same select medium to confirm the interaction and for further rescue and PCR amplification (Figure 3C).
Figure 3.
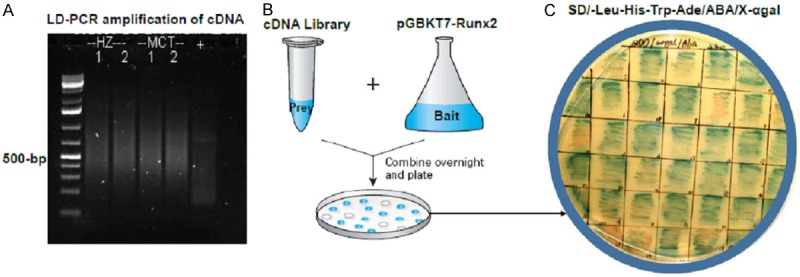
Construction and screening of cDNA library. A. Total RNAs from tissues of hypertrophic zone (HZ) and from hypertrophic MCT cells were reversely transcribed and PCR amplified using specific random primer (CDSIII/6). Most of the cDNA fragments (PCR products) are around 500-bp. B. The library cDNA and the PGBKT7-Runx2 bait plasmid were co-transformed and plate on selective media (SD/-Leu-His-Trp-Ade/ABA/X-αgal) for selection of target colonies. C. The target colonies showing potential interaction were further re-streaked on the same selective medium to confirm the interaction and for further analysis.
Rescue plasmids from yeast and PCR identification of inserts
Plasmids were rescued and ADLD-PCR was performed to identify inserts from the above colonies, which include genuine, false positive, and duplicate ones. The results showed that most of the colonies have inserts that may potentially encode target proteins (Figure 4A). The PCR products (inserts) were then purified for sequencing analysis and after comparison with the NCBI/GenBank databases, we identified several potential transcription factors or co-factors, including Col1a2, Edf1 (Endothelial differentiation factor), Lgals1 (Lectin, galactose binding, soluble 1-1), and Timp-2 as illustrated in Figure 4B, which shows partial sequence of exon 4 of Timp-2 mRNA transcript covering nucleotides from 682-737 (reverse direction).
Figure 4.

Yeast colony PCR and sequencing of inserts. A. The plasmids in above colonies grown in selective media were rescued and ADLD-PCR was performed to identify the inserts. The results showed that most of the colonies have inserts and range from 600 bp to 1 kb. M: DNA marker, N: negative PCR control. B. The PCR products (inserts) were sequenced and analyzed by comparison with the NCBI/GenBank databases. Illustrated is the chromatograph sequencing result showing sequence of Timp-2 gene covering exon 4 nucleotides 682-737 (reverse direction).
Differential expression of candidate genes in GenePaint
We have examined the expression pattern of the above candidate genes in GenePaint and compared with the hypertrophic chondrocyte-specific gene, Col10a1. As expected, Col10a1 (GenePaint Set ID: EB3371) was exclusively expressed in chondro-osseous junctions featuring hypertrophic zone of ribs of mouse embryos at E14.5 (Figure 5A). Genes Col1a2 (EB1096), Edf1 (EG465), and Lgals1 (EG486) all showed different levels of ubiquitous expression, while gene Lgals1 showed a moderately enhanced expression in the hypertrophic zone of rib (Figure 5B-D). We also checked gene Timp-2, but no data was shown in the database for Timp-2. These results suggest a diverse expression pattern of candidate genes that are identified from the cDNA library of hypertrophic chondrocytes.
Figure 5.
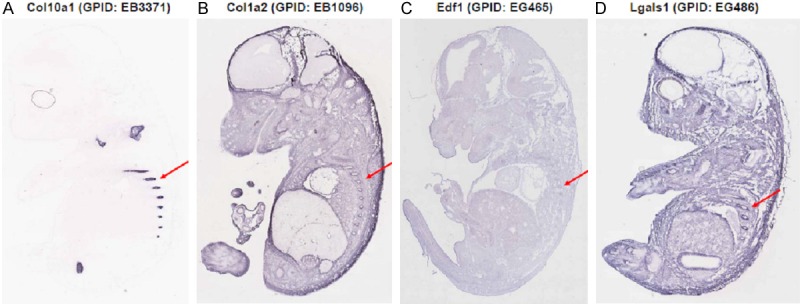
Expression pattern of candidate genes in GenePaint. The expression pattern of candidate genes was searched in the GenePaint database and compared with Col10a1. A. Col10a1 (GPID: EB3371) is exclusively expressed in the hypertrophic zone of mouse rib section (red arrow). B. Gene Col1a2 (GPID: EB1096) shows moderate ubiquitous expression including regions of hypertrophic zone (red arrow). C. Gene Edf1 (GPID: EG465) shows weak ubiquitous expression and is barely detectable in the hypertrophic zone (red arrow). D. Gene Lgals1 (GPID: EG486) showed stronger ubiquitous expression compared with Col1a2 and Edf1, while the hypertrophic zone showed enhanced expression although weaker than Col10a1. GPID: GenePaint Set ID.
Expression analysis of candidate genes interacting with Runx2
We have examined the mRNA levels of above candidate Runx2-interacting genes in MCT cells by semi-quantitative RT-PCR analysis. As illustrated in Figure 6A, Col10a1 is much more abundant in hypertrophic than in proliferative MCT cells. While Lgals1 is abundant in both MCT cells, Timp-2 showed much higher level of mRNA in hypertrophic than in proliferative MCT cells (Figure 6A). Meanwhile, Col2a1 and Edf1 was barely detectable neither in proliferative nor in hypertrophic MCT cells (data not shown). We have also performed real-time RT-PCR analysis of these genes and compared with Col10a1. The results showed that Col10a1 is more than 10-fold upregulated in hypertrophic MCT cells (P<0.01). Lgals1 and Timp-2 were also significantly upregulated in hypertrophic MCT cells (P<0.01), while no significant fold changes for Col1a2 was shown between hypertrophic and proliferative MCT cells (Figure 6B).
Figure 6.
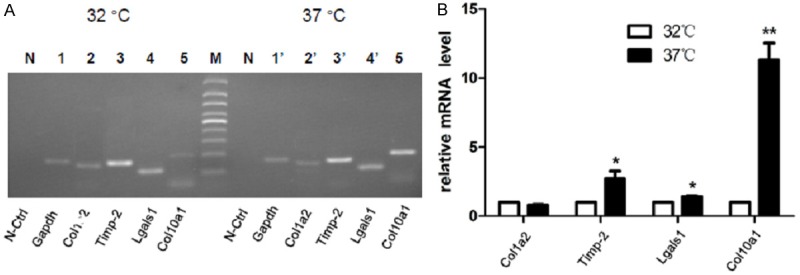
Expression analysis of candidate genes in MCT cells (A). Semi-quantitative RT-PCR analysis showed that Col10a1 is significantly upregulated in hypertrophic compared with proliferative MCT cells. Candidate genes Lgals1 is and Timp-2 also showed higher level of mRNA in hypertrophic than in proliferative MCT cells. Whereas Col2a1 and Edf1 was barely detectable neither in proliferative nor in hypertrophic MCT cells (data not shown). (B) Real-time RT-PCR analysis revealed that Col10a1 mRNA transcript is 10-fold higher in hypertrophic than in proliferative MCT cells. Genes Lgals1 (P<0.01) and Timp-2 (P<0.05) are also significantly upregulated in hypertrophic MCT cells, while no significant fold changes for Col1a2 was shown between hypertrophic and proliferative MCT cells Data were collected from 3 independent experiments with duplicate samples. Gapdh was used as an internal control of mRNA quantity and quality.
Discussion
RUNX2 has been shown as a multifunctional transcription factor that controls both chondrocyte and osteoblast differentiation during cartilage and bone development [10]. Chondrocyte hypertrophy represents a late and critical stage of cartilage development. Extensive in vitro and in vivo studies have previously demonstrated that Runx2 is the major player that regulates this process, as Runx2 null mice show disturbed chondrocyte differentiation and maturation [7]. The possible mechanisms have been attributed to Runx2 dysregulation of many target genes associated with chondrocyte differentiation and maturation, including Col1a1, Col1a2, Col10a1, Mmp13, Osteopontin, Bsp, and Collagenase 3 etc [11]. However, these genes are known to differentially express at different stages of cartilage development. In addition, chondrocyte hypertrophy and its marker genes are not unanimously affected in different long bone growth plates in Runx2 null mice, demonstrating the complexity of chondrocyte hypertrophy and its regulation during endochondral bone formation [7,8].
Our hypothesis was that multiple candidate genes interact with Runx2 together to regulate chondrocyte hypertrophy. To identify these genes, we have performed yeast two hybrid screening using Runx2 cDNA as a bait to screen cDNA libraries derived from hypertrophic chondrocytes. Runt domain, which covers amino acids 116-237, is highly conserved among the Runx family members and is known to be responsible for DNA binding and protein-protein interactions. Theoretically, it might be ideal to use the Runt domain as a bait for the two-hybrid screening. However, the non-Runt domain sequence (amino acids 263-351) of Runx2 has previously been successfully utilized in a Y2H screening, which identified TFIIA gamma (general transcription factor IIA gamma) as a Runx2-interacting factor facilitating osteoblast-specific gene expression [17]. Full-length cDNA of another gene (pGBKT7-podocin) has also been utilized for similar Y2H screening, and positive clones interacting with podocin have been obtained by screening a mouse kidney cDNA library [18]. These studies demonstrated the flexibility using different cDNA baits to reliably screen for target interacting proteins using Y2H approach. In this study, Runx2 cDNA fragment covering the whole ORF was used as a bait, and we successfully identified multiple candidate genes that potentially encode proteins interacting with Runx2 (two-hybrid). These genes include Edf1, Col1a2, Lgals1, and Timp-2.
To find out their correlation with chondrocyte hypertrophy, we have examined the expression patterns of the candidate genes with bioinformatics analysis using GenePaint. The expression patterns of genes in GenePaint are mostly displayed at E14.5, which is a critical developmental stage when the hypertrophic zone becomes obvious within the growth plate [15]. This also allows us to compare the expression patterns of target genes with Col10a1, a gene highly expressed in the hypertrophic zone corresponding to the chondro-osseous junctions of long bones. Compared with the specific expression of Col10a1 in clavicle, rib, and scapula chondro-osseous junctions (Figure 5A), ubiquitous expression was observed for candidate genes Edf1, Col1a2, and Lgals1. However, through detailed analysis, we notice that although gene Edf1 was barely detectable, genes Col1a2 and Lgals1 did show moderate to strong expression within the rib hypertrophic zone (Figure 5B-D). This is not surprising, as these genes were screened from cDNA libraries of hypertrophic chondrocytes.
To further characterize these candidate genes, we have examined their expression levels in established MCT cell model. The results showed that, along with the significant upregulation of Col10a1, we also detected significantly increased level of Lgals1 in hypertrophic MCT cells, while gene Col1a2 was weakly expressed in both proliferative and hypertrophic MCT cells, corresponding well with the expression pattern identified by GenePaint. It was previously shown that Lgals1, also known as Galectin-1, expressed both in articular and in growth plate cartilage, with significant higher level at the hypertrophic zone [19]. Interestingly, while the Timp family members Timp-1 (EH1095), Timp-3 (EH1096), and Timp-4 (EH2514) all showed expression within the GenePaint database, no data was shown for Timp-2. In our results, significantly increased level of Timp-2 was detected in hypertrophic MCT cells as demonstrated by semi-quantitative RT-PCR and confirmed by qRT-PCR analysis (Figure 6). This observation corresponds with previous findings that Timp-2 was weakly expressed in resting chondrocytes, started rising in proliferative and further elevated in hypertrophic chondrocytes [20]. TIMP-2 mRNA was also detected both in human normal and in osteoarthritic articular chondrocytes [21]. In a murine STR/ort model of osteoarthritis, up-regulated MMPs and TIMP-2 mRNA level was detected, but not at protein level for Timp-2 [22]. Intriguingly, it has been reported that TIMP2 was decreased, while TIMP1, as well as genes COL1A2, MMP2, MMP9, MMP13 were increased in OA bone relative to normal bone, suggesting a correlation of these cartilage genes with the radiographic severity of OA [23]. We notice that Runx2 has been shown to contribute to OA development, as Runx2 heterozygous mice are less susceptible to developing OA [24]. On the other hand, it has been shown that TIMP2 deficient mice develop accelerated osteoarthritis [25]. While further studies are needed, these observations support that the putative interactions between Runx2 and Timp-2, as well as Lgals1 may play a role in cartilage development, and possibly participate in the pathogenesis of osteoarthritis.
Acknowledgements
This work was supported by grants from the Youth National Natural Science Foundation of China (Project number: 81302319, FL), the Natural Science Foundation of Anhui Province (Project number: 1308085MH121, FL), the National Natural Science Foundation of China (Project number: 31271399, 81472047, and 81672229 QZ, JG, YL), and the innovation program of Jiangsu province, China (QZ).
Disclosure of conflict of interest
None.
References
- 1.Pacifici M, Golden EB, Oshima O, Shapiro IM, Leboy PS, Adams SL. Hypertrophic chondrocytes. The terminal stage of differentiation in the chondrogenic cell lineage? Ann N Y Acad Sci. 1990;599:45–57. doi: 10.1111/j.1749-6632.1990.tb42363.x. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A. 2014;111:12097–12102. doi: 10.1073/pnas.1302703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsang KY, Chan D, Cheah KS. Fate of growth plate hypertrophic chondrocytes: death or lineage extension? Dev Growth Differ. 2015;57:179–192. doi: 10.1111/dgd.12203. [DOI] [PubMed] [Google Scholar]
- 4.Lee B, Thirunavukkarasu K, Zhou L, Pastore L, Baldini A, Hecht J, Geoffroy V, Ducy P, Karsenty G. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat Genet. 1997;16:307–310. doi: 10.1038/ng0797-307. [DOI] [PubMed] [Google Scholar]
- 5.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Q, Sebald E, Zhou G, Chen Y, Wilcox W, Lee B, Krakow D. Dysregulation of chondrogenesis in human cleidocranial dysplasia. Am J Hum Genet. 2005;77:305–312. doi: 10.1086/432261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inada M, Yasui T, Nomura S, Miyake S, Deguchi K, Himeno M, Sato M, Yamagiwa H, Kimura T, Yasui N, Ochi T, Endo N, Kitamura Y, Kishimoto T, Komori T. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev Dyn. 1999;214:279–290. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 8.Kim IS, Otto F, Zabel B, Mundlos S. Regulation of chondrocyte differentiation by Cbfa1. Mech Dev. 1999;80:159–170. doi: 10.1016/s0925-4773(98)00210-x. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Q, Zhou G, Morello R, Chen Y, Garcia-Rojas X, Lee B. Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J Cell Biol. 2003;162:833–842. doi: 10.1083/jcb.200211089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komori T. Regulation of bone development and maintenance by Runx2. Front Biosci. 2008;13:898–903. doi: 10.2741/2730. [DOI] [PubMed] [Google Scholar]
- 11.Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339:189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 12.Geoffroy V, Kneissel M, Fournier B, Boyde A, Matthias P. High bone resorption in adult aging transgenic mice overexpressing cbfa1/runx2 in cells of the osteoblastic lineage. Mol Cell Biol. 2002;22:6222–6233. doi: 10.1128/MCB.22.17.6222-6233.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merciris D, Marty C, Collet C, de Vernejoul MC, Geoffroy V. Overexpression of the transcriptional factor Runx2 in osteoblasts abolishes the anabolic effect of parathyroid hormone in vivo. Am J Pathol. 2007;170:1676–1685. doi: 10.2353/ajpath.2007.061069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefebvre V, Garofalo S, de Crombrugghe B. Type X collagen gene expression in mouse chondrocytes immortalized by a temperature sensitive simian virus 40 large tumor antigen. J Cell Biol. 1995;128:239–245. doi: 10.1083/jcb.128.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visel A, Thaller C, Eichele G. GenePaint. org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32:D552–556. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu S, Jiang Y, Galson DL, Luo M, Lai Y, Lu Y, Ouyang HJ, Zhang J, Xiao G. General transcription factor IIA-gamma increases osteoblast-specific osteocalcin gene expression via activating transcription factor 4 and runt-related transcription factor 2. J Biol Chem. 2008;283:5542–5553. doi: 10.1074/jbc.M705653200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SJ, Lee BH, Kim DJ. Identification of proteins that interact with podocin using the yeast 2-hybrid system. Yonsei Med J. 2009;50:273–279. doi: 10.3349/ymj.2009.50.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsich E, Mozetic P, Ortolani F, Contin M, Marchini M, Vetere A, Pacor S, Semeraro S, Vittur F, Paoletti S. Galectin-1 in cartilage: expression, influence on chondrocyte growth and interaction with ECM components. Matrix Biol. 2008;27:513–525. doi: 10.1016/j.matbio.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Tchetina EV, Mwale F, Poole AR. Changes in gene expression associated with matrix turnover, chondrocyte proliferation and hypertrophy in the bovine growth plate. Acta Naturae. 2014;6:89–97. [PMC free article] [PubMed] [Google Scholar]
- 21.Zafarullah M, Su S, Martel-Pelletier J, DiBattista JA, Costello BG, Stetler-Stevenson WG, Pelletier JP. Tissue inhibitor of metalloproteinase-2 (TIMP-2) mRNA is constitutively expressed in bovine, human normal, and osteoarthritic articular chondrocytes. J Cell Biochem. 1996;60:211–217. doi: 10.1002/(sici)1097-4644(19960201)60:2<211::aid-jcb5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Flannelly J, Chambers MG, Dudhia J, Hembry RM, Murphy G, Mason RM, Bayliss MT. Metalloproteinase and tissue inhibitor of metalloproteinase expression in the murine STR/ort model of osteoarthritis. Osteoarthritis Cartilage. 2002;10:722–733. doi: 10.1053/joca.2002.0818. [DOI] [PubMed] [Google Scholar]
- 23.Clements DN, Fitzpatrick N, Carter SD, Day PJ. Cartilage gene expression correlates with radiographic severity of canine elbow osteoarthritis. Vet J. 2009;179:211–218. doi: 10.1016/j.tvjl.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Kamekura S, Kawasaki Y, Hoshi K, Shimoaka T, Chikuda H, Maruyama Z, Komori T, Sato S, Takeda S, Karsenty G, Nakamura K, Chung UI, Kawaguchi H. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 2006;54:2462–2470. doi: 10.1002/art.22041. [DOI] [PubMed] [Google Scholar]
- 25.Mi M, Shi S, Li T, Holz J, Lee YJ, Sheu TJ, Liao Q, Xiao T. TIMP2 deficient mice develop accelerated osteoarthritis via promotion of angiogenesis upon destabilization of the medial meniscus. Biochem Biophys Res Commun. 2012;423:366–372. doi: 10.1016/j.bbrc.2012.05.132. [DOI] [PubMed] [Google Scholar]


