Abstract
Increasing evidence indicates that dysregulation of miRNAs is involved in the initiation and progression of colorectal cancer (CRC). MicroRNA (miR)-613 has been reported to function as a tumor suppressor in many cancers. However, the precise role of miR-613 in CRC progression is unclear. This study aimed to investigate the role and underlying mechanism of miR-613 in growth and metastasis of CRC. Real-time quantitative PCR (qPCR) and western blot techniques were used to assess expression of miR-613 and formin-like 2 (FMNL2) in CRC cell lines and tissues. Luciferase reporter assays were conducted to investigate the association between miR-613 and FMNL2. Proliferation, wound healing, and transwell invasion assays, as well as flow cytometric analysis, were performed to evaluate the effect of miR-613 on proliferation, migration, invasion, and cell-cycle status, respectively, of CRC cells. We found that miR-613 was significantly downregulated in CRC cell lines and tissue samples, and correlated closely with TNM stage. miR-613 suppressed CRC cell proliferation, migration, and invasion, and induced cell-cycle arrest at G1 phase. FMNL2 was identified as a direct target of miR-613 in CRC cells. Importantly, FMNL2 overexpression rescued miR-613-induced suppression of proliferation, migration, and invasion of CRC cells. These results suggest that miR-613 functions as a tumor suppressor in the progression of CRC by regulating FMNL2.
Keywords: miR-613, colorectal cancer, progression, FMNL2
Introduction
Colorectal cancer (CRC) has become one of the most common types of malignant tumor in the world with more than one million new cases diagnosed every year [1]. Despite the use of many treatment strategies, the rate of survival of CRC patients is still very low [2]. The initiation and development of CRC is complex, involving multiple stages and molecules [3]. Therefore, an understanding of the molecular mechanisms underlying CRC tumorigenesis and progression is urgently needed to identify novel diagnostic markers and therapeutic targets and agents.
MicroRNAs (miRNAs) are small (19-25 nucleotides), endogenous noncoding RNAs that negatively regulate gene expression by binding to the 3’-untranslated region (UTR) of their target mRNAs [4]. miRNAs have been reported to play crucial roles in many diverse biological processes, including cell proliferation, differentiation, and apoptosis [5]. miRNAs have been suggested to serve an oncogenic or tumor-suppressor function by targeting oncogenes or tumor-suppressor genes, respectively [6-8]. It is noteworthy that miRNAs have been implicated in tumor initiation and progression of CRC [9,10].
miR-613 has been reported to be downregulated and serve as a suppressive miRNA in hepatocellular carcinoma [11,12], ovarian cancer [13], non-small cell lung cancer [14], esophageal sequamous cell carcinoma [15], papillary thyroid carcinoma [16], and prostate cancer [17]. However, the function and underlying molecular mechanism of miR-613 in CRC remains unclear. Therefore, the aim of the study was to investigate the biological function of miR-613 and identify its mechanism of action in CRC cells.
Materials and methods
Patients and tissue samples
A total of 32 pairs of CRC tissues and adjacent nontumor tissues were harvested from CRC patients who underwent surgery at the First Hospital of Jilin University (Changchun, China) between September 2013 and September 2015. Adjacent normal colorectal tissue was taken from 5 cm away from the tumor tissue. All samples were frozen immediately following surgery in liquid nitrogen and stored at -80°C until RNA extraction. Clinicopathological features of CRC patients are shown in Table 1. Written prior informed consent and approval were given by all patients. This study was approved by the Ethics Committee of Jilin University.
Table 1.
Correlation between clinicopathological features and miR-613 expression in 32 patients with colorectal cancer
| Variable | Cases (n) | miR-613 expression level | P | |
|---|---|---|---|---|
|
| ||||
| Low n (%) | High n (%) | |||
| Age (years) | 0.823 | |||
| <60 | 14 | 7 (50.0) | 7 (50.0) | |
| ≥60 | 18 | 10 (55.6) | 8 (43.4) | |
| Gender | 0.791 | |||
| Male | 21 | 11 (52.4) | 10 (47.6) | |
| Female | 11 | 6 (54.5) | 5 (45.5) | |
| TNM stage | <0.01 | |||
| I-II | 22 | 8 (36.4) | 14 (63.6) | |
| III-IV | 10 | 9 (90.0) | 1 (10.0) | |
| Tumor size | 0.082 | |||
| <5 cm | 19 | 8 (42.1) | 11 (57.9) | |
| ≥5 cm | 13 | 9 (69.2) | 4 (30.8) | |
| Lymph node metastasis | <0.01 | |||
| No | 23 | 8 (34.8) | 15 (55.2) | |
| Yes | 9 | 9 (100.0) | 0 (0.0) | |
Cell lines and transfection
Five human colorectal cancer cell lines (HCT116, HT29, SW480, SW620, and LoVo) and a normal colonic cell line (NCM460) were obtained from Shanghai Institute for Biological Sciences (Shanghai, China) and the American Type Culture Collection (ATCC; Manassas, VA, USA). All cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA), and 100 units/ml penicillin or 100 mg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2.
miR-613 mimic and corresponding negative control miR (miR-NC) were purchased from RiboBio (Guangzhou, China). The FMNL2 coding sequence was amplified by PCR from human liver cDNA, inserted into vector pCDNA3.1 (Invitrogen, Carlsbad, CA, USA), and confirmed by sequencing. Transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
Real-time quantitative PCR (qPCR)
Total RNA was extracted from the cells or tissues using TRIzol reagent (Invitrogen) following the manufacturer’s instructions and quantified with a NanoDrop ND-100 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The relative expression level of miR-613 was determined using the mirVana™ qRT-PCR microRNA Detection kit (Ambion, Austin, TX, USA) according to the manufacturer’s instructions using a 7900 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Primers for miR-613 and U6 (internal standard) were obtained from Ambion. For detection of FMNL2 mRNA, cDNAs were synthesized from total RNA using PrimeScript RT Reagent Kit (Takara, Otsu, Japan). FMNL2 mRNA was quantified using the standard SYBR-Green PCR Kit (Takara) following the manufacturer’s instructions and a 7900 Real-Time PCR System (Applied Biosystems). Primers for FMNL2 and β-actin (ACTB, internal standard) were used as described previously [18]. The relative expression level of FMNL2 mRNA or miR-613 was quantified using the 2-∆∆Ct method.
Cell proliferation assay
Cell proliferation was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, transfected cells in exponential growth phase were plated at a density of 2×103 cells/well in 96-well plates, and cell proliferation was determined 24, 48, and 72 h after seeding. Optical density at 490 nm (OD490) was determined using an ELx800 absorbance reader (BioTek Instruments, Winooski, VT, USA).
Cell-cycle assay
A cell-cycle assay was performed 48 h after transfection. Briefly, cells were harvested and fixed in ice-cold 70% ethanol overnight at -20°C. Cells were then incubated with RNase A at 37°C for 30 min and stained with propidium iodide (Sigma-Aldrich, St. Louis, MO, USA) for 30 min in the dark. Cell-cycle status was analyzed with a FACSCaliber instrument (BD Biosciences, San Jose, CA, USA).
Cell migration assay
Cell migration was determined using a wound healing assay. Briefly, transfected cells were seeded at 5×104 cells/well in six-well plates and cultured until they reached confluence. Monolayers were wounded by scratching once with a sterile plastic micropipette tip. Cultures were fixed and observed 0 and 24 h after wounding using an X71 inverted microscope (Olympus, Tokyo, Japan).
Cell invasion assay
Invasion assay was performed using Transwell invasion chambers (Corning, Tewksbury, MA, USA) containing a membrane with 8-μm pores according to the manufacturer’s instructions. Briefly, 2×105 transfected cells in serum-free DMEM were seeded in the upper chamber, which had been precoated with Matrigel (BD Biosciences). DMEM containing 10% FBS (600 μl) was added to the lower chamber as a chemoattractant. After 48 h of incubation at 37°C, cells on the lower surface of the membrane were fixed in 20% methanol and stained with 0.1% crystal violet. Photographs were taken and the invasive number were counted in five randomly selected fields under a X71 inverted microscope (Olympus, Tokyo, Japan).
Luciferase assays
Wild type (Wt) and mutant (Mut) 3’-untranslated regions (UTRs) of FMNL2 mRNA were synthesized, annealed, and inserted into vector psiCHECK-2 at the HindIII/SpeI site. For luciferase assays, SW480 cells were co-transfected with Wt or Mut FMNL2 3’-UTR plasmids and miR-613 or miR-NC using Lipofectamine 2000 according to the manufacturer’s protocol. Firefly and Renilla luciferase activities were measured consecutively using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) 48 h post-transfection.
Western blot analysis
Total protein was extracted from cells or tissues using PIPA lysis buffer (Beyotime, Jiangsu, China), and quantified with the Pierce bicinchoninic acid kit (Thermo Fisher Scientific, Waltham, MA, USA). Proteins were separated by SDS/PAGE through a 10% gel and transferred to polyvinylidene difluoride membranes (Millipore, Burlington, MA, USA) at 80 V for 2 h at 4°C. After blocking in 5% nonfat dry milk in Tris-buffered saline (TBS; 20 mM Tris-HCl, pH 7.5, 150 mM NaCl), the membranes were incubated with anti-FMNL2 antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-β-actin antibody (1:3000; Santa Cruz Biotechnology) in TBS overnight at 4°C. After three washes with TBS containing 0.1% Tween-20 (TBST), the membranes were incubated with goat anti-mouse IgG conjugated to horseradish peroxidase (1:3000, Santa Cruz Biotechnology) in TBS for 1 h at room temperature. Protein bands were detected by enhanced chemiluminescence (ECL) using Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific) and exposed to X-ray filmusing an ECL detection system (Thermo Fisher Scientific). β-actin was used as a control.
Statistical analysis
Statistical analysis was performed using SPSS 17 software (SPSS, Chicago, IL, USA) and GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Results are presented as mean ± standard deviation (SD) of at least three independent experiments. Significance was determined by two-tailed Student’s t-test or one-way ANOVA. Correlation between FMNL2 and miR-613 expression was assessed by Pearson correlation analysis. In all cases, P<0.05 was considered statistically significant.
Results
miR-613 expression was downregulated in CRC samples and cell lines
To investigate the status of miR-613 in CRC, we measured its expression in 32 pairs of CRC specimens and corresponding adjacent nontumor tissue using qPCR. It was found that miR-613 expression was significantly decreased in CRC tissues compared with the adjacent nontumor tissues (Figure 1A). To investigate the clinical relevance of miR-613 in CRC, we divided the 32 patients into a high miR-613-expressing group (n=17) and a low miR-613-expressing group (n=15) using the mean value (0.251±0.08) of miR-613 relative expression level as a cutoff. As shown in Table 1, miR-613 expression was correlated significantly with lymph node metastasis (P<0.01) and TNM stage (P<0.01), whereas there was no correlation with patient age, gender, or tumor size (all P>0.05). Moreover, in five CRC cell lines (HCT116, HT29, SW480, SW620, and LoVo), miR-613 expression was significantly downregulated compared with expression in the normal colonic cell line (NCM460) (Figure 1B). miR-613 expression was lowest in SW480 cells (Figure 1B), and, therefore, this line was selected for use as the model for the remainder of the study.
Figure 1.
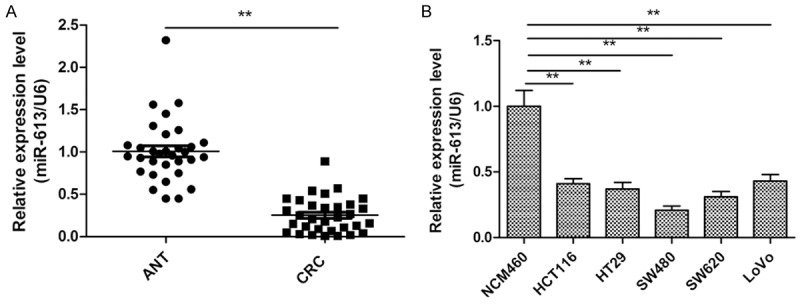
Expression of miR-613 in human colorectal cancer (CRC) tissue and cell lines. A. Relative miR-613 expression levels in 32 samples of primary CRC tissue and paired adjacent nontumor tissue (ANT) were detected by real-time quantitative PCR (qPCR) analysis. B. qPCR analysis of miR-613 expression in five human colorectal cancer cell lines (HCT116, HT29, SW480, SW620, and LoVo) and a normal colonic cell line (NCM460). *P<0.05, **P<0.01.
miR-613 inhibited cell proliferation and induced cell-cycle arrest at G1 in CRC cells
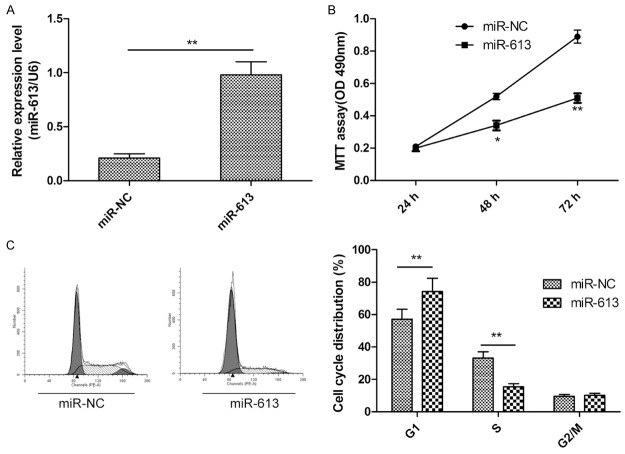
To examine the functional role of miR-613 in CRC cells, we transfected SW480 cells with miR-613 mimic or control miR-NC. We found that expression of miR-613 was significantly upregulated in SW480 cells transfected with miR-613 mimic compared to cells transfected with miR-NC (Figure 2A). MTT assay clearly showed that overexpression of miR-613 reduced proliferation of SW480 cells (Figure 2B). It is well known that cell proliferation is associated with cell-cycle distribution. Thus, cell-cycle status of SW480 cells transfected with miR-613 mimic or miR-NC was assessed. As expected, overexpression of miR-613 significantly decreased the percentage of cells in S phase and increased the percentage of cells in G1 phase (P<0.05, Figure 2C). These results suggested that miR-613 impaired CRC cell proliferation by inducing cell-cycle arrest at G1.
Figure 2.
miR-613 inhibited proliferation and induced cell-cycle arrest at G1 phase in CRC cells. A. qPCR validation of miR-613 expression levels in SW480 cells after transfection with miR-613 mimic or miR-NC. B. Cell proliferation was evaluated in SW480 cells by MTT assay at the indicated times after transfection with miR-613 mimic or miR-NC. C. Results of flow cytometric analysis of cell-cycle distribution of SW480 cells after transfection with miR-613 mimic or miR-NC (left) and quantitation (right). *P<0.05, **P<0.01.
miR-613 inhibited CRC cell migration and invasion
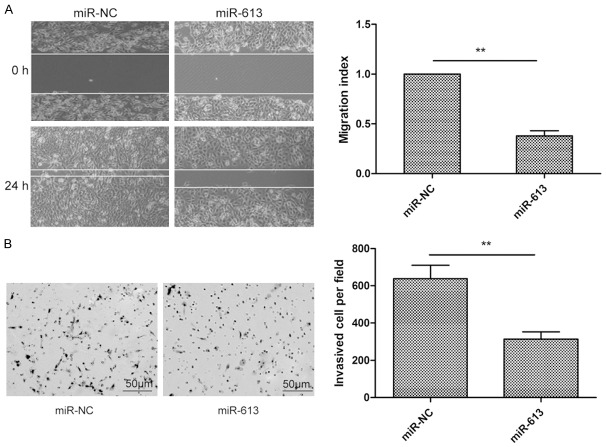
The role of miR-613 in CRC cell metastasis was then investigated by wound healing assay and Transwell invasion assay. As shown in Figure 3, SW480 cells transfected with miR-613 mimic showed significantly reduced migratory and invasive ability compared with that of cells transfected with miR-NC. These observations indicate that that miR-613 may inhibit CRC metastasis.
Figure 3.
miR-613 inhibited cell migration and invasion in CRC cells. A. Results of measurement of cell migration in SW480 cells by wound healing assay at the indicated times after transfection with miR-613 mimic or miR-NC (left) and quantitation (right). B. Results of measurement of invasion of SW480 cellsbyTranswell chamber invasion assay after transfection with miR-613 mimic or miR-NC (left) and quantitation (right). *P<0.05, **P<0.01.
miR-613 directly targets FMNL2 mRNA by binding to its 3’-UTR
To investigate the molecular mechanism underlying the antitumor activity of miR-613 in CRC cells, potential targets of miR-613 were predicted using bioinformatic software (TargetScan and miRanda). FMNL2, an oncogene, was selected as a potential target. To further explore whether FMNL2 responds to miR-613 through a direct interaction with the 3’-UTR in CRC, we cloned the Wt and Mut FMNL2 3’-UTRs (Figure 4A) into a luciferase reporter vector and assessed the effect of miR-613 on luciferase activity by dual-luciferase assay. The results showed that miR-613 significantly repressed the luciferase activity of the Wt-FMNL2 3’-UTR, whereas no significant change was observed in luciferase activity of the Mut-FMNL2 3’-UTR in SW480 cells (Figure 4B). To further confirm the potential role of miR-613 in regulation of FMNL2, we examined FMNL2 expression in SW480 cells after transfection with miR-613 mimic or miR-NC by qPCR and western analysis. Our results demonstrated that overexpression of miR-613 caused a notable decrease in FMNL2 expression at the mRNA and protein levels in SW480 cells (Figure 4C and 4D). These results suggested that FMNL2 is a direct target of miR-613 in CRC cells.
Figure 4.
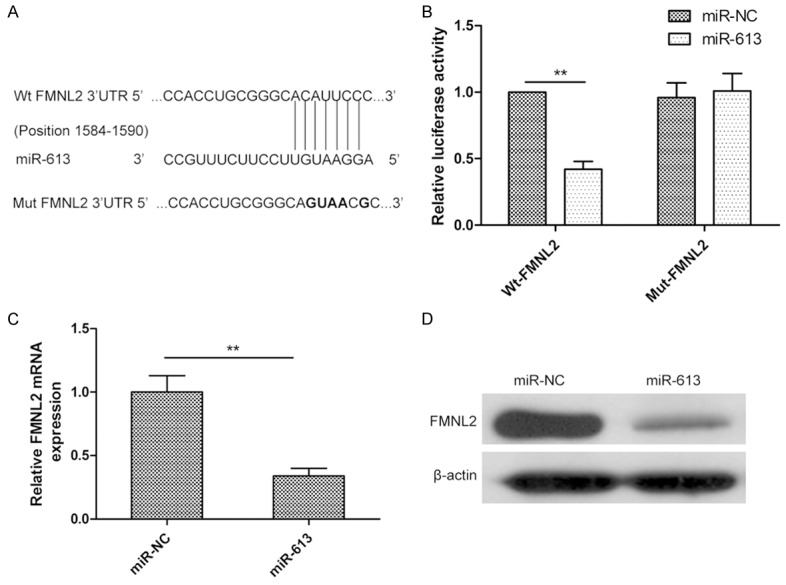
miR-613 directly targets FMNL2 mRNA by binding to its 3’-UTR. A. Predicted miR-613 target sequence in the 3’-UTR of FMNL2 mRNA and positions of five mutated nucleotides (bold) in the 3’-UTR of miR-613. Wt, wild type; Mut, mutant. B. Luciferase reporter assay of SW480 cells cotransfected with Wt or Mut FMNL2 3’-UTR reporter plasmid and miR-613 or miR-NC. C and D. FMNL2 expression at the mRNA and protein levels was detected in SW480 cells transfected with miR-613 or the miR-NC by qPCR and western analysis, respectively. β-actin served as the loading control. *P<0.05, **P<0.01.
miR-613 level negatively correlates with FMNL2 level in CRC tissue
To further examine the relationship between miR-613 and FMNL2, we assessed the expression level of FMNL2 mRNA in 32 pairs of CRC tissues and adjacent nontumor tissues by qPCR. Results showed that FMNL2 mRNA level was dramatically upregulated in CRC tissue compared to adjacent nontumor tissues (P<0.01, Figure 5A). Pearson correlation analysis further demonstrated that miR-613 level was negatively correlated with the expression level of FMNL2 mRNA in CRC tissue (r=-0.513, P=0.003, Figure 5B). Taken together, these data lead to the conclusion that reduced miR-613 expression, together with increased FMNL2 expression, commonly occurs in human CRC cells.
Figure 5.
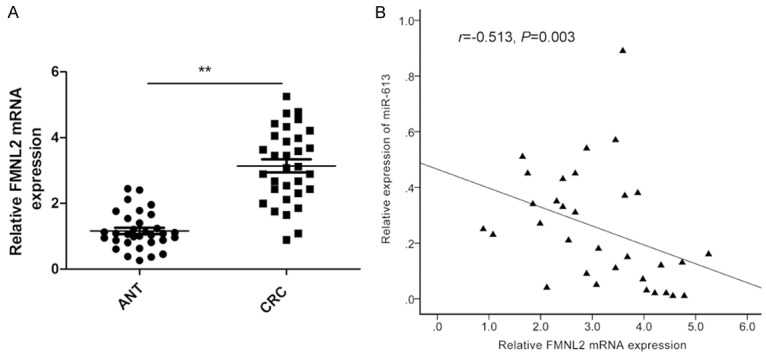
miR-613 level negatively correlates with FMNL2 mRNA level in CRC tissue. A. FMNL2 mRNA expression level in 32 samples of CRC tissues and paired adjacent nontumor tissues (ANT) was determined by qPCR. β-actin was used as an internal control. B. Pearson correlation analysis shows inverse relationship between FMNL2 and miR-613 expression levels in CRC tissues (n=32). *P<0.05, **P<0.01.
Overexpression of FMNL2 reversed the tumor-suppressive effect of miR-613 in CRC cells
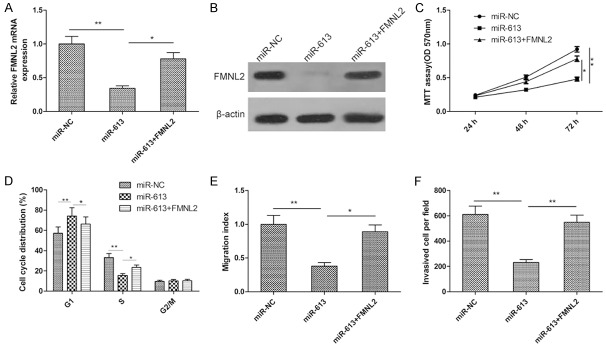
To evaluate whether FMNL2 is responsible for the functional effects of miR-613 in CRC cells, we generated FMNL2 expression plasmid pCDNA3.1-FMNL2 and used it to transfect SW480 cells overexpressing miR-613. Then, proliferation, cell-cycle status, and migratory and invasive ability of the cells were determined. We found that overexpression of FMNL2 restored expression of FMNL2 mRNA and protein that had been suppressed by overexpression of miR-613 (Figure 6A and 6B). Results of MTT assay showed that FMNL2 overexpression markedly rescued the suppression of cell proliferation induced by miR-613 (Figure 6C). Furthermore, overexpression of FMNL2 led to a significant increase in the proportion of miR-613-expressing cells in S phase and decreased the proportion in G1 (Figure 6D). Restoration of FMNL2 expression also significantly attenuated miR-613-induced suppression of cell migration and invasion in SW480 cells (Figure 6E and 6F). These data make it clear that miR-613 exerted its suppressive role in CRC cells by targeting FMNL2.
Figure 6.
Overexpression of FMNL2 reversed the tumor-suppressive effect of miR-613 in CRC cells. (A and B) SW480 cells were transfected with FMNL2 expression plasmid (pcDNA3.1-FMNL2) and high levels of miR-613, and FMNL2 mRNA (A) and protein (B) were determined by qPCR and western blot, respectively. β-actin served as loading control. (C-F) Proliferation, cell-cycle status, migration, and invasion of the cells were assessed by MTT assay, flow cytometry, and wound healing and transwell invasion assays, respectively. *P<0.05, **P<0.01.
Discussion
Aberrant expression of miRNAs has been suggested to play critical roles in the development and progression of different cancers through modulation of oncogenic and tumor-suppressor pathways [6,8]. Specifically with regard to CRC, it has already been reported that miRNAs are involved in CRC progression through regulation of cell-cycle status, proliferation, migration, apoptosis, and invasion [9,10]. For example, Li et al reported that restoring expression of miR-152 in CRC cells dramatically reduced cell proliferation, migration, and invasion and promoted apoptosis and caspase-3 activity in vitro, and also suppressed tumor growth in vivo by targeting phosphoinositide-3-kinase regulatory subunit 3 (PIK3R3) [19]. Han et al found that miR-874 inhibited proliferation, reduced colony formation, enhanced apoptosis, and decreased 5-fluorouracil resistance in CRC cells by targeting XIAP [20]. Wu et al demonstrated that miR-128 inhibited CRC cell proliferation, colony formation, migration, and invasion and promoted apoptosis in vitro, and also suppressed CRC xenograft tumor growth in vivo by targeting insulin receptor substrate 1 (IRS1) and indirectly regulating the AKT signaling pathway [21]. Here, we investigated the role of miR-613 in CRC growth and metastasis. Our results showed that miR-613 was downregulated in CRC tissue and cell lines, and that a low level of miR-613 was significantly associated with advanced TNM stage and lymph node metastasis. Investigation of cell functions showed that miR-613 significantly decreased CRC cell proliferation, migration, and invasion, and induced cell-cycle arrest at G1 phase. These data suggest that miR-613 might serve as a novel biomarker or therapeutic target for CRC.
miR-613 has been reported to be downregulated in several types of cancers [11-17]. miR-613 inhibits cancer cell growth, invasion, and migration, and promotes apoptosis by targeting several oncogenes, including SphK2 [16], DCLK1 [11], CDK4 [14], LXRα [12] and Fzd7 [17]. However, to date, the biological function and underlying mechanism of miR-613 in CRC remains unclear. Here, our findings that miR-613 overexpression significantly inhibited CRC cell proliferation, migration, and invasion, and promoted cell-cycle arrest at G1 phase suggest that miR-613 might also act as a tumor suppressor in CRC.
It is well known that understanding the biological functions and the underlying mechanisms of miRNAs relies on the identification of relevant target genes [7]. Here, using bioinformatic software (TargetScan and miRanda), we identified FMNL2 as a potential target of miR-613. FMNL2 is a member of the diaphanous-related formins (DRFs), which act as effectors of Rho family guanosine triphosphatases (GTPases) [22,23] and play key roles in a growing range of cellular processes, such as filopodium formation, cell migration, cytokinesis, cell adhesion, cell polarity, and tumor development [24]. FMNL2 has been shown to be upregulated in CRC tissue [18], a positive regulator of cell motility and metastasis in CRC cells in vitro [25], and to enhance CRC invasion by inducing epithelial-mesenchymal transition [26]. Importantly, several miRNAs, including miR-137 [27], miR-34a [28], miR-206 [29], and miR-145 [30], had been reported to be involved in FMNL2 regulation in CRC cells in vitro. Here, we further confirmed that FMNL2 is a direct target of miR-613 in CRC through dual-luciferase assay, real-time qPCR, and western analysis. We also demonstrated that FMNL2 mRNA was upregulated in CRC tissues and inversely correlated with miR-613 expression in CRC tissues. Overexpression of FMNL2 significantly reversed the tumor-suppressive effects of miR-613 on CRC proliferation, migration, and invasion. These findings suggest that miR-613 exerts tumor-suppressive effects in CRC, at least in part, by targeting FMNL2.
In conclusion, our findings suggest that miR-613 might serve as a novel biomarker of CRC and function as a tumor suppressor in CRC cells by targeting FMNL2. Further, miR-613 and FMNL2 are potential targets for the development of novel therapeutic agents to treat and prevent CRC and CRC metastasis.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Harris TJ, McCormick F. The molecular pathology of cancer. Nat Rev Clin Oncol. 2010;7:251–265. doi: 10.1038/nrclinonc.2010.41. [DOI] [PubMed] [Google Scholar]
- 3.Migliore L, Migheli F, Spisni R, Coppede F. Genetics, cytogenetics, and epigenetics of colorectal cancer. J Biomed Biotechnol. 2011;2011:792362. doi: 10.1155/2011/792362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valinezhad Orang A, Safaralizadeh R, Kazemzadeh-Bavili M. Mechanisms of miRNA-mediated gene regulation from common downregulation to mRNA-specific upregulation. Int J Genomics. 2014;2014:970607. doi: 10.1155/2014/970607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 6.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94:776–780. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 8.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong Y, Yu J, Ng SS. MicroRNA dysregulation as a prognostic biomarker in colorectal cancer. Cancer Manag Res. 2014;6:405–422. doi: 10.2147/CMAR.S35164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou JJ, Zheng S, Sun LF, Zheng L. MicroRNA regulation network in colorectal cancer metastasis. World J Biol Chem. 2014;5:301–307. doi: 10.4331/wjbc.v5.i3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Zhang H, Wang L, Zhang S, Tang M. miR-613 inhibits the growth and invasiveness of human hepatocellular carcinoma via targeting DCLK1. Biochem Biophys Res Commun. 2016;473:987–992. doi: 10.1016/j.bbrc.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Zhong D, Zhang Y, Zeng YJ, Gao M, Wu GZ, Hu CJ, Huang G, He FT. MicroRNA-613 represses lipogenesis in HepG2 cells by downregulating LXRalpha. Lipids Health Dis. 2013;12:32. doi: 10.1186/1476-511X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Zhang H. Diminished miR-613 expression as a novel prognostic biomarker for human ovarian cancer. Eur Rev Med Pharmacol Sci. 2016;20:837–841. [PubMed] [Google Scholar]
- 14.Li D, Li DQ, Liu D, Tang XJ. MiR-613 induces cell cycle arrest by targeting CDK4 in non-small cell lung cancer. Cell Oncol (Dordr) 2016;39:139–147. doi: 10.1007/s13402-015-0262-4. [DOI] [PubMed] [Google Scholar]
- 15.Guan S, Wang C, Chen X, Liu B, Tan B, Liu F, Wang D, Han L, Wang L, Huang X, Wang J, Yao B, Shi J, Chen P, Nesa EU, Song Q, Cheng Y. MiR-613: a novel diagnostic and prognostic biomarker for patients with esophageal squamous cell carcinoma. Tumour Biol. 2016;37:4383–4391. doi: 10.1007/s13277-015-4271-8. [DOI] [PubMed] [Google Scholar]
- 16.Qiu W, Yang Z, Fan Y, Zheng Q. MicroRNA-613 inhibits cell growth, migration and invasion of papillary thyroid carcinoma by regulating SphK2. Oncotarget. 2016;7:39907–39915. doi: 10.18632/oncotarget.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren W, Li C, Duan W, Du S, Yang F, Zhou J, Xing J. MicroRNA-613 represses prostate cancer cell proliferation and invasion through targeting Frizzled7. Biochem Biophys Res Commun. 2016;469:633–638. doi: 10.1016/j.bbrc.2015.12.054. [DOI] [PubMed] [Google Scholar]
- 18.Zhu XL, Liang L, Ding YQ. Overexpression of FMNL2 is closely related to metastasis of colorectal cancer. Int J Colorectal Dis. 2008;23:1041–1047. doi: 10.1007/s00384-008-0520-2. [DOI] [PubMed] [Google Scholar]
- 19.Li B, Xie Z, Li B. miR-152 functions as a tumor suppressor in colorectal cancer by targeting PIK3R3. Tumour Biol. 2016;37:10075–84. doi: 10.1007/s13277-016-4888-2. [DOI] [PubMed] [Google Scholar]
- 20.Han J, Liu Z, Wang N, Pan W. MicroRNA-874 inhibits growth, induces apoptosis and reverses chemoresistance in colorectal cancer by targeting X-linked inhibitor of apoptosis protein. Oncol Rep. 2016;36:542–550. doi: 10.3892/or.2016.4810. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Shi B, Huang K, Fan G. MicroRNA-128 suppresses cell growth and metastasis in colorectal carcinoma by targeting IRS1. Oncol Rep. 2015;34:2797–2805. doi: 10.3892/or.2015.4251. [DOI] [PubMed] [Google Scholar]
- 22.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 23.Higgs HN. Formin proteins: a domain-based approach. Trends Biochem Sci. 2005;30:342–353. doi: 10.1016/j.tibs.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Eisenmann KM, Harris ES, Kitchen SM, Holman HA, Higgs HN, Alberts AS. Dia-interacting protein modulates formin-mediated actin assembly at the cell cortex. Curr Biol. 2007;17:579–591. doi: 10.1016/j.cub.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Zhu XL, Zeng YF, Guan J, Li YF, Deng YJ, Bian XW, Ding YQ, Liang L. FMNL2 is a positive regulator of cell motility and metastasis in colorectal carcinoma. J Pathol. 2011;224:377–388. doi: 10.1002/path.2871. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Zhu X, Zeng Y, Wang J, Zhang X, Ding YQ, Liang L. FMNL2 enhances invasion of colorectal carcinoma by inducing epithelial-mesenchymal transition. Mol Cancer Res. 2010;8:1579–1590. doi: 10.1158/1541-7786.MCR-10-0081. [DOI] [PubMed] [Google Scholar]
- 27.Liang L, Li X, Zhang X, Lv Z, He G, Zhao W, Ren X, Li Y, Bian X, Liao W, Liu W, Yang G, Ding Y. MicroRNA-137, an HMGA1 target, suppresses colorectal cancer cell invasion and metastasis in mice by directly targeting FMNL2. Gastroenterology. 2013;144:624–635. e624. doi: 10.1053/j.gastro.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 28.Lu G, Sun Y, An S, Xin S, Ren X, Zhang D, Wu P, Liao W, Ding Y, Liang L. MicroRNA-34a targets FMNL2 and E2F5 and suppresses the progression of colorectal cancer. Exp Mol Pathol. 2015;99:173–179. doi: 10.1016/j.yexmp.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Ren XL, He GY, Li XM, Men H, Yi LZ, Lu GF, Xin SN, Wu PX, Li YL, Liao WT, Ding YQ, Liang L. MicroRNA-206 functions as a tumor suppressor in colorectal cancer by targeting FMNL2. J Cancer Res Clin Oncol. 2016;142:581–592. doi: 10.1007/s00432-015-2053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie H, Ren X, Xin S, Lan X, Lu G, Lin Y, Yang S, Zeng Z, Liao W, Ding YQ, Liang L. Emerging roles of circRNA_001569 targeting miR-145 in the proliferation and invasion of colorectal cancer. Oncotarget. 2016;7:26680–91. doi: 10.18632/oncotarget.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]