Abstract
Botulinum toxin (BoNT) can relieve muscle spasticity by blocking axon terminals acetylcholine release at the motor endplate (MEP) and is the safest and most effective agent for the treatment of muscle spasticity in children with cerebral palsy. In order to achieve maximum effect with minimum effective dose of BoNT, one needs to choose an injection site as near to the MEP zone as possible. This requires a detailed understanding about the nerve terminal distributions within the muscles targeted for BoNT injection. This study focuses on BoNT treatment in children with muscle spasms caused by cerebral palsy. Considering the differences between children and adults in anatomy, we used child cadavers and measured both the nerve entry points and nerve terminal sense zones in three deep muscles of the anterior forearm: flexor digitorum profundus (FDP), flexor pollicis longus (FPL), and pronator quadratus (PQ). We measured the nerve entry points by using the forearm midline as a reference and demonstrated intramuscular nerve terminal dense zones by using a modified Sihler’s nerve staining technique. The locations of the nerve entry points and that of the nerve terminal dense zones in the muscles were compared. We found that all nerve entry points are away from the corresponding intramuscular nerve terminal dense zones. Simply selecting nerve entry points as the sites for BoNT injection may not be an optimal choice for best effects in blocking muscle spasm. We propose that the location of the nerve terminal dense zones in each individual muscle should be used as the optimal target sites for BoNT injection when treating muscle spasms in children with cerebral palsy.
Keywords: Anterior forearm muscles, intramuscular nerve, botulinum toxin injection, cerebral palsy, muscle spasms
Introduction
Cerebral palsy is a non-progressive clinical syndrome of multiple movement dysfunctions caused by damage to the immature brain motor areas [1]. Its incidence in live births is 2.1/‰ [2]. Muscular spasticity can be caused by cerebral palsy and it affects daily living tremendously. Anti-spasm therapy is an important factor during rehabilitation of cerebral palsy. Clinically, a variety of regimens have been used to relieve muscle spasticity in children with cerebral palsy and to improve the motor function of their extremities. Botulinum toxin (BoNT) can relieve muscle spasticity by blocking acetylcholine release from the axon terminals at the motor endplate (MEP) [3,4] and is the safest and most effective agent for the treatment of muscle spasticity in children [5,6].
Forearm muscle spasticity in children with cerebral palsy can cause hand deformity and seriously affect motor function of the upper extremity. Intramuscular injection of BoNT in the anterior compartment of the forearm can reduce spasticity and improve the basic functional movements [7-9]. The anti-muscle spasm effect of BoNT is dose-dependent and BoNT is very expensive [10]. Therefore, it is beneficial to patients by using the minimum effective dose at an injection site as near to the MEP zone as possible [11-14]. Thus, determining the accurate location of the MEP zones in forearm muscles is a prerequisite for a better outcome of BoNT injection. However, the anatomical locations of the MEP zone in most spastic muscles, including the forearm muscles, remain unknown [15].
The MEP zone is a junction site that connects the motor neuron terminals with skeletal muscle fibers. Sihler’s nerve stain is a whole-mount nerve staining technique that is used to visualize intramuscular nerve distribution [16]. This method allows a clear three dimensional visualization of the distribution pattern of terminal nerve branches in skeletal muscles [17]. Sihler’s stain can be used to locate the dense zone (i.e., MEP zone) of intramuscular nerve terminals and is the most important breakthrough in recent years to identify the target sites for BoNT injection [13,14,18,19]. In a study utilizing Sihler’s nerve staining technique on the intramuscular nerve distribution patterns of deep muscles in the anterior compartment of the forearm [19-21], Peker et al. observed the intramuscular nerve distribution pattern of flexor pollicis longus (FPL) but did not provide detailed information about how to determine the BoNT injection site on the body surface [21]. Won et al. utilized this technique in combination with body surface landmarks to locate the dense zone of intramuscular nerve terminals in flexor digitorum profundus (FDP) [19]. However, their studies were conducted in adult cadavers. Sihler’s stain has not been used to locate the dense zone of intramuscular nerve terminals of anterior forearm deep muscles in children.
In this study, a modified Sihler’s intramuscular nerve staining technique was used to investigate the intramuscular nerve distribution pattern of deep muscles in the anterior forearm of child cadavers. The location of the dense zones of intramuscular nerve terminals in reference to body surface bony landmarks is determined. This study provides a guideline for selecting the BoNT injection site in children with muscle spasticity caused by cerebral palsy.
Materials and methods
Localization of nerve entry points of the deep muscles of the anterior forearm
This study was approved by Zunyi Medical University, Human Subject Study Committee. Twenty two formalin-fixed child cadavers (10 males and 12 females; age 2-5 years; mean age: 3.7 years) were used in this study. No evidence of injuries or neuromuscular diseases were noticed in any forearms. All cadavers were placed in the supine position with arms abducted and forearms supinated. After removing the skin and superficial fascia, the specimens were dissected to expose the FDP, FPL or pronator quadratus (PQ) and the nerves going into them. On the epimysium, the sites where the anterior interosseous nerve and ulnar nerve branches enter the muscles were identified as the nerve entry points. The forearm midline was defined as a line drawing connecting the midpoint of the intercondylar line (the line connecting the medial epicondyle and lateral epicondyle of the humerus) and the midpoint of the interstyloid line (the line connecting the styloid process of the radius and that of the ulna). A vernier caliper was used to measure the forearm midline length. This line was measured in each sample and used as a reference for the locations of nerve entry points and for the muscle bellies in FDP, FPL and PQ. The latter were determined by measuring the distances between the muscle bellies and the forearm midline and then expressed as a percentage of the length of the forearm midline.
Intramuscular nerve distribution patterns and localization of nerve terminal-dense zone of the FDP, FPL and PQ
The FDP, FPL or PQ was excised en bloc and marked; fascia and adipose tissue on the muscular surface were removed, extra-muscular nerve trunks were preserved. The modified Sihler’s intramuscular nerve staining method was used to process these muscles, including depigmentation, decalcification, staining, destaining, neutralization and clearing, until the stained muscle samples became transparent and intramuscular nerves became visible with blue and purple color. The whole process took 4 months. Upon completion, the specimens were returned to their original anatomical locations of the same cadaver and the distributions of intramuscular nerve branches were measured and documented. The length of the muscle belly, as well as the distance between intramuscular nerve terminal-dense areas and each end of the muscle belly were individually measured using a vernier caliper. The locations of intramuscular nerve terminal-dense areas were recorded as a percentage of the length of the muscle belly. After the specimens were photographed and diagrammed, the locations of intramuscular nerve terminal-dense zones were recorded as a percentage of the length of the forearm midline. The lengths of each stained muscle belly were normalized to the lengths of its original length measured before processing for staining.
All of the experimental data were analyzed using SPSS17.0 statistical software. The α level was set at 0.05. The Wilcoxon signed-rank test was used to assess differences between both sides.
Results
Localization of nerve entry points of the FDP, FPL and PQ
The FDP has two nerve entry points: 1) The ulnar nerve runs between the flexor carpi ulnaris and the FDP and gives off 1 or 2 branches entering the muscle from the anterior surface on the ulnar side; 2) The median nerve passes through the pronator teres, enters the deep surface of the flexor digitorum superficialis and gives off the anterior interosseous nerve, which in turn gives off 3 or 5 branches entering the FDP from the anterior surface of the muscle belly at the proximal end on the radial side. The anterior interosseous nerve also gives off 2 or 3 branches, which enter the FPL from the medial (ulnar) aspect at proximal end. The final branch of the anterior interosseous nerve travels between the FDP and the FPL and enters the PQ at the middle point along the upper-edge. The locations of these nerve entry points are summarized in Table 1.
Table 1.
The percentage of the distances from the nerve entry points to the intercondylar line relative to the length of the forearm midline
| Nerve Entry Points | MIN (%) | MAX (%) | Percentage (%) |
|---|---|---|---|
| FDP (AIONB) | 21.05 | 38.8 | 30.55±5.14 |
| FDP (UNB) | 12.16 | 24.03 | 18.19±2.94 |
| FPL | 28.60 | 45.72 | 36.40±4.78 |
| PQ | 70.62 | 82.34 | 74.91±3.29 |
N=44 Forearm; AIONB, anterior interosseous nerve branches; UNB, ulnar nerve branches; MIN, minimum value; MAX, maximum value.
The length of the forearm midline is 12.28±1.61 cm. The proximal ends and distal ends of FDP, FPL, and PQ are at 6.57-91.59%, 32.76-100% and 73.81-100%, respectively, in reference to the forearm midline measured from the intercondylar line.
Intramuscular nerve distribution patterns and localization of nerve terminal-dense zones in the FDP
The FDP receives double innervation from the ulnar nerve and the anterior interosseous nerve. The anterior interosseous nerve gives off 3 or 5 main branches, which enter the FDP on the radial aspect from the superficial surface of its proximal belly. After entering the muscle, these branches run along the second and third digital tendons, from the superficial layer to the deep layer and from proximal to distal. In addition, the branch that innervates the third muscle belly may supply the lower muscle belly of the ring finger. The ulnar nerve gives off 1 or 2 main branches, which enter the FDP on the ulnar aspect from the superficial surface of its proximal belly. After entering the muscle, these branches supply the fourth and fifth digital muscle bellies from the proximal end to the distal end; in some cases, they may supply the proximal end of the third muscle belly. A large Y-shaped nerve pattern at the proximal end and many small Y-shaped nerve patterns at the distal end were observed between the branch of the anterior interosseous nerve that supplies the third digital muscle belly and the branch of the ulnar nerve that supplies the fourth digital muscle belly (Figure 1).
Figure 1.
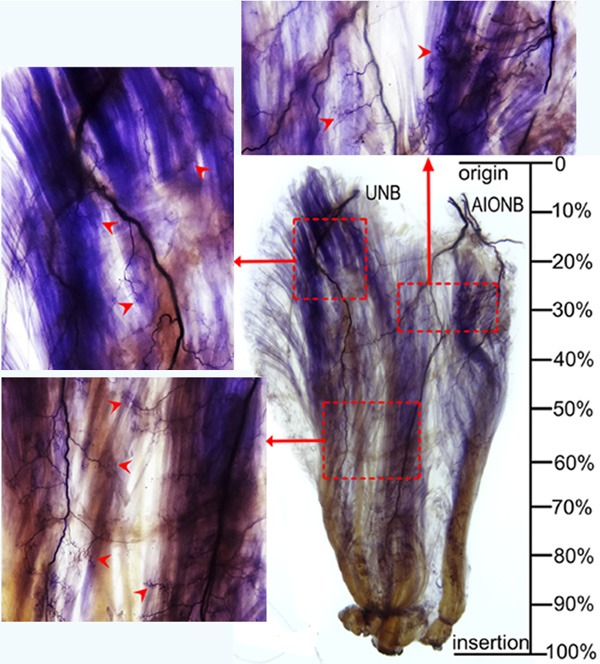
Intramuscular nerve distribution in the FDP (right, deep side), the red dotted box represents the dense zone, and arrows indicate nerve terminal branches (AIONB, anterior interosseous nerve branches; UNB, ulnar nerve branches).
The distribution range of intramuscular nerve branches in the FDP is at 9.02% to 87.06% of the length of the forearm midline. The dense intramuscular nerve terminals present a tree-like shape. There appear to have three dense intramuscular nerve terminal zones. The first zone is located at the upper and radial aspect of the FDP and is formed by the distribution of the anterior interosseous nerve at the upper part of the second and third digital muscle bellies. The second zone is located at the upper and ulnar aspect of the FDP and is formed by the distribution of the ulnar nerve at the upper part of the fourth and fifth digital muscle bellies. The third zone is located at the upper-lower belly of the FDP and is formed by the distribution of the anterior interosseous nerve and ulnar nerve at the third and fourth digital muscle bellies (Table 2 and Figure 2).
Table 2.
The percentage of the distances from the nerve terminal-dense zones to the proximal end of muscle belly (N1) and the nerve terminal-dense zones to the intercondylar line relative to the length of the forearm midline (N2), (mean ± SD)
| Muscle | Nerve terminal-dense zones | N1 | N2 | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| MIN (%) | MAX (%) | Percentage (%) | MIN (%) | MAX (%) | Percentage (%) | ||
| FDP | Upper radial aspect | 25.82 | 34.12 | 29.85±2.62 | 36.94 | 43.99 | 40.36±2.23 |
| Upper ulnar aspect | 12.27 | 27.68 | 21.37±4.43 | 25.41 | 38.41 | 33.14±3.77 | |
| Lower middle muscle belly | 48.25 | 63.81 | 57.74±4.40 | 56.00 | 69.23 | 63.54±3.66 | |
| FPL | Middle muscle belly | 35.79 | 55.66 | 44.73±5.57 | 56.83 | 70.19 | 62.84±3.74 |
| PQ | Middle ulnar aspect | 26.86 | 62.24 | 45.17±11.48 | 81.65 | 90.84 | 84.24±3.01 |
N=44 Forearm; MIN, minimum value; MAX, maximum value.
Figure 2.
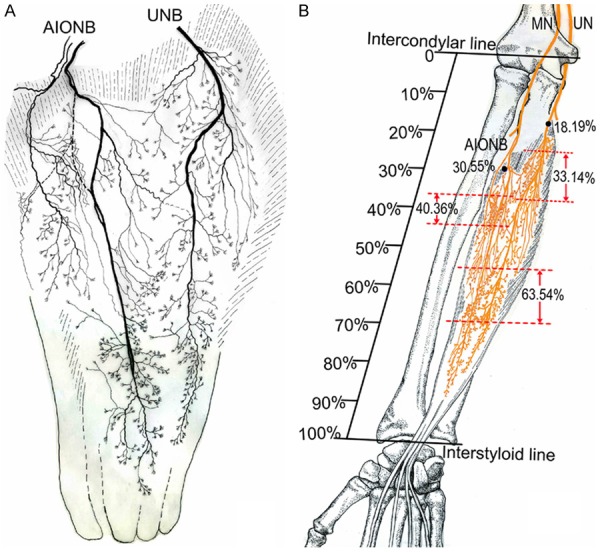
Intramuscular nerve pattern in FDP (right, deep side). A: Drawing demonstrating the FDP is supplied by the AIONB and UNB. B: The dashed line represents the percentage of the dense zone of intramuscular nerves of the FDP relative to the forearm length. The black dots represent the percentage of the nerve entry point of the FDP relative to the forearm length (AIONB, anterior interosseous nerve branches; UNB, ulnar nerve branches; UN, ulnar nerve; MN, median nerve).
Intramuscular nerve distribution patterns and localization of nerve terminal-dense zones in the FPL
The FPL is supplied by branches of the anterior interosseous nerve. The anterior interosseous nerve gives off 3 first-generation branches, which enter the FPL from the ulnar aspect of its proximal end and are distributed in a 3-dimensional manner. There are 2 main forms of intramuscular nerve distribution patterns: 1) One of the first-generation nerve branches, relatively smaller in diameter, mainly supplies the proximal 1/3 of the muscle, and the other two, relatively thicker in diameter, descend along the superficial or deep surface of the muscle belly on either side of the tendon and supply the distal 2/3 of the muscle. 2) The size of the three first-generation branches are similar. Two of them descend along the superficial or deep surface of the muscle belly on either side of the tendon and supply the proximal 2/3 of the muscle; the other branch descends along the deep surface of the muscle belly on the tendon contralateral edge and supplies the distal 1/3 of the muscle. Many minor branches arise from the three first-generation branches, travel toward tendons of the muscle and supply the superficial or deep surface of muscle belly on both sides of the tendons (Figure 3). The distribution range of intramuscular nerve branches in the FPL is at 15.59% to 74.81% of the length of the forearm midline. Its dense zone of intramuscular nerve terminals is located at the middle of the muscle belly (Table 2 and Figure 4).
Figure 3.
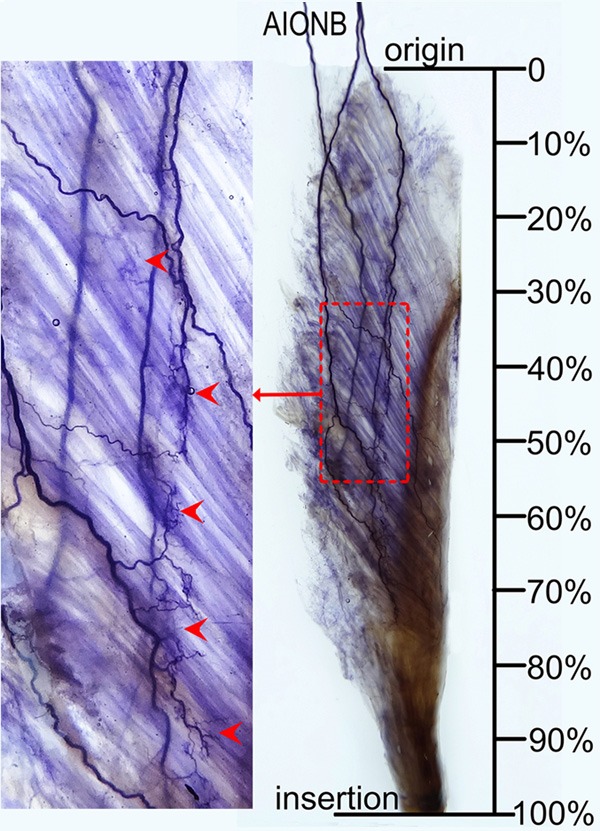
Intramuscular nerve distribution in the FPL (right, superficial side), the red dotted square represents the nerve terminal-dense zone, and arrows indicate nerve terminal branches (AIONB, anterior interosseous nerve branches).
Figure 4.
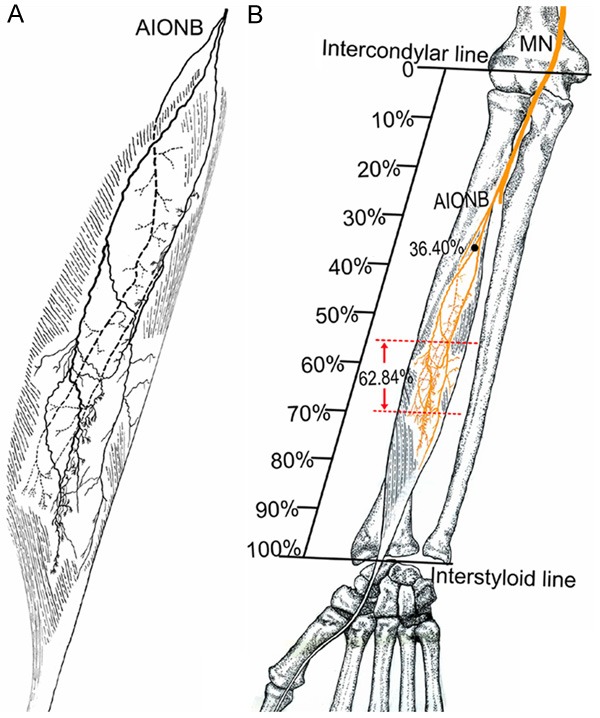
Intramuscular nerve pattern in the FPL (right, superficial side). A: Drawing demonstrating the FPL is supplied by the AIONB. B: The red dashed line represents the percentage of the dense zone of intramuscular nerves of the FPL relative to the forearm length; the dots represent the percentage of the nerve entry point of the FPL relative to the forearm length (AIONB, anterior interosseous nerve branches; MN, median nerve).
Intramuscular nerve distribution patterns and localization of nerve terminal-dense zones in the PQ
The PQ includes a superficial layer of muscle and a deep layer of muscle. The deep layer is located in the deep surface of the ulnar aspect of this muscle. The PQ is supplied by the final branch of the anterior interosseous nerve which enters this muscle from its upper edge and supplies the deep surface of the ulnar aspect of this muscle. Two main branches arise from the anterior interosseous nerve before or when it enters the muscle to supply the proximal or distal part of the muscle in a tree-branch-like distribution. One main branch supplies the proximal part and travels diagonally from the ulnar aspect to the radial aspect on the deep surface giving off 3 or 5 secondary branches that supply the superficial and deep muscular fibers of the proximal ulnar aspect and 2 or 4 branches that supply the superficial muscular fibers of the proximal radial aspect. The other main branch descends at the deep surface of the ulnar aspect to the distal part and gives off 1 to 3 secondary branches that supply the superficial and deep muscular fibers of the distal ulnar aspect. Then, this branch continues to pass through the deep muscular fibers diagonally from the ulnar to the radial aspect and gives off 3 to 5 branches that supply the superficial muscular fibers of the distal radial aspect. The 2 main branches that supply the superficial muscular fibers have communication sites at the middle of the radial aspect. Thus, the intramuscular nerve branches distribute as circular shape on the radial side. The dense zone of intramuscular nerve terminals is located at the center of the circle in the middle ulnar aspect of this muscle (Figure 5). The detailed location is listed in Table 2 and shown in Figure 6.
Figure 5.
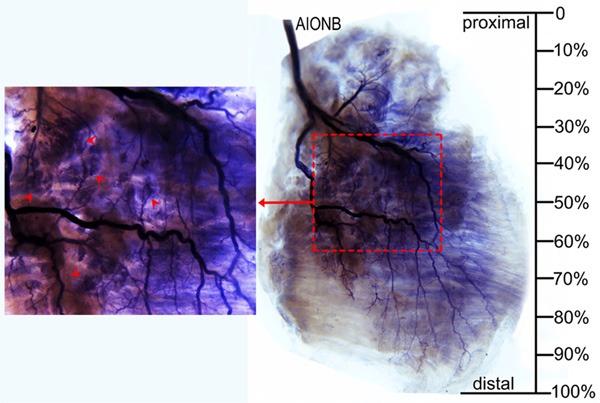
Intramuscular nerve distribution in the PQ (right, deep side), the red dotted square represents the nerve terminal-dense zone, and arrows indicate nerve terminal branches (AIONB, anterior interosseous nerve branches).
Figure 6.
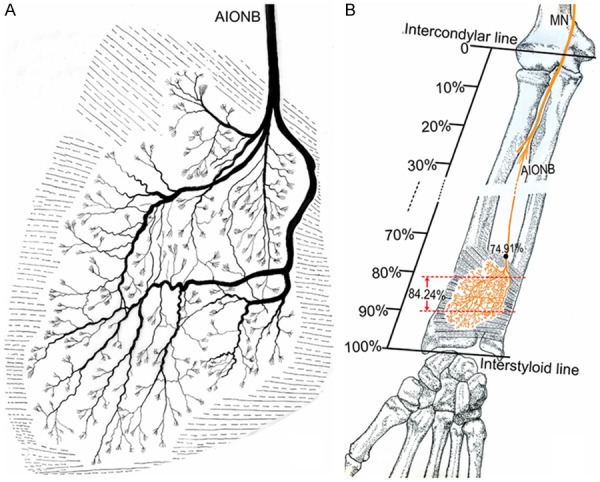
Intramuscular nerve pattern in the PQ (right, deep side). A: Drawing demonstrating the PQ is supplied by the AIONB. B: The red dashed line represents the percentage of the dense zone of intramuscular nerves of the PQ relative to the forearm length; the dots represent the percentage of the nerve entry point of the PQ relative to the forearm length (AIONB, anterior interosseous nerve branches; MN, median nerve).
Discussion
Forearm muscle spasticity in children with cerebral palsy can result in extremity deformations such as forearm pronation and wrist or finger flexion, which affect upper extremity function [19]. BoNT injection into anterior compartment muscles of the forearm can release spasticity and improve upper extremity function [7-9]. However, it has always been a challenge to select the appropriate BoNT injection site for the target muscle. In recent years, although the application of electromyograms, electrical stimulation, and ultrasound-guided positioning techniques were used to determine an injection site, fine tuning of injection techniques are still needed. Kinnett conducted a retrospective study on BoNT injection in children and addressed the limitations of the current localization techniques in accurately determining the injection site on the involved muscle because children have smaller muscles and belong to a special, uncooperative patient group. Therefore, a detailed forearm muscle anatomy is needed to help determining the safest and most effective injection site of BoNT in children [4].
Instead of using the MEP zone, some researchers have considered the “movement points” (motor nerve entry points) of the forearm muscles [11,22,23] as the BoNT injection site for children due to the shortage of fresh human specimens that can be used to determine the MEP zone [24]. However, the movement points are not the actual MEP zones [13-15]. In this study, both the movement points (nerve entry points) and the dense zones (i.e., MEP zones) of intramuscular nerve terminals were investigated. Our results have shown that the location of the MEP zone in each muscle is not consistent with the location of the corresponding movement point. The movement point is not the MEP zone because there is a certain distance between them. The movement point and the MEP zone in the FPL are at 36.40% and 62.84% respectively of the length of the forearm, and the distance between them is the longest recorded in this study. The movement point and the MEP zone in the PQ are at 74.91% and 84.24% respectively of the length of the forearm, and the distance between them is the shortest. This result indicates that the distance between the movement point and the MEP zone varies between muscles. In muscles with a shorter distance between the movement point and the MEP zone, BoNT injection into the movement point may have a certain blocking effect because of the diffusion of the medication [4]. This explains why BoNT injection into the movement point may also have a therapeutic effect. However, this observation does not support the idea that the movement point is the best target site for BoNT injection because the BoNT dose must be higher to allow effective diffusion to the MEP zone in the muscles with a longer distance between the injection site and the MEP zone. The increased dose will cause unwanted side effects and increased financial burden on patients. The nerve entry points on the FDP in this study and previous studies are compared in Table 3 [11,19,23,25]. Our results are consistent with the study by Bhadra but not with other studies done on adult human cadavers. Considering the similarly proportioned distributions of body landmarks between children and adults, the inconsistency between the studies may be due to different measuring methods or individual techniques.
Table 3.
Mean positions of the nerve entry points of FDP from the intercondylar line
To find the optimal BoNT injection sites, some researchers, using microdissection, found that the dense zones of the MEP were distributed between the proximal and distal ends of intramuscular nerve terminals [11,22]. However, there were certain limitations to these studies because it was almost impossible to visualize the neuromuscular junction in dissection. Therefore, these results cannot accurately locate the MEP zones [26], rather, only provide anatomical data that help to locate BoNT injection sites close to MEP zones. Sihler’s intramuscular nerve staining technique clearly demonstrated the distribution pattern of the intramuscular nerve terminals in 3 dimensions and then can determine the MEP location without damaging muscle fibers. This technique has been confirmed in studies by Amirali et al. [15]. Using a microdissection technique, Lepage et al. measured the distances from the nerve terminal-dense zones (starting from the proximal ends and distal ends respectively) in FDP, FPL and PQ to the medial epicondyle of the humerus [11]. We used the same measuring method but our results show that the ranges of these distances are narrower compared with Lepage’s results, indicating that the MEP locations determined by microdissection are somewhat different from that obtained by Sihler’s nerve staining technique. The possible reasons may be that the smaller intramuscular nerve terminals visualized by our staining method cannot be visualized in microdissection.
The FDP includes 3 intramuscular nerve terminal dense zones but 4 muscle bellies. This means one of the muscle bellies has to have dual nerve innervations. When carrying out BoNT injection in this muscle, selective blockage of certain muscle bellies can prevent the weakening of the entire muscular strength for finger flexion. Won et al. also utilized the Sihler’s nerve staining technique and body surface landmarks to locate the intramuscular nerve terminal dense zones in FDP from adult human cadavers [19]. They reported that 62.5% communication sites between the median nerve and ulnar nerve in FDP were located at the distal muscle belly and were 61.6% of the length of the forearm midline away from the intercondylar line of the humerus [20]. In our study, the nerve terminal dense zones in the middle-lower muscle belly are 63.54% of the length of the forearm midline from the intercondylar line of the humerus. The dense zone locations were very similar between the two studies. These results indicate that a better anti-spasm effect can be achieved if BoNT is injected at the nerve terminal dense zones in the middle-lower muscle belly of FDP when treating episodes of spasticity in children with cereal palsy.
For FPL, our study found that the intramuscular nerve dense zones were located in the middle muscle belly. This result is consistent with Peker’s findings [21], indicating the middle muscle belly in the FPL is the target site of BoNT injection. However, Peker et al. did not provide detailed information about surface landmarks for BoNT injection.
The intramuscular nerve terminal dense zones in PQ have not been reported in previous studies. Our results show that the nerve terminal dense zones in PQ are located on the ulnar aspect of its middle part. This distribution may be related to its 2 layered structure (superficial and deep layers). Because the deep layer muscle fibers are mainly located at the deep surface of the ulnar aspect, the BoNT injection at the middle ulnar aspect can achieve better effect in PQ.
In summary, when BoNT injection is used to relieve spasticity of FDP, FPL or PQ in children with cereal palsy, the optimal target sites for injection are at the lower-middle part of the FDP, the middle part of the FPL and the middle ulnar aspect of the PQ. This should help to achieve the best BoNT efficacy, to prevent side effects, and to reduce the financial burden of patients.
Acknowledgements
We wish to Dr. Yu Wang, Department of Drawing Office, Zunyi Medical University, who helped us to draft pictures. This work was supported by the National Natural Science Foundation (Grant No.31360256) of China.
Disclosure of conflict of interest
None.
Abbreviations
- BoNT
botulinum toxin
- FDP
flexor digitorum profundus
- FPL
flexor pollicis longus
- MEP
motor endplate
- PQ
pronator quadrates
References
- 1.Eunson P. The long-term health, social, and financial burden of hypoxic-ischaemic encephalopathy. Dev Med Child Neurol. 2015;57(Suppl 3):48–50. doi: 10.1111/dmcn.12727. [DOI] [PubMed] [Google Scholar]
- 2.Morgan C, Darrah J, Gordon AM, Harbourne R, Spittle A, Johnson R, Fetters L. Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Dev Med Child Neurol. 2016;58:900–9. doi: 10.1111/dmcn.13105. [DOI] [PubMed] [Google Scholar]
- 3.Delnooz CC, Veugen LC, Pasman JW, Lapatki BG, van Dijk JP, van de Warrenburg BP. The clinical utility of botulinum toxin injections targeted at the motor endplate zone in cervical dystonia. Eur J Neurol. 2014;21:1486–e1498. doi: 10.1111/ene.12517. [DOI] [PubMed] [Google Scholar]
- 4.Kinnett D. Botulinum toxin A injections in children: technique and dosing issues. Am J Phys Med Rehabil. 2004;83:S59–64. doi: 10.1097/01.phm.0000141131.66648.e9. [DOI] [PubMed] [Google Scholar]
- 5.Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Delgado MR, Hirtz D, Aisen M, Ashwal S, Fehlings DL, McLaughlin J, Morrison LA, Shrader MW, Tilton A, Vargus-Adams J. Practice parameter: pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2010;74:336–343. doi: 10.1212/WNL.0b013e3181cbcd2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copeland L, Edwards P, Thorley M, Donaghey S, Gascoigne-Pees L, Kentish M, Cert G, Lindsley J, McLennan K, Sakzewski L, Boyd RN. Botulinum toxin A for nonambulatory children with cerebral palsy: a double blind randomized controlled trial. J Pediatr. 2014;165:140–146. e144. doi: 10.1016/j.jpeds.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 7.Shaw LC, Price CI, van Wijck FM, Shackley P, Steen N, Barnes MP, Ford GA, Graham LA, Rodgers H, Bo TI. Botulinum Toxin for the Upper Limb after Stroke (BoTULS) Trial: effect on impairment, activity limitation, and pain. Stroke. 2011;42:1371–1379. doi: 10.1161/STROKEAHA.110.582197. [DOI] [PubMed] [Google Scholar]
- 8.Speth LA, Leffers P, Janssen-Potten YJ, Vles JS. Botulinum toxin A and upper limb functional skills in hemiparetic cerebral palsy: a randomized trial in children receiving intensive therapy. Dev Med Child Neurol. 2005;47:468–473. doi: 10.1017/s0012162205000903. [DOI] [PubMed] [Google Scholar]
- 9.Lowe K, Novak I, Cusick A. Low-dose/high-concentration localized botulinum toxin A improves upper limb movement and function in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 2006;48:170–175. doi: 10.1017/S0012162206000387. [DOI] [PubMed] [Google Scholar]
- 10.Hexsel D, Hexsel C, Siega C, Schilling-Souza J, Rotta FT, Rodrigues TC. Fields of effects of 2 commercial preparations of botulinum toxin type A at equal labeled unit doses: a double-blind randomized trial. JAMA Dermatol. 2013;149:1386–1391. doi: 10.1001/jamadermatol.2013.6440. [DOI] [PubMed] [Google Scholar]
- 11.Lepage D, Parratte B, Tatu L, Vuiller F, Monnier G. Extra- and intramuscular nerve supply of the muscles of the anterior antebrachial compartment: applications for selective neurotomy and for botulinum toxin injection. Surg Radiol Anat. 2005;27:420–430. doi: 10.1007/s00276-005-0012-9. [DOI] [PubMed] [Google Scholar]
- 12.Van Campenhout A, Bar-On L, Desloovere K, Huenaerts C, Molenaers G. Motor endplate-targeted botulinum toxin injections of the gracilis muscle in children with cerebral palsy. Dev Med Child Neurol. 2015;57:476–483. doi: 10.1111/dmcn.12667. [DOI] [PubMed] [Google Scholar]
- 13.Won SY, Rha DW, Kim HS, Jung SH, Park ES, Hu KS, Kim HJ. Intramuscular nerve distribution pattern of the adductor longus and gracilis muscles demonstrated with Sihler staining: guidance for botulinum toxin injection. Muscle Nerve. 2012;46:80–85. doi: 10.1002/mus.23273. [DOI] [PubMed] [Google Scholar]
- 14.Yi KH, Rha DW, Lee SC, Cong L, Lee HJ, Lee YW, Kim HJ, Hu KS. Intramuscular nerve distribution pattern of ankle invertor muscles in human cadaver using sihler stain. Muscle Nerve. 2016;53:742–747. doi: 10.1002/mus.24939. [DOI] [PubMed] [Google Scholar]
- 15.Amirali A, Mu L, Gracies JM, Simpson DM. Anatomical localization of motor endplate bands in the human biceps brachii. J Clin Neuromuscul Dis. 2007;9:306–312. doi: 10.1097/CND.0b013e31815c13a7. [DOI] [PubMed] [Google Scholar]
- 16.Xie P, Jiang Y, Zhang X, Yang S. The study of intramuscular nerve distribution patterns and relative spindle abundance of the thenar and hypothenar muscles in human hand. PLoS One. 2012;7:e51538. doi: 10.1371/journal.pone.0051538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie P, Qin B, Yang F, Yu T, Yu J, Wang J, Zheng H. Lidocaine Injection in the Intramuscular Innervation Zone Can Effectively Treat Chronic Neck Pain Caused by MTrPs in the Trapezius Muscle. Pain Physician. 2015;18:E815–826. [PubMed] [Google Scholar]
- 18.Mu L, Sanders I. Sihler’s whole mount nerve staining technique: a review. Biotech Histochem. 2010;85:19–42. doi: 10.1080/10520290903048384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Won SY, Hur MS, Rha DW, Park HD, Hu KS, Fontaine C, Kim HJ. Extra- and intramuscular nerve distribution patterns of the muscles of the ventral compartment of the forearm. Am J Phys Med Rehabil. 2010;89:644–652. doi: 10.1097/PHM.0b013e3181d8a116. [DOI] [PubMed] [Google Scholar]
- 20.Won SY, Choi DY, Lee JG, Yoon KH, Kwak HH, Hu KS, Kim HJ. Intramuscular communicating branches in the flexor digitorum profundus: dissection and Sihler’s staining. Surg Radiol Anat. 2010;32:285–289. doi: 10.1007/s00276-010-0634-4. [DOI] [PubMed] [Google Scholar]
- 21.Peker T, Gulekon N, Turgut BH, Anil A, Karakose M, Mungan T, Danisman N. Observation of the relationship between the shape of skeletal muscles and their nerve distribution patterns: a transparent and microanatomic study. Plast Reconstr Surg. 2006;117:165–176. doi: 10.1097/01.prs.0000186539.80555.27. [DOI] [PubMed] [Google Scholar]
- 22.Hwang K, Jin S, Hwang SH, Lee KM, Han SH. Location of nerve entry points of flexor digitorum profundus. Surg Radiol Anat. 2007;29:617–621. doi: 10.1007/s00276-007-0260-y. [DOI] [PubMed] [Google Scholar]
- 23.Roberts C, Crystal R, Eastwood DM. Optimal injection points for the neuromuscular blockade of forearm flexor muscles: a cadaveric study. J Pediatr Orthop B. 2006;15:351–355. doi: 10.1097/01202412-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Song DH, Chung ME, Han ZA, Kim SY, Park HK, Seo YJ. Anatomic localization of motor points of wrist flexors. Am J Phys Med Rehabil. 2014;93:282–286. doi: 10.1097/PHM.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 25.Bhadra N, Keith MW, Peckham PH. Variations in innervation of the flexor digitorum profundus muscle. J Hand Surg Am. 1999;24:700–703. doi: 10.1053/jhsu.1999.0700. [DOI] [PubMed] [Google Scholar]
- 26.Moon JY, Hwang TS, Sim SJ, Chun SI, Kim M. Surface mapping of motor points in biceps brachii muscle. Ann Rehabil Med. 2012;36:187–196. doi: 10.5535/arm.2012.36.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]


