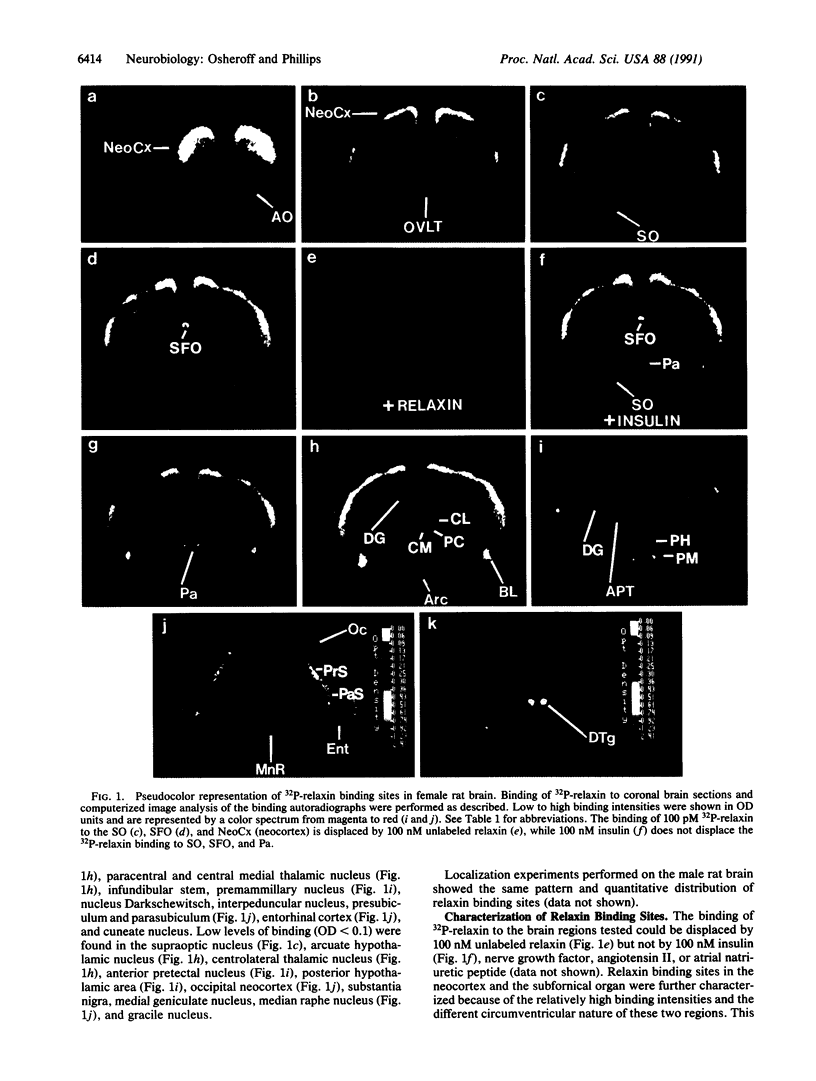
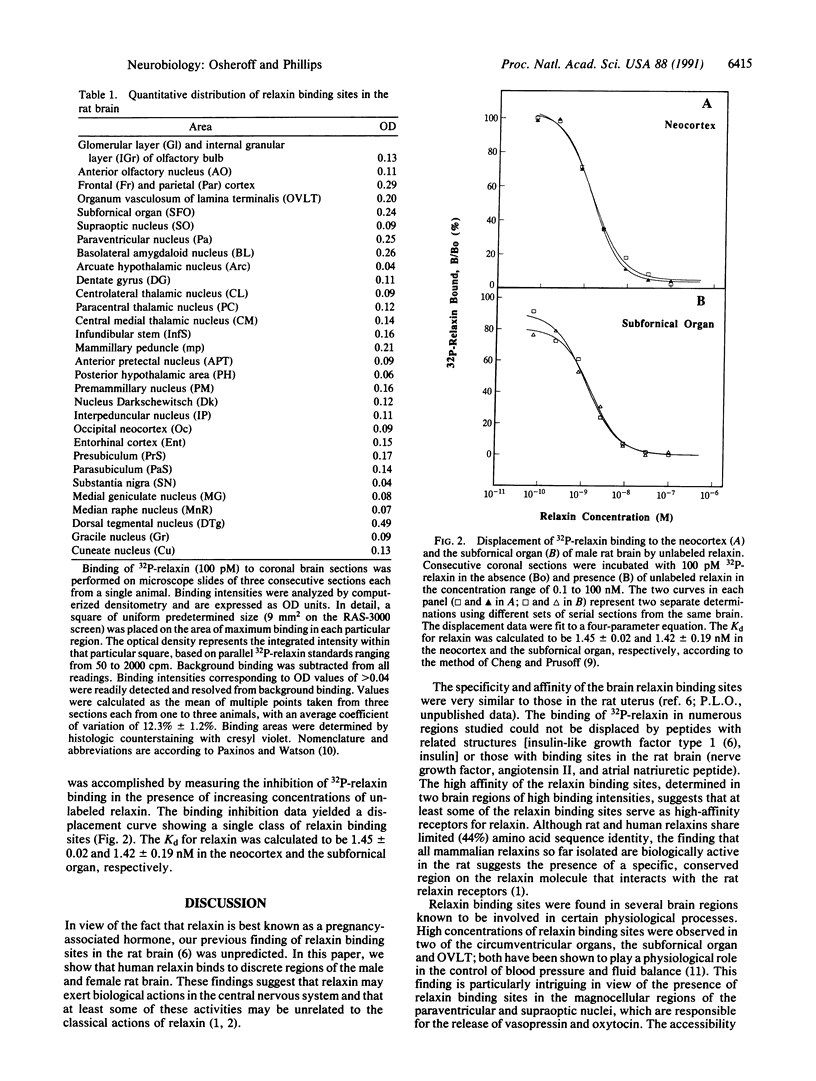
Abstract
Relaxin is a member of the insulin family of polypeptide hormones and exerts its best understood actions in the mammalian reproductive system. Using a biologically active 32P-labeled human relaxin, we have previously shown by in vitro autoradiography specific relaxin binding sites in rat uterus, cervix, and brain tissues. Using the same approach, we describe here a detailed localization of human relaxin binding sites in the rat brain. Displaceable relaxin binding sites are distributed in discrete regions of the olfactory system, neocortex, hypothalamus, hippocampus, thalamus, amygdala, midbrain, and medulla of the male and female rat brain. Characterization of the relaxin binding sites in the subfornical organ and neocortex reveals a single class of high-affinity sites (Kd = 1.4 nM) in both regions. The binding of relaxin to two of the circumventricular organs (subfornical organ and organum vasculosum of the lamina terminalis) and the neurosecretory magnocellular hypothalamic nuclei (i.e., paraventricular and supraoptic nuclei) provides the anatomical and biochemical basis for emerging physiological evidence suggesting a central role for relaxin in the control of blood pressure and hormone release. We conclude that specific, high-affinity relaxin binding sites are present in discrete regions of the rat brain and that the distribution of some of these sites may be consistent with a role for relaxin in control of vascular volume and blood pressure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahokas R. A., Sibai B. M., Anderson G. D. Lack of evidence of a vasodepressor role for relaxin in spontaneously hypertensive and normotensive pregnant rats. Am J Obstet Gynecol. 1989 Sep;161(3):618–622. doi: 10.1016/0002-9378(89)90365-7. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Durham D. A. Bidirectional transport of interleukin-1 alpha across the blood-brain barrier. Brain Res Bull. 1989 Dec;23(6):433–437. doi: 10.1016/0361-9230(89)90185-8. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J. Saturable transport of peptides across the blood-brain barrier. Life Sci. 1987 Sep 14;41(11):1319–1338. doi: 10.1016/0024-3205(87)90606-0. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Dayanithi G., Cazalis M., Nordmann J. J. Relaxin affects the release of oxytocin and vasopressin from the neurohypophysis. 1987 Feb 26-Mar 4Nature. 325(6107):813–816. doi: 10.1038/325813a0. [DOI] [PubMed] [Google Scholar]
- Essig M., Schoenfeld C., D'Eletto R. T., Amelar R., Steinetz B. G., O'Byrne E. M., Weiss G. Relaxin in human seminal plasma. Ann N Y Acad Sci. 1982;380:224–230. doi: 10.1111/j.1749-6632.1982.tb18045.x. [DOI] [PubMed] [Google Scholar]
- Fishman J. B., Rubin J. B., Handrahan J. V., Connor J. R., Fine R. E. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J Neurosci Res. 1987;18(2):299–304. doi: 10.1002/jnr.490180206. [DOI] [PubMed] [Google Scholar]
- Jones S. A., Summerlee A. J. Effects of chronic infusion of porcine relaxin on oxytocin release in lactating rats. J Endocrinol. 1987 Aug;114(2):241–246. doi: 10.1677/joe.0.1140241. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Niall H. D. Relaxin. Vitam Horm. 1984;41:79–115. doi: 10.1016/s0083-6729(08)60088-6. [DOI] [PubMed] [Google Scholar]
- Kramer S. M., Gibson U. E., Fendly B. M., Mohler M. A., Drolet D. W., Johnston P. D. Increase in cyclic AMP levels by relaxin in newborn rhesus monkey uterus cell culture. In Vitro Cell Dev Biol. 1990 Jun;26(6):647–656. doi: 10.1007/BF02624216. [DOI] [PubMed] [Google Scholar]
- Loumaye E., De Cooman S., Thomas K. Immunoreactive relaxin-like substance in human seminal plasma. J Clin Endocrinol Metab. 1980 Jun;50(6):1142–1143. doi: 10.1210/jcem-50-6-1142. [DOI] [PubMed] [Google Scholar]
- MILLER J. W., KISLEY A., MURRAY W. J. The effects of relaxin-containing ovarian extracts on various types of smooth muscle. J Pharmacol Exp Ther. 1957 Aug;120(4):426–437. [PubMed] [Google Scholar]
- Matsuo K., Niwa M., Kurihara M., Shigematsu K., Yamashita S., Ozaki M., Nagataki S. Receptor autoradiographic analysis of insulin-like growth factor-I (IGF-I) binding sites in rat forebrain and pituitary gland. Cell Mol Neurobiol. 1989 Sep;9(3):357–367. doi: 10.1007/BF00711415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn F. A., Quirion R., Saavedra J. M., Aguilera G., Catt K. J. Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1575–1579. doi: 10.1073/pnas.81.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado-Simmen R. C., Bryant-Greenwood G. D., Greenwood F. C. Characterization of the binding of 125I-relaxin to rat uterus. J Biol Chem. 1980 Apr 25;255(8):3617–3623. [PubMed] [Google Scholar]
- Mercado-Simmen R. C., Bryant-Greenwood G. D., Greenwood F. C. Relaxin receptor in the rat myometrium: regulation by estrogen and relaxin. Endocrinology. 1982 Jan;110(1):220–226. doi: 10.1210/endo-110-1-220. [DOI] [PubMed] [Google Scholar]
- Mercado-Simmen R. C., Goodwin B., Ueno M. S., Yamamoto S. Y., Bryant-Greenwood G. D. Relaxin receptors in the myometrium and cervix of the pig. Biol Reprod. 1982 Feb;26(1):120–128. doi: 10.1095/biolreprod26.1.120. [DOI] [PubMed] [Google Scholar]
- Mumford A. D., Parry L. J., Summerlee A. J. Lesion of the subfornical organ affects the haemotensive response to centrally administered relaxin in anaesthetized rats. J Endocrinol. 1989 Sep;122(3):747–755. doi: 10.1677/joe.0.1220747. [DOI] [PubMed] [Google Scholar]
- O'Byrne K. T., Eltringham L., Clarke G., Summerlee A. J. Effects of porcine relaxin on oxytocin release from the neurohypophysis in the anaesthetized lactating rat. J Endocrinol. 1986 Jun;109(3):393–397. doi: 10.1677/joe.0.1090393. [DOI] [PubMed] [Google Scholar]
- Osheroff P. L., Ling V. T., Vandlen R. L., Cronin M. J., Lofgren J. A. Preparation of biologically active 32P-labeled human relaxin. Displaceable binding to rat uterus, cervix, and brain. J Biol Chem. 1990 Jun 5;265(16):9396–9401. [PubMed] [Google Scholar]
- Phillips M. I. Functions of angiotensin in the central nervous system. Annu Rev Physiol. 1987;49:413–435. doi: 10.1146/annurev.ph.49.030187.002213. [DOI] [PubMed] [Google Scholar]
- Porter D. G., Downing S. J., Bradshaw J. M. Relaxin inhibits spontaneous and prostaglandin-driven myometrial activity in anaesthetized rats. J Endocrinol. 1979 Nov;83(2):183–192. doi: 10.1677/joe.0.0830183. [DOI] [PubMed] [Google Scholar]
- Quirion R., Dalpé M., De Lean A., Gutkowska J., Cantin M., Genest J. Atrial natriuretic factor (ANF) binding sites in brain and related structures. Peptides. 1984 Nov-Dec;5(6):1167–1172. doi: 10.1016/0196-9781(84)90183-9. [DOI] [PubMed] [Google Scholar]
- STEINETZ B. G., BEACH V. L., KROC R. L., STASILLI N. R., NUSSBAUM R. E., NEMITH P. J., DUN R. K. Bioassay of relaxin using a reference standard: a simple and reliable method utilizing direct measurement of interpubic ligament formation in mice. Endocrinology. 1960 Jul;67:102–115. doi: 10.1210/endo-67-1-102. [DOI] [PubMed] [Google Scholar]
- Sills M. A., Nguyen K. Q., Jacobowitz D. M. Increases in heart rate and blood pressure produced by microinjections of atrial natriuretic factor into the AV3V region of rat brain. Peptides. 1985 Nov-Dec;6(6):1037–1042. doi: 10.1016/0196-9781(85)90425-5. [DOI] [PubMed] [Google Scholar]
- St-Louis J., Massicotte G. Chronic decrease of blood pressure by rat relaxin in spontaneously hypertensive rats. Life Sci. 1985 Oct 7;37(14):1351–1357. doi: 10.1016/0024-3205(85)90251-6. [DOI] [PubMed] [Google Scholar]
- Stewart D. R., Celniker A. C., Taylor C. A., Jr, Cragun J. R., Overstreet J. W., Lasley B. L. Relaxin in the peri-implantation period. J Clin Endocrinol Metab. 1990 Jun;70(6):1771–1773. doi: 10.1210/jcem-70-6-1771. [DOI] [PubMed] [Google Scholar]
- Summerlee A. J., O'Byrne K. T., Jones S. A., Eltringham L. The subfornical organ and relaxin-induced inhibition of reflex milk ejection in lactating rats. J Endocrinol. 1987 Nov;115(2):347–353. doi: 10.1677/joe.0.1150347. [DOI] [PubMed] [Google Scholar]
- Summerlee A. J., O'Byrne K. T., Paisley A. C., Breeze M. F., Porter D. G. Relaxin affects the central control of oxytocin release. Nature. 1984 May 24;309(5966):372–374. doi: 10.1038/309372a0. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Weiss G. Relaxin in the male. Biol Reprod. 1989 Feb;40(2):197–200. doi: 10.1095/biolreprod40.2.197. [DOI] [PubMed] [Google Scholar]
- van Houten M., Posner B. I., Kopriwa B. M., Brawer J. R. Insulin-binding sites in the rat brain: in vivo localization to the circumventricular organs by quantitative radioautography. Endocrinology. 1979 Sep;105(3):666–673. doi: 10.1210/endo-105-3-666. [DOI] [PubMed] [Google Scholar]
- van Houten M., Schiffrin E. L., Mann J. F., Posner B. I., Boucher R. Radioautographic localization of specific binding sites for blood-borne angiotensin II in the rat brain. Brain Res. 1980 Mar 31;186(2):480–485. doi: 10.1016/0006-8993(80)90995-6. [DOI] [PubMed] [Google Scholar]