Abstract
The objective of this paper was to investigate the suitability of the miniature pig as a large animal model of cochlear implantation (CI). Micro-CT scanning and three-dimensional reconstructions of the inner ear were completed in six animals. Photographs of the procedures and measurements of the inner ear were made. The CI procedure was simulated in 10 animals. Electrically evoked auditory brain stem responses (EABRs) and radiographic images were evaluated during or after the CI procedure. Morphological examination and measurements of inner ears of the miniature pigs were completed by micro-CT scanning. The height of the scala tympani was 873.12 µm in the 1st turn, 641.46 µm in the 2nd turn, 445.13 µm in the third turn and 339.19 µm in the fourth turn. The length of the cochlea was 38.6 mm, larger than other animal models (7.2 mm in rats and 22 mm in macaque, for example) and similar to that in the human (36 mm). Commercial electrodes used in humans (870 µm at the end and 630 µm at the tip in diameter) were implanted in the pig’s cochlea, through which normal eABRs were obtained. Radiographic images after the CI procedure revealed electrodes located in the scala tympani of the first and second turns. Compared with traditional animal models, greater similarities of the inner ear between miniature pigs and humans make this animal a potentially useful model for CI studies.
Keywords: Miniature pig, cochlear implantation, animal model, Micro-CT, electrically evoked auditory brain stem responses
Introduction
Artificial cochlear implantation (CI) is an effective treatment for sensorineural deafness. Since the 1970s, when artificial cochlea technology began, artificial cochleae have continually been replaced by new ones. Animal testing is especially important in validating the necessary steps in the development of a new cochlea. Traditional animal models for CI studies include cats, guinea pigs and other animals with cochlear structures that differ greatly from those of humans (Table 1) [1-4]. A new cochlear electrode can be implanted into the animal’s cochlea with a special transformation, but the experimental data cannot fully reflect the real situation in humans. Therefore, we need to continue looking for more appropriate animal ear models that share greater similarities with humans.
Table 1.
Comparison of cochlear of animal models
| Species Cochlear | Human | Marmoset | Macaque | Cat | Guinea Pig | Rat | Miniature pig |
|---|---|---|---|---|---|---|---|
| Turns | 2.5-3 | 2.84 | 2.75 | 3-3.5 | 4 | 2.2 | 3.5 |
| Scala Tympani Length (mm) | 36 | 16.5 | 22 | 23 | 16.2 | 7.2 | 38.58125 |
Animal models for traditional CI include cats, guinea pigs, and other animals with cochlear structures that differ greatly from those of humans (Marc, et al, 2011; Klinke, et al, 1999; Agterberg, et al, 2010; Luke, et al, 2012).
Among experimental animals, pigs are the species most similar to humans after primates. Pigs have high similarity with humans in terms of the morphology and structure of their auditory organs. Studies have found that the structure of the middle ear cavity and pharyngeal lymphatic organization are highly consistent between pigs and humans, making pigs a very suitable model of otitis [5]. Because the thickness and strength of pig cochlear bone wall are very close to those of human, pig cochlea has been widely applied in cochlear fenestration [6], tomographic examination of fine cochlear structures [7], and surgical technologies research. Our earlier research found that the size and shape of pig cochlea are very similar to those of humans. The inner ear shape is nearly mature, similar to humans, and the pig has normal hearing at birth [8]. Therefore, pigs as a large animal model have promising application in the field of otology. In this study, we investigated the suitability of the miniature pig as a new animal model of CI.
Materials and methods
Sixteen Chinese miniature pigs of both sexes aged 6 to 8 weeks were used in this investigation. Six pigs were used for measurement of the ear and 10 used for CI.
The pigs were obtained from the Chinese Agricultural University in Beijing. This species was derived from a small-size swine species from Guizhou Province, China, in 1985. Its genetic background is well understood. Its features include an inherent small size, early sexual maturity, rapid breeding, and ease of management [9]. Its baseline biochemical and hematological parameters have been clearly established [10].
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the Institutional Animal Care and Use Committee of China Agricultural University in Beijing. This study had been approved by this committee. All efforts were made to minimize suffering, including: 1. Animal housing and care follows good practice; 2. Discomfort, distress and pain are minimized using appropriate anesthesia and analgesia; 3. Humane endpoints are defined and implemented; 4. Protocols involving animal use undergo ethical review; 5. Investigators and all personnel who handle and use animals are appropriately trained and qualified; 6. Euthanasia is carried out according to good practice.
Anesthesia and specimen preparation
Twelve pigs were anesthetized using ether inhalation. Six animals were immediately sacrificed by exsanguination from the axillary artery and dissected within 12 h postmortem. The head was removed at the C2 level. Ten animals were used to simulate CI procedure.
Micro-CT scan and three-dimensional (3D) reconstruction of temporal bone
In six animals, a 4×4×6 cm cube of tissue containing the entire tympanic cavity and petrous bone including the inner ear was obtained from each side. The 12 tissue blocks were scanned using a micro-CT system (SkyScan 1172, Belgium). Full technical details of the micro-CT parameters, including measurement by CTvox, modeling by CTAn, and reconstruction by Nrecon, are given elsewhere [11-13]. The data were processed using the VGStudio Max software, which allowed segmentation and reconstruction and generated animation clips of the ear. An ear model including the external auditory canal, mastoid, tympanic membrane (TM), ossicles, petrous bone (inner ear), and soft tissue (including muscles and nerve), was established. Measurement of the inner ear, including the height of every turn, the height of the scala tympani, and the perimeter of every turn, was completed.
Measurement of the area of the scala tympani on HE staining sections
The area of the scala tympani was measured in 4 cochleae. Light microscopy (Olympus SZX10) images of the modiolus were achieved and depicted against a scale. The images were transformed using the Mimics 17.00 software in JPEG format for measurement of the length and cross-area of the scala tympani of every turn, which was repeated four times.
Simulated CI procedure in 10 animals
CI system
A cochlear implant (CS-10A; Nurotron, China) with no modification (either the speech processor or the implant) for animal experiment was used in the study. This type of implant has been used in numerous patients in China. The 24-channel electrode array of the CS-10A has a length of 20.5 mm and a maximum diameter of 0.87×0.83 mm at the base and 0.57×0.63 mm at the tip.
CI procedure
Following a postauricular incision, a hand drill (OSSEODOC; Bien-Air Surgery SA, Switzerland) with a flexible shaft and appropriate cutting burs was used to enlarge the external auditory canal until the TM was exposed. The tympanic cavity and cochlea were exposed by enlarging the posterior wall of the external ear canal, as in myringoplasty in humans. The facial nerve, if encountered in this course, was partially dissociated downward for protection. Care was taken to avoid damage to the delicate structures of the TM and ossicular chain. A subperiosteal pocket of skull back to the ear was created for positioning the implant induction coil. Then a bone pocket tailored to the size of the device being implanted was created, and the induction coil was fixed to the cortex with a fixation suture. The electrode was implanted in the scala tympani through the penetration in the basal turn. The outer induction coil was sutured accordingly in the scalp and the speech processor was fixed in the back as shown in Figure 4. The surgical time of this approach was 2-3 hours plus half an hour for electrode insertion. Procedures were recorded on photographs using an Olympus DP72 camera attached to a ZEISS microscope and processed by DP2SW software.
Figure 4.
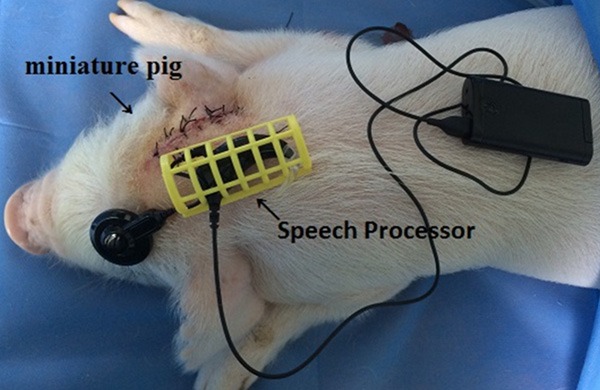
Location of speech processor in a miniature pig.
Evaluation of electrically evoked auditory brain stem responses (EABRs)
After the electrodes were placed in the scala tympani, electrical stimulation with biphasic, charge-balanced pulses (200 μs/phase, monopolar stimulation using the apical-most electrode of the implant and an indifferent electrode placed in the muscles at the neck) was performed using an optically isolated current source. The presentation rate was 13 stimuli per second in all animals. EABRs were recorded at varying intensities. Stimulus levels were computed from peak-to-peak amplitudes of the pulses. The highest current level was reached when facial nerve stimulation appeared. Recordings with facial nerve stimulation resulting in muscle contractions were contaminated with muscle activities and discarded from further processing.
Postoperative images and auditory thresholds after CI insertion
A CT scan and radiograph of the temporal bone were obtained in all 10 pigs. The electrode was revealed clearly in the first and second turns of the cochlea. The auditory thresholds before and after CI insertion were evaluated. ABRs were evoked with clicks and/or 5-ms tone pips (0.5 ms rise-fall, at 30/sec) at 1, 4, 8, 16 or 20 kHz. The response was amplified, filtered, and averaged using the Intelligent Hearing System (Intelligent Hearing System Corp., Miami, FL). The sound level was raised in 20- and/or 5-dB steps. At each level, 1,024 responses were averaged. Hearing threshold was determined by visual inspection.
Results
Micro-CT scan and 3D reconstruction
The ear was scanned and reconstructed using micro-CT scans in six pigs. The scan and 3D reconstruction combined with microdissection of the middle and inner ears were very helpful in revealing the anatomy of the miniature pig ear, as shown in Figures 1 and 2. The 3D reconstruction provided more accurate and clearer views of miniature pig ear structures and their spatial relations than did 2D microdissection.
Figure 1.
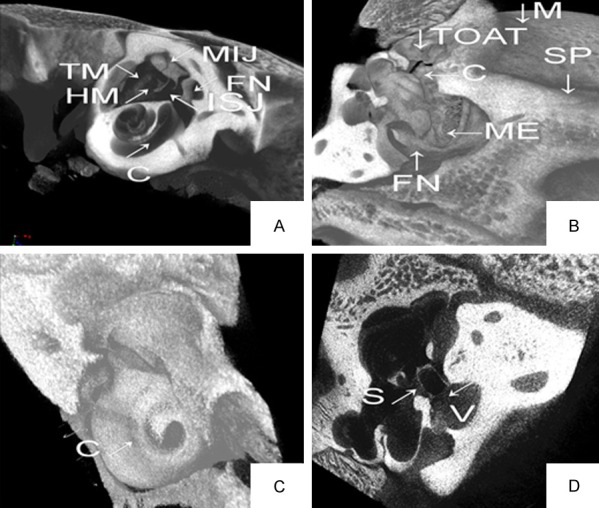
Micro-CT scans and 3-D reconstruction of the miniature pig ear. A: 3-D reconstruction of the middle ear and part of cochlea using Micro-CT scans. B: 3-D reconstruction of the middle ear. C: 3-D reconstruction of the cochlea. D: 3-D reconstruction of the stapes. MIJ-incudomalleolar joint, ISJ-incudostapedial joint, V-vestibule, SP-styloid process, TOAT-tympanic opening of auditory tube, C-cochlea, M-mastoid, S-stapes, FN-facial nerve, TM-tympanic membrane, HM-handle of malleus.
Figure 2.
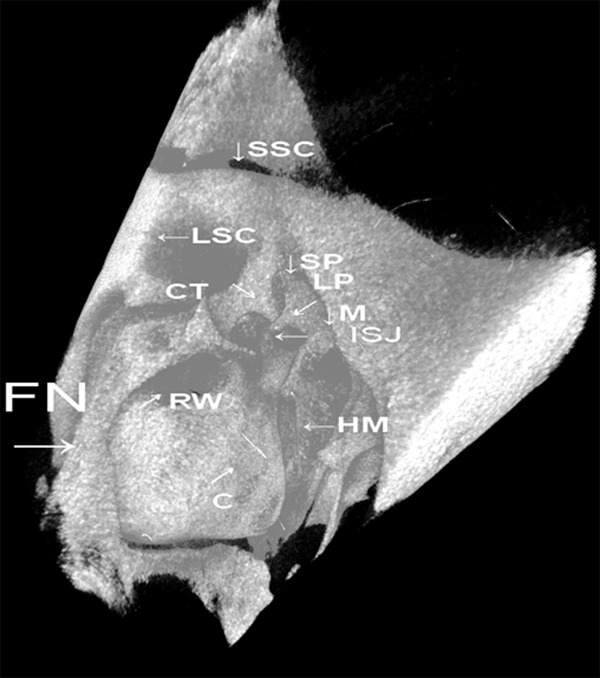
3-D reconstruction of the media wall of the middle ear. MIJ-incudomalleolar joint, ISJ-incudostapedial joint, V-vestibule, SP-styloid process, TOAT-tympanic opening of auditory tube, C-cochlea, M-mastoid, S-stapes, FN-facial nerve, TM-tympanic membrane, HM-handle of malleus.
Measurement of the inner ear
Measurement of the inner ear was completed using software including the CTvox (measurement), CTAn (modeling) and Nrecon (reconstruction). The height of the scala tympani at every turn was measured on a reconstructed section of the cochlea along a virtual line parallel to the modiolus. Points “b” and “c” were crossover points of the line with the bottom of the scala tympani and the top of the scala vestibular, whereas Point “a” was at the osseous spiral lamina level. The height of the scala tympani was the distances between Points a-b and a-c (Figure 3), and the data are shown in Tables 1 and 2. The height of the scala tympani was 873.12 µm in the 1st turn, 641.46 µm in the 2nd turn, 445.13 µm in the third turn and 339.19 µm in the fourth turn. The length of the cochlea was 38.6 mm, larger than other typical animal models and similar to that obtained in the human (Table 1). The standard commercial electrode used in humans (CS-10A) was easily implanted in the pig’s cochlea.
Figure 3.
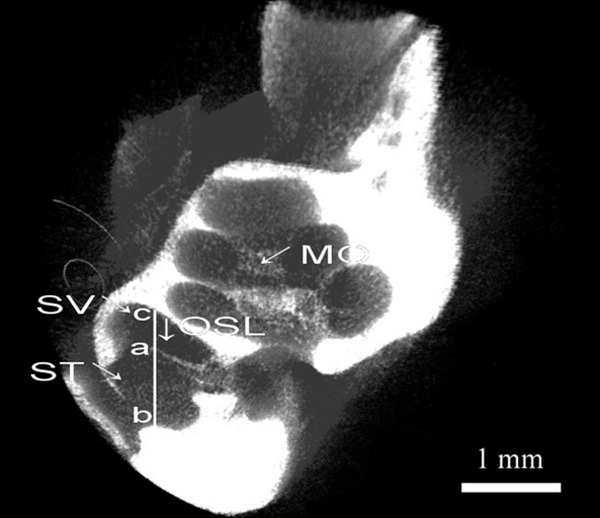
Reconstructed cochlear sections using Micro-CT scans. ST-scala tympani, SV-scala vestibular, OSL-osseous spiral lamina, MO-modiolus.
Table 2.
Measurement of the cochlea (μm)
| No. of Turn | Turn Height | Height of Scala Tympani | Turn Perimeter |
|---|---|---|---|
| 1 | 1243.57 | 873.24 | 16512.30 |
| 2 | 859.32 | 641.46 | 11402.41 |
| 3 | 753.54 | 445.13 | 8553.11 |
| 4 | 532.14 | 339.19 | 2113.43 |
The 12 cochlear were scanned using a micro-CT system (SkyScan 1172, Belgium). The data were processed by the program VGStudio Max, which allowed segmentation and reconstruction and generated animation clips of the ear. An ear model was established and measurement of the inner ear, including the height of every turn, the height of the scala tympani, and the perimeter of every turn, was completed.
Measurement of the section area of scala tympani on HE sections
In order to verify the feasibility of insertion of human CI electrodes, the section area of scala tympani was measured on HE sections. The area in the basal turn was 2.475 mm2 and greater than the diameter of the CS-10A electrode used in the human, indicating feasibility of insertion of human CI electrodes in the miniature pig (Table 3).
Table 3.
The measurements of areas of the scala tympani (mm2) in four cochleae
| Cochleaes | The basal turn | The second turn | The third turn |
|---|---|---|---|
| 1 | 2.45 | 0.093 | 0.064 |
| 2 | 2.46 | 0.094 | 0.062 |
| 3 | 2.43 | 0.096 | 0.065 |
| 4 | 2.42 | 0.093 | 0.069 |
| Average | 2.44 | 0.094 | 0.065 |
Four cochleae were examined to measure the area of the scala tympani. The images of modiolus histological sectionsby light microscope (Olympus SZX10) were imported into the Mimics software 17.00 in JPEG format, then measured the scale length and the cross-areas of scala tympani of every turn by its measuring kit directly four times.
Simulated CI procedure in 10 animals
CI procedure
The pig skull appeared more compact with a significantly smaller brain capacity than in humans. Anatomically, the temporal bone was located in the same position as in humans, including the squamous, mastoid, and tympanic (which was not obvious) parts as well as the styloid process. The mastoid air cell system lay medial to the temporomandibular joint and inferior to the tympanic cavity. The external ear canal was very long and orientated strictly upward and backward. Although the locations of the mastoid and external ear canal in the miniature pig differ significantly from those in humans, this did not appear to influence the CI procedure. The miniature pig cochlea had 3.5 turns. Eleven to 14 active electrodes could be implanted in the basal and second turns, which yielded very satisfactory results.
CI trial simulation (Figure 4) and scala tympani measurement on micro-CT scans showed an appropriate scala tympani size for the CI electrode used in humans, which needed no additional modification for the animal trials.
Evaluation of EABRs
EABRs in tested animals revealed a morphology characteristic of acoustically evoked brain stem responses (Figure 5). In most animals, waves III and V could be consistently differentiated. Additional waves appeared at high stimulus levels. When at least one wave was present, usually wave V, it was demonstrated that the electrodes worked efficiently. The latency of wave III was around 2.5 ms and that of wave V was around 3.6 ms. The prolongation of wave latencies with decreasing stimulating current levels was not as significant as that seen in acoustic ABR test.
Figure 5.
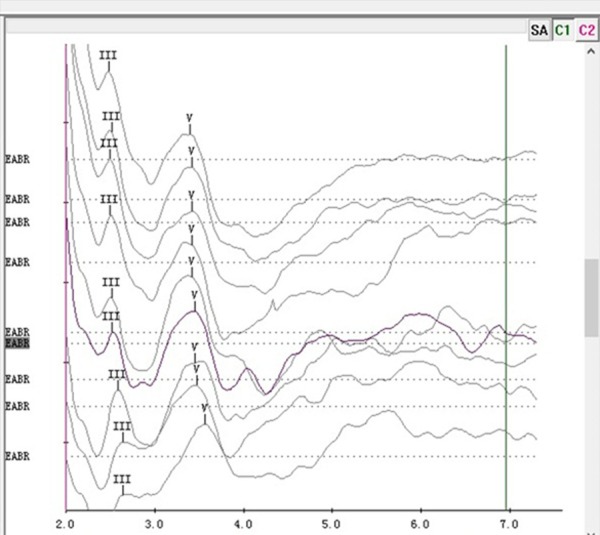
Electrically evoked brainstem evoked responses (EABRs) in the miniature pig revealed a morphology characteristic of acoustically evoked brainstem response, at nine different current levels (highest current level at the top).
Postoperative images and auditory thresholds after CI insertion
In the 10 temporal bones radiographed, the electrode was revealed clearly in the first and second turns of the cochlea (Figure 6). Auditory thresholds immediately after CI surgery were evaluated (Table 4). No recognizable waveforms were seen up to 100 dB SPL sound for 4 to 32 kHz, in contrast to the normal ABR thresholds of 35 to 40 dB SPL in pigs. Auditory thresholds one week after CI could be found in Table 4.
Figure 6.
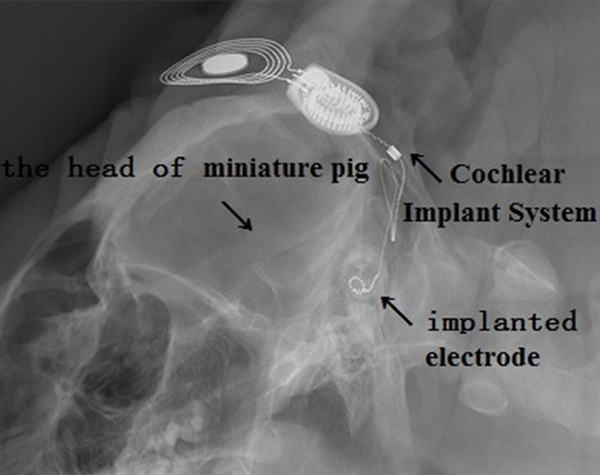
Temporal bone radiographs in the ten pigs undergone CI. The electrode is revealed clearly in the first and second cochlear turns.
Table 4.
The thresholds of the miniature pig the after CI insertion (dB SPL)
| Frequencies | Preoperation | Instant test postoperation | One week postoperation |
|---|---|---|---|
| Click | 32±4.2 | >100 | 77±3.1 |
| 1000 HZ | 38±2.4 | >100 | 79±4.5 |
| 4000 HZ | 37±3.5 | >100 | 79±4.2 |
| 8000 HZ | 47±2.6 | >100 | 94±2.8 |
| 16000 HZ | 31±5.2 | >100 | 114±5.7 |
| 20000 Hz | 33±2.7 | >100 | 112±3.7 |
Discussion
Pigs and miniature pigs as animal models for medical studies
Animal models of human diseases have always played a central role in biomedical research for the exploration and development of new therapies [2]. However, the evolutionary gap between humans and many of the animal models (such as rodents) has always hampered direct applicability of the knowledge gained for human therapy [2]. A crucial prerequisite for the development of safe preclinical protocols in biomedical research is the use of a suitable animal model that would allow for human-related validation of valuable research information gathered from experimentation with lower mammals [2]. Thus, a suitable animal model is very important in biomedical research. Rodents and nonhuman primates (for ethical reasons) are not always suitable animal models.
As a domesticated eutherian (placental) mammal, the pig has evolved similarly to humans and represents a taxon with diverse selected phenotypes [14]. The pig also represents an evolutionary clade distinct enough from primates and rodents to provide considerable power in the understanding of genetic complexity. The similarities of numerous physiological functions between pigs and humans have stimulated a wide range of biomedical studies. However, traditional pigs tend to be large in size and difficult to manage.
Different types of miniature pigs have been bred, including the Chinese experimental miniature pig [10]. Its characteristics include inherent small size, early sexual maturity, rapid breeding, and ease of management [9]. Compared with rodents, the advantage is obvious-the miniature pig shares more similarities to humans and thus results of biomedical research using the pig are more relevant. Moreover, there are fewer ethical concerns with pigs than with nonhuman primates. Additionally, the miniature pig is more cost-effective than traditional pigs. Miniature pigs have been extensively used as a large animal model in many biomedical experiments and studies of artificial organs [5-8]. The physiology and morphology of the ear of the miniature pig, including conventional histological analyses using HE staining sections used by our team, have been investigated [8]. In this study, we assessed the suitability of the miniature pig as a new animal model for CI.
Measurement of the scala tympani
Knowledge of the dimensions of the scala tympani in both humans and experimental animals is necessary for the continued development of cochlear implants. Such information would ensure that scala tympani electrode arrays are not inserted past a point in the cochlea dimensionally smaller than the electrode array. This knowledge will also provide necessary anatomic information required for the development of improved scala tympani electrode arrays [15]. Dimensions of the cochlea in humans and laboratory animals have been reported by several authors [16-22]. However, these reports have some limitations, and the methods are complicated. We designed a new and simple method of measuring the scala tympani by micro-CT according to the following theory.
Previous evidence [23-27] from electrode insertion studies in human temporal bones suggested that the banded electrode array of the cochlear implant (Nucleus; Cochlear, Centennial, CO, USA) lay along the outer wall of the scala tympani. Recent acute electrical stimulation studies in cats showed that EABR thresholds decreased and the dynamic range increased as the banded electrode array was moved from the outer wall of the scala tympani toward the modiolus [28]. These results suggest that an electrode array placed close to the modiolus would be capable of exciting a greater number of discrete groups of auditory nerve fibers. Additionally, the scala tympani is somewhat smaller in height than in width [22]. Therefore, we believe that the osseous spiral lamina is a limiting factor for the size of more effective electrode arrays. If the electrode array is smaller than the distance from Point a to b, it can be inserted into the scala tympani closer to the modiolus. The distance from Point a to b (Figure 3) as the height of the scala tympani is more relevant. We believe that our method, which utilizes micro-CT, is simple and valid. The banded scala tympani electrode array from Cochlear has a maximum diameter of 0.87×0.83 mm and tapers to a tip diameter of 0.57×0.63 mm. This electrode array has been designed for insertion to a depth of 20.5 mm from the round window. The data in Table 2 reveal that the scala tympani of the miniature pig is suitable for the electrode array, which needs not to be modified for animal trials. And In order to verify the feasibility of insertion of human CI electrodes, the cross area of scala tympani was measured on HE sections and was shown to be greater than the CS-10A electrode used in the human. The results support the feasibility of insertion of human CI electrodes in the miniature pig (Table 3). The smaller cross area of the second may be related with collapsed vestibular membrane.
Simulation of CI procedure in the miniature pig
The CI procedure was simulated in the miniature pig (Figure 4). The EABR results after electrodes were implanted in the scala tympani (Figure 5) proved that the electrodes worked efficiently. Temporal bone radiographs (Figure 6) clearly showed that the electrode was in the first and second turns of the cochlea. Immediate ABRs showed no recognizable waveforms up to 100 dB SPL for 4 to 32 kHz (Table 4). Potential mechanisms of instant hearing loss following CI surgery include direct mechanical trauma to the basilar membrane or osseous spiral lamina. ABR thresholds one week after CI revealed residual hearing, indicating that damage by CI insertion did not cause complete hearing destruction (Table 4). The level of residual hearing at one week following CI surgery may depend on the level of restoration of cochlear internal environment, although further work is needed to determine the exact mechanisms.
Our CI simulation and the micro-CT scala tympani measurement results show that the size of the miniature pig scala tympani can receive CI electrodes used in humans without the need for modification. This helps validate CI animal trials, which would allow for continuous improvement of electrode array designs.
Compared with traditional animal models, similarities between miniature pig and human inner ears make this animal a potentially useful model for applications in CI research.
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (973 Program) (#2012CB967900), the Major State Basic Research Development Program of China (973 Program) (#2011CBA01000), and the National Natural Science foundation of China (31101701).
Disclosure of conflict of interest
None.
References
- 1.Hoffstetter M, Lugauer F, Kundu S, Wacker S, Perea-Saveedra H, Lenarz T, Hoffstetter P, Schreyer AG, Wintermantel E. Middle ear of human and pig: a comparison of structures and mechanics. Biomed Tech (Berl) 2011;56:159–165. doi: 10.1515/BMT.2011.011. [DOI] [PubMed] [Google Scholar]
- 2.Klinke R, Kral A, Heid S, Tillein J, Hartmann R. Recruitment of the auditory cortex in congenitally deaf cats by long-term cochlear electrostimulation. Science. 1999;285:1729–33. doi: 10.1126/science.285.5434.1729. [DOI] [PubMed] [Google Scholar]
- 3.Agterberg MJ, Versnel H, de Groot JC, van den Broek M, Klis SF. Chronic electrical stimulation does not prevent spiral ganglion cell degeneration in deafened guinea pigs. Hear Res. 2010;269:169–79. doi: 10.1016/j.heares.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Johnson LA, Della Santina CC, Wang X. Temporal bone characterization and cochlear implant feasibility in the common marmoset (Callithrix jacchus) Hear Res. 2012;290:37–44. doi: 10.1016/j.heares.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pracy JP, White A, Mustafa Y, Smith D, Perry ME. The comparative anatomy of the pig middle ear cavity: a model for middle ear inflammation in the human. J Anat. 1998;192:359–68. doi: 10.1046/j.1469-7580.1998.19230359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coulson CJ, Taylor RP, Reid AP, Griffiths MV, Proops DW, Brett PN. An autonomous surgical robot for drilling a cochleostomy: preliminary porcine trial. Clin Otolaryngol. 2008;33:343–7. doi: 10.1111/j.1749-4486.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 7.Sepehr A, Djalilian HR, Chang JE, Chen Z, Wong BJ. Optical coherence tomography of the cochlea in the porcine model. Laryngoscope. 2008;118:1449–51. doi: 10.1097/MLG.0b013e318173dd6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo W, Yi H, Ren L, Chen L, Zhao L, Sun W, Yang SM. The morphology and electrophysiology of the cochlea of the miniature pig. The Anatomical Record. 2015;298:494–500. doi: 10.1002/ar.23095. [DOI] [PubMed] [Google Scholar]
- 9.Yu SM, Wang CW, Zhao DM, Zhang QC, Pei DZ. Raising and pathogen purification of Chinese experimental mini-pig. Lab Anim Sci Admin. 2003;20:44–46. [Google Scholar]
- 10.Chen Y, Qin S, Ding Y, Li S, Yang G, Zhang J, Li Y, Cheng J, Lu Y. Reference values of biochemical and hematological parameters for Guizhou minipigs. Exp Biol Med (Maywood) 2011;236:477–482. doi: 10.1258/ebm.2011.010283. [DOI] [PubMed] [Google Scholar]
- 11.Uzun-Coruhlu H, Curthoys IS, Jones AS. Attachment of the utricular and saccular maculae to the temporal bone. Hear Res. 2007;233:77–85. doi: 10.1016/j.heares.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Epley JM. The canalith repositioning procedure: for treatment of benign paroxysmal positioning vertigo. Otolaryngol Head Neck Surg. 1992;107:399–404. doi: 10.1177/019459989210700310. [DOI] [PubMed] [Google Scholar]
- 13.Curthoys IS, Uzun-Coruhlu H, Wong CC, Jones AS, Bradshaw AP. The configuration and attachment of the utricular and saccular maculae to the temporal bone. New evidence from microtomography-CT studies of the membranous labyrinth. Ann N Y Acad Sci. 2009;1164:13–8. doi: 10.1111/j.1749-6632.2008.03729.x. [DOI] [PubMed] [Google Scholar]
- 14.Rothschild MF, Ruvinsky A. Genetics of the pig. Wallingford, UK: CAB international; [Google Scholar]
- 15.Hatsushika S, Shepherd RK, Tong YC, Clark GM, Funasaka S. Dimensions of the scala tympani in the human and cat with reference to cochlear implants. Ann Otol Rhinol Laryngol. 1990;99:871–6. doi: 10.1177/000348949009901104. [DOI] [PubMed] [Google Scholar]
- 16.Wever EG. The width of the basilar membrane in man. Ann Otol Rhinol Laryngol. 1938;47:37–47. [Google Scholar]
- 17.Hardy M. The length of organ of Corti in man. Am J Anat. 1938;62:291–311. [Google Scholar]
- 18.Fernandez C. Dimensions of the cochlea (guinea pig) J Acoust Soc Am. 1952;24:519–23. [Google Scholar]
- 19.Schuknecht HF. Techniques for study of cochlear function and pathology in experimental animals. Arch Otolaryngol. 1953;58:377–97. doi: 10.1001/archotol.1953.00710040399001. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi M, Mahon RG Jr, Konishi S. Comparative measurements of cochlear apparatus. J Speech Hear Res. 1968;11:229–35. doi: 10.1044/jshr.1102.229. [DOI] [PubMed] [Google Scholar]
- 21.Igarashi M, Takahashi M, Alford BR. Cross-sectional area of scala tympani in human and cat. Arch Otolaryngol. 1976;102:428–9. doi: 10.1001/archotol.1976.00780120076011. [DOI] [PubMed] [Google Scholar]
- 22.Zrunek M, Lischka M, Hochmair-Desoyer I, Burian K. Dimensions of the scala tympani in relation to the diameters of multichannel electrodes. Arch Otorhinolaryngol. 1980;229:159–65. doi: 10.1007/BF02565517. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd RK, Clark GM, Pyman BC, Webb RL. Bandedintracochlear electrode array: evaluation of insertion trauma in human temporal bones. Ann Otol Rhinol Laryngol. 1985;94:55–9. doi: 10.1177/000348948509400112. [DOI] [PubMed] [Google Scholar]
- 24.Franz BK-HG, Clark GM. Refined surgical technique for insertion of banded electrode array. Ann Otol Rhinol Laryngol. 1987;96:15–7. [Google Scholar]
- 25.Clifford AR, Gibson WPR. Anatomy of the round window with respect to cochlear implant surgery. Ann Otol Rhinol Laryngol. 1987;96:17–9. [Google Scholar]
- 26.Kennedy OW. Multichannel intracochlear electrodes: mechanism of insertion trauma. Laryngoscope. 1987;97:42–9. doi: 10.1288/00005537-198701000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Clark GM, Shepherd RK, Franz BK, Dowell RC, Tong YC, Blamey PJ, Webb RL, Pyman BC, McNaughtan J, Bloom DM, et al. The histo-pathology of the human temporal bone and auditory central nervous system following cochlear implantation in a patient. Correlation with psychophysics and speech perception results. Acta Otolaryngol Suppl. 1988;448:1–65. doi: 10.3109/00016488809098972. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd RK, Maffi CL, Hatsushika S, Javel E, Tong YC. Temporal and spatial coding in auditory prostheses. In: Rowe MJ, Aitkin LM, editors. Information processing in mammalian auditory and tactile systems. New York, NY: Wiley-Liss; pp. 281–93. [Google Scholar]


