Abstract
This study aims to explore the role of extra-cellular matrix metalloproteinase inducer (EMMPRIN) in the drug resistance of the pseudomonas aeruginosa (PA). The BALB/c mice were transfected with PA, then the mice were infected with the siRNA of EMMPRIN to silence the EMMPRIN gene. The EMMPRIN mRNA and protein were detected by using RT-PCR and western blot, respectively. In order to examine the function of EMMPRIN in drug resistance of PA, the BALB/c and C57BL/6 mice were treated with EMMPRIN siRNA. The cytokines, EMMPRIN and MMP9 were examined by the RP-PCR and ELISA, respectively, undergoing the silence of EMMPRIN siRNA. Moreover, the western blot assay was also used to test the phosphorylated MAPK in the murine macrophages after silenced by the EMMPRIN siRNA. The EMMPRIN was activated, with lipopolysaccharide stimulation and treated with the MAPK inhibitor, to evaluate whether the MAPK participates in the EMMPRIN-triggered drug resistance. The results indicated that the EMMPRIN expression was elevated in the infected BALB/c at 3 or 5 days post-infection. Silence of EMMPRIN Enhanced the Production of pro-inflammatory cytokines in PA keratitis. Silence of EMMPRIN significantly up-regulated Th1-type cytokines IFN-γ, IL-12, and IL-18, but down-regulated Th2-type cytokines IL-4, IL-5, and IL-10. MMP9 was increased in the cells with rEMMPRIN treatment. EMMPRIN inhibits pro-inflammatory cytokine production via a MAPK signaling pathway. In conclusion, EMMPRIN promotes host resistance against pseudomonas aeruginosa infection via MAPK signaling pathway.
Keywords: EMMPRIN, pseudomonas aeruginosa, durg resistance, MAPK
Introduction
The bacterial infections induced by the pseudomonas aeruginosa (PA) and other Gram-negative (G-) bacteria mainly illustrated as a rapidly development and progression [1-3]. There are many characteristics, including mecrosis, inflammatory epithelial edema and stromal ulceration, above of which can culminate in significant stromal function loss and the destruction of the tissue [4,5]. For the chronic inflammation and infection in numerous tissue, including heart, lung, liver and connective tissue, the morphologic biomarkers mainly represent as the destruction or progressive damage of the extra-cellular matrix (ECM) components [5].
Matrix metalloproteinases (MMPs) belongs to the Zn2+-dependent endo-peptidases protein family, which keeps and remodels the architecture of the tissue [6-8]. The previous studies [6-8] reported that the MMPs play very wide range roles in the biological processes of cells, such as repair and regeneration of the tissue, and the embryonic development. As all is known, the MMPs always associated with the destructive processes and some of the human diseases, such as chronic obstructive lung disease (COPD), corneal infection, cancer metastasis/progression and the multiple sclerosis. The extra-cellular MMPs inducer (EMMPRIN), also called as CD147 molecule (basigin in mice), is a kind of highly glycosylated type I transmembrane glycoprotein, which may interact with the other membrane protein by activating the intra-membrane response [9-12]. The overall amino acid sequence identity between mouse and rat EMMPRIN is 94%, between human and mouse EMMPRIN is 58% and between mouse and chicken is 45%. The Ig domain is very critical for stimulating the fibroblast MMP production by human EMMPRIN [13-15]. It is believed that EMMPRIN has a valuable function in the tissue progression, cell differentiation, tissue development and the remodeling of some normal tissue such as retina, cornea, liver, lung, thymus, and even the nervous system [16-18].
We hypothesize that EMMPRIN promotes drug resistance against pseudomonas aeruginosa infection. In our study, we used the PA to induce the corneal perforation in the C57BL/C mice (B6), and the infection was effectively resolved (which classified as the resistant, no corneal perforation). The present study illustrated the presence of EMMPRIN in the corneal perforation mice, and illustrated the regulatory function for EMMPRIN regarding the MMP-9 protein expression.
Materials and methods
Materials
The female BALB/c mice (8 years old) and C57BL/6 (B6) mice (8 years old) were purchased from Animal Center of Wuhan University (Wuhan, China). The PA strain (19660) was purchased from ATCC (Manassas, VA). Pseudomonas isolation agar was purchased from Aldrich-Sigma (CA, USA), The thioglycollate medium was purchased from Tiangen company (Beijing, China). The mouse anti-ERK1/2 and mouse anti-β-actin were Santa Cruz Co. In. (CA, USA). The rabbit anti-mouse IgG was purchased from Cell Signaling Technology (Danvers, MA).
siRNA injection
The BALB/c mice (n = 5/group) were injected sub-conjunctivally with 5 μL saline, which contains 8 μM of EMMPRIN siRNA one day before infection. Then, the mice were sub-conjunctivally topically with 5 μL saline containing 4 μM EMMPRIN siRNA, once time on the day of PA infection (d0) and twice time per day post the PA infection. The control mice were also treated with the same processes, but with a non-specific (non-targeting) scrambled siRNA. The EMMPRIN siRNAs synthesized in this study were the complex RNA, which composes of 20 to 25 nt siRNAs and could knock-down the EMMPRIN gene expression, but the scrambled siRNA could not.
Peritoneal macrophages isolation
1.0 mL Brewer’s thioglycollate medium (3%) was injected into the BALB/c 5 days before the peritoneal macrophages cell harvest. The Peritoneal exudate cells were collected and stained by using the trypan blue (0.1%). Then, the viable cells were enumerated by utilizing a hemacytometer. Consequently, the cells were seeded (106 cells per well) into the 12-well plates and incubated for two hours at 37°C. Four hours later, the non-adherent peritoneal cells were discarded and the macrophages were isolated (with the purity higher than 90%), which was used in the in vitro experiments.
Recombinant EMMPRIN peptide treatment
1 μg (dissolved in to the 5 μL saline) recombinant EMMPRIN was sub-conjunctivally injected into the B6 mice (n = 5/group). Then, the IP injections (1 μg rhTestican-1 dissolved in the 100 μL PBS) were injected into the mice subsequently. The control mice were treated with the solution of PBS, which contains 0.1% BSA.
Real-time PCR
The corneas (n = 5/group/time) isolated from the infected or non-infected B6, BALB/c, and the siRNA-treated mice at 1, 3, 5, and 7 days, respectively. The harvested corneas were treated with lysis buffer and the total RNA isolation reagent (Tiangen, Beijing, China) was used to obtain the total RNA. The cDNA was synthesized by using the cDNA synthesis kit (Invitrogen, CA, USA) according to the manufacturer’s instruments. The transcript levels or mRNA levels of EMMPRIN were examined by using the real-time PCR (RT-PCR). The primers for EMMPRIN were designed on computer (PrimerQuest SM; Integrated DNA Technologies, Coralville, IA), and synthesized by employing the SuperArray Bioscience Corporation (Frederick, MD, US). The relative standard curve method was used to calculate the relative mRNA levels, and compare the amount of target normalized to an endogenous reference. In this study, the β-actin was employed as the endogenous reference. The primers used in this study was listed in Table 1.
Table 1.
Sequence of all the primers used for PCR assay
| Gene | Primers | Gene ID | Amplification size | |
|---|---|---|---|---|
| EMMPRIN | Forward | CGGAGTATGAGGTGGACTCAGAA | NM_001075371 | 63 bp |
| Reverse | GCTCCGGAAGGAAGATGCA | |||
| EMMPRIN1 | Forward | TCCAAAACACGACTCACCTGTG | NM_001075371 | 579 bp |
| Reverse | GCTTCCGCCTCTTCTCGTAGAT | |||
| EMMPRIN2 | Forward | GTCTGCAAATCGGACTCCTT | NM_001075371 | 120 bp |
| Reverse | TCTTCGTGGTCTCCTCZAGAG | |||
| EMMPRIN3 | Forward | ATCATCTTCATCTACGAGAAGA | NM_001075371 | 240 bp |
| Reverse | AAGCAGACCGTGTTGTACATTC | |||
| MMP-9 | Forward | TGAATCAGCTGGCTTTTGTG | NM_013599.4 | 60 bp |
| Reverse | GTGGATAGCTCGGTGGTGTT | |||
| IL-10 | Forward | AGCTGGACAACATACTGCTAACCGAC | NM_010548.2 | 246 bp |
| Reverse | CTTGATTTCTGGGCCATGCTTCTCTG | |||
| IL-12 | Forward | GGTCACACTGGACCAAAGGGACTATG | NM_001311141.1 | 260 bp |
| Reverse | ATTCTGCTGCCGTGCTTCCAAC | |||
| IL-18 | Forward | GCCTGTGTTCGAGGATATGACTGA | NM_008360.1 | 356 bp |
| Reverse | TTCACAGAGAGGGTCACAGCCA | |||
| TNF-α | Forward | CACAGAAAGCATGATCCGCGAC | NM_001278601.1 | 295 bp |
| Reverse | TCGCACAAGCAGGAATGAGAAGAG | |||
| IL-6 | Forward | GCTACCAAACTGGATATAATCAGGA | NM_001314054.1 | 154 bp |
| Reverse | CCAGGTAGCTATGGTACTCCAGAA | |||
| INF-γ | Forward | ACGTGAATAAGGATCCTGTGGG | M28995.1 | 180 bp |
| Reverse | CCCTGATCATCTCACAGGACAG | |||
| β-actin | Forward | GATTACTGCTCTGGCTCCTAGC | NM_007393.5 | 138 bp |
| Reverse | GACTCATCGTACTCCTGCTTGC | |||
Cytokines assay
The cells were homogenized in the PBS solution (1:2, w/v), which contains protease inhibitors (with the concentration of 1%). Then, the cells were centrifuged at the speed of 12000 ×g at 4°C for 15 min. The supernatants were retained, and analyzed by utilizing the ELISA assay to examine the cytokines according to the ELISA kit instruction.
Western blot
The cells were lyzed by using the lysis buffer, and centrifuged at the speed of 5 000 ×g at 4°C for 5 min. The supernatants was analyzed by using the BCA protein assay kit (Biyuntian, Beijing, China). The protein sample (50 μg) was loaded and separated on a 15% SDS-poly-acrylamide gel (SDS page). Then, the separated protein on the gel was transferred onto the nitrocellulose membrane, and blocked with the 5% fetal bovine serum (BSA) overnight at 4°C. Then, the nitrocellulose membrane was washed with the PBS for three times, and 5 min per time. The washed nitrocellulose membranes were incubated with the goat anti-mouse testican-1 (1:1000) polyclonal antibody at 4°C overnight. The membranes were washed with PBST for 3 times, and 5 min per time, and then incubated with the donkey anti-goat IgG-HRP polyclonal antibody (1:2000). Finally, the membranes were washed with PBST and detected by using the ECL kit. In this study, we used the human recombinant testican-1 (95% homology with mouse testican-1; R&D Systems) as the positive control for the western blot assay. And the Testican-1 blocking peptide was used as the negative control for western blot assay (Santa Cruz Biotechnology, USA).
Statistical analysis
The data in this study was analyzed by using the SPSS 19.0 software. The data in this study was presented as the mean ± SD. The differences between the two groups tested by using the student’s t-test. The data were considered as the significant with the P value less than 0.05. All of the experiments in this study were repeated at least three times.
Results
EMMPRIN expression in response to PA infection
Given the role of EMMPRIN during the process of inflammation, the particular protease of the individual corneal samples was also examined by using the real-time PCR (RT-PCR), under the normal conditions, 0 day, 1 day, 3 days, 5 days, and 7 days post the infection in BALB/c and B6 mice. The results indicated that the EMMPRIN2 was up-regulated significantly in BALB/c cornea compared to the B6 at 3 days, 5 days, and 7 days post the infection (Figure 1A). However, the EMMPRIN1 (Figure 1B) and EMMPRIN3 (Figure 1C) were not up- or down-regulated in BALB/c cornea and the B6 cornea. The ELISA results showed that EMMPRIN protein expression was significantly increased in the cornea of BALB/c compared to the B6 mice at the every time points (Figure 1D). At 1 day post the infection, EMMPRIN could be detected, even at very low levels. By 5 and 7 days post the infection, the results indicated higher EMMPRIN in BALB/c mice.
Figure 1.
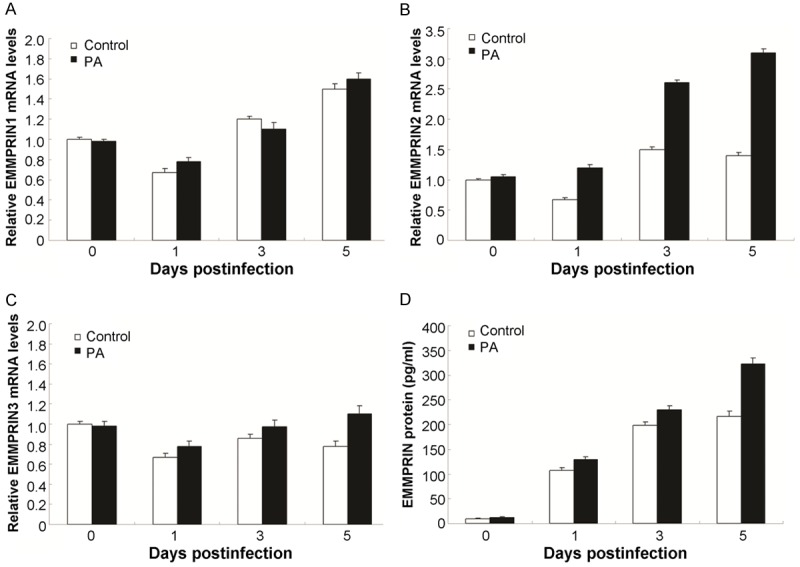
Expression of EMMPRIN in response to PA infection. A. EMMPRIN2 mRNA was examined in the infected lung epithelial cells compared with the control ones at 0, 1, 3, and 5 days. B. EMMPRIN1 mRNA was examined in the infected lung epithelial cells compared with the control ones at 0, 1, 3, and 5 days. C. EMMPRIN3 mRNA was examined in the infected lung epithelial cells compared with the control ones at 0, 1, 3, and 5 days. D. EMMPRIN protein levels in the lung epithelial cells of B6 and BALB/c mice by ELISA method.
Silence of EMMPRIN increases the proinflammatory cytokines levels in the PA keratitis
In this study, the pro-inflammatory cytokines were examined by using the RT-PCR assay and the ELISA assay, respectively. The TNF-α (Figure 2A), IL-6 (Figure 2B), and IFN-γ (Figure 2C) were significantly increased in the EMMPRIN silence group compared to the control group. Furthermore, ELISA results also indicated that same changes of TNF-α (Figure 2D), IL-6 (Figure 2E), and IFN-γ (Figure 2F) in both group.
Figure 2.
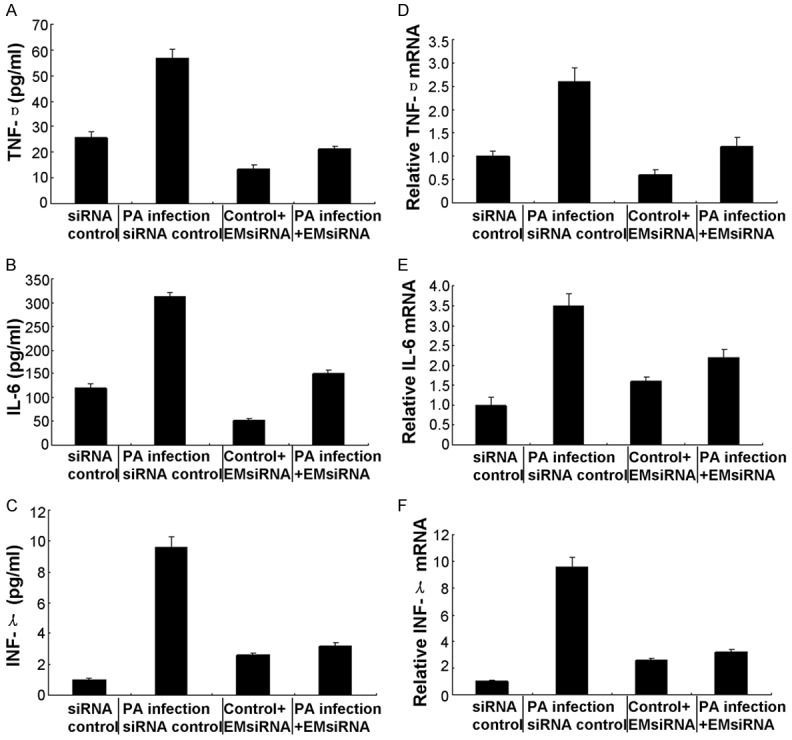
Expression of proinflammatory cytokines after in vivo silence of EMMPRIN. Real-time PCR data demonstrated that at 1 and 5 days, silence of EMMPRIN decreased the mRNA levels of proinflammatory cytokines, including TNF-α (A), IL-6 (B), and IFN-γ (C) in the PA-infected BALB/c mouse, compared with control treatment. ELISA data further demonstrated that at both 1 and 5 days post-infection, protein expression levels of TNF-α (D), IL-6 (E), and IFN-γ (F) were decreased in EMMPRIN-silenced versus control-treated BALB/c mouse lung. Data represented three individual experiments each with five animals per group per time per assay.
EMMPRIN silence increases the nacterial load and PMN infiltration post the PA infection
The effect of EMMPRIN silence on the bacterial load and PMN infiltration was also evaluated in this study. The viable bacteria in the infected corneas was examined by observing the bacterial plate counts at 1 and 5 days post the infection. The results illustrated that the EMMPRIN silence increased the bacterial load at 5 days post the infection (Figure 3A, P<0.001). However, there were no changes at 1 day post the infection (P>0.05). Furthermore, the PMN infiltration (Figure 3B) was also increased in the corneas of the EMMPRIN silence mouse at both of 1 and 5 days post the infection (P<0.001). Moreover, the mRNA levels of CRAMP, NOX2, ROS generation in EMMPRIN silence group was also significantly decreased, which suggests that the EMMPRIN is required in both of the oxygen-dependent and oxygen-independent bacterial associated killing.
Figure 3.
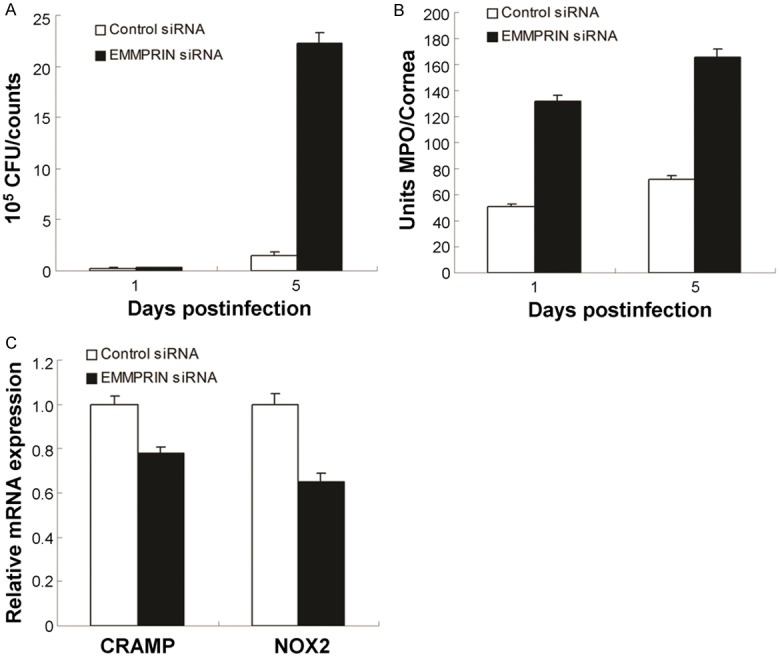
Silence of EMMPRIN enhanced bacterial load and PMN infiltration after PA infection. A. Bacterial load was enhanced in EMMPRIN siRNA versus control-treated corneas at 5 days post-infection. B. The number of infiltrated PMNs as detected by MPO assay was elevated in the corneas at 1 and 5 days post-infection. C. mRNA levels of CRAMP and NOX2 were decreased in EMMPRIN-silenced versus control-treated corneas at 5 days postinfection. Data represent three individual experiments each with five animals per group per time per assay.
Proinflammatory cytokines and Th1-/Th2-type production post EMMPRIN silence in vitro
We also tested the role of EMMPRIN in macrophage cells from BALB/c mice, which were transfected with the EMMPRIN siRNA or the scrambled control siRNA for 24 h. Then, the mice were challenged with the LPS derived from PA (with the concentration of 1 μg/mL) for 18 h. The PCR data showed that the Th1-type cytokines IFN-γ, IL-12, and IL-18 were also increased in EMMPRIN silence group (Figure 4A), however, the Th2-type cytokines IL-4, IL-5, and IL-10 were significantly decreased (Figure 4B) in the cells after LPS stimulation. Inflammatory cytokines IFN-γ increased and IL-4 decreased by employing in vitro culture of murine macrophages when EMMPRIN was knocked down (Figure 4C).
Figure 4.
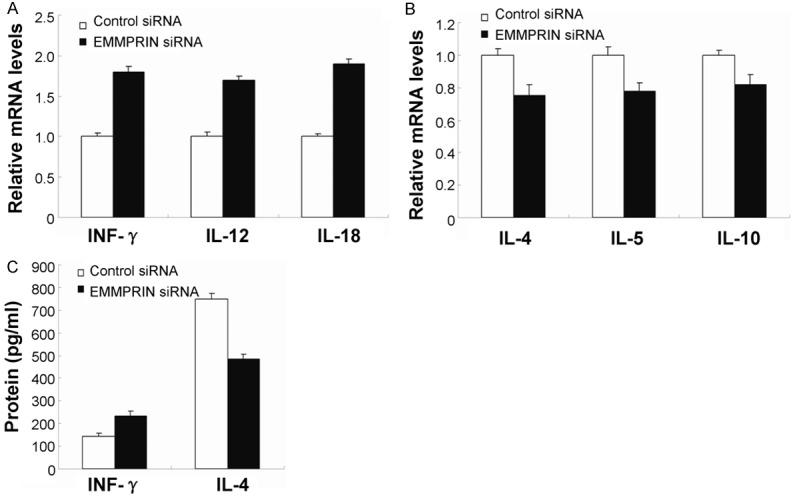
Expression of Th1/Th2 and proinflammatory cytokines after in vitro silence of EMMPRIN. PCR data demonstrated that in LPS stimulated RAW264.7 cells, silence of EMMPRIN increased expression of Th1 cytokines, such as IFN-γ, IL-12, and IL-18 (A) and decreased expression of Th2 cytokines, including IL-4, IL-5, and IL-10 (B), when compared with control treatment. (C) IFN-γ and IL-4 protein were analyzed by ELISA. Data represented three individual experiments.
EMMPRIN decreases proinflammatory cytokine levels through the MAPK signaling pathway
In order to assess the signaling pathway participated in the EMMPRIN-mediated immune regulation, the recombinant EMMPRIN was used to incubate the murine macrophage cells, which experiment could trigger the EMMPRIN signaling pathway. The data of RT-PCR (Figure 5A) and ELISA (Figure 5B) showed that ERK inhibitor U0126 inhibited pro-inflammatory cytokines TNF-in BALB/c peritoneal macrophages stimulated EMMPRIN. MMP9 was increased in the cells with rEMMPRIN treatment (Figure 5C). In order to further confirm the effects of MAPK signaling on the EMMPRIN-modulated immune response, the peritoneal macrophages isolated from the BALB/c mice were incubated with the inhibitors for ERK with LPS stimulation. The western blot results showed that the ERK signaling was involved in the EMMPRIN activation (Figure 5D). These data indicated that EMMPRIN promoted the proinflammatory cytokines levels through triggering the MAPK signaling pathway.
Figure 5.

EMMPRIN increased production of pro-inflammatory cytokines via MAPK signaling pathway. A. TNF-α, IFN-γ and IL-6 mRNA levels in the macrophages with mouse recombinant EMMPRIN or Erk inhibitor U0126 by real time RT-PCR. B. TNF-α, IFN-γ and IL-6 protein levels in the macrophages with mouse recombinant EMMPRIN or Erk inhibitor U0126 by ELISA. C. MMP9 protein levels in the macrophages with mouse recombinant EMMPRIN or Erk inhibitor U0126 by ELISA. D. Phosphorylated and total protein levels of ERK in mouse macrophages were examined by Western blot before and after treatment with recombinant EMMPRIN or ERK inhibitor U0126.
Discussion
According to the previous studies, we found that both of the reconstitution of the ECM and restoration of tissue homeostasis are very critical components for the host response to the infections or the diseases [19,20]. In the pathogenesis of the chronic inflammation and pathologic disease states, the extra-cellular matrices always with a progressive degradation characteristic [21,22]. Actually, the changed levels of ECM is main cause of the bystander tissue damage, which has been clearly illustrated of or results in the B6 mice disease pathogenesis during the infection. In this study, the EMMPRIN was destructed and the mechanism was also examined in the mouse. Meanwhile, in the BALB/c mice corneas, we also found the reorganization and the stromal healing clearly.
In our research, the EMMPRIN expression were increased significantly at the later time points post-infection. Whereas at 1 day, EMMPRIN2 mRNA and protein levels were decreased in the cornea of the infected BALB/c. According to the result of PA-induced EMMPRIN expression, we speculated that there was may be a promising relationship between the EMMPRIN and the PA keratitis. that the results showed that the EMMPRIN silence or inhibition could enhance the IFN-γ, IL-12, and IL-18 production, however, the EMMPRIN inhibition could decrease the IL-4, IL-5, and IL-10 expression in the corneas of the PA-infected BALB/c mouse. The above results suggested that EMMPRIN could enhance the host resistance against the PA infection via increasing the Th2-type immunol responses. Our study also indicated that the PA infection usually only cause a slight disease severity, meanwhile, the infected corneas could be healed within the 5 days after the infection. There, we speculated that the PA keratitis may be associated with the Th1- and Th2-type responses in the corneas.
In this study, we hypothesize that the enhanced EMMPRIN expression in the wound-healing phase may be due to the triggered EMMPRIN expression in the corneal epithelial cells and the induced EMMPRIN expression by the epithelial-derived signals pathway. The EMMPRIN silence enhanced the bacterial load in the corneas of the PA-infected BALB/c mouse. The bacterial load could aggravate the corneal damage and the disease development.
Moreover, our study illustrated that the phosphorylated Erk were increased in PA-infected corneas. Whereas when pretreatment with the Erk inhibitors, which could retain the EMMPRIN-mediated diseases of the pro-inflammatory cytokines. These results showed that the MAPK signaling pathway may be participated in the EMMPRIN associated immune regulation. These results are consistent with previous studies [23,24] that the MAPK inhibition could enhance the serum INF-γ, TNF-α, and IL-6 levels, and trigger the susceptibility to the sepsis in clinical.
In conclusion, the present study explored a novel investigation for the EMMPRIN function, further studied its regulatory effects in PA infection. Moreover, our results also illustrated that the EMMPRIN expression also plays an important role in the wound healing.
Acknowledgements
The study was granted by Scientific and technological project of Henan Province (Grant No. 132102310244), granted by the Henan University of Traditional Chinese Medicine Graduate Innovation Fund Project (Grant No. 201210) and also granted by the Education Department of Henan Province Science and Technology Key Project (Grant No. 14A320028).
Disclosure of conflict of interest
None.
References
- 1.Kernacki KA, Barrett RP, Hobden JA, Hazlett LD. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. J Immunol. 2000;164:1037–1045. doi: 10.4049/jimmunol.164.2.1037. [DOI] [PubMed] [Google Scholar]
- 2.Kernacki KA, Goebel DJ, Poosch MS, Hazlett LD. Early cytokine and chemokine gene expression during Pseudomonas aeruginosa corneal infection in mice. Infect Immun. 1998;66:376–379. doi: 10.1128/iai.66.1.376-379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res. 2002;21:1–14. doi: 10.1016/s1350-9462(01)00015-5. [DOI] [PubMed] [Google Scholar]
- 4.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 5.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo T, Takino T, Miyamori H, Thompson EW, Sato H. Substrate choice of membrane-type 1 matrix metalloproteinase is dictated by tissue inhibitor of metalloproteinase-2 levels. Cancer Sci. 2007;98:569–568. doi: 10.1111/j.1349-7006.2007.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Invest Ophthalmol Vis Sci. 2007;48:4360–4367. doi: 10.1167/iovs.06-1234. [DOI] [PubMed] [Google Scholar]
- 8.McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- 9.Redzic JS, Armstrong GS, Isern NG, Jones DN, Kieft JS, Eisenmesser EZ. The retinal specific CD147 Ig0 domain: from molecular structure to biological activity. J Mol Biol. 2011;411:68–82. doi: 10.1016/j.jmb.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin Z, Dai L, Slomiany MG, Toole BP, Parsons C. Direct activation of emmprin and associated pathogenesis by an oncogenic herpesvirus. Cancer Res. 2010;70:3884–3889. doi: 10.1158/0008-5472.CAN-09-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe A, Yoneda M, Ikeda F, Terao-Muto Y, Sato H, Kai C. CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells. J Virol. 2010;84:4183–4193. doi: 10.1128/JVI.02168-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pushkarsky T, Yurchenko V, Laborico A, Bukrinsky M. CD147 stimulates HIV-1 infection in a signal-independent fashion. Biochem Biophys Res Commun. 2007;363:495–499. doi: 10.1016/j.bbrc.2007.08.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt R, Redecke V, Breitfeld Y, Wantia N, Miethke T, Massberg S, Fischel S, Fischel S, Neumann FJ, Schomig A, May AE. EMMPRIN (CD 147) is a central activator of extracellular matrix degradation by Chlamydia pneumoniae-infected monocytes. Implications for plaque rupture. Thromb Haemost. 2006;95:151–158. [PubMed] [Google Scholar]
- 14.Zhou J, Zhu P, Jiang JL, Zhang Q, Wu ZB, Yao XY, Tang H, Lu N, Yang Y, Chen ZN. Involvement of CD147 in overexpression of MMP-2 and MMP-9 and enhancement of invasive potential of PMA-differentiated THP-1. BMC Cell Biol. 2005;6:25. doi: 10.1186/1471-2121-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi EY, Kim D, Hong BK, Kwon HM, Song YG, Byun KH, Park HY, Whang KC, Kim HS. Upregulation of extracellular matrix metalloproteinase inducer (EMMPRIN) and gelatinases in human atherosclerosis infected with Chlamydia pneumoniae: the potential role of Chlamydia pneumoniae infection in the progression of atherosclerosis. Exp Mol Med. 2000;34:391–400. doi: 10.1038/emm.2002.56. [DOI] [PubMed] [Google Scholar]
- 16.Pushkarsky T, Zybarth G, Dubrovsky L, Yurchenko V, Tang H, Guo H, Toole B, Sherry B, Bukrinsky M. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc Natl Acad Sci U S A. 2001;98:6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X, Song Z, Zhang S, Nanda A, Li G. CD147: A Novel Modulator of Inflammatory and Immune Disorders. Curr Med Chem. 2014;21:2138–2145. doi: 10.2174/0929867321666131227163352. [DOI] [PubMed] [Google Scholar]
- 18.Malesevic M, Gutknecht D, Prell E, Klein C, Schumann M, Nowak RA, Simon JC, Schiene-Fischer C, Saalbach A. Anti-inflammatory effects of extracellular cyclosporins are exclusively mediated by CD147. J Med Chem. 2013;56:7302–7311. doi: 10.1021/jm4007577. [DOI] [PubMed] [Google Scholar]
- 19.Berger EA, Vistisen KS, Barrett RP, Hazlett LD. Effect of VIP on corneal reconstitution and homeostasis following pseudomonas aeruginosa induced keratitis. Invest Ophthalmol Vis Sci. 2012;53:7432–7439. doi: 10.1167/iovs.12-9894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang HC, Fogo AB. Mechanisms of disease reversal in focal and segmental glomerulosclerosis. Adv Chronic Kidney Dis. 2014;21:442–447. doi: 10.1053/j.ackd.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attia M, Huet E, Gossard C, Menashi S, Tassoni MC, Martelly I. Early events of overused supraspinatus tendons involve matrix metalloproteinases and EMMPRIN/CD147 in the absence of inflammation. Am J Sports Med. 2013;41:908–917. doi: 10.1177/0363546512473817. [DOI] [PubMed] [Google Scholar]
- 22.Liu T, Zhai H, Xu Y, Dong Y, Sun Y, Zang X, Zhao J. Amniotic membrane traps and induces apoptosis of inflammatory cells in ocular surface chemical burn. Mol Vis. 2012;18:2137–2146. [PMC free article] [PubMed] [Google Scholar]
- 23.Kook SH, Choi KC, Lee YH, Cho HK, Lee JC. Raphanus sativus L. seeds prevent LPS-stimulated inflammatory response through negative regulation of p38 MAPK-NF-kB pathway. Int Immunopharmacol. 2014;23:726–734. doi: 10.1016/j.intimp.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Su Z, Lin R, Chen Y, Shu X, Zhang H, Nie R, Wang J, Xie S. Knokdown of EMMPRIN improves adverse remodeling mediated by IL-18 in the post-infarcted heart. Am J Transl Res. 2015;7:1908–1916. [PMC free article] [PubMed] [Google Scholar]


