Abstract
Object: To determine the potential of bone marrow-derived mesenchymal stem cells (BMSCs) for immunomodulatory mechanism in mice model of allergic rhinitis (AR). Methods: BMSCs were isolated and the surface markers and stemness were analyzed. The effect of BMSCs was evaluated in BALB/c mice that were randomly divided into three groups (control group, ovalbumin (OVA) group, OVA+BMSCs group). BMSCs were administered intravenously to OVA sensitized mice on days 1, 7, 14 and 21, and subsequent OVA challenge was conducted daily from days 22 to 35. Several parameters of allergic inflammation were assessed. Results: Mesenchymal stem cells can be successfully isolated from bone marrow of mice. Intravenous injection of BMSCs significantly reduced allergic symptoms, eosinophil infiltration, OVA-specific immunoglobulin E (IgE), T-helper 2 (Th2) cytokine profile (interleukin (IL)-4, IL-5 and IL-13) and regulatory cytokines (IL-10). In addition, level of Th1 (IFN-γ) was significantly increased. Conclusion: Administration of BMSCs effectively reduced allergic symptoms and inflammatory parameters in the mice model of AR. BMSCs treatment is potentially an alternative therapeutic modality in AR.
Keywords: Cell therapy, allergic rhinitis, immunosuppression, cytokines, mesenchymal stem cell
Introduction
Allergic rhinitis is a common chronic reversible inflammatory disease of the nasal passages inducing rhinorrhoea, nasal obstruction, nasal itching and sneezing [1]. AR affects up to 20% of adults in the United States [2] and is characterized by an influx of eosinophils and Th2 excessive activation [3]. There is growing evidence that the Th2 cytokines such as IL-3, IL-4, IL-5 and IL-13 down-regulated by T cells were on increase in AR patients [4]. AR aggravates other conditions, such as sinusitis, asthma and increase health-care cost [5]. Several new treatment modalities are attempted for reversing the established Th2 response, and numerous small-scale stem cell therapies are currently underway for allergic diseases [4].
Mesenchymal stem cells (MSCs) are ubiquitous multipotent cells capable of differentiating into several mesenchymal lineages, such as bone, cartilage, muscle and adipose tissue [6,7]. The experimental and clinical evidence indicate that MSCs could be effective anti-inflammatory cells for several diseases, including multiple asthma, graft-vs.-host disease, Crohn’s disease, multiple sclerosis and other inflammatory disorders [8-11]. In addition to the potential for therapeutic applications in tissue engineering and regenerative medicine [12,13], a growing body of evidence has demonstrated that MSCs exhibit strong immunomodulation potential, making them attractive candidates for the development of novel allogeneic cell-based therapeutic approaches in the treatment of a variety of immune diseases [14-16]. MSCs can modulate dendritic cell maturation [17], suppress natural killer cell function [18,19] and inhibit the allogeneic T cell response by altering the cytokine secretion profile of dendritic cells and T cells induced by an allogeneic immune reaction [18].
Few researches have investigated the immunomodulatory effects of BMSCs obtained from mice. In this study, we addressed the immunomodulatory effects of BMSCs on AR, providing a basis of further clinical applications of BMSCs on treating allergic diseases.
Materials and methods
Four-week-old male BALB/c mice were obtained from the Laboratory Animal Center of China Medical University. All experimental animal procedures used in this study were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Ethics Review Committee for Animal Experimentation of the China Medical University.
Extraction, isolation, and characterization of BMSCs
BMSCs were extracted from male BALB/c mice at 4 weeks of age, 18-20 g and were collected and cultured as described previously [18]. Briefly, under anesthesia with intravenous sodium pentobarbital (40 mg/kg), mice were euthanized and the bone marrow was flushed out of the femurs and tibias with Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA). The cells were washed once with DMEM and were centrifuged (400 g for 15 minutes), resuspended in Dulbecco’s modified Eagle’s medium, added to Ficoll-Hypaque (Histopaque 1083; Sigma-Aldrich, USA). The mononuclear cell fraction was washed for 3 times with DMEM. The cell pellets were plated in 25 cm2 culture flasks (Corning, USA) filled with 5 ml DMEM containing 10% FBS and 100 μg/ml penicillin/streptomycin. Cells were maintained in a humidified tissue culture incubator (37°C, 5% CO2) and the medium was changed subsequently every 3 days for further cultivation. When BMSCs reached 90% confluence, the cells were passaged by 0.25% trypsin and 0.05% EDTA (Gibco, USA) for analysis or transplantation. This study used BMSCs at their third passage.
To induce osteogenic differentiation, cells were cultured for 2 weeks in osteogenic medium (low-glucose DMEM supplemented with 10% FBS, 10 mM β-glycerophosphate, 0.1 mM dexamethasone, and 50 μg/ml ascorbic acid), as described previously [20]. Early mineralization was detected using Alizarin Red S. Cells were fixed with 70% ethanol and washed for 3 times with distilled water. BMSCs were incubated in 2% alizarin red solution for 15 minutes at room temperature and washed for 3 times with distilled water. For adipogenic differentiation, cells were cultured in adipogenic differentiation medium (high-glucose DMEM containing 10% FBS, 100 μg/ml 3-isobutyl-1-methylxanthine, 100 μM indomethacin, 10 μM bovine insulin, and 1 μM dexamethasone), and Oil Red-O staining was performed after 14 days [21]. The cells were fixed at room temperature with 70% ethanol for 15 minutes and incubated in 2% Oil Red O reagent for 1 hour at room temperature. 70% ethanol was employed to wash excess stain, followed by several changes of distilled water. For phenotypic characterization, approximately 105 cells were incubated for 30 min with monoclonal antibodies to CD29, CD44, CD45 and CD34 labeled with fluorescein isothiocyanate or phycoerythrin. Cells were analyzed using fluorescence-activated cell sorter (FACSCalibur, BD Biosciences, USA) and Cell Quest software. The absence of CD34 and CD45 and the presence of CD44 and CD29 were used to identify MSCs.
Allergen sensitization and nasal challenge
The sensitization and antigen challenge for the murine model of AR were induced as previously described with minor modification [22]. Briefly, under pathogen-free conditions, mice were sensitized using OVA (Sigma-Aldrich, St. Louis, MO, USA) as follows. Ovalbumin (40 µg/kg) OVA diluted in sterile phosphate-buffered saline (PBS) was administered along with 1 mg aluminum in a total volume of 200 μl PBS (alum adjuvant, 40 mg/kg) to mice four times by intraperitoneal injection on days 1, 7, 14, and 21. This was followed by daily intranasal challenge with OVA diluted with sterile normal saline intranasally (20 µl of 25 mg/ml OVA per mouse) from days 22 to 35. Twenty-four hours after the last OVA challenge, blood, spleen tissues, and nasal cavity tissues were collected from each mouse. Mice were divided into three groups, with eight mice in each group. In control group, mice were sensitized, treated, and challenged with PBS. In OVA group mice were sensitized with OVA and Alum and challenged with OVA, but instead of injection of BMSCs, PBS was injected. In OVA+BMSCs group, mice were sensitized with OVA plus Alum, injected BMSCs, and challenged with OVA. In BMSCs group, 0.5×106 into 100 µl PBS of BMSCs were administered intravenously on days 22 to 35 of the experimental period (Figure 1).
Figure 1.
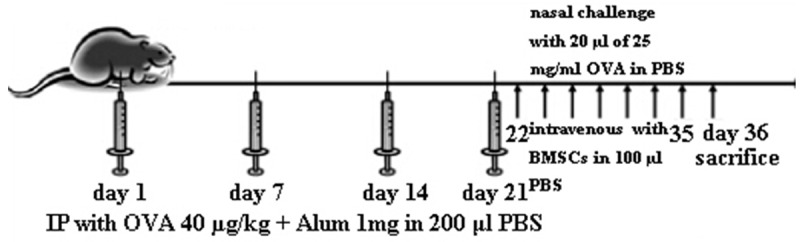
Allergic rhinitis model. On days 1, 7, 14, and 21, mice received an intraperitoneal (ip) injection of 40 µg/kg of ovalbumin (OVA) and 1 mg of alum in 200 µl of PBS. One week after the last OVA sensitization (day 21), a series of 14 daily intranasal (in) OVA (20 µl of 25 mg/ml) challenges were administered. The mice were killed 24 h after the last nasal challenge for further analyses.
Allergic nasal symptoms
Twenty four hours after the final nasal challenge with OVA, the frequencies of sneezing and nasal rubbing were recorded for each mouse by blinded observers, over a 15 min interval. The mice were then killed 24 h after the last nasal challenge for further analyses [23].
Nasal histology
Twenty four hours after the final OVA nasal challenge, nasal cavity tissues were removed from the mice, fixed in 4% paraformaldehyde, embedded in paraffin. Samples were cut into 4 µm cross sections and were stained with hematoxylin and eosin. The numbers of eosinophils were counted in the nasal septal mucosa under a light microscope (400 magnification).
Real-time RT-PCR for IL-4, IL-5, IL-10, IL-13 and IFN-γ in the nasal mucosa
Total RNA was isolated from the nasal mucosa using TriZol reagent (Invitrogen, USA). Complementary DNA (cDNA) was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories; CA). For analysis of IL-4 (Mm00445258_g1), IL-5 (Mm00439646_m1), IL-13 (Mm4331182-m1), IL-10 (Mm00439616_m1), IFN-γ (Mm99999071_m1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Mm03302249_g1). Amplification of IL-4, IL-5, IL-10, IL-13, IFN-γ, and GAPDH cDNA was carried out in MicroAmp optical 96-well reaction plates (Applied Biosystems). The reaction was performed using an Applied Biosystem 7500 Real-Time PCR System (Applied Biosystems). The average transcript levels of genes were then normalized to GAPDH. The relative mRNA gene expression was calculated by using the 2-∆∆Ct method.
Expression of cytokines in the spleen
24 hours after the last OVA challenge, the spleen was removed and collected in a tissue culture petri dish containing 3 ml of culture medium. 5 ml syringe was used to crush the tissue. Then, the crushed tissue was treated with lysis buffer containing potassium bicarbonate and ammonium chloride to deplete erythrocytes. Spleen single-cell suspensions (105 cells/well in 96-well culture plates) were cultured in RPMI 1640 medium supplemented with 10% FBS and stimulated with OVA (100 ng/ml). The cells were incubated in a CO2 incubator at 37C for 72 h. IL-4, IL-5, IL-10, IL-13 and interferon (IFN)-γ levels were measured by ELISA using an ELISA kit (Abcam, UK) followed the instruction of manufacturer.
Measurement of OVA-specific IgE and total IgE
Serum levels of OVA-specific IgE, IgG1 and IgG2 were measured by solid-phase enzyme-linked immunosorbent assay (ELISA) in accordance with the manufacturer’s instructions. Bound immunoglobulin isotypes were detected with specific secondary antibody (biotin-conjugated rat anti-mouse IgE, IgG1 and IgG2a Abs were purchased from BD Pharmingen, San Jose, CA, USA).
Statistical analysis
Data are presented as mean standard error of the mean. Comparisons between groups were made by the Kruskall-Wallis test followed by Dun’s test using the SPSS software package version 13.0 (SPSS Inc., Chicago, IL) and P < 0.05 was considered as statistical significant.
Results
Morphology and characterization of ADSCs
BMSCs isolated from femurs and tibias had the ability to self-renew and adhere to plastic and expanded in culture without losing differentiation potential. Flow cytometric analysis demonstrated that BMSCs were positive for CD29, CD44, but negative for CD45 and CD34 (Figure 2A-D) as reported previously [24]. These cells could be induced to differentiate into mature adipocytes, which were confirmed by intracellular lipid vacuoles after Oil Red-O staining (Figure 2E). Osteogenic differentiation was confirmed by the deposition of calcium as demonstrated by alizarin red staining (Figure 2F).
Figure 2.
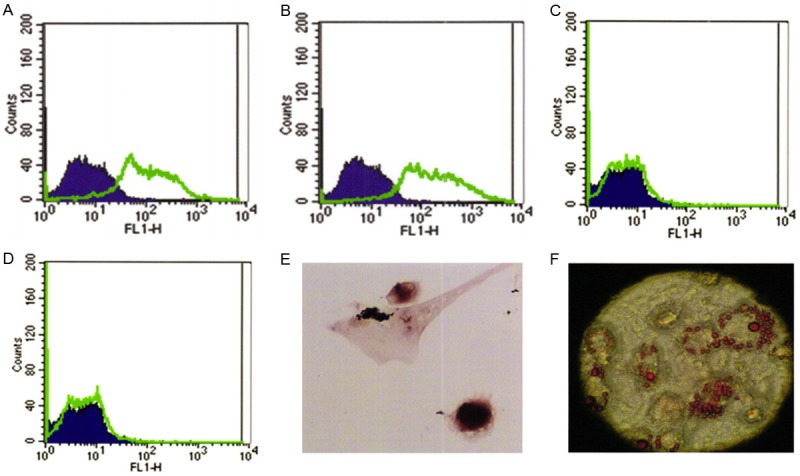
Surface markers and induced differentiation of BMSCs at passage 3. A. CD29. B. CD44. C. CD45. D. CD34. E. Alizarin red staining after osteogenic induction. F. Oil Red-O staining after adipogenic induction.
BMSCs reduced the nasal symptoms in OVA-sensitized mice
To investigate the effect of BMSCs on nasal symptoms, the frequency of sneezing and nasal rubbing was counted for 15 min after the last OVA sensitization. As shown in Figure 3, ovalbumin sensitized animals showed manifestation of allergic rhinitis symptoms as compared to non-sensitized mice. However, BMSCs significantly decreased the number of nasal rubbing and sneezing compared to the group undergoing treatment with OVA only demonstrating that BMSCs inhibits OVA-induced allergic nasal symptoms (Figure 3E, 3F). Histopathologic analysis of the nasal mucosa obtained from the OVA group revealed greatly typical pathologic features of AR in the submucosa which was infiltrated with numerous inflammatory cells. There were significant differences in the gross appearance of cross sections between BMSCs-treated mice and control mice. BMSCs protected the nasal mucosa against damages and greatly reduced infiltration of eosinophils (Figure 3A-D). This data suggested that BMSCs could prevent the rhinitis symptoms.
Figure 3.
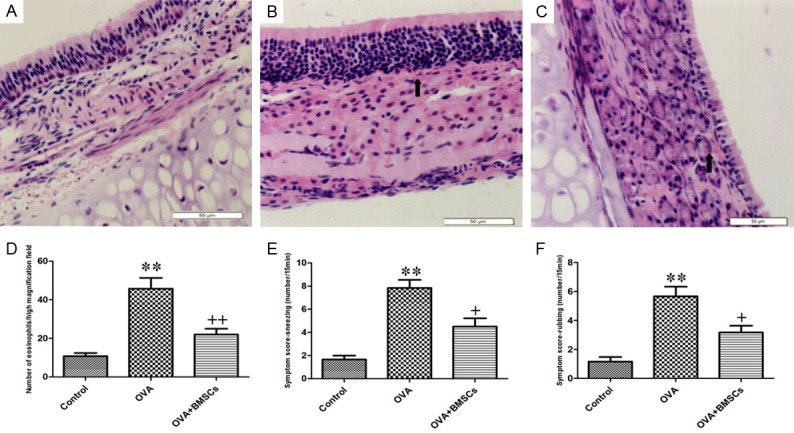
Comparison of nasal mucosa histopathology, eosinophilic infiltration and nasal symptoms among three groups. A. Control group. B. OVA group. C. OVA+BMSCs group. D. Eosinophil counts in the nasal mucosa. E. Sneezing. F. Nasal rubbing. *P < 0.05; **P < 0.01 vs control group. +P < 0.05; ++P < 0.01 vs OVA group.
Effect on nasal cytokine mRNA expressions
To investigate further the immunomodulatory effects of BMSCs on the T-cell phenotype, we examined the effects of BMSCs on mRNA expression levels of cytokine production (IFN-γ representing the Th1 phenotype; IL-4, IL-5 and IL-13 representing the Th2 phenotype; and IL-10 representing the Treg phenotype) in the nasal mucosa (Figure 4A). The treatment of BMSCs significantly reduced IL-10 levels compared with the OVA group (P < 0.01). On the other hand, the level of IFN-γ was significantly increased and the levels of IL-4, IL-5 and IL-13 were significantly decreased in the BMSCs group compared with the OVA group (P < 0.05).
Figure 4.
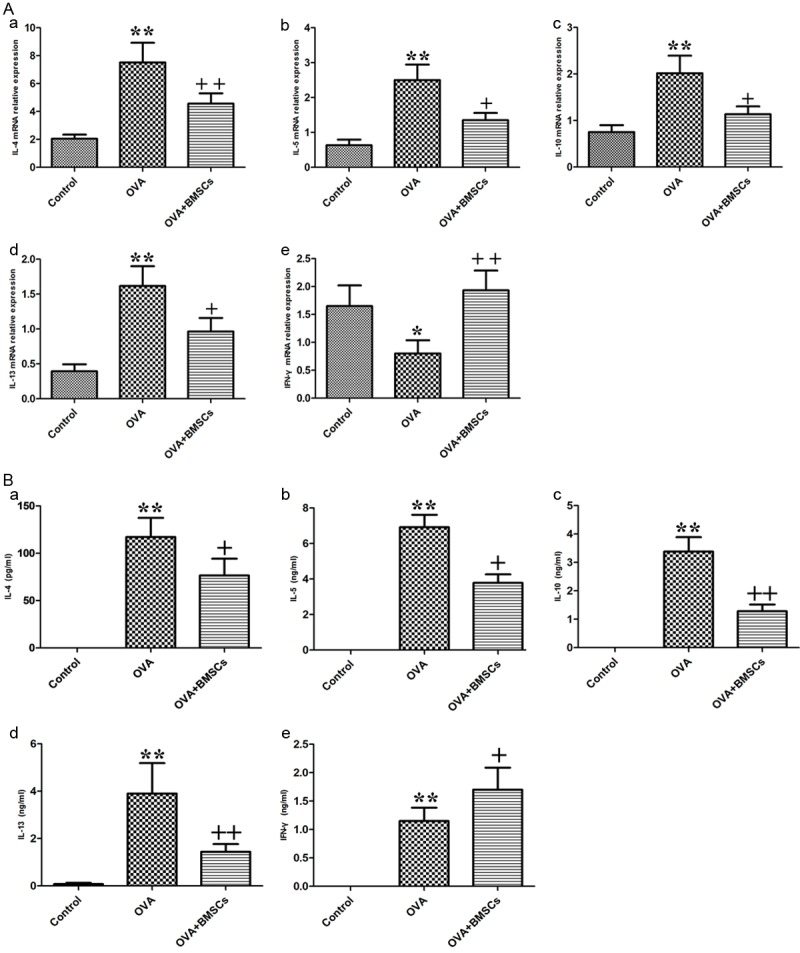
Effects of BMSCs engraftment on levels of inflammatory cyokines. A. Cytokine mRNA expression levels in the nasal mucosa (IL-4, IL-5, IL-10, IL-13, and IFN-γ). B. Systemic cytokine levels (IL-4, IL-5, IL-10, IL-13, and IFN-γ) in the splenocyte. *P < 0.05; **P < 0.01 vs control group. +P < 0.05; ++P < 0.01 vs OVA group.
Effect on systemic cytokine profile
The systemic cytokine profile from spleen culture also demonstrated a similar trend (Figure 4B) as shown in the nasal mucosa. IL-4, IL-5, and IL-13 protein concentrations were significantly increased and the level of IFN-γ was significantly decreased in the OVA group compared with the BMCSs group (P < 0.05). IL-10 levels in BMSCs group were significantly decreased compared with the control group (P < 0.01).
Effect on immunoglobulin levels
The OVA group had increased serum OVA specific IgE, IgG1, and IgG2a levels compared with the control group. The BMSCs group had significantly decreased OVA-specific IgE, and IgG1 levels (P < 0.01). However, the OVA-specific IgG2a level did not change significantly in both treatment group compared with the OVA group (Figure 5).
Figure 5.
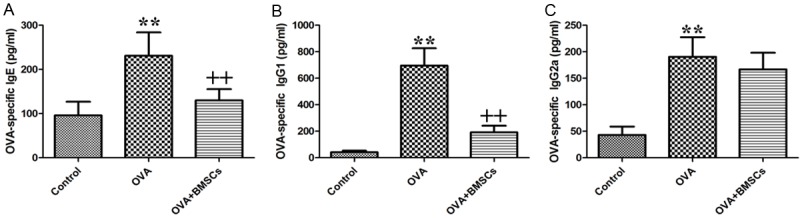
Effects of BMSCs on serum OVA-specific antibodies. (A) OVA-specific IgE. (B) IgG1. And (C) IgG2a. *P < 0.05; **P < 0.01 vs control group. +P < 0.05; ++P < 0.01 vs OVA group.
Discussion
The objective of this study was to investigate the effect of BMSCs on mice model of AR. In our study, we showed that BMSCs had significant inhibitory effects on allergic inflammation, which is consistent with other studies on AR [4,8,25-27]. We have shown that BMSCs inhibited allergic nasal symptoms, including rubbing and sneezing, and reduced the numbers of eosinophils in nasal mucosa. BMSCs also decreased Th2 cytokine (IL-4, IL-5 and IL-13) levels and regulatory cytokines (IL-10) significantly. In contrast, levels of Th1 (IFN-γ) increased significantly after BMSCs treatment. Furthermore, OVA-specific IgE and IgG1 levels were significantly decreased by BMSCs treatment.
Over the past decade, MSCs have attracted remarkable interest due to their stemness of differentiating into various cell type, which makes them attractive for regenerative medicine [24]. In addition, MSCs possess powerful immunomodulatory ability which support their therapeutic use for immune mediated diseases [28,29]. Although MSCs have not been used in the clinic, these results demonstrate the potential beneficial role of these cells for the treatment of allergic airway diseases. The symptoms of AR is by eosinophilic dependent inflammation and T-helper 2 (Th2) excessive activation [30]. Evidence has shown that the Th2 cytokines which include IL-4, IL-5 and IL-13 down-regulated by T cells were elevated in AR patients [4]. It was reported that these interleukins could inhibit the functions of Th1 immune response [31]. IL-10 also plays a crucial role in down-regulate the activities of Th1 [32]. IFN-γ is the principal Th1 effector cytokine which affects Th1/Th2 differentiation and triggers the production of macrophages and also inhibits Th2 cell proliferation [33]. In this study, the levels of Th2 cytokines (IL-4, IL-5, and IL-13) were significantly decreased after the addition of BMSCs, in then meantime IL-10 levels were significantly reduced. The eosinophilic inflammation controlled by these interleukins is also reduced by BMSCs treatment. These results support the proposal that shift from Th2 to Th1 occurs after BMSCs injection. In contrast, IFN-γ was increased in the presence of BMSCs compared to OVA group which provide another evidence of the ability of BMSCs on shifting Th2 to Th1 immune response to allergens. The balance between Th1 and Th2 is crucial to avoid AR. The shift from Th2 to Th1 caused by BMSCs injection may be a feasible way to control AR, because the excessive activation of Th2 was identified to be a leading cause of AR.
Since immunoglobulins play major roles in mediating allergy and inflammatory reactions, we investigated the expressions of several main immunoglobulin antibodies (IgE, IgG1 and IgG2a) that have been implicated in B-cell immune responses controlled by cytokines from Th cells. As shown in Figure 5, the level of IgE, a Th2 dependent antibody was significantly increased in OVA group than that in control group. The injection of BMSCs had a statistically significant tendency to reduce the secretion of IgE, suggesting BMSCs may down-regulate Th2 immune responses. Administration of BMSCs also significantly decreased OVA-induced IgG1 serum levels. No impressive difference of IgG2a levels was observed between OVA and BMSCs groups. This reveals the Th1 immune response may be priming by the mounting of IgG1 production [34]. In another words, increase of IgG1 and the maintenance of IgG2 suggests the shift of Th2 to Th1 immune response. This shift may be responsible for BMSCs to regulate immunoglobulin production in allergic disease.
Although the experimental design was similar to that of conventional studies using rodent AR models and BMSCs infusion, there were some limitations associated with the current study, because not all the possible ranges of variables were investigated. First, we did not provide control for the effects of BMSCs on mice without induction of OVA. However, our found that BMSCs did not aggravate injury and can alleviate injury. Therefore, it would be expected that these influence on these animals would be limited. Second, the exact mechanisms underlying the observed improvement in the model of AR through BMSCs administration are likely to be more complex and remain to be elucidated.
Based on the collective results, administration of BMSCs significantly reduced inflammatory parameters and allergic symptoms in the model of AR. BMSCs treatment decreased nasal eosinophil infiltration, Th2 cytokine and OVA-specific IgE secretion in the nasal mucosa. Therefore, BMSCs treatment is a potential therapeutic modality in AR.
Disclosure of conflict of interest
None.
References
- 1.Ciprandi G, Cirillo I, Klersy C, Marseglia GL, Caimmi D, Vizzaccaro A. Nasal obstruction is the key symptom in hay fever patients. Otolaryngol Head Neck Surg. 2005;133:429–435. doi: 10.1016/j.otohns.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 2.Settipane RA. Demographics and epidemiology of allergic and nonallergic rhinitis. Allergy Asthma Proc. 2001;22:185–189. [PubMed] [Google Scholar]
- 3.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun YQ, Deng MX, He J, Zeng QX, Wen W, Wong DS, Tse HF, Xu G, Lian Q, Shi J, Fu QL. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells. 2012;30:2692–2699. doi: 10.1002/stem.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106:S12–16. doi: 10.1016/j.anai.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow-and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 7.Delorme B, Charbord P. Culture and characterization of human bone marrow mesenchymal stem cells. Methods Mol Med. 2007;140:67–81. doi: 10.1007/978-1-59745-443-8_4. [DOI] [PubMed] [Google Scholar]
- 8.Cho KS, Park HK, Park HY, Jung JS, Jeon SG, Kim YK, Roh HJ. IFATS collection: Immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells. 2009;27:259–265. doi: 10.1634/stemcells.2008-0283. [DOI] [PubMed] [Google Scholar]
- 9.Vaes B, Van’t Hof W, Deans R, Pinxteren J. Application of MultiStem((R)) Allogeneic Cells for Immunomodulatory Therapy: Clinical Progress and Pre-Clinical Challenges in Prophylaxis for Graft Versus Host Disease. Front Immunol. 2012;3:345. doi: 10.3389/fimmu.2012.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyer SS, Co C, Rojas M. Mesenchymal stem cells and inflammatory lung diseases. Panminerva Med. 2009;51:5–16. [PubMed] [Google Scholar]
- 11.Kapoor S, Patel SA, Kartan S, Axelrod D, Capitle E, Rameshwar P. Tolerance-like mediated suppression by mesenchymal stem cells in patients with dust mite allergy-induced asthma. J Allergy Clin Immunol. 2012;129:1094–1101. doi: 10.1016/j.jaci.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 12.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klingemann H, Matzilevich D, Marchand J. Mesenchymal Stem Cells - Sources and Clinical Applications. Transfus Med Hemother. 2008;35:272–277. doi: 10.1159/000142333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466–2477. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- 15.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 16.Rafei M, Campeau PM, Aguilar-Mahecha A, Buchanan M, Williams P, Birman E, Yuan S, Young YK, Boivin MN, Forner K, Basik M, Galipeau J. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 17.English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115:50–58. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 19.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 20.Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 21.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 22.Saito H, Matsumoto K, Denburg AE, Crawford L, Ellis R, Inman MD, Sehmi R, Takatsu K, Matthaei KI, Denburg JA. Pathogenesis of murine experimental allergic rhinitis: a study of local and systemic consequences of IL-5 deficiency. J Immunol. 2002;168:3017–3023. doi: 10.4049/jimmunol.168.6.3017. [DOI] [PubMed] [Google Scholar]
- 23.Rhee CS, Libet L, Chisholm D, Takabayashi K, Baird S, Bigby TD, Lee CH, Horner AA, Raz E. Allergen-independent immunostimulatory sequence oligodeoxynucleotide therapy attenuates experimental allergic rhinitis. Immunology. 2004;113:106–113. doi: 10.1111/j.1365-2567.2004.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 25.Desai MB, Gavrilova T, Liu J, Patel SA, Kartan S, Greco SJ, Capitle E, Rameshwar P. Pollen-induced antigen presentation by mesenchymal stem cells and T cells from allergic rhinitis. Clin Transl Immunology. 2013;2:e7. doi: 10.1038/cti.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho KS, Park MK, Kang SA, Park HY, Hong SL, Park HK, Yu HS, Roh HJ. Adipose-derived stem cells ameliorate allergic airway inflammation by inducing regulatory T cells in a mouse model of asthma. Mediators Inflamm. 2014;2014:436476. doi: 10.1155/2014/436476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, LeClair L, Poynter ME, Steele C, Rincon M, Weiss DJ. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells. 2011;29:1137–1148. doi: 10.1002/stem.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 29.Eslaminejad MB, Nikmahzar A, Taghiyar L, Nadri S, Massumi M. Murine mesenchymal stem cells isolated by low density primary culture system. Dev Growth Differ. 2006;48:361–370. doi: 10.1111/j.1440-169X.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 30.Fu QL, Chow YY, Sun SJ, Zeng QX, Li HB, Shi JB, Sun YQ, Wen W, Tse HF, Lian Q, Xu G. Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy. 2012;67:1215–1222. doi: 10.1111/j.1398-9995.2012.02875.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998;95:3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly JL, Lyons A, Soberg CC, Mannick JA, Lederer JA. Anti-interleukin-10 antibody restores burn-induced defects in T-cell function. Surgery. 1997;122:146–152. doi: 10.1016/s0039-6060(97)90003-9. [DOI] [PubMed] [Google Scholar]
- 33.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 34.Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, Makarova N, Perez DR, Macdonald GH, McCullers JA. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol. 2006;13:981–990. doi: 10.1128/CVI.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


