Abstract
Pyroptosis is a programmed cell death associated with caspase-1 and accompanied by the secretion of a large number of pro-inflammatory cytokines. In the acute stage of sepsis, the release of several pro-inflammatory cytokines aggravates hepatic cell death, and acute liver injury is aggravated with the progress of the disease, resulting in acute liver failure with a very high mortality rate. The present study investigated the effect of inhibiting hepatic cell pyroptosis on the septic acute liver injury. Septic acute liver injury mice model was established by cecal ligation and puncture (CLP model). The liver tissues were assessed for inflammatory infiltration by HE, serum concentrations of ALT, AST, IL-1β, and IL-18 were examined by ELISA, hepatic cell pyroptosis was determined by flow cytometry, and expressions of caspase-1 and NLRP3 were assessed by Western blot. CLP-induced acute liver injury was distinct at 24 h post-operation, with the highest hepatic cell pyroptosis rate. The pyroptosis rate and liver injury indexes were positively correlated. Western blot showed that the expressions of pyroptosis-related proteins, caspase-1, and NLRP3, were increased. Normal mouse hepatic cells were cultured in vitro and LPS+ATP introduced to establish the cell model of septic acute liver injury. The expressions of caspase-1, NLRP3, IL-1β, and IL-18 in LPS+ATP group were significantly higher than the control group by Western blot and ELISA. The inhibitors of NLRP3 (Glyburide) and caspase-1 (AC-YVAD-CMK) alone or in combination were used to pre-treat the hepatic cells, which revealed that the pyroptosis rate was decreased and the cell damage alleviated. The in vivo assay in rats showed that post inhibitor treatment, the 10-days survival was significantly improved and the liver damage reduced. Therefore, inhibiting the hepatic cell pyroptosis could alleviate CLP-induced acute liver injury, providing a novel treatment target for septic acute liver injury.
Keywords: Sepsis, acute liver injury, pyroptosis
Introduction
Sepsis is the primary cause of death in the intensive care unit (ICU), with an incidence of 50-80%, its rapid onset and progression have led to a major concern to be resolved in a clinical setting [1,2]. In the acute phase of sepsis, the pattern recognition receptor and its downstream signaling pathway mediate a strong inflammatory response, which can activate a variety of inflammatory cells, release a large number of pro-inflammatory cytokines, in order to kill and remove pathogens. However, excessive release of inflammatory mediators may lead to uncontrolled inflammation and induce systemic inflammatory response syndrome, which further damages the tissues and organs of patients [3-5]. As a vital immune organ and the energy metabolism center of the body, liver is one of the most frequently affected target tissues in the acute phase of sepsis. The structure, function, and metabolism changes in liver could affect the progress of sepsis, and severe liver injury can also lead to multiple organ dysfunction syndrome (MODS) [6]. Therefore, exploring the pathological mechanism and effective treatment for sepsis is highly significant to prevent the secondary multiple organ failures and improve the prognosis of sepsis.
Present, although several studies suggest that excessive inflammatory response is the main pathogenesis of acute liver injury in sepsis, the use of anti-inflammatory drugs alone does not improve the acute liver injury remarkably [7-10]. Thus, further investigation of the molecular mechanisms regulating the inflammatory balance in sepsis-induced acute liver injury is essential. Related studies show that multiple cell death pathways play a major role in the regulation of blood coagulation, complement system, pro-inflammatory cytokines, and anti-inflammatory cytokines [11]. Apoptosis is a programmed cell death, which has been shown to play a regulatory role in a wide variety of biological processes. Previous studies have indicated that in the acute phase of sepsis, the excessive inflammatory response can induce apoptosis, and also, apoptosis can promote the inflammatory reaction [12,13]. Chida et al. found aggravated hepatic cell apoptosis in acute liver injury induced by sepsis, and the inhibition of apoptosis could efficiently alleviate the acute liver injury [14].
Pyroptosis is a novel programmed cell death that has been discovered and verified recently. The phenomenon is characterized by caspase-1 dependence and is associated with the release of a large number of pro-inflammatory cytokines. Currently, the leading mechanism of pyroptosis is speculated as NLRP3- the inflammoasome of immune cells in the body, which is activated by pathogens. It further induces the local aggregation of inactive caspase precursor-1 (Pro-caspase-1) and promotes its hydrolysis into active caspase activity-1 (Caspase-1), which can shear the inactive IL-1β precursor and IL-18 precursor into active IL-1β and IL-18, causing pyroptosis [15-17]. Several studies have shown that pyroptosis plays a regulatory role in many infectious and noninfectious diseases [18-20]; however, little is known about its functions in septic acute liver injury.
Based on the animal and cell models of septic acute liver injury, the present study explored the relationship between pyroptosis and septic acute liver injury by HE staining, flow cytometry, ELISA, and Western Blot, to find the potential treatment target for alleviating the acute liver injury induced by sepsis.
Materials and methods
Animals
Male C57BL/6 mice, aged 6-8 weeks, 18-22 g, were purchased from SHANGHAI SLAC LABORATORY ANIMAL CO. LTD. Mice were housed at 21-23°C with a 12 h light cycle with free access to food and water in the Shanghai Pulmonary Hospital. All animal experiments were performed according to the guidelines for the care and use of animals and approved by the Animal Care and Use Committee of the Tongji University School of Medicine (TUSM, Shanghai, China).
CLP model
Sepsis was induced by cecal ligation and puncture (CLP) as described previously [21]. Mice were completely anesthetized with 0.75% pentobarbital solution (10 µg/g) and a midline abdominal incision was performed. The cecum was ligated at the 1/2 of the distal end and was perforated by sterile needles no. 7 to induce polymicrobial peritonitis. The abdominal wall was sutured in two layers and injected subcutaneously with 1 mL 0.9% sodium chloride solution for fluid resuscitation. The animals in the sham group underwent laparotomy and bowel manipulation without ligation and perforation. All mice had free access to food and water after recovery from anesthesia.
Experimental design
Animal model
The animals were male C57BL/6 mice: sham and CLP groups (n = 5 per time point). Blood was collected by an intracardiac puncture at the indicated time points after surgery. Whole blood was stored 2 h at room temperature, serum isolated by centrifugation at 5000 rpm for 15 min, and preserved at -80°C until further usage. The livers were harvested at 6, 12, or 24 h after CLP (or sham operation); one part was fixed in 4% paraformaldehyde and processed for hematoxylin-eosin (HE) staining, some other parts were snap-frozen in liquid nitrogen and stored at -80°C for subsequent biochemical analysis and the remaining parts were homogenized into single cell suspension for flow cytometry.
Caspase inhibitor experiments included WT mice exposed to CLP (n = 6 each), and the sham controls (n = 3 each). The CLP mice were peritoneally injected with the caspase-1 inhibitor, AC-YVAD-CMK (100 μg per mouse, R&D Systems, Minneapolis, MN, USA), and NLRP3 inhibitor, Glyburide (500 mg per mouse, R&D Systems), (n = 3) or 0.1% DMSO (n = 3) 30 min before CLP. Blood samples of all the mice were collected after 24 h in the sham group or after CLP. The livers were harvested at 24 h after CLP (or sham operation), and the samples were treated as mentioned above. All the 30 mice were challenged to obtain the survival curve (sham, CLP, and CLP plus inhibitor, n = 10 each group).
Cell model
The isolated hepatic cells from WT mouse were cultured in the indicated medium. The cells were treated with 100 ng/mL LPS for 3 h, then stimulated with 5 mmol/L ATP for 36 h, and/or supplemented with caspase-1 inhibitor and/or NLRP3 inhibitor. The supernatants were collected and frozen as above for enzyme-linked immunosorbent assay (ELISA). The cells were harvested for flow cytometry or Western blot.
HE staining
Liver tissue was fixed in 4% formaldehyde for at least 24 h. The sections (4 μm) were paraffin embedded using standard techniques and stained with hematoxylin-eosin.
ELISA
IL-1β, IL-18, AST, and ALT levels in serum or supernatant were determined by ELISA kits (R&D Systems) according to the manufacturer’s instructions.
Flow cytometry
The single cell suspension of the livers or the cultured cells was washed twice and 1×106 cells were suspended in 50 µL PBS supplemented with 1% FBS. The cells were stained with FLICA (FAM-VAD-FMK655, ImmunoChemistry Technologies) for 40 min at 37°C, followed by the addition of 7AAD (BD PharmingenTM) before loading. The stained cells were analyzed with a FACS Calibur flow cytometer and the data with FlowJo software.
Western blot analysis
The liver tissues or cultured hepatic cells were homogenized in RIPA buffer in the presence of PMSF (Beyotime Institute of Biotechnology, Jiangsu, China) and clarified by centrifugation at 12000×g for 15 min at 4°C. The protein concentration in the supernatant was measured by the Bradford method. Equivalent amounts of denatured protein were separated on 6-12% SDS/PAGE and transferred to poly (vinylidene difluoride) membranes (Millipore, Shanghai, China). The membranes were blocked with 5% skimmed milk in NaCl/Tris for 2 h and incubated at 4°C overnight with the indicated primary antibodies. For detection of immunoreactive bands, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies; the bands were visualized with the phototope-horseradish peroxidase Western blot detection system (Cell Signaling Technologies, Beverly, MA, USA), and quantified by densitometry using ImageJ. The primary antibodies and concentrations were as follows: anti-NLRP3 (1:1000; Abcam, Cambridge, MA, USA), anti-active caspase-1 p10 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-β-actin (1:1000; Abcam).
Cell isolation
The hepatic cells were obtained using an in situ collagenase (Sigma, St. Louis, MO, USA) perfusion technique as described previously [22]. The cells were purified by three differential sedimentations at 50×g for 2 min.
Statistical analysis
SPSS18.0 software (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA) were used for statistical analysis. All data were expressed as mean ± SD. The statistical analyses were performed using Student’s t-test, one-way ANOVA with posthoc tests, or the Pearson’s correlation coefficient. The survival curve was estimated by the Kaplan-Meier method. The significant differences were considered at P < 0.05 and P < 0.01.
Results
Correlation between hepatic cell pyroptosis and the degree of acute liver injury induced by sepsis
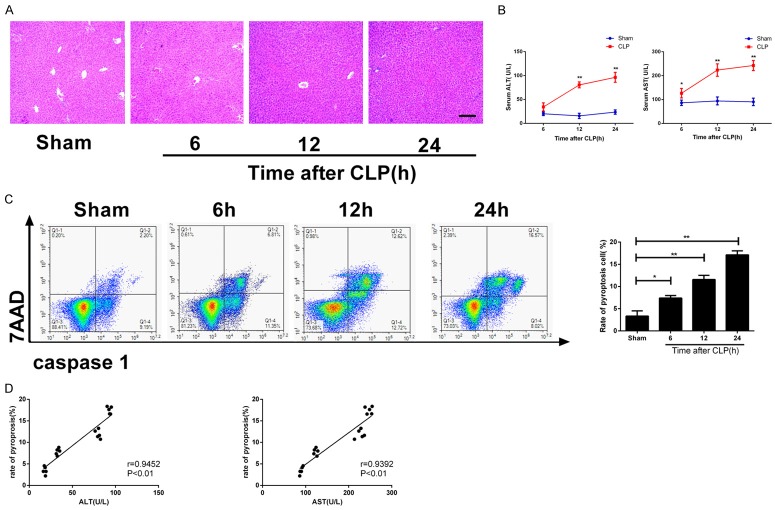
CLP-induced acute liver injury model was established. The HE staining results of liver tissues showed that the inflammatory cell infiltration degree of the liver in CLP group was severe than that in the sham operation group at 6, 12, and 24 h post-operation, and the degree of inflammatory cell infiltration was in a time-dependent manner (Figure 1A). ELISA results showed that at 6, 12, and 24 h after modeling, the serum concentrations of ALT and AST in the CLP group were higher than those in the sham operation group, and the concentrations elevated in a time-dependent manner (*P < 0.05, **P < 0.01; Figure 1B). Flow cytometry of hepatic cells showed that at 6, 12, and 24 h after modeling, the pyroptosis rate of hepatic cells in CLP group was higher than that of the sham operation group, and the rate was also in a time-dependent manner (*P < 0.05, **P < 0.01; Figure 1C). ALT and AST concentrations were positively correlated with the degree of hepatic cell pyroptosis in acute liver injury (r = 0.9452, P < 0.01; r = 0.9392, P < 0.01; Figure 1D).
Figure 1.
The degree of septic acute liver injury is associated with hepatic cell pyroptosis. Serum and liver tissue samples were collected at 6, 12, and 24 h after modeling. A. HE staining of liver tissues from each group (100×); B. Serum concentrations of ALT and AST; C. Hepatic cell pyroptosis examined by flow cytometry, left: two-dimensional graph, right: histogram; D. Correlation analysis of serum ALT and AST concentrations and hepatic cell pyroptosis rate. *P < 0.05; **P < 0.01, vs. sham operation group.
Increased expression of pyroptosis-related proteins indicated severe cell damage in acute liver injury
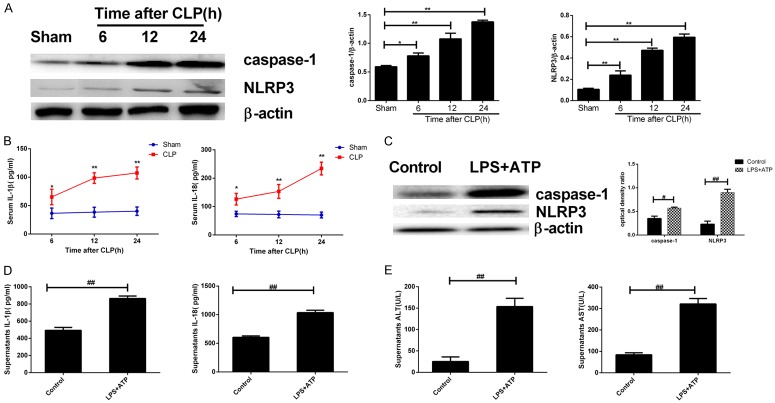
CLP-induced rat model of acute liver injury was successfully constructed. The Western blot results of total protein of hepatic cells showed that at 6, 12, and 24 h after modeling, caspase-1 and NLRP3 expressions in hepatic cells of CLP group were higher than those in the sham operation group, and the increase was in a time-dependent manner (Figure 2A). ELISA revealed that at 6, 12, and 24 h after modeling, the serum concentrations of IL-18 and IL-1β in the CLP group were significantly higher than those in the sham operation group, and the concentrations increased in a time-dependent manner (*P < 0.05, **P < 0.01; Figure 2B). Normal mouse hepatic cells were isolated and cultured in vitro, and LPS+ATP was used to construct the cell model of septic acute liver injury. The cell supernatant was extracted, and the total protein was extracted for Western blot. The result showed that compared to the control group, the expression of caspase-1 and NLRP3 in hepatic cells of LPS+ATP group were increased (Figure 2C). ELISA was used to detect the concentration of cytokines in the supernatant, and the results showed that in comparison with the control group, the concentrations of IL-1β, IL-18, ALT, and AST in LPS+ATP group were significantly increased (##P < 0.01; Figure 2D, 2E).
Figure 2.
Expression of pyroptosis-related proteins increased in septic acute liver injury accompanied by worsened cell damage. Serum and total liver proteins were extracted at different time points after modeling (A, B); Isolation and culture of normal mouse hepatic cells to establish the cell model of septic liver injury (C, D). (A) The expressions of pyroptosis-related proteins in each group examined by Western blot, left: Western blot; right: quantitative analysis representation; (B) The concentrations of serum IL-1β and IL-18 in each group examined by ELISA; (C) The expressions of pyroptosis-related proteins were examined by Western blot, left: Western blot; right: quantitative analysis representation, and expressed as the optical density ratio to β-actin; (D, E) The concentrations of IL-1β and IL-18 in the supernatant (D) and ALT & AST (E) examined by ELISA. *P < 0.05, **P < 0.01, vs. sham operation group; ## P < 0.01, vs. blank control group.
Inhibitors of pyroptosis-related proteins can reduce the pyroptosis and damage of hepatic cells
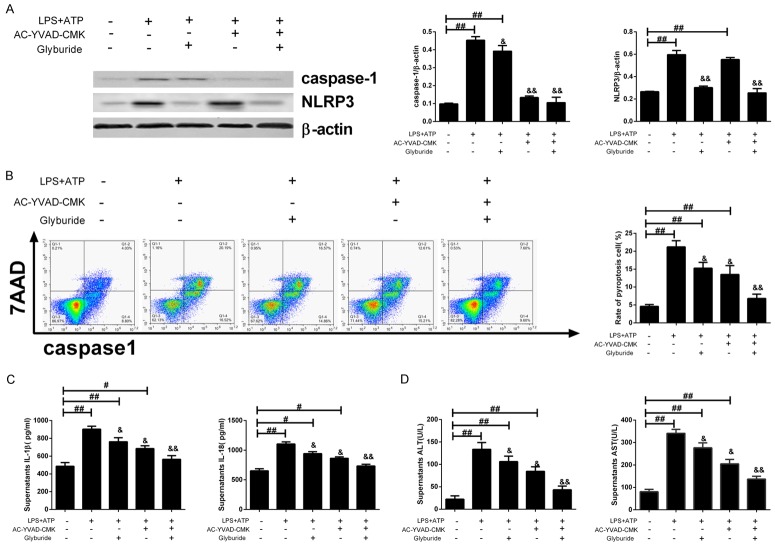
Normal mouse hepatic cells were isolated and cultured in vitro, and the inhibitors of caspase-1 (AC-YVAD-CMK) and NLRP3 (Glyburide), as well as, the combined inhibitors were used for the pretreatment of hepatic cells before constructing the cell model of septic acute liver injury. The cell supernatant was collected, and the total protein was extracted for Western blot, and the results revealed that the expressions of caspase-1 and NLRP3 were decreased under the pre-treatment of inhibitors (Figure 3A). Flow cytometry was used to detect the pyroptosis rate of hepatic cells, and the result showed that compared to the LPS+ATP group, the pyroptosis rate after caspase-1 and NLRP3 inhibition was significantly decreased, especially in the combined inhibition group (inhibition of both caspase-1 and NLRP3, see Figure 3B). ELISA was used to detect the concentrations of cytokines in the supernatant, which demonstrated that in comparison with the LPS+ATP group, the concentrations of IL-1β, IL-18, ALT, and AST after inhibition of caspase-1 and NLRP3 were significantly decreased, especially in the combined inhibition group (&P < 0.05, &&P < 0.01; Figure 3C, 3D).
Figure 3.
Pyroptosis-related protein inhibitors can reduce the pyroptosis and cell damage in the cell model. Normal mouse hepatic cells were prepared and capase-1 inhibitor (AC-YVAD-CMK), NLRP3 inhibitor (Glyburide) alone or in combination were used for the pretreatment of prepared cells before establishing the cell model of septic acute liver injury: (A) The expressions of pyroptosis-related proteins were examined by Western blot, left: Western blot; right: quantitative analysis representation; (B) Hepatic cell pyroptosis was evaluated by flow cytometry, left: two-dimensional graphs, right: histogram; (C, D) The concentrations of IL-1β and IL-18 in the supernatant (C) and ALT & AST (D) were examined by ELISA. #P < 0.05, ##P < 0.01, vs. blank control group; &P < 0.05, &&P < 0.01, vs. LPS+ATP group.
Inhibitors of pyroptosis-associated proteins can alleviate acute liver injury induced by sepsis
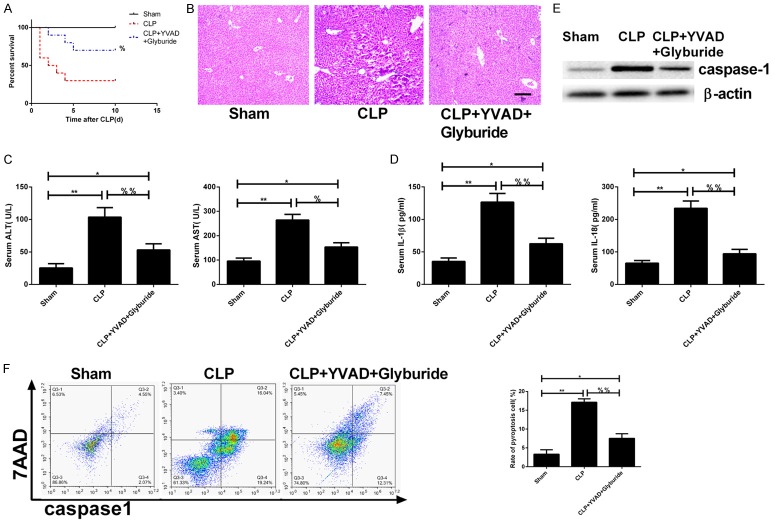
The inhibitors (AC-YVAD-CMK and Glyburide) were intraperitoneally injected into experimental rats before constructing the rat model of CLP-induced acute liver injury. The survival rate was monitored after 10 days of injection. The results showed that compared to the CLP group, the survival rate in the inhibition group (CLP+YVAD+Glyburide) was significantly improved (%P < 0.05; Figure 4A). 24 h after establishing the model, the HE staining of liver tissues revealed a distinct alleviation in the inflammatory infiltration in the inhibition group (Figure 4B). ELISA showed that compared to the CLP group, the concentrations of IL-1β, IL-18, ALT, and AST in the inhibition group were significantly decreased (%P < 0.05; %%P < 0.01; Figure 4C, 4D). The Western blot of total hepatic cell protein demonstrated that the expressions of caspase-1 and NLRP3 in the inhibition group were decreased (Figure 4E). Flow cytometry was used to detect the cell pyroptosis 24 h after modeling, and the result showed that compared to CLP, the pyroptosis rate in the inhibition group was significantly decreased (%%P < 0.01; Figure 4F).
Figure 4.
Pyroptosis-associated protein inhibitors can alleviate acute liver injury induced by sepsis. Inhibitors of capase-1 (AC-YVAD-CMK), NLRP3 (Glyburide) alone or in combination were administrated to mouse via intraperitoneal injection before CLP: (A) The 10 days survival rate in each group; (B) The HE staining of liver tissues (100×) of each group; (C, D) The concentrations of IL-1β and IL-18 in the serum (C) and ALT & AST (D) were examined by ELISA; (E) The expressions of pyroptosis-related proteins were examined by Western blot; (F) Hepatic cell pyroptosis was examined by flow cytometry, left: two-dimensional graphs, right: histogram. *P < 0.05, **P < 0.01, vs. sham operation group; %P < 0.05, %%P < 0.01, vs. CLP group.
Discussion
Sepsis is a complex inflammatory disorder syndrome caused by a pathogenic microorganism and its products. The delayed treatment of sepsis may cause severe sepsis and septic shock, and even result in MODS [23,24], which is characterized by acute liver injury [25]. Thus, it is of great significance to explore the pathophysiology and mechanism of acute liver injury induced by sepsis.
The excessive inflammatory reaction in acute stage of sepsis is considered as crucial pathogenesis of acute liver injury in patients with sepsis. Previous studies have indicated that apoptosis, as a major form of programmed cell death, plays a major role in the inflammatory response of liver injury induced by sepsis [14]. However, whether pyroptosis, another important form of programmed cell death, is involved in acute liver injury induced by sepsis is not yet clarified. Based on the animal and cell models of septic acute liver injury, we explored the relationship between pyroptosis and septic acute liver injury via multiple biological technologies and found a potential target to alleviate acute liver injury using pyroptosis-related protein inhibitor.
Several studies have indicated that Caspase-1-dependent pyroptosis is widely involved in the occurrence and development of infectious diseases, nervous system diseases, and atherosclerotic diseases [18-20]. The establishment of the mouse model of sepsis with CLP in the present study demonstrated that the liver injury aggravated in a time-dependent manner in the acute phase of sepsis and climaxed at 24 h post-ligation. Simultaneously, the hepatic cell pyroptosis also increased in a time-dependent manner with the highest rate at 24 h after modeling; the severity of liver pyroptosis rate was correlated to the liver damage. In the study of acute liver injury caused by trauma and hemorrhagic shock, Menzel et al. found that caspase-1 can reduce the liver injury and inflammatory response [26]. Labbe et al. proved that pyroptosis-induced cell damage during infection is conducive to the effective removal of pathogenic microorganisms, limit the growth of bacteria, and efficiently protect the body from infection. On the other hand, while inducing cell death, pyroptosis could also activate other inflammatory response and induce inflammatory infiltration, which is the foundation of the pathological process of endotoxemia [27]. These conclusions are in accordance with the findings of our study. Herein, we showed that Caspase-1 released the signal during the process of pyroptosis, and induced the immune response of inflammation, in which the NLRP inflammatory cytokines participated [28]. Zhao et al. found that the activated NLRP1 and NLRP3 during pyroptosis could promote the secretion of IL-1β [29]. We isolated the hepatic cells of CLP mice to detect the expressions of pyroptosis-associated proteins NLRP3, Caspase-1, IL-1β, and IL-18, and observed that those protein expressions increased in acute liver injury induced by sepsis. In the in vitro cell model of septic acute liver injury by LPS+ATP, we found that the expressions of pyroptosis-associated proteins and inflammatory cytokines also increased significantly, and the concentrations of hepatic cell injury-related ALT and AST also increased in the supernatant. These results were consistent with the findings of Cao et al. that NLRP3 activation could aggravate acute renal injury [30].
Wu et al. demonstrated that the use of Caspase-1 inhibitor, AC-YVAD-CMK, could ease the pyroptosis of alveolar macrophages and alleviate acute lung injury [31]. Cao et al. used Caspase-1 inhibitor, AC-YVAD-CMK, to reduce CLP-induced acute kidney injury [30]. In vitro experiments revealed that after the use of inhibitors of Caspase-1 (AC-YVAD-CMK) and NLRP3 (Glyburide), caspase-1 and NLRP3 activity as well as hepatic cell pyroptosis rates were decreased, and the pyroptosis-related inflammatory cytokines, IL-1β and IL-18, were also decreased. Concurrently, the concentrations of supernatant ALT and AST were also reduced significantly. The most obvious decrease was found in the combinatory use of both caspase-1 and NLRP3 inhibitors. The in vivo experiment showed that the survival rate of CLP mice was improved after using caspase-1 and NLRP3 inhibitors, whereas the HE staining of liver tissues revealed a reduced infiltration of inflammatory cells, as well as the serum ALT and AST levels. In addition, flow cytometry proved that hepatic cell pyroptosis was reduced after inhibiting the pyroptosis-related proteins and IL-1β and IL-18. In the course of sepsis, hepatic cell skeleton change and pyroptosis increase are the leading mechanisms of functional decline, thereby resulting in weakened phagocytosis and removal of endotoxin and various pathogens, and further strengthening the endotoxin-induced damage on liver. Excessive activation induced by hepatic cell pyroptosis can cause the release of a large number of inflammatory cytokines, such as TNFα, IL-6, and IL-1β to participate in the inflammatory immune reaction and further worsen the hepatic cell damage. In 1996, Rouquet et al. used an ICE (caspase-1) inhibitor to reduce cell apoptosis mediated by Fas [32] Then in 2001, Cookson et al. first time used “pyroptosis” to explain the caspase-1 dependent cell death; the results of this study corroborated with the findings in 1996 [33].
Conclusion
The process of septic acute liver injury, hepatic cell pyroptosis is a form of programmed cell death, aggravating the acute liver injury. Inhibiting the liver pyroptosis by NLRP3 and caspase-1 inhibitors could reduce the degree of septic acute liver injury, which may provide a new potential therapeutic target in the treatment of the condition.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (Nos. 81272142 and 81671947), the Shanghai Natural Science Foundation (No. 14ZR1407000) and Open Projects of Jiangsu Province (KJS1403).
Disclosure of conflict of interest
None.
References
- 1.Andries O, De Filette M, De Smedt SC, Demeester J, Van Poucke M, Peelman L, Sanders NN. Innate immune response and programmed cell death following carrier-mediated delivery of unmodified mRNA to respiratory cells. J Control Release. 2013;167:157–166. doi: 10.1016/j.jconrel.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107:57–64. doi: 10.1093/bja/aer093. [DOI] [PubMed] [Google Scholar]
- 3.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Poll T, Opal SM. Host-pathogen interactions in sepsis. Lancet Infect Dis. 2008;8:32–43. doi: 10.1016/S1473-3099(07)70265-7. [DOI] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 6.Yan GT, Xue H, Lin J, Hao XH, Zhang K, Wang LH. Changes of hepatic function in sepsis mice and protective effects of Leptin on it. Acad J Sec Mil Med Univ. 2006;27:268–271. [Google Scholar]
- 7.Ely EW, Kleinpell RM, Goyette RE. Advances in the understanding of clinical manifestations and therapy of severe sepsis: an update for critical care nurses. Am J Crit Care. 2003;12:120–133. quiz 134-125. [PubMed] [Google Scholar]
- 8.Xu Z, Huang Y, Mao P, Zhang J, Li Y. Sepsis and ARDS: the dark side of histones. Mediators Inflamm. 2015;2015:205054. doi: 10.1155/2015/205054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tukov FF, Luyendyk JP, Ganey PE, Roth RA. The role of tumor necrosis factor alpha in lipopolysaccharide/ranitidine-induced inflammatory liver injury. Toxicol Sci. 2007;100:267–280. doi: 10.1093/toxsci/kfm209. [DOI] [PubMed] [Google Scholar]
- 10.Zhang HB, Luo HC, Xin XJ, Zeng AZ. Up-regulated reg proteins induced by Interleukin-22 treatment ameliorate acute liver injury in rat model. Int J Clin Exp Med. 2015;8:1253–1258. [PMC free article] [PubMed] [Google Scholar]
- 11.Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 12.Pires-Neto RC, Morales MM, Lancas T, Inforsato N, Duarte MI, Amato MB, de Carvalho CR, da Silva LF, Mauad T, Dolhnikoff M. Expression of acute-phase cytokines, surfactant proteins, and epithelial apoptosis in small airways of human acute respiratory distress syndrome. J Crit Care. 2013;28:111.e119–111.e115. doi: 10.1016/j.jcrc.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Jaeschke H. Inflammation in response to hepatocellular apoptosis. Hepatology. 2002;35:964–966. doi: 10.1053/jhep.2002.0350964. [DOI] [PubMed] [Google Scholar]
- 14.Chida Y, Sudo N, Takaki A, Kubo C. The hepatic sympathetic nerve plays a critical role in preventing Fas induced liver injury in mice. Gut. 2005;54:994–1002. doi: 10.1136/gut.2004.058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauernfeind F, Hornung V. Of inflammasomes and pathogens--sensing of microbes by the inflammasome. EMBO Mol Med. 2013;5:814–826. doi: 10.1002/emmm.201201771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 18.Cervantes J, Nagata T, Uchijima M, Shibata K, Koide Y. Intracytosolic Listeria monocytogenes induces cell death through caspase-1 activation in murine macrophages. Cell Microbiol. 2008;10:41–52. doi: 10.1111/j.1462-5822.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Sozen T, Hasegawa Y, Chen W, Zhang JH. Caspase-1 inhibitor prevents neurogenic pulmonary edema after subarachnoid hemorrhage in mice. Stroke. 2009;40:3872–3875. doi: 10.1161/STROKEAHA.109.566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West MA, Keller GA, Cerra FB, Simmons RL. Killed Escherichia coli stimulates macrophage-mediated alterations in hepatocellular function during in vitro coculture: a mechanism of altered liver function in sepsis. Infect Immun. 1985;49:563–570. doi: 10.1128/iai.49.3.563-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christaki E, Anyfanti P, Opal SM. Immunomodulatory therapy for sepsis: an update. Expert Rev Anti Infect Ther. 2011;9:1013–1033. doi: 10.1586/eri.11.122. [DOI] [PubMed] [Google Scholar]
- 24.Giamarellos-Bourboulis EJ, Raftogiannis M. The immune response to severe bacterial infections: consequences for therapy. Expert Rev Anti Infect Ther. 2012;10:369–380. doi: 10.1586/eri.12.2. [DOI] [PubMed] [Google Scholar]
- 25.Wang P, Chaudry IH. Mechanism of hepatocellular dysfunction during hyperdynamic sepsis. Am J Physiol. 1996;270:R927–938. doi: 10.1152/ajpregu.1996.270.5.R927. [DOI] [PubMed] [Google Scholar]
- 26.Menzel CL, Sun Q, Loughran PA, Pape HC, Billiar TR, Scott MJ. Caspase-1 is hepatoprotective during trauma and hemorrhagic shock by reducing liver injury and inflammation. Mol Med. 2011;17:1031–1038. doi: 10.2119/molmed.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labbe K, Saleh M. Cell death in the host response to infection. Cell Death Differ. 2008;15:1339–1349. doi: 10.1038/cdd.2008.91. [DOI] [PubMed] [Google Scholar]
- 28.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhao D, Wu Y, Zhuang J, Xu C, Zhang F. Activation of NLRP1 and NLRP3 inflammasomes contributed to cyclic stretch-induced pyroptosis and release of IL-1beta in human periodontal ligament cells. Oncotarget. 2016 doi: 10.18632/oncotarget.11944. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Y, Fei D, Chen M, Sun M, Xu J, Kang K, Jiang L, Zhao M. Role of the nucleotide-binding domain-like receptor protein 3 inflammasome in acute kidney injury. FEBS J. 2015;282:3799–3807. doi: 10.1111/febs.13379. [DOI] [PubMed] [Google Scholar]
- 31.Wu DD, Pan PH, Liu B, Su XL, Zhang LM, Tan HY, Cao Z, Zhou ZR, Li HT, Li HS, Huang L, Li YY. Inhibition of alveolar macrophage pyroptosis reduces lipopolysaccharide-induced acute lung injury in mice. Chin Med J (Engl) 2015;128:2638–2645. doi: 10.4103/0366-6999.166039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouquet N, Pages JC, Molina T, Briand P, Joulin V. ICE inhibitor YVADcmk is a potent therapeutic agent against in vivo liver apoptosis. Curr Biol. 1996;6:1192–1195. doi: 10.1016/s0960-9822(02)70688-x. [DOI] [PubMed] [Google Scholar]
- 33.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]