Abstract
As the most common malignant tumor, gastric cancer had persistently high occurrence and mortality rate worldwide. Unfavorable treating outcome occur due to distal metastasis, making the inhibition of angiogenesis and managing tumor metastasis being crucial factors for affecting prognosis. Vascular endothelial growth factor-C (VEGF-C) is one important angiogenesis factor and mainly facilitates proliferation and differentiation of vascular endothelial cells in angiogenesis. It has been indicated in development and occurrence in gastric cancer, while its expression and correlation with microvascular density (MVD)/lymph node metastasis are still unclear. A total of 52 gastric tumor and 25 normal tissue samples were recruited for quantifying mRNA and protein expression of VEGF-C by real-time PCR and Western blotting. MVD and lymph tube density were quantified for further analysis of the correlation between VEGF-C and pathological parameters including clinical stage and lymph node metastasis. Both mRNA and protein levels of VEGF-C were significantly elevated in gastric tissues (p<0.05). In lymph node metastasis cases, VEGF-C was further potentiated compared to non-metastatic group (p<0.05). VEGF-C expression was positively correlated with MVD, lymph tube density and clinical stage (p<0.05) but not with age, sex or differentiation grade. VEGF-C expression is closely correlated with lymph node metastasis of gastric cancer. It may participate in the progression of gastric cancer via facilitating angiogenesis and lymph node metastasis, thus can be used in predicting prognosis of patients with gastric carcinoma.
Keywords: Gastric cancer, VEGF-C, microvascular density, lymph node metastasis, correlation analysis
Introduction
Gastric cancer (GC) is one of the most common malignant tumors in digestive tract, with high incidence and mortality rate worldwide [1,2]. With the transition of life style and diet habit, the occurrence rate of GC in China is rapidly increased, with younger patient being the major population [3]. Major treatment for GC includes surgery combined with chemo- or radio-therapy. With advancement of medical sciences, variety of methods has been developed against GC. However, persistently high metastatic and recurrent rate lead to unfavorable prognosis and no improvement of 5-year survival rate [4,5]. There are multiple factors governing the prognosis of GC. The occurrence and development of malignancy involves a complicated process including multiple genes, factors and steps. However, nutrient supply is ultimately required for growth, infiltration and metastasis of malignant tumors via newly formed vessels in tumors [6]. Therefore one school proposed that the inhibition of tumor growth can be used for anti-tumor treatment [7]. The development of GC is highly dependent on angiogenesis, with infiltration of tumor cells into adjacent tissues causing organ dysfunction and damage. Tumor cells can also have distal metastasis via blood and lymph fluid, leading to systemic dispersing and metastatic cancer [8,9]. Therefore it is of critical importance for effectively inhibiting angiogenesis and managing the occurrence of metastasis to improve the prognosis of GC.
As one of the most important angiogenesis facilitating factor, vascular endothelial growth factor (VEGF) is highly expressed in endothelial cells and can increase vessel permeability, in addition to degrading extracellular matrix, facilitating proliferation, differentiation, migration and motility of endothelial cells for the formation of cavity structure [10]. During tumor occurrence, VEGF can facilitate the formation of new vessels [11]. VEGF-C is one isotopic dimer of VEGF by disulfide bond. It is also named as lymph tube endothelial growth factor due to the facilitation of lymph tube formation [12]. Previous studies have confirmed the role of VEGF-C in occurrence and development of gastric cancer [13,14]. The correlation between VEGF-C expression in GC and MVD/lymph node metastasis, however, remains unclear yet.
Materials and methods
Research objects
A total of 52 GC patients (27 males and 25 females, aging between 43 and 78 years old, average age = 56.0±7.2 years) who received surgical resection in Shandong Provincial Hospital affiliated to Shandong University from January 2014 to December 2014 were recruited in this study. Based on pathological sub-typing, there were 23 cases of mucinous adenocarcinoma, 22 cases of tubular adenocarcinoma and 7 cases of other adenocarcinoma. Based on differentiation grade, there were 37 cases of moderate to high differentiation cases and 15 cases of low differentiation tumor. A total of 34 patients had lymph node metastasis while the other 18 patients had no metastasis. TNM staging revealed 2, 11, 17 and 22 cases of stage I, II, III and IV patients. Meanwhile 25 normal gastric tissue samples from gastric ulcer surgery were collected. All patients were primarily diagnosed without any chemo-, radio- or biological treatment before surgery. Those patients were excluded from this study: (1) With history of cardio/cerebrovascular disease; (2) Severe heart/kidney failure; (3) Accompanied with hematological tumors or systemic immune disorder; (4) Infectious or inflammation disease; (5) Those who did not complete follow-up; (6) Mortality due to accidents; (7) Incomplete clinical information. This study has been pre-approved by the ethical committee of Shandong Provincial Hospital affiliated to Shandong University and has obtained written consents from all patients or families. Tumor and normal tissues were collected during the surgery and were kept in liquid nitrogen for further use.
Equipment and reagent
DMSO and MTT powder were purchased from Gibco (US). Trypsin-EDTA was purchased from Sigma (US). PVDF membrane was purchased from Pall Life Sciences (US). EDTA was purchased from Hyclone (US). Western blotting kits were provided by Beyotime (China). ECL kit was purchased from Amersham Biosciences (US). Rabbit anti-human VEGF-C monoclonal antibody and mouse anti-rabbit IgG conjugated with horseradish peroxidase were purchased from Cell signaling (US). RNA extraction kit and reverse transcription kit were purchased from Axygen (US). Other common reagents were purchased from Sangon (China). Labsystem Version 1.3.1 microplate reader was purchased from Bio-rad (US). Ultrapure work station was provided by Sutai (China).
Real-time PCR
Tissues were homogenized and extracted for mRNA using Trizol kit. Reverse transcription was performed using assay kit. Real-time PCR was used to detect the expression of target gene using specific primers synthesized by Yingjun Bio (China, see Table 1 for sequences) under the following conditions: 52°C 1 min, followed by 35 cycles each containing 90°C 30 sec, 58°C 50 sec and 72°C 35 sec. Fluorescent quantitative PCR was employed for collecting data. CT values were calculated based on GAPDH internal reference gene for plotting standard curves. Quantitative analysis was performed by 2-ΔCt method.
Table 1.
Primer sequences
| Target gene | Forward primer 5’-3’ | Reverse primer 5’-3’ |
|---|---|---|
| GADPH | AGTGCCGTCTCCTCAGCATAG | CGACTTGCTTGACGTGGGTAG |
| VEGF-C | GTGCTAAGCCCCTAAAATGAG | GCTATAGCGACTCTCCGGTA |
Western blotting
Tissues were homogenized and lysed on ice for 15~30 min. Cells were ruptured by ultrasound for 5 s (4 times) and were centrifuged at 10 000 g for 15 min at 4°C. Supernatants were saved and quantified for separation in 10% SDS-PAGE. Proteins were then transferred to PVDF membrane by semi-dry method. Non-specific binding sites were blocked by 5% defatted milk powder for 2 h. VEGF-C monoclonal antibody (1:1,000) diluted for 4°C overnight incubation. On the next day, PBST was used to rinse the membrane, followed by dark incubation with goat anti-rabbit secondary antibody (1:2,000) for 30 min. ECL reagent was added for developing the membrane following PBST washing. X-ray was used to expose the fil. Quantity One software was used to scan X-ray film for measuring band density. Each experiment was repeated four times (N = 4) for statistical analysis.
MVD and lymph tube density quantification
Mias2000 imaging analyzer was used to determine the maximal MVD site within tumors under low-magnification objectives, followed by the quantification under high-power. Microvessel was identified as those structures that did not connect to others, with exclusion of those vessels with significant muscular layer. A total of four fields were counted for obtaining average MVD value of tumors. The quantification of micro lymph tube density adopted the same methodology as those used for MVD [15].
Statistical analysis
SPSS16.0 software was used to process all collected data, in which measurement data were presented as mean ± standard deviation (SD). The comparison among multiple groups was done by one-way analysis of variance (ANOVA). Between-group comparison of means was performed using LSD analysis. The correlation analysis was performed by Spearman’s correlation. A statistical significance was defined when p<0.05.
Results
The expression of VEGF-C mRNA in gastric tissues
Real time PCR was employed to test the expression of VEGF-C mRNA in gastric tissues from normal, GC with no lymph node metastasis and GC with lymph node metastasis tissues. Results (Figure 1) showed significantly elevated expression of VEGF-C mRNA in GC tissues compared to normal ones (p<0.05). Among those GC samples, lymph node metastasis further displayed elevated VEGF-C mRNA levels (p<0.05 compared to non-metastatic tissues).
Figure 1.
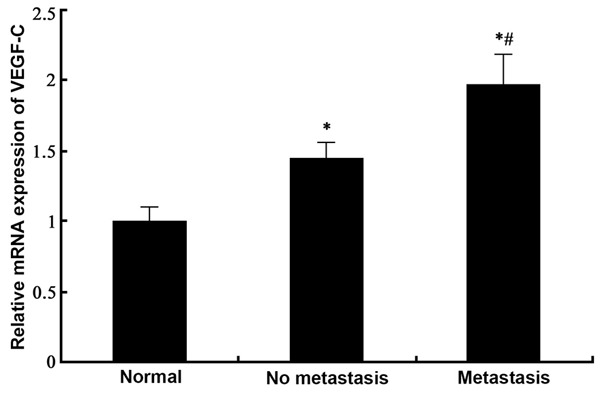
Expression of VEGF-C mRNA in gastric cancer tissues. #, p<0.05 compared to no metastasis group.
VEGF-C protein expression in GC tissues
Western blotting was employed to test the expression of VEGF-C protein in gastric tissues from normal, GC with no lymph node metastasis and GC with lymph node metastasis tissues. Results (Figures 2, 3) showed similar results as those in mRNA level, as significantly elevated expression of VEGF-C proteins existed in GC tissues compared to normal ones (p<0.05). Among those GC samples, lymph node metastasis presented elevated VEGF-C protein levels (p<0.05 compared to non-metastatic tissues).
Figure 2.
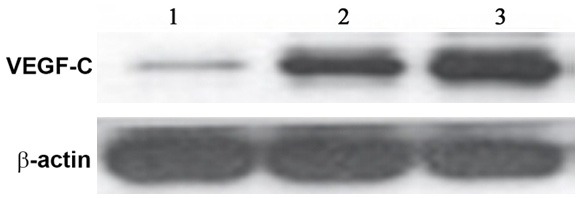
Western blotting bands of VEGF-C proteins. Lane 1, control; Lane 2, GC tissues without lymph node metastasis; Lane 3, GC tissues with lymph node metastasis.
Figure 3.
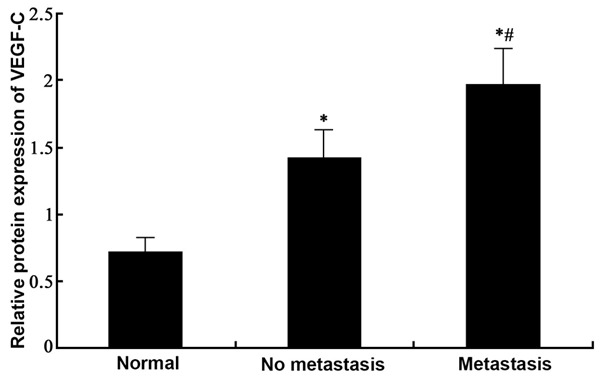
Expression of VEGF-C proteins in GC tissues. #, p<0.05 compared to no metastasis group.
Correlation between lymph tube density and clinical features of GC
We further checked the density of lymph tubes in GC tissues and analyzed its correlation with clinical features of GC. Results (Table 2) showed significant correlation between lymph tube density with clinical feature of GC patients. There was statistically significant difference of values across different clinical stages and lymph node metastasis (p<0.05) but not in differentiation grades.
Table 2.
Correlation between lymph tube density and clinical features of GC
| Parameters | N | Lymph tube density | P value |
|---|---|---|---|
| TNM stage | |||
| 1-Stage I-II | 13 | 5.10±2.75 | <0.05 |
| 2-Stage III-IV | 39 | 12.41±2.91 | |
| Lymph node metastasis | |||
| 1-Yes | 18 | 6.21±3.31 | <0.05 |
| 2-No | 34 | 11.75±4.17 | |
| Differentiation grade | |||
| 1-Moderate to high | 37 | 7.41±2.35 | >0.05 |
| 2-Low | 15 | 8.47±3.16 |
MVD and clinical feature of GC
To analyze the correlation between MVD and clinical feature of GC patients, as similar to those in the scenario between lymph tube density and GC, MVD was significantly different across TNM stages and lymph node metastasis (p<0.05) but not in differentiation grades (Table 3).
Table 3.
MVD and GC clinical features
| Parameters | N | MVD | P value |
|---|---|---|---|
| TNM stage | |||
| 1-Stage I-II | 13 | 6.13±2.41 | <0.05 |
| 2-Stage III-IV | 39 | 11.32±2.75 | |
| Lymph node metastasis | |||
| 1-Yes | 18 | 7.24±3.33 | <0.05 |
| 2-No | 34 | 10.51±3.18 | |
| Differentiation grade | |||
| 1-Moderate to high | 37 | 6.89±2.67 | >0.05 |
| 2-Low | 15 | 7.95±3.61 |
VEGF-C and GC clinical features
In a further analysis about the correlation between VEGF-C expression and clinical feature of GC, we found no statistically significant correlation between mRNA or protein levels of VEGF-C and sex, age or differentiation grade of patients. A positive relationship, however, was found between VEGF-C and TNM stage or lymph node metastasis of tumors (p<0.05, Table 4).
Table 4.
Correlation between VEGF-C and clinical features of GC
| Index | N | VEGF-C mRNA | VEGF-C protein | ||
|---|---|---|---|---|---|
|
| |||||
| r value | P value | r value | P value | ||
| Sex | |||||
| 1-Male | 27 | 0.17 | >0.05 | 0.13 | >0.05 |
| 2-Female | 25 | ||||
| Age (years) | |||||
| 1-≥60 | 31 | 0.29 | >0.05 | 0.11 | >0.05 |
| 2-<60 | 21 | ||||
| TNM stage | |||||
| 1-Stage I-II | 13 | 0.73 | <0.05 | 0.69 | <0.05 |
| 2-Stage III-IV | 39 | ||||
| Lymph node metastasis | |||||
| 1-No | 18 | 0.86 | <0.05 | 0.77 | <0.05 |
| 2-Yes | 34 | ||||
| Differentiation grade | |||||
| 1-Moderate to high | 37 | 0.24 | >0.05 | 0.21 | >0.05 |
| 2-Low | 15 | ||||
Correlation among VEGF-C, MVD and lymph tube density
We further analyzed the correlation between VEGF-C and MVD or lymph tube density in GC tissues. Results (Table 5) showed positive correlation between mRNA/protein expression of VEGF-C and MVD or lymph tube density of GC (p<0.05).
Table 5.
Correlation between VEGF-C, MVD and lymph tube density
| Indexes | VEGF-C mRNA | VEGF-C protein | ||
|---|---|---|---|---|
|
| ||||
| r value | P value | r value | P value | |
| MVD | 0.75 | <0.05 | 0.89 | <0.05 |
| Lymph tube density | 0.81 | <0.05 | 0.76 | <0.05 |
Discussion
China is one prevalence area of GC, which had insidious onset. Thus patients were often at terminal stage at the time of first diagnosis and were accompanied with lymph node metastasis. Unfavorable treatment efficacy was thus obtained in GC, leading to worse survival period and life quality. During the occurrence, infiltration and progression of GC, lymph node metastasis and tumor angiogenesis are critical factors. Therefore to find the specific biological marker for GC metastasis is helpful to evaluate the invasion and metastasis of tumors, which will benefit diagnosis, treatment and prognosis of GC [16-18]. Current reports have suggested VEGF as an important angiogenesis facilitating factor with elevated expression in most of malignant tumor tissues. VEGF can also modulate the behavior of metastatic lesion in addition to facilitating tumor angiogenesis, becoming one research focus in evaluating tumor metastasis. VEGF family includes VEGF-A, VEGF-B, VEGF-C and VEGF-D. Previous study found that VEGF-C could specifically bind to VEGF receptor-2 (VEGFR-2) and VEGFR-3 to facilitate motility of endothelial cells and lymph tube proliferation [19]. Via binding to VEGFR-2, VEGF-C can facilitate proliferation of micro vessels around tumor tissues via synergistic effects with angiotensin-2. Via binding to VEGFR-3, VEGF-C could potentiate the proliferation of lymph tube around tumors, supplying sufficient nutrient for tumors via newly formed micro vessels, and could enhance tumor metastasis [20]. We thus firstly analyzed the expression of VEGF-C in GC and found significantly elevated expression regarding either mRNA or protein levels in tumor tissues. Furthermore, in those GC patients with lymph node metastasis, VEGF-C expression was further potentiated. Compared to those without metastasis, the difference was of statistical significance. All these results supported the close correlation between VEGF-C and progression and development of VEGF-C.
MVD has been used to reflect the status of de novo blood vessel formation, and was widely applied as an index for tumor angiogenesis. Study has shown that with elevated MVD, tumor is rapidly growing with potential invasion, metastasis and recurrence [21]. The density of lymph tube can reflect the status of micro-environment of tumor cells. They can modulate biological behaviors of tumor cells including proliferation, growth, metastasis and invasion. During the expansion of tumor size, edema in interstitial cavity was significant with higher lymph tube density and expansion, both of which lead to lymph node metastasis of tumor cells [22]. This study found that, similar patterns existed for MVD and lymph tube density in the correlation analysis of clinical features in GC patients, as they were all correlated with TNM stage or lymph node metastasis but not with differentiation grade. Further analysis regarding the correlation between VEGF-C and clinical feature of GC patients showed no correlation with sex, age or differentiation grade. VEGF-C, however, was positively correlated with TNM stage and lymph node metastasis. Another correlation analysis between VEGF-C and MVD or lymph tube density found a positive relationship. All these results indicated that VEGF-C might participate in the angiogenesis of tumors and lymph nodes, for further facilitating tumor metastasis and dispersion.
In summary, VEGF-C expression was closely correlated with lymph node metastasis of GC. It may participate in the progression of GC via facilitating tumor angiogenesis and lymph node metastasis. VEGF-C thus can be used to evaluate the prognosis of GC patients and provide evidences for optimizing clinical treatment strategy.
Acknowledgements
This work was supported by the Shandong Province Scientific and Technological project (Grant. No. 2012G0021830).
Disclosure of conflict of interest
None.
References
- 1.Li D, Li Z, Xiong J, Gong B, Zhang G, Cao C, Jie Z, Liu Y, Cao Y, Yan Y, Xiong H, Qiu L, Yang M, Chen H, Jiang S, Yang X. MicroRNA-212 functions as an epigenetic-silenced tumor suppressor involving in tumor metastasis and invasion of gastric cancer through down-regulating PXN expression. Am J Cancer Res. 2015;5:2980–2997. [PMC free article] [PubMed] [Google Scholar]
- 2.Kanat O, O’Neil B, Shahda S. Targeted therapy for advanced gastric cancer: A review of current status and future prospects. World J Gastrointest Oncol. 2015;7:401–410. doi: 10.4251/wjgo.v7.i12.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Lin J, Guo ZQ, Lin WS, Zhou ZF, Huang CZ, Chen Q, Ye YB. MHC I-related chain a expression in gastric carcinoma and the efficacy of immunotherapy with cytokine-induced killer cells. Am J Cancer Res. 2015;5:3221–3230. [PMC free article] [PubMed] [Google Scholar]
- 4.Yang C, Zhang H, Zhang S, Liu L, Ma B, Lou J, Sun X, Zhang B. Oculomotor Paralysis, Postorbital Pain, and Hypopituitarism as First Presentations of Metastatic Gastric Cancer in the Pituitary Flourished by Internal Carotid Aneurysm: A Case Report. Medicine (Baltimore) 2015;94:e2317. doi: 10.1097/MD.0000000000002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiyokoba R, Yagi H, Yahata H, Kawano Y, Kaneki E, Okugawa K, Sonoda K, Kato K. Tumor-To-Tumor Metastasis of Poorly Differentiated Gastric Carcinoma to Uterine Lipoleiomyoma. Case Rep Obstet Gynecol. 2015;2015:352369. doi: 10.1155/2015/352369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerrini GP, Soliani P, D’Amico G, Benedetto FD, Negri M, Piccoli M, Ruffo G, Orti-Rodriguez RJ, Pissanou T, Fusai G. Pancreaticojejunostomy Versus Pancreaticogastrostomy After Pancreaticoduodenectomy: An Up-to-date Meta-Analysis. J Invest Surg. 2016;29:175–84. doi: 10.3109/08941939.2015.1093047. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A, Tiwari G, Tiwari R, Srivastava R. Factorial designed 5-fluorouracil-loaded microsponges and calcium pectinate beads plugged in hydroxypropyl methylcellulose capsules for colorectal cancer. Int J Pharm Investig. 2015;5:234–246. doi: 10.4103/2230-973X.167688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su HJ, Zhang Y, Zhang L, Ma JL, Li JY, Pan KF, You WC. Methylation status of COX-2 in blood leukocyte DNA and risk of gastric cancer in a high-risk Chinese population. BMC Cancer. 2015;15:979. doi: 10.1186/s12885-015-1962-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito S, Ito Y, Misawa K, Shimizu Y, Kinoshita T. Neoadjuvant chemotherapy followed by surgery in gastric cancer patients with extensive lymph node metastasis. World J Clin Oncol. 2015;6:291–294. doi: 10.5306/wjco.v6.i6.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang ZQ, Han YZ, Nian Q, Chen G, Cui SQ, Wang XY. Tumor Invasiveness, Not Lymphangiogenesis, Is Correlated with Lymph Node Metastasis and Unfavorable Prognosis in Young Breast Cancer Patients (≤35 Years) PLoS One. 2015;10:e0144376. doi: 10.1371/journal.pone.0144376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naruse T, Yanamoto S, Yamada SI, Takahashi H, Matsushita Y, Imayama N, Ikeda H, Shiraishi T, Fujita S, Ikeda T, Asahina I, Umeda M. Immunohistochemical study of vascular endothelial growth factor-C/vascular endothelial growth factor receptor-3 expression in oral tongue squamous cell carcinoma: Correlation with the induction of lymphangiogenesis. Oncol Lett. 2015;10:2027–2034. doi: 10.3892/ol.2015.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia L, Du Y, Li T, Lv Y, Wang Y, Zhang Y, Zhou X, Liu W. Differential expression of vascular endothelial growth factor-A, -C and -D for the diagnosis and prognosis of cancer patients with malignant effusions. Oncol Lett. 2015;10:667–674. doi: 10.3892/ol.2015.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao R, Chang Y, Liu Z, Liu Y, Guo S, Yu J, Wang J. Effect of vascular endothelial growth factor-C expression on lymph node metastasis in human cholangiocarcinoma. Oncol Lett. 2015;10:1011–1015. doi: 10.3892/ol.2015.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang HH, Qi F, Cao YH, Zu XB, Chen MF. Expression and clinical significance of microRNA-21, maspin and vascular endothelial growth factor-C in bladder cancer. Oncol Lett. 2015;10:2610–2616. doi: 10.3892/ol.2015.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura Y, Morohashi S, Yoshizawa T, Suzuki T, Morohashi H, Sakamoto Y, Koyama M, Murata A, Kijima H, Hakamada K. Clinicopathological significance of vascular endothelial growth factor, thymidine phosphorylase and microvessel density in colorectal cancer. Mol Med Rep. 2016;13:1551–1557. doi: 10.3892/mmr.2015.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim J, Ryu JH, Kim EJ, Ham S, Kang D. Inhibition of Vascular Endothelial Growth Factor Receptor 3 Reduces Migration of Gastric Cancer Cells. Cancer Invest. 2015;33:398–404. doi: 10.3109/07357907.2015.1047509. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Li W, Chen T, Yang J, Luo L, Zhang L, Sun B, Liang R. Retrospective analysis of the clinicopathological characteristics of gastrointestinal neuroendocrine neoplasms. Exp Ther Med. 2015;10:1084–1088. doi: 10.3892/etm.2015.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie HX, Xu ZY, Tang JN, Du YA, Huang L, Yu PF, Cheng XD. Effect of Huaier on the proliferation and apoptosis of human gastric cancer cells through modulation of the PI3K/AKT signaling pathway. Exp Ther Med. 2015;10:1212–1218. doi: 10.3892/etm.2015.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tawada M, Hayashi S, Ikegame Y, Nakashima S, Yoshida K. Possible involvement of tumor-producing VEGF-A in the recruitment of lymphatic endothelial progenitor cells from bone marrow. Oncol Rep. 2014;32:2359–2364. doi: 10.3892/or.2014.3499. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Li HG, Wen JM, Peng TS, Zeng H, Wang LY. Expression of CD44v3, erythropoietin and VEGF-C in gastric adenocarcinomas: correlations with clinicopathological features. Tumori. 2014;100:321–327. doi: 10.1700/1578.17216. [DOI] [PubMed] [Google Scholar]
- 21.Pires FR, da Silva PJ, Natal RF, Alves FA, Pinto CA, Rumayor A, Miranda AM, de Almeida OP. Clinicopathologic features, microvessel density, and immunohistochemical expression of ICAM-1 and VEGF in 15 cases of secondary syphilis with oral manifestations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:274–281. doi: 10.1016/j.oooo.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki K, Yabushita H, Ueno T, Wakatsuki A. Role of hypoxia-inducible factor-1alpha, carbonic anhydrase-IX, glucose transporter-1 and vascular endothelial growth factor associated with lymph node metastasis and recurrence in patients with locally advanced cervical cancer. Oncol Lett. 2015;10:1970–1978. doi: 10.3892/ol.2015.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]


