Abstract
Objective: To investigate the effects of etomidate and propofol on immune function in patients with lung adenocarcinoma. Methods: Sixty patients who were scheduled for lung cancer surgery under general anesthesia were studied. The patients were randomly divided into an etomidate total intravenous anesthesia group (group E) and a propofol total intravenous anesthesia group (group P), with 30 cases in each group. Results: Within group comparison: The percentage of CD4+ in the two groups was significantly reduced at 24 hours post-operation (T2) compared with the percentage before surgery, whereas the percentage of CD8+ was higher at T2. Between group comparison: The CD4+ percentage of group E was higher than that of group P (P < 0.05) at T2, whereas the CD8+ percentage was lower than that of group P (P < 0.05) at T1. Conclusion: Using etomidate for anesthesia has less of an effect on immune function in patients with lung adenocarcinoma.
Keywords: Etomidate, propofol, immune function, general anesthesia, lung adenocarcinoma
Introduction
Lung cancer results in high morbidity and mortality; it is characterized by malignant tumors with a poor prognosis, which grow rapidly and destroy infiltrated tissues and organs and distant metastases [1]. Surgical resection is the main treatment for lung cancer, but many studies have shown that cancer patients often exhibit immunosuppression, decreased immunity for tumor recurrence and a suitable environment for metastasis, thus affecting prognosis [2]. Preoperative anesthesia management and related outcomes in patients with malignancies have increasingly attracted the attention of anesthesiologists. Anesthesia and other relevant factors, including use of preoperative narcotic drugs, can affect the immune function of cancer patients [3-6], thus affecting the outcome.
Regarding the effects of anesthetic techniques on tumor growth and metastasis, a large number of studies have suggested that general anesthesia combined with epidural anesthesia, as compared with general anesthesia alone, can reduce the extent of postoperative immunosuppression. From 1984 to 2008, the Merquiol study reviewed the 5-year survival rate of a total of 271 cases of throat and jaw cancer patients, and found that patients receiving cervical epidural anesthesia combined with general anesthesia exhibited a significantly higher survival rate than did patients receiving general anesthesia alone (68 vs 37%). The study has suggested that general anesthesia combined with epidural anesthesia improves the 5-year survival rate of patients with malignant tumors [3]. Wan-Kun Chen has analyzed anesthesia methods and tumor types and has found that the use of general anesthesia combined with epidural anesthesia (anesthesia or nerve block) in cancer surgery patients is superior to the use of general anesthesia alone. The following benefits of epidural anesthesia have been suggested: (1) epidural anesthesia (anesthesia or nerve block) provides good preoperative analgesia; (2) epidural anesthesia reduces the incidence of general anesthesia complications; (3) this reduction may lead to the use of immunosuppressive drugs, such as certain general anesthetics and opioids; (4) epidural anesthesia (anesthesia or nerve block) plays a role in inhibiting the stress response to noxious stimuli by blocking central conduction; and (5) as confirmed in animal experiments, nerve block can be controlled by the degree of stress to avoid excessive stress-induced immune suppression, thereby reducing the immune suppression caused by postoperative infection, delayed recovery and tumor metastasis [7]. Therefore, epidural anesthesia combined with general anesthesia may improve cancer patient outcomes.
Cata [4] has observed natural killer (NK) cell and T cell subsets in patients with non-small cell lung cancer, and found that in preoperative samples compared with preoperative samples, the number of functional T cell and NK cell subsets, as well as the CD4+/CD8+ levels, significantly decreased. However, Cata observed no significant differences in TH1/TH2 ratios. The study has shown that patient immune function decreases after surgery, whereas epidural anesthesia combined with general anesthesia has no protective effect on immune function. However, various researchers have observed that epidural anesthesia combined with general anesthesia affects survival time in gynecologic stages IIIC and IV ovarian cancer patients with tumor recurrence. Their studies have shown that epidural anesthesia combined with general anesthesia, compared with the simple application of complete anesthesia, does not result in statistically significant differences in patients’ tumor recurrence and survival time [5].
In contrast to the two views described above, the 1999-2008 program, in which different anesthesia regimens were delivered to small cell liver cancer patients undergoing radiofrequency ablation, has indicated that the rate of tumor recurrence in anesthesia patients receiving an epidural is lower than it is in patients receiving anesthesia. However, significant differences in survival time were not found for the different anesthesia methods. Therefore, the study results suggest that local anesthesia may be beneficial for tumor prognosis in certain types of tumors [6].
Conclusions from previous studies are not consistent, thus suggesting that the effects of anesthesia methods on cancer patient outcomes are complex, and many factors are involved. As a result, it is difficult to reach consistent conclusions, but it is clear that different anesthesia methods affect tumor outcomes.
The majority of studies have suggested that narcotic drugs, including intravenous anesthetics, inhalation anesthetics and analgesics, affect tumor growth and metastasis. Among intravenous anesthetics, propofol is the most commonly used in clinical anesthesia. In recent years, researchers have published considerable information regarding propofol and tumor surgery patient outcomes. With respect to inhalation anesthetics, the protective effects of propofol on immune function are more significant, and propofol exhibits a good inhibitory effect on tumor recurrence and metastasis. Studies have shown that propofol inhibits vascular proliferation and regeneration of esophageal squamous cell carcinoma and metastasis lesions by reducing ERK-VEGF/MMP-9 signaling [8]. In addition, a study of propofol in patients with hepatocellular carcinoma has found that propofol significantly increases the expression of miR-199a and down-regulates the expression of MMP-9. In addition, miR-199a up-regulates the expression of MMP-9 and also has a feedback inhibitory effect on MMP-9 expression; thus, reduced MMP-9 expression may play a role in suppressing cancer cell invasion [9]. Propofol also synergizes with chemotherapeutic drugs in inducing apoptosis in cancer cells [10]. Studies have shown that propofol inhibits ovarian tumor cell proliferation while enhancing the role of paclitaxel-induced apoptosis in cancer cells.
Most previous studies have suggested that inhalation anesthetics inhibit NK cell activity, which is conducive to tumor growth and metastasis. Isoflurane and halothane have been found to inhibit NK cell activity in animal studies, and sevoflurane causes the release of cytokines (IL-1 and TNF) and may also have additional effects [11]. Animal studies have suggested that nitrous oxide may promote the growth of liver, lung and colon cancer. However, no significant correlation of this treatment with the prognosis of cancer patients has been found [12]. Some researchers have also compared the effects of inhalation anesthesia and intravenous anesthesia on immune function in patients with oral malignancy. One study has found that immune suppression is not as potent in patients receiving intravenous anesthesia; thus, the intravenous anesthetic drug-related effects on the suppression of immune activity may be stronger for inhaled anesthetics [13].
In contrast with propofol, opioids promote tumor cell proliferation, differentiation, metastasis and angiogenesis. This effect may be related to opioid receptor regulation; therefore, some researchers have hypothesized that opioid receptor antagonists might have anti-tumor effects [14]. In addition, Dylan has indicated that the increased use of opioids is associated with shortened survival in prostate cancer patients. However, the effects of opioids on cancer patient outcomes must be further confirmed in prospective randomized studies [15].
However, some researchers have put forward different views. One study has found that opioid receptor activation suppresses tumor proliferation and differentiation in breast cancer patients [16]. Yamamizu has suggested that κ receptor activation via inhibition of VEGF signal expression inhibits tumor tissue angiogenesis [17].
In addition, the widely used non-barbiturate intravenous anesthetic etomidate has a rapid onset, short duration of action and minimal effects on hemodynamics characteristics, particularly for the induction of anesthesia in critically ill patients. Thus, etomidate has been increasingly used to maintain anesthesia for minor surgeries [18]. However, for traumatic surgeries, including thoracotomy, the use of etomidate for the maintenance of anesthesia and its effects on immune function remain uncharacterized. Here, we sought to compare the effects of etomidate and propofol in the maintenance of anesthesia on T lymphocyte subsets in lung adenocarcinoma patients to explore the effects of different maintenance anesthesia medications on patients’ immune function.
Materials and methods
Case selection
This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Tianjin Chest Hospital, Tianjin, China. In total, 60 patients scheduled for elective lung adenocarcinoma surgery under general anesthesia with good cardiac and lung function were studied. The patients were graded as ASA I-II. The patients were 48 to 67 years old (33 male cases, 27 female cases). All of the patients agreed on the methods of anesthesia. Patients with a history of immune system diseases were excluded. Patients undergoing radiotherapy and chemotherapy, taking immunosuppressive agents or undergoing perioperative blood transfusions were also excluded. The exclusion criteria included a history of immune system diseases; radiotherapy; chemotherapy; corticosteroids and other immunosuppressive drug therapies; and preoperative, intraoperative and postoperative (within 3 days) allogeneic blood transfusions.
Block methods
Sixty patients were divided into two groups by using a random sampling method: the propofol target controlled infusion group (P group) and the continuous infusion of etomidate group (E group). General anesthesia combined with thoracic epidural anesthesia was administered to both groups. However, one group (group P) was subjected to target-controlled infusion with propofol, whereas the other group (group E) was subjected to etomidate continuous infusion. The two groups of patients were treated with general anesthesia combined with thoracic epidural anesthesia.
Anesthetic management
Prior to anesthesia (0.5 hours), the patients were administered 0.01 mg/kg of intramuscular midazolam and 0.1 mg/kg of morphine. After each patient entered the operating room, routine monitoring of ECG (II, V leads), pulse oximetry and bispectral index (BIS) (VISTA, USA) were performed. In addition, row radial artery catheterization was used to monitor arterial blood pressure. When each patient was in a lateral position, the thoracic epidural catheterization puncture point T4-5 was selected, and T3-8 were maintained under anesthesia as well. Anesthesia was induced with intravenous rapid induction drugs: 0.1 mg/kg of midazolam, 3 mg/kg of etomidate (production batch number: 20141202, Jiangsu En Hua Pharmaceutical Co., Ltd.), 0.6 mg/kg of rocuronium and 1 μg/kg of sufentanil. For tracheal intubation, a bronchoscope was orally inserted under direct vision for double-lumen endotracheal intubation and mechanical ventilation with a tidal volume of 8-10 ml/kg and a respiratory rate of 10 to 12 times/min. Maintenance of anesthesia: Group P was administered propofol via target controlled infusion with a 1-3 μg/ml target plasma propofol concentration. Group E received a continuous infusion of etomidate, and the rate of infusion was 10-20 µg/kg*min. Two patients using a muscle relaxant were intermittently administered 0.2 mg/kg of cisatracurium. The epidural analgesia was administered intermittently using 0.375% ropivacaine (5 ml, AstraZeneca). A BIS value of 40 to 60 was maintained during surgery in both groups. Spontaneous breathing, strong coughing, strong swallowing and regaining consciousness occurred at the end of the surgery. The blood gas analysis was normal, and no airway obstruction was observed. The tidal volume was consistently greater than 8 ml/kg extubation after the administration of continuous epidural analgesia.
Samples collected and indicator tests
Five milliliters of blood was collected from the patients before anesthesia (T0), immediately after the operation (T1) and at 24 (T2) and 72 (T3) hours post-operation. Flow cytometry was used to assess the CD4+ and CD8+ percentages and the CD4+/CD8+ levels. The mean arterial pressure (MAP) was also recorded at each time point. In addition, heart rate (HR), BIS, the recording time, awake extubation time, intraoperative and postoperative twitching, injection pain, nausea, vomiting and other adverse reactions were also recorded.
Statistical analysis
The data were analyzed using the statistical software package SPSS 17.0 and summarized as means ± standard errors. A one-way ANOVA was used between groups, and a repetitive measure analysis of variance was used within groups at the 5% level.
Results
General patient data
In this study, the propofol group (P) and the etomidate infusion group (E) each included 30 patients. The age (55 ± 5 vs 58 ± 5), body weight (59.7 ± 9.8 vs 61.2 ± 7.2), gender (17/13 vs 16/14), ASA classification (14/16 vs 15/15) and other aspects of the two groups were not significantly different. The operation times of the two groups were approximately 3 h (171.8 ± 15.3 vs 163.5 ± 17.5), and the amount of bleeding during surgery (191.5 ± 28.3 vs 205.4 ± 20.7) was not significantly different, as noted in Table 1.
Table 1.
General patient data
| Group | Age | Body weight (kg) | Gender (Male/Female) | ASA Grade (I/II) | Operation time (min) | Amount of bleeding (ml) |
|---|---|---|---|---|---|---|
| P | 55 ± 5 | 59.7 ± 9.8 | 17/13 | 14/16 | 171.8 ± 15.3 | 191.5 ± 28.3 |
| E | 58 ± 5 | 61.2 ± 7.2 | 16/14 | 15/15 | 163.5 ± 17.5 | 205.4 ± 20.7 |
Mean arterial pressure
The MAP, HR and pulse oxygen saturation (SpO2) were compared at each time point. The MAP, HR and SpO2 values did not significantly differ between the groups (P > 0.05), as shown in Figure 1.
Figure 1.
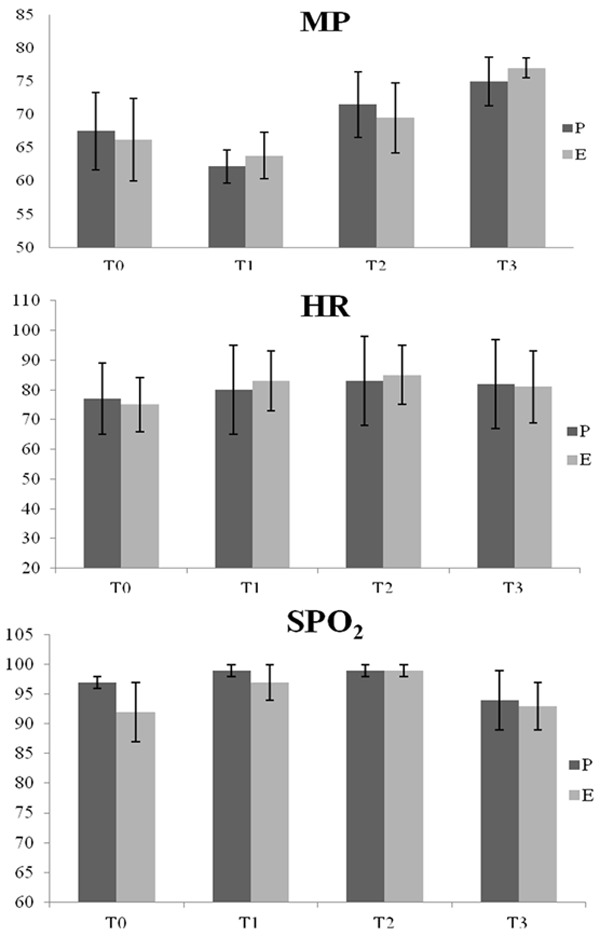
Information from group P and group E patients at each time point. HR, MAP and SpO2 comparisons are reported as the mean ± standard deviation. At the same time points, no significant differences in HR, MAP or SpO2 were observed.
Adverse events [cases (%)]
As shown in Table 2, group P did not exhibit the twitching phenomenon induced by anesthesia, but two patients in group E twitched (0 vs 6.7%). Nausea occurred in 4 and 5 cases (13.3 vs 16.7%) in groups P and E, respectively. Three cases of vomiting were noted in both groups (10 vs 10%). More patients reported injection in group P than in group E (16.7 vs 6.7%, P < 0.05), and the incidence of thrombophlebitis was higher in group P than in group E (6.7 vs 3.3%, P < 0.05).
Table 2.
The two groups of patients after surgery and the occurrence of adverse reactions
| Group | No. | Muscle trembling | Nausea | Vomiting | Injection pain | Thrombophlebitis |
|---|---|---|---|---|---|---|
| P | 30 | 0 (0.0%)a | 4 (13.3%) | 3 (10.0%) | 5 (16.7%)a | 2 (6.7%)a |
| E | 30 | 2 (6.7%)b | 5 (16.7%) | 3 (10.0%) | 2 (6.7%)b | 1 (3.3%)b |
Numbers with different letters (a, b) are significantly different.
Patients with CD4+ (%), CD8+ (%) and CD4+/CD8+
Within group comparison: CD4+ significantly decreased at 24 h post operation (T2) (34.46 ± 3.93 vs 26.01 ± 8.06, P < 0.05), and CD8+ at T2 increased (29.01 ± 5.82 vs 36.57 ± 8.52; 25.24 ± 3.97 vs 35.89 ± 7.38, P < 0.05), compared with preoperative (T0) values. No significant differences in T1 and T3 were noted compared with T0. Between groups comparison: After 24 h (T2), the CD4+ percentage was higher in group E than in group P (26.01 ± 8.06 vs 38.21 ± 13.09, P < 0.05, respectively). In the immediate postoperative (T1) period, the CD8+ percentage was lower in group E than in group P (27.26 ± 1.71 vs 22.65 ± 3.61, P < 0.05, respectively). The remaining values were not significantly different between the two groups, as shown in Figure 2.
Figure 2.
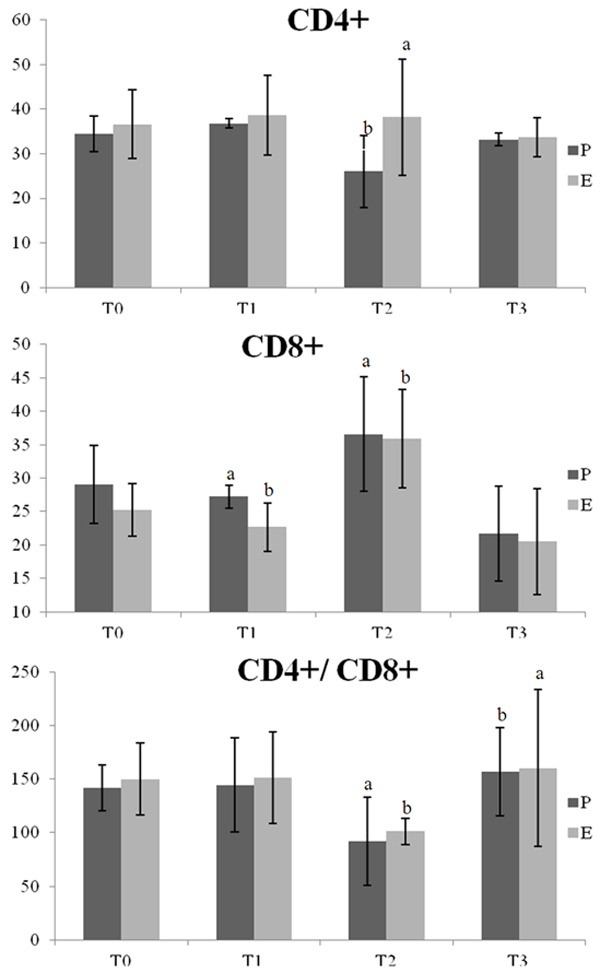
CD4+ (%), CD8+ (%) and CD4+/CD8+ group P and group E patients at various time points. In-group comparisons: Two T2 CD4+ patients exhibited a significant decrease in CD4+ (P < 0.05) compared with T0, and CD8+ increased at T0 (P < 0.05). No significant differences in T1 and T3 were observed compared with T0. Between the two groups: The T2 CD4+ percentage was higher in group E compared with group P (P < 0.05). The percentage of CD8+ cells at T1 was lower in group E compared with group P (P < 0.05). The differences in the remaining points were not statistically significant.
Discussion
Etomidate is a non-barbiturate intravenous anesthetic that adjusts γ-aminobutyric acid A (GABA) receptors to produce anesthesia [19]. The drug exhibits a rapid onset short duration and has a mild effect on hemodynamic features. Based on recent etomidate research, an increasing number of researchers believe that etomidate’s inhibition of adrenal cortex function is transient in nature and can be maintained in secure applications in anesthesia [20].
In this study, an etomidate infusion rate of 10-20 µg/kg/min was maintained within the desired range of BIS values for most surgical patients, and the effect of the propofol target concentration of 1-3 μg/ml was readily achievable [21]. No differences in MAP, HR and SpO2 were noted in the etomidate and propofol groups. Although secondary to epidural analgesia, which offers relatively stable perioperative hemodynamic changed, the conclusions from our study are consistent with those of Shah [22]. That study did not report BIS and hemodynamic fluctuations cases but has suggested that the continuous infusion of etomidate is safe for the maintenance of anesthesia. In addition, propofol had a similar effect.
Twitching is the most common complication of etomidate-induced anesthesia, and this complication may prevent the use of sedatives, analgesics and muscle relaxants [23]. In this study, the induction of anesthesia with composite applications of midazolam and sufentanil promoted stable sleep in patients. After the first application of rocuronium and before the application of etomidate, the muscle tremor incidence was 6.7%, which is significantly lower than the previously reported incidence [24].
In this study, the incidence of injection pain (6.7%) was significantly reduced in the etomidate group compared with the propofol group (16.7%). Thus, other composite applications, such as midazolam and sufentanil, may be used. Specifically, etomidate ester solvent emulsions contain 20% long-chain triglycerides, close to physiological osmolality. The propofol solvent is a long-chain triglyceride and is likely to be the cause of patient pain on injection. Compared with propofol, etomidate exhibits almost no vascular stimulation. Additional composite applications include sedative and analgesic drugs. Reductions in the injection rate and proper injection site selection are also important for the relief of pain upon injection [25].
This observation suggests that etomidate for lung resection can allow the maintenance of general anesthesia, to further maintain hemodynamic stability, an adequate depth of sedation and a low incidence of adverse reactions of the target. Weng and others have reported that etomidate maintains anesthesia with stable hemodynamics, rapid waking and a low incidence of adverse event characteristics, and that it can be safely used in the maintenance of anesthesia [26].
The body’s immune response to cancer is very complex and mainly involves cellular immunity, humoral immunity and cytokines. Cellular immunity is an important part of the immune system. CD4+ and CD8+ cells are important T lymphocyte effector cells: CD4+ cells help B cells differentiate to produce antibodies and activate other cellular immune responses, and CD8+ cells suppress the immune function and inhibit antibody secretion, synthesis and proliferation. Changes in the CD4+/CD8+ ratio indicate immune function disorder. T lymphocyte subsets may serve as an indicator of immune function in lung adenocarcinoma patients [27].
Surgical trauma and anesthesia drugs can affect immune function in patients with lung cancer [28]. The perioperative selection of the correct method of anesthesia and anesthetic drugs may protect patients’ immune function, treatment and prognosis; thus, cancer patients should play an active role in the choice of anesthesia. Some studies have shown [29] that propofol inhibits vascular proliferation and regeneration of esophageal squamous cell carcinoma and metastasis lesions through reduced ERK-VEGF/MMP-9 signaling. Several studies have shown [30-31] that the protective effect of propofol on immune function is significant and that propofol exhibits an inhibitory effect on tumor recurrence and metastasis. As an anesthetic maintenance medication, the effect of etomidate on immune function was assessed. Levels of CD4+ cells were significantly lower 24 h after surgery than before surgery, whereas levels of CD8+ cells were significantly higher after surgery than before surgery. These results suggest that after lung cancer patients undergo a surgical procedure, their immunity further declines. At 72 h, CD4+ and CD8+ cells gradually returned to preoperative levels, suggesting that the patient’s immune function gradually recovered. Compared with propofol, a higher percentage of CD4+ cells were observed in group P than in the etomidate group 24 h after intravenous maintenance, thus suggesting that etomidate has a minimal effect on CD4+ cells. In addition, immune function recovered more rapidly after surgery in these patients. At the remaining time points, no significant differences in the effects of etomidate on the T cell subsets were observed, and the effect of etomidate on immune function in patients was similar to that of propofol. This study shows that the use of anesthesia for surgery and anesthesia medication affects immune function via a transient suppression that is quickly restored. For maintaining anesthesia, the use of etomidate has no significant inhibitory effect on immune function in patients.
Ji’s studies [32-33] have shown that etomidate exhibits minimal inhibition of circulatory function and that it is a good drug to maintain intravenous anesthesia. However, these studies did not observe its effects on immune function. Our research suggests that etomidate is ideal for lung cancer patients because it offers hemodynamic stability during the maintenance of anesthesia, and no significant adverse reactions were observed perioperatively. Regarding immune function maintenance, etomidate maintains patient CD4+ and CD8+ cell levels. Similarly, propofol has no inhibitory effect on the CD4+/CD8+ ratio.
Acknowledgements
This project was financially supported by the Tianjin Medical Association of Anesthesiology Young Researchers Nurture Fund Project (TJMZJJ-2014-01) of China.
References
- 1.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, Marcos-Gragera R, Stiller C, Azevedo e Silva G, Chen WQ, Ogunbiyi OJ, Rachet B, Soeberg MJ, You H, Matsuda T, Bielska-Lasota M, Storm H, Tucker TC, Coleman MP CONCORD Working Group. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rybojad P, Jabłonka A, Wilczyńska B, Tabarkiewicz J. Surgery decreases number of cells secreting cytotoxic mediators and increases secretion of interleukin 10 in patients with lung cancer. Eur J Surg Oncol. 2013;39:1269–1277. doi: 10.1016/j.ejso.2013.07.083. [DOI] [PubMed] [Google Scholar]
- 3.Merquiol F, Montelimard AS, Nourissat A, Molliex S, Zufferey PJ. Cervical epidural anesthesia is associated with increased cancer-free survival in laryngeal and hypopharyngeal cancer surgery:a retrospective propensity-matched analysis. Reg Anesth Pain Med. 2013;38:398–402. doi: 10.1097/AAP.0b013e31829cc3fb. [DOI] [PubMed] [Google Scholar]
- 4.Cata JP, Bauer M, Sokari T, Ramirez MF, Mason D, Plautz G, Kurz A. Effects of surgery,general anesthesia, and perioperative epidural analgesia on the immune function of patients with non-small cell lung cancer. J Clin Anesth. 2013;25:255–262. doi: 10.1016/j.jclinane.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Lacassie HJ, Cartagena J, Brañes J, Assel M, Echevarría GC. The relationship between neuraxial anesthesia and advanced ovarian cancer-related outcomes in the Chilean population. Anesth Analg. 2013;117:653–660. doi: 10.1213/ANE.0b013e3182a07046. [DOI] [PubMed] [Google Scholar]
- 6.Lai R, Peng Z, Chen D, Wang X, Xing W, Zeng W, Chen M. The effects of anesthetic technique on cancer recurrence in percutaneous radiofrequency ablation of small hepatocellular carcinoma. Anesth Analg. 2012;114:290–296. doi: 10.1213/ANE.0b013e318239c2e3. [DOI] [PubMed] [Google Scholar]
- 7.Chen WK, Miao CH. The Effect of Anesthetic Technique on Survival in Human Cancers: A Meta-Analysis of Retrospective and Prospective Studies. PLoS One. 2013;8:e56540. doi: 10.1371/journal.pone.0056540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu YB, Du QH, Zhang MY, Yun P, He CY. Propofol suppresses proliferation, invasion and angiogenesis by down-regulating ERK-VEGF/MMP-9 signaling in Eca-109 esophageal squamous cell carcinoma cells. Eur Rev Med Pharmacol Sci. 2013;17:2486–2494. [PubMed] [Google Scholar]
- 9.Zhang J, Zhang D, Wu GQ, Feng ZY, Zhu SM. Propofol inhibits the adhesion of hepatocellular carcinoma cells by upregulating microRNA-199a and downregulating MMP-9 expression. Hepatobiliary Pancreat Dis Int. 2013;12:305–309. doi: 10.1016/s1499-3872(13)60048-x. [DOI] [PubMed] [Google Scholar]
- 10.Wang P, Chen J, Mu LH, Du QH, Niu XH, Zhang MY. Propofol inhibits invasion and enhances paclitaxel- induced apoptosis in ovarian cancer cells through the suppression of the transcription factor slug. Eur Rev Med Pharmacol Sci. 2013;17:1722–1729. [PubMed] [Google Scholar]
- 11.Zhang L, Zhang J, Yang L, Dong Y, Zhang Y, Xie Z. Isoflurane and sevofluane increase interleukin-6 levels through the nuclear factor-kappa B pathway in neuroglioma cells. Br J Anaesth. 2013;110:82–91. doi: 10.1093/bja/aet115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105:106–115. doi: 10.1093/bja/aeq164. [DOI] [PubMed] [Google Scholar]
- 13.Guptill V, Cui X, Khaibullina A, Keller JM, Spornick N, Mannes A, Iadarola M, Quezado ZM. Disruption of the transient receptor potiential vanilloid can affect survival, bacterial clearance,and cytokine gene expression during murine sepsis. Anesthesiology. 2011;114:1190–1199. doi: 10.1097/ALN.0b013e318212515b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao L, Lin S, Lin J. The effects of anesthetics on tumor progression. Int J Physiol Pathophysiol Pharmacol. 2013;5:1–10. [PMC free article] [PubMed] [Google Scholar]
- 15.Zylla D, Gourley BL, Vang D, Jackson S, Boatman S, Lindgren B, Kuskowski MA, Le C, Gupta K, Gupta P. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer. 2013;119:4103–4110. doi: 10.1002/cncr.28345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kharmate G, Rajput PS, Lin YC, Kumar U. Inhibition of tumor promoting signals by activation of SSTR2 and opioid receptors in human breast cancer cells. Cancer Cell Int. 2013;13:93–106. doi: 10.1186/1475-2867-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamizu K, Furuta S, Hamada Y, Yamashita A, Kuzumaki N, Narita M, Doi K, Katayama S, Nagase H, Yamashita JK, Narita M. к Opioids inhibit tumor angiogenesis by suppressing VEGF signaling. Sci Rep. 2013;14:3213. doi: 10.1038/srep03213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng D, Huang M, Jiang R, Zhan R, Yang C. Clinical study of etomidate emulsion combined with remifentanil in general anesthesia. Drug Des Devel Ther. 2013;20:771–776. doi: 10.2147/DDDT.S45979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moody EJ, Knauer CS, Granja R, Strakhovaua M, Skolnick P. Distinct structural requirements for direct and indirectactions of the anaesthetic etomidate at GABAA receptors. Toxicol Lett. 1998;100-101:209–215. doi: 10.1016/s0378-4274(98)00187-8. [DOI] [PubMed] [Google Scholar]
- 20.Vanacker B, Wiebalck A, Van Aken H, Sermeus L, Bouillon R, Amery A. Quality of induction and adrenocortical function: a clinical comparison of Etomidate-Lipuro and Hypnomidate. Anaesthesist. 1993;42:81–89. [PubMed] [Google Scholar]
- 21.Banihashem N, Alijanpour E, Basirat M, Shokri Shirvany J, Kashifard M, Taheri H, Savadkohi S, Hosseini V, Solimanian SS. Sedation with etomidate-fentanyl versus propofol-fentanyl in colonoscopies: A prospective randomized study. Caspian J Intern Med. 2015;6:15–19. [PMC free article] [PubMed] [Google Scholar]
- 22.Shah SB, Chowdhury I, Bhargava AK, Sabbharwal B. Comparison of hemodynamic effects of intravenous etomidate versus propofol during induction and intubation using entropy guided hypnosis levels. J Anaesthesiol Clin Pharmacol. 2015:180–185. doi: 10.4103/0970-9185.155145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luan HF, Zhao ZB, Feng JY, Cui JZ, Zhang XB, Zhu P, Zhang YH. Prevention of etomidate-induced myoclonus during anesthetic induction by pretreatment with dexmedetomidine. Braz J Med Biol Res. 2015;48:186–190. doi: 10.1590/1414-431X20144100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He L, Ding Y, Chen H, Qian Y, Li Z. Dezocine pretreatment prevents myoclonus induced by etomidate: a randomized, double-blinded controlled trial. J Anesth. 2015;29:143–145. doi: 10.1007/s00540-014-1854-2. [DOI] [PubMed] [Google Scholar]
- 25.Safavi M, Honarmand A, Sahaf AS, Sahaf SM, Attari M, Payandeh M, Iazdani A, Norian N. Magnesium sulfate versus Lidocaine pretreatment for prevention of pain on etomidate injection: A randomized, double-blinded placebo controlled trial. J Res Pharm Pract. 2015;4:4–8. doi: 10.4103/2279-042X.150044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weng D, Huang M, Jiang R, Zhan R, Yang C. Clinical study of etomidate emulsion combined with remifentanil in general anesthesia. Drug Des Devel Ther. 2013;7:771–776. doi: 10.2147/DDDT.S45979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganesan AP, Johansson M, Ruffell B, Yagui-Beltrán A, Lau J, Jablons DM, Coussens LM. Tumor-infiltrating regulatory T cells inhibit endogenous cytotoxic T cell responses to lung adenocarcinoma. J Immunol. 2013;191:2009–2017. doi: 10.4049/jimmunol.1301317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei L, Meng QG, Bi ZG. Result of a randomized clinical trial comparing different types of anesthesia on the immune function of patients with osteosarcoma undergoing radical resection. Panminerva Med. 2013;55:211–216. [PubMed] [Google Scholar]
- 29.Xu YB, Du QH, Zhang MY, Yun P, He CY. Propofol suppresses proliferation, invasion and angiogenesis by down-regulating ERK-VEGF/MMP-9 signaling in Eca-109 esophageal squamous cell carcinoma cells. Eur Rev Med Pharmacol Sci. 2013;17:2486–2494. [PubMed] [Google Scholar]
- 30.Zhang J, Zhang D, Wu GQ, Feng ZY, Zhu SM. Propofol inhibits the adhesion of hepatocellular carcinoma cells by upregulating microRNA-199a and downregulating MMP-9 expression. Hepatobiliary Pancreat Dis Int. 2013;12:305–309. doi: 10.1016/s1499-3872(13)60048-x. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Chen J, Mu LH, Du QH, Niu XH, Zhang MY. Propofol inhibits invasion and enhances paclitaxel- induced apoptosis in ovarian cancer cells through the suppression of the transcription factor slug. Eur Rev Med Pharmacol Sci. 2013;17:1722–1729. [PubMed] [Google Scholar]
- 32.Keim SM, Erstad BL, Sakles JC, Davis V. Etomidate for procedural sedation in the emergency department. Pharmacotherapy. 2002;22:586–592. doi: 10.1592/phco.22.8.586.33204. [DOI] [PubMed] [Google Scholar]
- 33.Ji L, Jin-Bao L, Xiao-Ming D. Feasibility of TCI etomidate for total intravenous anesthesia. J Clin Anesthesiol. 2009;25:389–391. [Google Scholar]


