Abstract
The dysfunction of peripheral immune tolerance plays an important role in the pathogenesis of allergic diseases. Recent reports indicate that micro RNA (miR)-98 is associated with the process of aberrant immune responses. This study aims to test a hypothesis that miR-98 is associated with the pathogenesis of airway allergy via interfering with the development of regulatory B cells (Breg). In this study, patients with airway allergy were recruited into this study. The frequency of Bregs was assessed by flow cytometry. The levels of miR-98 in peripheral B cells were determined by RT-qPCR. A cell-culture model of B cells was developed to test the role of miR-98 in the repressing of interleukin (IL)-10 in B cells. The results showed that the levels of IL-10 in peripheral B cells were significantly lower in patients with airway allergy as compared with healthy subjects. High levels of miR-98 (one of the miR-98 members) were detected in peripheral B cells of patients with airway allergy, which was mimicked by stimulating B cells with IL-4. Histone acetyltransferase p300 was involved in the IL-4-induced miR-98 expression. miR-98 mediated the IL-4-inhibited IL-10 expression in B cells. In conclusion, miR-98 affects the expression of IL-10 in B cells and may be a novel therapeutic target for the treatment of allergic diseases.
Keywords: Allergy, airway, micro RNA, interleukin-10, B cell
Introduction
Airway allergy includes allergic asthma and allergic rhinitis. T helper (Th)2 polarization plays an important role in the pathogenesis of airway allergy. The over production of Th2 cytokines, including interleukin (IL)-4, IL-5, IL-13, etc., is detected in the local tissue, which induces skewed immune response and inflammation in the airway mucosa [1]. The Th2 cytokines are also responsible for inducing IgE production by B cells [2]. Besides producing IgE, a fraction of B cells produce IL-10 that is designated regulatory B cells (Breg) [3]. Bregs are capable of suppressing other immune cell activities [3]. Decrease in the frequency of IL-10-producing Bregs has been observed in patients with immune diseases [4-6]. The underlying mechanism by which the expression of IL-10 in B cells is compromised is unclear.
MicroRNA (miR) is a class of noncoding RNA molecules. Their length is ranging from 18 to 24 nucleotides and is encoded by endogenous genes [7]. They are involved in transcriptional regulation of gene expression in animals, plants and virus. The miR gene can be a single copy, multi copy or gene existing in the genome [8]. Published data indicate that miRs are associated with the pathogenesis of allergic diseases [9,10] or with the regulation of allergic diseases [11]. The underlying mechanism remains to be further investigated. Recent reports indicate that the miR-98 suppresses the expression of IL-10 [12]. Based on the information above, we hypothesize that miR-98 may be associated with the pathogenesis of allergic diseases. Thus, we performed this study; we observed high levels of miR-98 in peripheral B cells of patients with airway allergy, which was negatively correlated with the expression of IL-10 in B cells.
Materials and methods
Patients
Patients with allergic asthma or/and allergic rhinitis were recruited into this study. The diagnosis of asthma and allergic rhinitis was carried out at Shenzhen Maternity & Child Health Hospital and Shenzhen Longgang ENT Hospital by physicians based on the disease history of asthma and allergic rhinitis, allergen skin prick test, serum specific IgE (>0.35 kU/L). Patients with severe autoimmune diseases and using immune suppression agents in recent two months were excluded. The demographic data of the patients are presented in Table 1. Healthy volunteers were also recruited into this study based on allergic disease history, serum total IgE levels were less than 0.35 kU/L. The experimental procedures were approved by the Human Ethic Committee at Shenzhen University. All the experiments were performed in accordance with the approved guidelines. An informed written consent was obtained from each subject.
Table 1.
Demographic data
| Item | Value |
|---|---|
| Age | 26.3 ± 12.7 |
| Weight | 52.4 ± 24.7 |
| Height | 158.7 ± 35.2 |
| Gender (M/F) | 10/10 |
| Asthma/allergic rhinitis | 14 (70%) |
| Asthma | 10/10 |
| FEV1 (%-pred.) | 95.2 ± 10.8 |
| FVC (%-pred.) | 86.8 ± 6.5 |
| Specific IgE (IU/L) | 93.6 ± 23.6 |
The values are presented as mean ± SD. Asthma/allergic rhinitis: Patients with both asthma and allergic rhinitis. Asthma: Patients with asthma. FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; specific IgE, specific IgE for dust mite.
SPT was performed in all subjects with the following allergen extracts (ALK-Abelló, Hørsholm, Denmark): D. pteronyssinus, D. farinae, grass pollen mix, cat and dog dander, American cockroach, mould mix, tree pollen mix and weed pollens. The results were checked 25 minutes after SPT. The judgement of a positive result was the prick spot became a wheal and fleck surrounding the wheal.
Collection of blood samples
Blood samples (25 ml per subject) were collected from each subject via ulnar vein puncture. Peripheral blood mononuclear cells (PBMC) were isolated from the blood samples by gradient density centrifugation. CD19+ B cells were purified from the PBMCs by magnetic cell sorting with a reagent kit (Miltenyi Biotech) following the manufacturer’s instructions. The purity of the isolated B cells was checked by flow cytometry (≥95%).
B cell culture
B cells were cultured with RPMI1640 medium supplemented with 100 U/ml penicillin, 10% fetal bovine serum, 0.1 mg/ml streptomycin and 2 mM L-glutamine. The cell viability was checked with the Trypan blue exclusion assay.
Assessment of precursor and mature miRNAs by quantitative real-time PCR (qPCR)
Total RNA was extracted from the B cells by Trizol (Invitrogen) according to the manufacturer’s instructions. Complementary DNA was synthesized from total RNA samples using the NCode Vilo miRNA cDNA Synthesis Kit (Life Technologies). Real-time PCR was performed with the mini Opticon real-time PCR system (Bio-Rad) using SYBR Green qPCR Master Mix (Invitrogen) with miRNA-specific primers and a universal qPCR primer according to the manufacturer’s protocol for the NCode VILO Kit. All miRNAs were normalized to a small nucleolar RNA, RNU48. Primers used in the present study were provided by the Enke Biotech (Shenzhen, China). For quantification, the fold-change of miRNA in experimental relative to control samples was determined by the 2-∆∆Ct method.
Assessment of serum IL-4 levels by enzyme-linked immunosorbent assay (ELISA)
The sera were isolated from the blood samples collected from the patients. The IL-4 levels of the serum were evaluated by ELISA with a reagent kit (R&D Systems) following the manufacturer’s instructions.
Preparation of protein extracts
Cells were lysed with lysis buffer at 4°C for 15 min, and centrifuged at 500×g for 10 min at 4°C. The supernatant (cytosolic extract) was collected for further experiments. The pellet was added with nuclear extract buffer and incubated for 15 min at 4°C, followed by centrifugation at 13,000×g for 10 min at 4°C. The supernatant (the nuclear extract) was collected for further experiments.
ChIP (chromatin immunoprecipitation assay)
ChIP assay was performed with the reagents (Sigma Aldrich) following the manufacturer’s instructions. The B cells were fixed for 15 min with 1% formalin, lysed and sonicated. Cell lysates were precleared by incubation with protein G-agarose for 2 h at 4°C. The samples were centrifuged for 5 min at 13,000 rpm; the supernatant was collected and incubated overnight at 4°C with 2 μg of specific antibodies or isotype IgG (Santa Cruz Biotech). The precipitated antibody-chromatin complex was collected by incubation with protein G-agarose beads for 1 h at 4°C, and then washed and eluted in elution buffer. The samples were reverse crosslinking with a reagent kit at 65°C for 4 h, and then digested with proteinase K for 1 h at 45°C to remove proteins from DNA, the DNA was recovered by phenol/chloroform extraction and ethanol precipitation. The DNA or input was analyzed by qPCR with the following miR-98 promoter primers: gcttacagtgcaggtagtga and ggagcacttagggcagtaga. The results were presented as fold change against the input.
Immunoblotting
B cells were collected from each experiment; the proteins were extracted from B cells, separated by SDS-PAGE and transferred onto a PVDF membrane. After blocking with 5% skim milk for 30 min, the membrane was incubated with antibodies of interest overnight at 4°C, and followed by incubating with the second antibodies (labeled with peroxidase) for 1 h at room temperature. Washing with Tris-buffered saline-Tween 20 was performed 3 times after each time of incubation. The immune blots was developed with ECL (enhanced chemiluminescence) and photographed with an imaging device.
RNA interference (RNAi) of miR-98
RNAi of miR-98 was performed in B cells with the shRNA reagents provided by GeneChem (Shanghai, China) following the manufacturer’s instructions. The sequences of miR-98 shRNA were AGTTGCACTACAAGAAGAATG. RT-qPCR was performed to evaluate the RNAi effects.
Statistics
The difference between two groups was determined by Student t test or ANOVA if more than two groups. Data are presented as mean ± SD. The Pearson Correlation Assay was performed to determine the correlation between two groups. The significant criterion was set P<0.05.
Results
Low levels of IL-10 and high levels of miR-98 were detected in peripheral B cells of patients with airway allergy
B cells were isolated from the samples from patients with airway allergy and healthy subjects and processed for cell extracts. The levels of IL-10 mRNA and miR-98 were analyzed in the extracts. As shown by Figure 1, the levels of miR-98 were significantly higher in the allergy group than in the healthy group, while the IL-10 levels were significantly lower in the allergy group than in the healthy group. A correlation assay was performed with the data of IL-10 and miR-98 in the B cells. The results showed a significant negative correlation between IL-10 and miR-98 (r = -0.78995, P<0.01). The results implicate that miR-98 may be associated with the low levels of IL-10 in B cells of patients with airway allergy.
Figure 1.
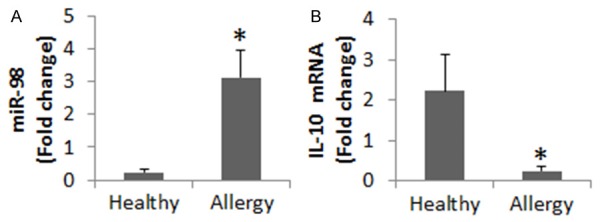
Assessment of miR-98 and IL-10 in peripheral B cells. Peripheral B cells from healthy subjects (n = 25) and patients of airway allergy (n = 25) were analyzed by RT-qPCR. The bar graphs show the miR-98 levels (A) and IL-10 mRNA levels (B). Data of bars are presented as mean ± SD. *, P<0.01, compared with the healthy group. Samples from individual patients were analyzed separately.
IL-4 is associated with the increase in miR-98 in B cells
We next investigated a possible role of IL-4 in the regulation of miR-98 expression in B cells. We assessed the serum levels of IL-4. The results showed that the levels of IL-4 were significantly higher in the allergy group than in the healthy group (Figure 2A), which was positively correlated (r = 0.79481, P<0.01) with the miR-98 levels in peripheral B cells. B cells were isolated from blood samples of healthy subjects and stimulated with IL-4 in the culture for 48 h. The B cells were analyzed by RT-qPCR. The results showed that exposure to IL-4 significantly increased the expression of miR-98 in B cells in an IL-4 dose-dependent manner (Figure 2B). The results implicate that IL-4 can up regulate the expression of miR-98 in peripheral B cells.
Figure 2.
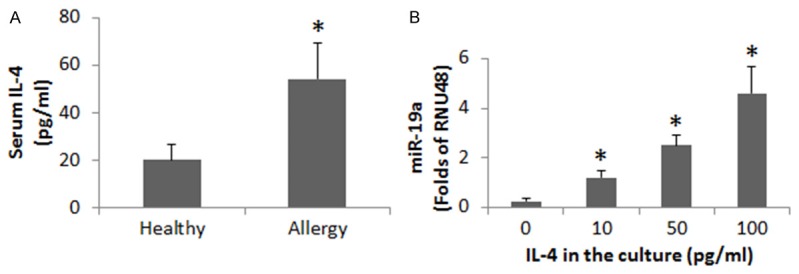
Determination of the correlation between serum IL-4 and miR-98 in B cells. (A) The bars indicate the serum levels of IL-4 (assessed by ELISA) in 25 healthy subjects and 25 patients of airway allergy. (B) The bars show the miR-98 levels in B cells (by RT-qPCR); the B cells were isolated from 6 healthy subjects and cultured in the presence of recombinant IL-4 (the concentrations of IL-4 are denoted on the X axis) for 48 h. Data of bars are presented as mean ± SD. *, P<0.01, compared with the healthy group (A) or dose “0” group (B). Samples from individual subjects were analyzed separately.
P300 mediates IL-4-increased miR-98 in B cells
We further investigated the role of p300 in the induction of miR-98 in B cells by IL-4. In the same approach of Figure 2A, we stimulated B cells (from healthy subjects) with IL-4 in the culture for 48 h. The nuclear extracts of B cells were analyzed by ChIP. The results showed that exposure to IL-4 significantly increased the levels of p300, H3K4 and RNA polymerase II at the miR-98 promoter locus of the B cells (Figure 3A-C). We also analyzed the miR-98 in the cytosolic extracts. The results showed that the levels of miR-98 were also increased markedly (Figure 3D). The results demonstrate that p300 is involved in the IL-4 facilitate the miR-98 gene transcription in B cells. To corroborate the results, we added an inhibitor of p300 to the culture. Indeed, the IL-4-induced miR-98 expression was abolished (Figure 3D).
Figure 3.
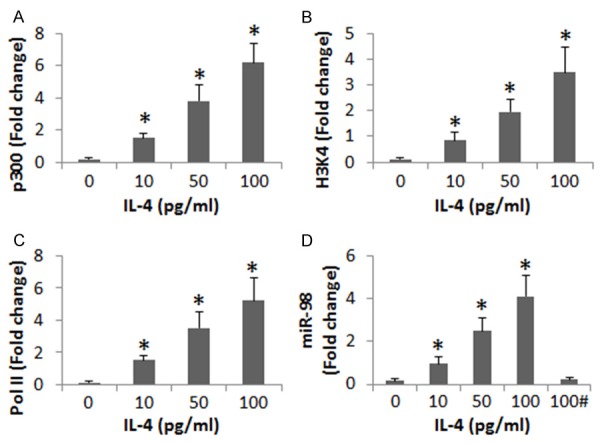
IL-4 induces chromatin remolding at miR-98 promoter locus to increase miR-98 expression in B cells. Peripheral B cells were collected from 6 healthy subjects and stimulated with IL-4 in the culture at graded doses (as denoted on the X axis of each panel) for 48 h. The B cells were analyzed by ChIP (A-D). The bars indicate the levels of p300 (A), H3K4 (B) and RNA polymerase II (Pol II; C) at the miR-98 promoter locus. (D) The bars indicate the miR-98 levels in the B cells. Data of bars are presented as mean ± SD. *, P<0.01, compared with the dose “0” group. Samples from individual subjects were analyzed separately (The data are summarized from 6 independent experiments). #, the presence of garcinol (an inhibitor of p300; 10 μM).
miR-98 mediates IL-4-inhibited IL-10 expression in B cells
The data of Figure 1 implicate that miR-98 is associated with the suppression of IL-10 expression in peripheral B cells of patients with airway allergy. To test this, we collected peripheral B cells from healthy subjects and stimulated with LPS. It increased the expression of IL-10 in the B cells. The increase in IL-10 could be blocked in the presence of IL-4. To test if miR-98 is involved in the IL-4-induced inhibition of IL-10 in B cells, we knocked down the miR-98 gene in B cells. The miR-98-deficient B cells were exposed to LPS and IL-4. Indeed, the B cells expressed high levels of IL-10 (Figure 4A-C). To corroborate the results, we constructed miR-98 plasmids to be transfected into B cells. The expression of miR-98 in the B cells was increased about 4 folds after transfection (Figure 4D). The LPS-induced expression of IL-10 in B cells was inhibited by the miR-98 overexpression (Figure 4E).
Figure 4.
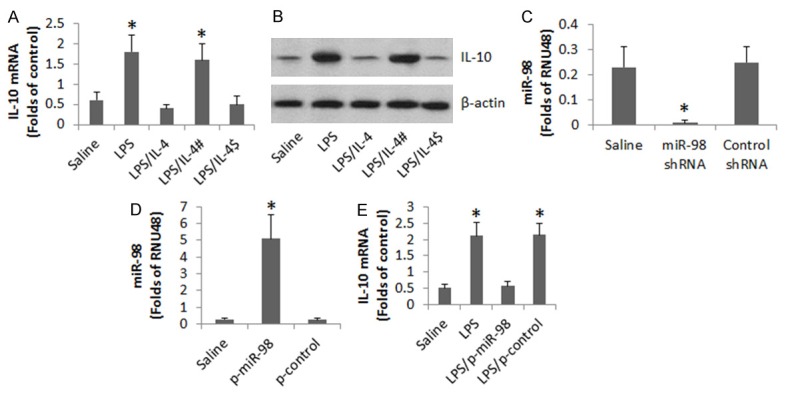
Assessment of the role of miR-98 in the IL-4-suppressed IL-10 expression in B cells. Peripheral B cells were collected from 3 healthy subjects. The B cells were treated in the culture as denoted on the X axis of (A). LPS = 500 ng/ml. IL-4 = 200 pg/ml. #, miR-98-deficient B cells. $, B cells were treated with control shRNA. (A) The bars indicate the levels of IL-10 mRNA in the B cells. (B) The immune blots indicate the protein levels of IL-10 in the B cells. (C) The bars indicate the miR-98 RNAi results. (D) The bars indicate the results of overexpression of miR-98 in B cells. p-miR-98: miR-98 plasmids. p-control: Control plasmids. (E) The bars indicate the mRNA levels of IL-10 in B cells. Data of bars are presented as mean ± SD. *, P<0.01, compared with the saline group. The data are representatives of 3 independent experiments.
Discussion
The present data show that miR-98 plays a critical role in the suppression of IL-10 in peripheral B cells in patients with airway allergy. The results showed that the levels of miR-98 were higher, the IL-10 levels were lower, in peripheral B cells of patients with airway allergy. miR-98 mediated the effect of IL-4 in the repression of IL-10 gene transcription in B cells. We also observed that p300 was a key molecule in the induction of miR-98; blocking p300 abolished the IL-4-induced miR-98 expression.
IL-10 is an immune regulatory cytokine. It can be produced by regulatory T cells or regulatory B cells to fulfill immune regulatory duties. Our data indicate that the IL-10 expression is much less in peripheral B cells in patients with airway allergy than that in healthy subjects, indicating the production of IL-10 by B cells is compromised somehow. A similar phenomenon was also observed by us in previous studies. In a food allergy animal model study, we observed low frequency of IL-10+ B cell in food allergy mice [13]. Others also found that the frequency of IL-10+ B cell in patients with immune disorders were less than healthy persons and correlated it with different clinical and immunological characteristics of the diseases [14].
To identify the causative factors in the inhibition of IL-10 in B cells is of significance. The present data show that the expression of miR-98 by B cells is negatively correlated with the expression of IL-10 in B cells. The factor implicates that miR-98 is involved in the suppression of IL-10 in B cells. miR-98 has been found playing roles in a number of immune disorders. Wang et al found that miR-98/b level in pancreatic cancer tissues was conversely correlated with TP53 and TP53INP1 expression, indicating miR-19 could be a molecular marker of pancreatic cancer [15]. Simpson et al found that miR-98 expression in CD4+ T cells was enhanced in asthma patient [16] that is somewhat in line with the present data, indicating that miR-98 is involved in the regulation of the activities of the major immune cells, T cells and B cells.
IL-4 is the signature cytokine in Th2 polarization. Over production of IL-4 is a common phenomenon in a number of Th2 polarization-related diseases, such as allergy. The present data also show high serum levels of IL-4 in patients with airway allergy. The data indicate that peripheral B cells are stimulated constantly by IL-4 at a high concentration in the blood system. Further experiments showed that IL-4 could block IL-10 expression, in which miR-98 played a critical role. Furthermore, we also identified that p300 was involved in the suppression of IL-10 in B cells mediated by miR-98, which was abolished by the presence of an inhibitor of p300, implicating that the p300 inhibitors have therapeutic potential in the treatment of allergic disorders.
Acknowledgements
This study was supported by grants from the innovation of science and Technology Commission of Shenzhen Municipality (JCYJ20160429091935720, JCYJ20160422101725667, JCYJ20140418095735611, and ZDSYS201506050935272, CXZZ20140902151802864), the Natural Science Foundation of China (31400856, 81373176, 31570932, 81400001, 81571790 and 81501573).
Disclosure of conflict of interest
None.
References
- 1.Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386:1086–1096. doi: 10.1016/S0140-6736(15)00157-9. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15:35–50. doi: 10.1038/nrd4624. [DOI] [PubMed] [Google Scholar]
- 3.Rieger A, Bar-Or A. B-cell-derived interleukin-10 in autoimmune disease: regulating the regulators. Nat Rev Immunol. 2008;8:486–487. doi: 10.1038/nri2315-c1. [DOI] [PubMed] [Google Scholar]
- 4.Bird L. Immunometabolism: regulatory B cells weigh in. Nat Rev Immunol. 2014;14:6–7. doi: 10.1038/nri3588. [DOI] [PubMed] [Google Scholar]
- 5.Noh G, Lee JH. Regulatory B cells and allergic diseases. Allergy Asthma Immunol Res. 2011;3:168–177. doi: 10.4168/aair.2011.3.3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 8.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 9.Rebane A. microRNA and Allergy. Adv Exp Med Biol. 2015;888:331–352. doi: 10.1007/978-3-319-22671-2_17. [DOI] [PubMed] [Google Scholar]
- 10.Newcomb DC, Cephus JY, Boswell MG, Fahrenholz JM, Langley EW, Feldman AS, Zhou W, Dulek DE, Goleniewska K, Woodward KB, Sevin CM, Hamilton RG, Kolls JK, Peebles RS Jr. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J Allergy Clin Immunol. 2015;136:1025–1034. e1011. doi: 10.1016/j.jaci.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo X, Han M, Liu J, Wang Y, Luo X, Zheng J, Wang S, Liu Z, Liu D, Yang PC, Li H. Epithelial cell-derived micro RNA-146a generates interleukin-10-producing monocytes to inhibit nasal allergy. Sci Rep. 2015;5:15937. doi: 10.1038/srep15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Chen Q, Song Y, Lai L, Wang J, Yu H, Cao X, Wang Q. MicroRNA-98 negatively regulates IL-10 production and endotoxin tolerance in macrophages after LPS stimulation. FEBS Lett. 2011;585:1963–1968. doi: 10.1016/j.febslet.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Xu LZ, Peng K, Wu W, Wu R, Liu ZQ, Yang G, Geng XR, Liu J, Liu ZG, Liu Z, Yang PC. Specific immunotherapy in combination with Clostridium butyricum inhibits allergic inflammation in the mouse intestine. Sci Rep. 2015;5:17651. doi: 10.1038/srep17651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barsotti NS, Almeida RR, Costa PR, Barros MT, Kalil J, Kokron CM. IL-10-producing regulatory B cells are decreased in patients with common variable immunodeficiency. PLoS One. 2016;11:e0151761. doi: 10.1371/journal.pone.0151761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Wang L, Mo Q, Jia A, Dong Y, Wang G. A positive feedback loop of p53/miR-19/TP53INP1 modulates pancreatic cancer cell proliferation and apoptosis. Oncol Rep. 2016;35:518–523. doi: 10.3892/or.2015.4361. [DOI] [PubMed] [Google Scholar]
- 16.Simpson LJ, Patel S, Bhakta NR, Choy DF, Brightbill HD, Ren X, Wang Y, Pua HH, Baumjohann D, Montoya MM, Panduro M, Remedios KA, Huang X, Fahy JV, Arron JR, Woodruff PG, Ansel KM. A microRNA upregulated in asthma airway T cells promotes TH2 cytokine production. Nat Immunol. 2014;15:1162–1170. doi: 10.1038/ni.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]


