Abstract
Objective: The identification of the biological function of M1 macrophages and the mechanism underlying their role in valvular interstitial cell (VIC) calcification may provide therapeutic targets for the prevention of aortic valve calcification (AVC). This study investigated the mechanism by which M1 macrophages and macrophage-derived microvesicles (MVs) affected the calcification of VICs. An additional aim was to investigate the involvement of the miR-214 pathway in this process. Methods: The M1 or M2 macrophage phenotype in human calcific aortic valve was confirmed by gene expression analysis of M1 or M2 macrophage markers. Two macrophage cell lines (BMDMs and RAW 264.7 macrophages) were transformed into M1 macrophages by lipopolysaccharide (LPS) stimulation. To investigate the mechanism by which M1 macrophages promoted VIC calcification, the generated M1 macrophages and macrophage-derived MVs were co-cultured with VICs and VICs were then used for calcification or signals analysis. In addition, a hypercholesterolemic apoE-/- AVC murine model was used to evaluate the therapeutic efficacy of miR-214 specific-siRNA (miR-214 inhibitor). Results: Macrophages in calcific aortic valves showed M1-directed polarization. In the VICs co-cultured with LPS-stimulated M1 macrophages and macrophage-derived MVs, VIC calcification was enhanced, and the expression of TWIST1, a direct target of miR-214, was downregulated. We showed that knockdown of TWIST1 serves as a responding molecule for miR-214 and reversed the anti-calcification action of miR-214 inhibitor, mediating signal delivery by the M1 macrophage-derived MVs to VICs and promoting VIC calcification. When M1 macrophages co-cultured with VICs, TWIST1 overexpression in M1 macrophages had no effect on the expression of TWIST1 in VICs. As shown by intravenous therapy, knockdown of miR-214 in mice seemed to improve AVC in apoE-/- mice with high-cholesterol (HC)-diet induced AVC. Conclusions: These findings suggested that M1 macrophages promoted AVC by the delivery of miR-214 to valvular interstitial cells via macrophage-derived MVs and subsequent downregulation of TWIST1 of valvular interstitial cells.
Keywords: Aortic valve calcification, microvesicles, osteocalcin, miR-214, TWIST1
Introduction
Aortic valve calcification (AVC) has a significant clinical impact, as it is the third leading cause of adult heart disease and the most common form of acquired valvular disease at present [1]. Recent clinical data suggested that AVC is involved a process of osteogenesis differentiation, which is associated with the regulation of osteogenesis-related factors [2]. Although the mechanistic pathways involved in the development of AVC disease remain largely unknown, examination of human calcific aortic valves and in vitro experiments with aortic valve interstitial cells (VICs) demonstrated that several factors, particularly macrophages, affected the calcification process [3,4].
The accumulation of macrophages in aortic valvular lesions provided the first histopathological evidence of the contribution of inflammation to cardiovascular calcification [5]. Depending on the microenvironment, macrophages can differentiate into either a proinflammatory M1 subtype, also known as a classically activated subtype, or an anti-inflammatory alternatively activated subtype (M2) [6]. Proinflammatory M1 macrophage polarization is accompanied by the production of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-12, and elevated levels of inflammation-associated proteins, such as inducible nitric oxide synthase (iNOS) and monocyte chemoattractant protein-1 (MCP-1) [6]. In contrast, in M2 macrophage-directed polarization, the anti-inflammatory cytokine TGF-β and markers of alternative activation, including arginase-1 (Arg-1) and Yml, are activated [7]. The roles of M1/M2 macrophage subtypes in AVC and the potential mechanism by which macrophages influence the process of VIC calcification remain unknown.
MicroRNAs (miRNAs) are a class of highly conserved small noncoding RNAs that have pivotal roles in post-transcriptional modulation of gene expression. The control of gene expression by miRNA was shown to be involved in regulating important pathophysiological processes, including cellular activity, differentiation, and inflammatory responses [8]. Research showed that the miRNA miR-214 played an important role in the inflammatory response to disease [9,10]. Increased expression of miR-214 was shown to be essential for M1-directed polarization of RAW 264.7 macrophages [11]. Recent evidence suggested that miR-214 was a mechanosensitive gene in the aortic valve and that it seemed to be associated with pathogenesis of AVC in porcine aortic valve [12]. Previous studies also reported that miR-214 influenced bone formation by affecting osteogenic differentiation and osteoclastogenesis through targeting several genes in various cell types [13-15]. However, thus far, no direct evidence for the role of miR-214 in VIC calcification during AVC has been presented. Based on the aforementioned literature, we speculated that miR-214 might be involved in macrophage-induced VIC calcification.
In the present study, we initially compared the expression of M1/M2 macrophage subtypes and miR-214 in calcific aortic valves and noncalcific aortic valves. We then investigated the role of the dominant macrophage subtype in the calcification of VICs and the functional involvement of miR-214 in this process.
Material and methods
Sample collection
All the samples were obtained after receiving written informed consent. Calcific aortic valves were isolated from patients with aortic valve stenosis and calcification undergoing aortic valve replacement surgery at the Affiliated Hospital of Nantong University from 1 January 2014 to 6 June 2014. Pathomorphological changes were confirmed at the time of aortic valve replacement. Noncalcific aortic valves isolated from aortic valve insufficiency patients served as controls. The study protocols were approved by the institutional ethics committee of the Affiliated Hospital of Nantong University.
Each aortic valve sample was frozen separately in liquid nitrogen for quantitative real-time PCR or Western blot analysis (Figure 1). The relative genes expression of aortic valve samples represents that of VICs attributed to the main cell type in samples is VIC. M1 polarization was indicated by increased mRNA expression of M1 genes, including iNOS, TNF-α, IL-6, IL-12, and monocyte chemoattractant protein-1 (MCP-1). M2 polarization was indicated by a change in the mRNA expression of Arg-1, TGF-β, and Yml.
Figure 1.
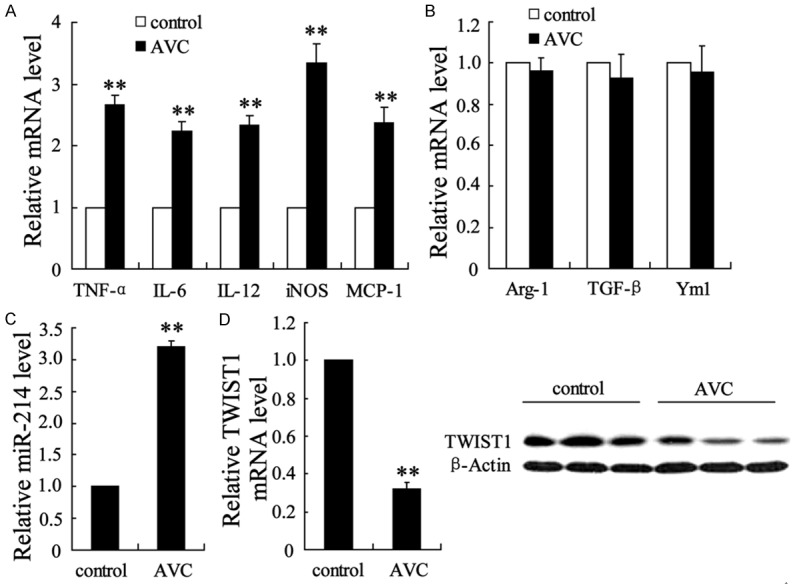
Macrophage phenotypes and genotypes of the calcific aortic valve. Normal aortic valves (n = 10) and calcific aortic valves (n = 10) were isolated from humans and subjected to molecular analysis. qRT-PCR was performed to determine the mRNA levels of (A) five markers of M1 macrophages (TNF-α, IL-6, IL-12, iNOS, and MCP-1), (B) three M2 macrophage markers (Arg-1, TGF-β, and Ym1), and (C) miR-214. (D) TWIST1 mRNA and protein expression levels were evaluated using qRT-PCR and Western blot analyses, respectively. The data are presented as the mean ± SD. **P < 0.01 compared with a normal aortic valve.
Quantitative real-time PCR
For mRNA and miR-214 expression in VICs, RNAs were isolated from the aortic valve samples or cells using Trizol (Invitrogen, Carlsbad, CA, U.S.) according to the manufacturer’s protocols. The RNAs were reverse transcribed into cDNA product using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, U.S.). Then, using a TaqMan Universal PCR Master Mix (Applied Biosystems), quantitative PCR (qPCR) was performed in 50 μl of reaction volume containing 0.1 μg of cDNA, 300 nM of each primer, and 20 nM of probes. Gene-specific primers and TaqMan probe sets for TNF-α, IL-6, IL-12, iNOS, monocyte chemotactic protein-1, Arg-1, TGF-β, Yml, TWIST1, osteocalcin, and miR-214 were purchased from Applied Biosystems. The expression level of β-actin or U6 was used as an internal control. The relative expression of genes was normalized to internal control and calculated using the 2-ΔΔCT method.
Western blot
The expression of intracellular proteins in VICs was determined by Western blotting under experimental conditions. In brief, total proteins were lysed from cells using a PIPA Lysis and Extraction Buffer (Thermo Fisher Scientific), and the protein concentration of the cell lysates was measured using a BCA Protein Assay Kit (Thermo Scientific Pierce, U.S.). A volume equal to 40 µg of total protein was separated by SDS-PAGE on a 5-10% gradient gel and then transferred to a PVDF membrane (Millipore, U.S.) in the presence of a transfer buffer in an ice bath. The membrane was blocked with 5% milk powder at room temperature for 2 h before probing the protein bands with specific antibodies against TWIST1 (dilution in 1:1000; Abcam, Cambridge, MA, U.S.) or osteocalcin (Santa Cruz, CA, U.S.). The expression level of the β-actin housekeeping gene was used as a control.
Culture and M1 polarization of BMDMs and RAW 264.7 macrophages
Murine BMDMs and RAW 264.7 cell lines were grown in Dulbecco modified Eagle medium (DMEM) medium, supplemented with 10% FBS (Hyclone, Logan, UT, U.S.) in an atmosphere of 5% CO2 and 37°C. The culture medium was replaced every 48 h. To activate macrophages with an M1 phenotype, the BMDMs and RAW 264.7 macrophages were stimulated with 100 ng/ml of lipopolysaccharide (LPS). After incubation for 24 h, the cells were harvested for M1 macrophage marker analysis, co-culture with VICs, or microvesicle (MV) derivation.
Isolation of MVs
MVs were isolated from the culture medium of BMDMs or RAW 264.7 macrophages by differential centrifugation. In brief, the supernatant medium was collected and centrifuged at 500 g and then at 1500 g to spin down the mature cells and cell debris. The supernatant was collected for filtration using a 0.22-μm filter The MVs were finally obtained by centrifugation of the filtered fluid at 130,000 g for 70 min. All the aforementioned steps were performed at 4°C. The protein concentration of the MVs was quantified by the BCA method. The levels of MVs were determined by measuring the total protein content, which was recorded in micrograms of total protein in the MVs.
Alkaline phosphatase (ALP) activity detection
ALP activity was measured to quantify osteoblast differentiation in the primary cultures of VICs. Briefly, the VICs were seeded into 48-well plates at a concentration of 5 × 104 cells per well and cultured for up to six days in DMEM containing 10% FBS and increasing concentrations of leptin. At various times thereafter, the cells were harvested and lysed with 1% Triton X-100. The ALP activity of the cells was assessed at 405 nm using a p-nitrophenol phosphate substrate kit (Sigma-Aldrich). The ALP values (units/ng protein) were normalized to those of proteins using a Bio-Rad DC protein assay (Bio-Rad).
VIC culture
Primary aortic VICs were isolated from noncalcified human aortic valves obtained from donor hearts during heart transplant or autopsies, as described previously [16]. After carefully removing nonleaflet tissues, endothelium layer of aortic valves leaflets in the aortic surface was gently scraped by surgical blade. The leaflets were then placed in 0.25% Trypsin at 37°C for 5 min. The tissues were then cut into approximately 3 mm-sized pieces and underwent further digestion for 2 h at 37°C. The primary VICs obtained were cultured in DMEM medium, supplemented with penicillin, streptomycin, and 10% FBS at 37°C in an atmosphere of humidity and 5% CO2. Fresh medium was replaced every 3~5 days. The purity of the cells was confirmed by microscopic examination and showed spindle-shape and oval nuclei.
Recombinant TWIST1 expression
Full-length TWIST1 cDNA was obtained and digested with XhoI/EcoRI. pcDNA-TWIST1 was generated by integration of restriction fragments to pcDNA3.1 vector (invitrogen) by Sangon Biotech (Shanghai, China). For transient TWIST1 expression, the VICs were transfected with pcDNA-TWIST1 using TurboFect Transfection Reagent (Thermo Scientific). Control vectors (pcDNA) were transfected as controls.
RNA interference
Human TWIST1 siRNA and a siRNA control (negative control [NC]) were purchased from Thermo Scientific. The VICs were seeded in a 48-well culture board and transfected with 200 nM of siRNA Lipofectamine 2000 (Life Technology) for 48 h, according to the manufacturer’s protocols.
For silencing miR-214 of macrophage, the macrophages were seeded (1 × 106/well) in 6-well plates 24 h prior to transfection and the miR-214 inhibitor (Ambion, Carlsbad, CA, U.S.) was added at the final concentration (100 nM for each well) after mixing with Lipofectamine 2000 (Invitrogen) according manufacturer’s protocols. The transfection of NC-miR-214 inhibitor served as the control. After 48 h transfection, cells were harvested, followed by PCR and Western blot analysis. To evaluate the impact of knockdown of miR-214 on the formation of AVC in vivo, miR-214 inhibitor was intravenously injected into a hypercholesterolemic apoE-/- murine model.
Hypercholesterolemic apoE-/- murine model
The AVC in vivo model was induced by high-cholesterol (HC) diet administration. Male apoE-/- mice aged 6-8 weeks were selected for the experiments. Based on the experimental protocols, the animals were randomly assigned to a normal diet (n = 10), a HC diet (0.2% cholesterol, 20% fat; n = 10), an NC-injection and HC diet (n = 10), and a miR-214 inhibitor-injection and HC diet. miR-214 inhibitor was injected via caudal vein at.
After 20 weeks, the animals were sacrificed, and the aortic valve tissues were isolated for analysis. The calcification area of the aortic valve leaflets was determined using Alizarin red staining. All the experiments were performed in an animal facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Statistical analysis
An analysis of variance was used to compare the data in the experimental group with those of the control. The significance of differences was determined using one-way ANOVA and Tukey-Kramer tests. Bonferroni multiple-comparison post-hoc tests were performed to analyze differences between groups. All the data are expressed as the mean ± standard deviation (SD). A p value less than 0.05 was considered statistically significant.
Results
Macrophage polarization and differential expression of miR-214 and TWIST1 in calcific aortic valves
Calcific aortic valves were obtained from patients with aortic stenosis and calcification, and control aortic valves were obtained from patients with aortic incompetence. The calcific aortic valves showed M1 macrophage-directed polarization, as indicated by increased mRNA levels of the expression of five M1 macrophage markers (TNF-α, IL-6, IL-12, iNOS, and MCP-1) (Figure 1A) in comparison with those of the control. Although the expression level of M2 macrophages was slightly reduced in calcific aortic valves, as indicated by decreased M2 macrophage-associated Arg-1, TGF-β, and Yml gene expression, this finding was not statistically significant (Figure 1B). These data pointed to a close correlation between M1 macrophage polarization and AVC. In addition, miR-214, a macrophage polarization-associated miRNA [11], was significantly upregulated (2.2-fold compared with the control) in the calcific aortic valves (Figure 1C). The expression of TWIST1 was attenuated in mRNA and proteins (Figure 1D).
Change of miR-214 expression in LPS-induced M1 BMDMs and RAW 264.7 macrophages
To explore the correlation between the ectopic expression of miR-214 and M1 macrophage polarization, we examined the expression level of miR-214 in BMDM and RAW 264.7 macrophage cell lines under LPS-induced M1-directed polarization. Figure 2A shows the enhanced expression of M1 macrophage-associated TNF-α, IL-6, IL-12, iNOS, and MCP-1 genes, as well as the increased level of miR-214 in BMDMs. As shown in Figure 2B, the expression of these genes was similar in the RAW 264.7 cell line, with a positive correlation between M1 macrophage polarization and miR-214 expression.
Figure 2.
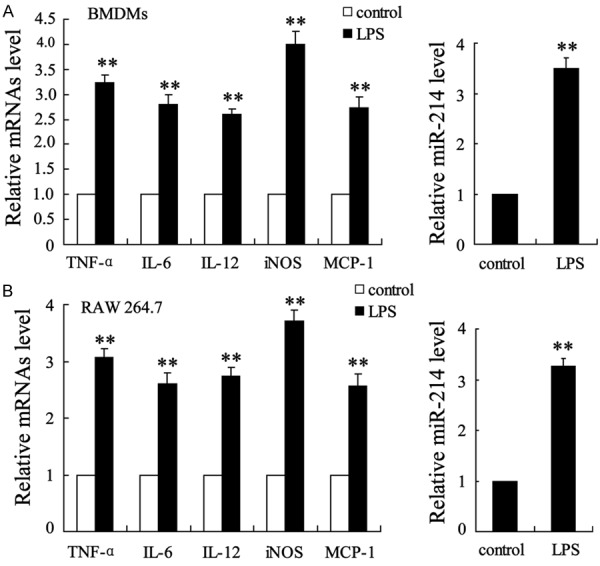
Expression of miR-214 in LPS-stimulated M1 BMDMs and RAW 264.7 macrophages. M1-directed polarization of macrophages was stimulated by LPS in BMDMs and RAW 264.7 macrophages. Cells with no treatment acted as controls. After incubation for 24 h, the expression levels of TNF-α, IL-6, IL-12, iNOS, and MCP-1 mRNA and miR-214 were determined in (A) BMDMs and (B) RAW 264.7 macrophages. The data are presented as the mean ± SD. **P < 0.01 compared with the control.
Impact of MVs derived from M1 macrophages on the calcification of VICs
To investigate the role of M1 macrophages in aortic calcification, we evaluated the calcification of VICs by determining ALP activity and differently expressed osteocalcin in VICs isolated from non-calcifying valves after a period of co-culture with LPS-stimulated M1 macrophages. Compared with the control (no LPS treatment), VICs co-incubated with LPS-stimulated BMDMs and LPS-RAW 264.7 macrophages exhibited substantial ALP activity (Figure 3A) and expressed higher osteocalcin mRNA and protein levels (Figure 3B). Meanwhile, MVs isolated from LPS-stimulated BMDMs and LPS-stimulated RAW 264.7 macrophages showed the same effect as the cell itself and caused the increase in ALP activity (Figure 3C) and the expression of osteocalcin mRNA and the osteocalcin protein (Figure 3D).
Figure 3.
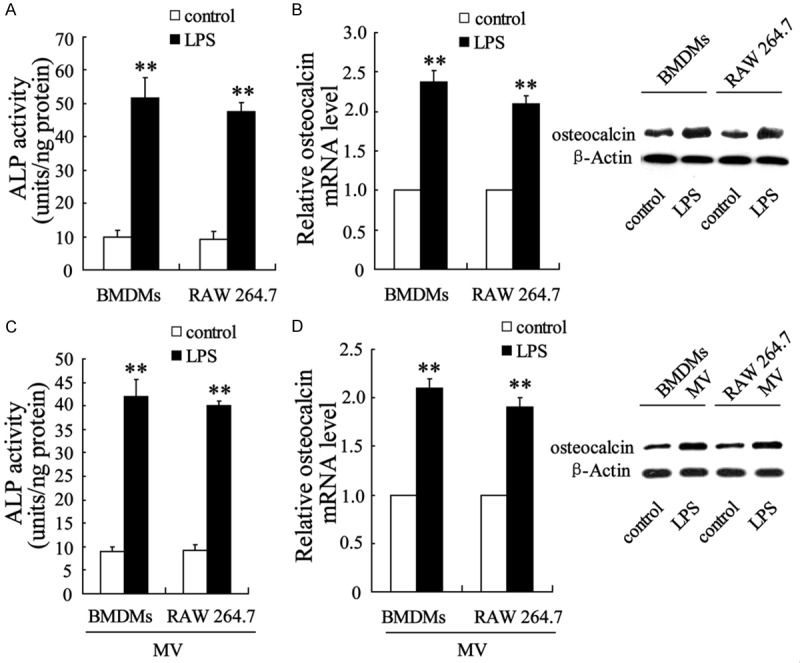
LPS-stimulated M1 BMDMs and RAW 264.7 macrophages enhanced procalcification gene expression in VICs. After incubation for 24 h with LPS, BMDMs or RAW 264.7 macrophages were co-cultured with VICs in the presence of LPS, and (A) the activity of ALP and (B) osteocalcin mRNA and protein expression levels in VICs were examined. VICs were co-cultured with MVs isolated from LPS-M1 BMDMs and RAW 264.7 macrophages, and (C) ALP activity and (D) osteocalcin mRNA and protein expression were then analyzed. The data are presented as the mean ± SD. **P < 0.01 compared with the control.
MVs derived from M1 macrophages enhanced TWIST1 expression in VICs
We next determined the expression of TWIST1 in VICs that had been co-cultured with M1 macrophages and M1 macrophage-derived MVs. As shown in Figure 4A, TWIST1 mRNA and TWIST1 protein were significantly downregulated. In addition, TWIST1 showed marked downregulation in MV-incubated VICs (Figure 4B).
Figure 4.
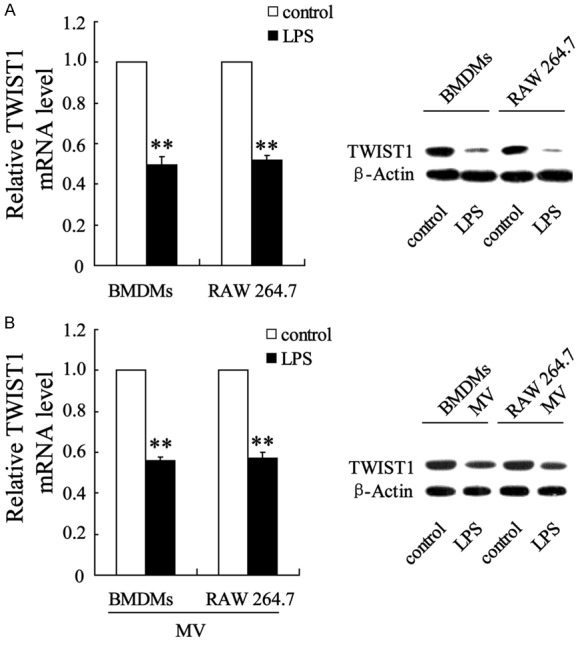
LPS-stimulated M1 BMDMs and RAW 264.7 macrophages enhanced TWIST1 gene expression in VICs. TWIST1 mRNA and protein expression were determined in VICs after co-culture with (A) LPS-stimulated M1 BMDMs, RAW 264.7 macrophages, and (B) M1-derived MVs. The data are presented as the mean ± SD. **P < 0.01 compared with the control.
TWIST1 in VICs responded to miR-214 of M1 macrophage-derived MVs and interacted with miR-214 to control M1 macrophage-induced VIC calcification
To explore the role of signal transfer from miR-214 to TWIST1 in mediating M1 macrophage-induced VIC calcification, MVs derived from miR-214-silenced BMDMs and RAW 264.7 macrophages were co-incubated with VICs that were or were not transfected with si-TWIST1, and VIC calcification was examined. MVs from the miR-214-silenced M1 macrophages effectively enhanced the mRNA and protein expression of TWIST1 in VICs (Figure 5A). Moreover, VICs had lower ALP activity (Figure 5B) and expressed significantly lower levels of osteocalcin (Figure 5C). These procalcification effects of MVs were abrogated by VICs pretreated with si-TWIST1 (Figure 5B and 5C). These data pointed to a signal transfer function of miR-214/TWIST1 in M1 macrophage-induced VIC calcification.
Figure 5.
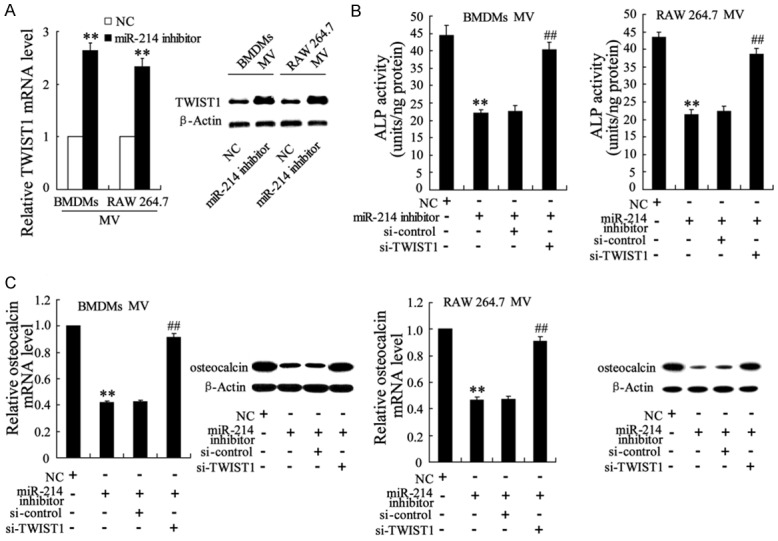
TWIST1 in VICs responded to miR-214 of M1 macrophage-derived MVs, and its response to miR-214 controlled M1 macrophage-induced VIC calcification. BMDMs and RAW 264.7 macrophages were pretreated with a miR-214 inhibitor or negative control (NC) and then exposed to LPS to induce M1 macrophages. MVs isolated from this medium were co-cultured with VICs, VICsi-TWIST1, or a VICsi-control. (A) TWIST1 mRNA and protein expression levels, (B) ALP activity, and (C) Osteocalcin mRNA and protein expression levels in VICs were measured. The data are presented as the mean ± SD. **P < 0.01 compared with the NC, ##P < 0.01 compared with the miR-214 inhibitor +si-control.
MVs derived from TWIST1-overexpressed M1 macrophages had no influence on VIC calcification
We next upregulated TWIST1 in BMDMs and RAW 264.7 macrophages by pcDNA-TWIST1 transfection and then evaluated the role of the derived MVs in VIC calcification. By qRT-PCR and Western blot analyses, we observed that the pcDNA-TWIST1-macrophage-derived MVs had no effect on TWIST1 mRNA and protein expression of VICs in comparison with pcDNA-macrophage-derived MVs (Figure 6A). The ALP activity (Figure 6B) and expression level of osteocalcin remained the same as that of empty pcDNA plasmid-transfected macrophages, suggesting that TWIST1 overexpression of M1 macrophages exerted no impact on M1 macrophage-induced VIC calcification.
Figure 6.
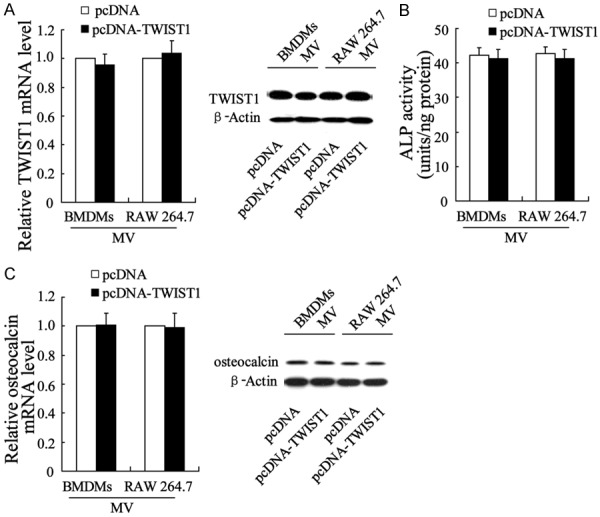
Lack of effect of MVs derived from M1 macrophages overexpressing TWIST1 on the calcification of VICs. MVs were isolated from medium containing BMDMspcDNA-TWIST1, RAW 264.7pcDNA-TWIST1, BMDMspcDNA, or RAW 264.7pcDNA and co-cultured with VICs. (A) TWIST1 mRNA and protein expression levels, (B) ALP activity, and (C) Osteocalcin mRNA and protein expression levels in VICs were evaluated.
An intravenous injection of the miR-214 inhibitor improved AVC in hypercholesterolemic apoE-/- mice
The apoE-/- mice were fed a HC diet to induce AVC. To confirm the role of miR-214 in the process of aortic valve calcification, the mice received a miR-214 inhibitor via intravenous therapy. As shown in Figure 7A, compared with the NC treatment, the miR-214 inhibitor injection substantially reduced AVC in the hypercholesterolemic apoE-/- mice. In addition, the mRNA and protein levels of TWIST1 were significantly upregulated in the aortic valves of the miR-214 inhibitor-treated mice (Figure 7B).
Figure 7.
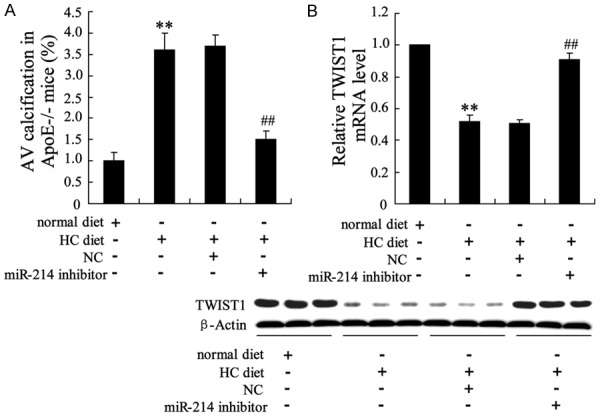
miR-214 inhibitor injection improved AVC in hypercholesterolemic apoE-/- mice. A: Percentage of AVC in apoE-/- mice fed a HC diet relative to that of the NC injection apoE-/- mice and miR-214 inhibitor injection apoE-/- mice fed a normal diet. B: TWIST1 mRNA and protein expression levels in the aortic valves of apoE-/- mice. **P < 0.01 compared with normal diet, ##P < 0.01 compared with HC diet +NC.
Discussion
Macrophages are important cells of innate immunity. They are found in all tissues and in the aortic valve, where they limit tissue damage or promote inflammation-induced tissue repair [17]. The pathophysiology of macrophages depends on their phenotype (M1 or M2). M1- or M2-induced polarization allows macrophages to adapt to different tissue environments. In the present study, the enhanced expression level of M1 markers (TNF-α, IL-6, IL-12, iNOS, and MCP-1) confirmed the dominant role of the M1 macrophage phenotype in AVC. The results point to the functional involvement and potential mechanism of M1 macrophages in AVC.
Although the process that induces mineral dysregulation in early aortic valve disease requires further exploration, molecular imaging has enabled simultaneous visualization of the roles of various cells and enzymes in the early stages of mineralization in vivo, supporting the concept of inflammation-dependent development of AVC-related disease [18]. A previous study demonstrated that inflammatory cytokines induced by macrophages and inflammation-induced cellular stress responses contributed to AVC associated with angiocardiopathy [19]. We used LPS, a potent bioactive factor that can induce the M1 macrophage phenotype [20], to transform BMDMs and RAW 264.7 macrophages into the M1 subtype. These LPS-induced M1 macrophages produced higher levels of TNF-α, IL-6, IL-12, iNOS, and MCP-1, as shown in previous studies of human AVC [6]. Moreover, when co-cultured with VICs, LPS-induced M1 macrophages contributed to osteogenic differentiation of VICs, supporting the functional involvement of M1 macrophages in AVC. The pathology of AVC was due to VIC osteogenic differentiation, as indicated by increased ALP activity and osteogenesis-related osteocalcin expression.
The data in the present study showed that MVs derived from M1 macrophages mediated the pro-osteogenic differentiation process of VICs. Extracellular MVs are small (30-1000 nm) membrane-bound particles, which are released from eukaryotic cells and play pivotal roles in mediating cell-to-cell communication [21]. Cell-specific MVs transport bioactive molecules between cells, thus mediating cell activation, phenotypic modification, and reprogramming of cell functions [22,23]. Our data suggested that the co-culture of MVs with VICs also promoted the osteogenic differentiation of VICs.
Additionally, we found that the upregulation of miR-214 of aortic valve samples was accompanied with both AVC and M1 macrophage polarization. To investigate the role of miR-214 by M1 macrophages-derived MVs in the calcification of VICs, miR-214 inhibitor was transfected to macrophages in prior to M1 polarization induced by LPS. We found that MVs from the miR-214 inhibited M1 RAW 264.7 or miR-214 inhibited M1 BMDM suppressed the osteogenic differentiation of VICs, pointing to an important role for miR-214 in the pro-AVC impact of M1 macrophages and the signaling transfer of M1 macrophage-derived MVs to VICs. These data indicated that miR-214 silencing may be a potential therapeutic target in AVC and that miR-214 may play a role in pro-osteogenic differentiation in VICs. The discordance between the present data and the findings of a previous study, which showed that miR-214 suppressed osteogenic differentiation [13], might be due to the different cell types and pathobiological environment. Notably, our data showed that the potential target of miR-214 (i.e., TWIST1) was upregulated in response to knockdown of miR-214 as reported in other studies [24,25]. Previous research demonstrated that TWIST1 was expressed in diseased aortic valves and that it was downregulated during valve leaflet remodeling [26]. As an important transcription factor, TWIST1 was also shown to be associated with inflammatory gene expression [27] and osteoblastic transdifferentiation of human VICs by functionally antagonizing the osteogenesis differentiation marker RUNX2 [26,28]. We found that knockdown of TWIST1 in VICs reversed the inhibitory impact of miR-214 silencing on the osteogenic differentiation of VICs, suggesting that TWIST1 inhibited osteogenic differentiation of VICs, which is in accordance with the findings of a previous study [28]. Our data also indicated that TWIST1 in VICs responded to MV-delivered miR-214 signals from macrophages. TWIST1 overexpression in macrophages had no effect on the osteogenic differentiation of VICs and no effect on M1 macrophages (Figure 5). Moreover, the functional involvement of miR-214/TWIST1 in the osteogenic differentiation of VICs was confirmed by intravenous treatment of mice with a miR-214 inhibitor, which significantly suppressed AVC and resulted in the upregulation of TWIST1.
This is the first study to demonstrate that miR-214/TWIST1 mediated M1 macrophage-induced calcification of VICs, thereby potentially contributing to AVC. Signals transferred from M1 macrophages to VICs were mediated by MV-delivered miR-214 to VICs and the TWIST1 pathway. The aforementioned pathway may serve as one mechanism underlying inflammation-driven valvular disease.
Acknowledgements
This study was approved by “Six Big Talent Peak” Project of Jiangsu Province (No: 2011-WS-063) and Nantong Municipal Commission of Science and Technology (No: MS 22015077, No: MS 2015027).
Disclosure of conflict of interest
None.
References
- 1.Chen XN, Chen ZJ, Ma XB, Ding B, Ling HW, Shi ZW, Chen N. Aortic Artery and Cardiac Valve Calcification are Associated with Mortality in Chinese Hemodialysis Patients: A 3.5 Years Follow-up. Chin Med J (Engl) 2015;128:2764–2771. doi: 10.4103/0366-6999.167315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res. 2011;108:1392–1412. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joghetaei N, Akhyari P, Rauch BH, Cullen P, Lichtenberg A, Rudelius M, Pelisek J, Schmidt R. Extracellular matrix metalloproteinase inducer (CD147) and membrane type 1-matrix metalloproteinase are expressed on tissue macrophages in calcific aortic stenosis and induce transmigration in an artificial valve model. J Thorac Cardiovasc Surg. 2011;142:191–198. doi: 10.1016/j.jtcvs.2010.09.051. [DOI] [PubMed] [Google Scholar]
- 4.Ohukainen P, Syvaranta S, Napankangas J, Rajamaki K, Taskinen P, Peltonen T, Helske-Suihko S, Kovanen PT, Ruskoaho H, Rysa J. MicroRNA-125b and chemokine CCL4 expression are associated with calcific aortic valve disease. Ann Med. 2015;47:423–429. doi: 10.3109/07853890.2015.1059955. [DOI] [PubMed] [Google Scholar]
- 5.New SE, Aikawa E. Cardiovascular calcification: an inflammatory disease. Circ J. 2011;75:1305–1313. doi: 10.1253/circj.cj-11-0395. [DOI] [PubMed] [Google Scholar]
- 6.Wang R, Chen W, Ma Z, Li L, Chen X. M1/M2 macrophages and associated mechanisms in congenital bicuspid aortic valve stenosis. Exp Ther Med. 2014;7:935–940. doi: 10.3892/etm.2014.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herranz S, Traves PG, Luque A, Hortelano S. Role of the tumor suppressor ARF in macrophage polarization: Enhancement of the M2 phenotype in ARF-deficient mice. Oncoimmunology. 2012;1:1227–1238. doi: 10.4161/onci.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kin K, Miyagawa S, Fukushima S, Shirakawa Y, Torikai K, Shimamura K, Daimon T, Kawahara Y, Kuratani T, Sawa Y. Tissue- and plasma-specific MicroRNA signatures for atherosclerotic abdominal aortic aneurysm. J Am Heart Assoc. 2012;1:e000745. doi: 10.1161/JAHA.112.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada H, Kohanbash G, Lotze MT. MicroRNAs in immune regulation--opportunities for cancer immunotherapy. Int J Biochem Cell Biol. 2010;42:1256–1261. doi: 10.1016/j.biocel.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao L, Liu YW, Yang T, Gan L, Yang N, Dai SS, He F. The mutual regulation between miR-214 and A2AR signaling plays an important role in inflammatory response. Cell Signal. 2015;27:2026–2034. doi: 10.1016/j.cellsig.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Lu S, Gao Y, Huang X, Wang X. Cantharidin exerts anti-hepatocellular carcinoma by miR-214 modulating macrophage polarization. Int J Biol Sci. 2014;10:415–425. doi: 10.7150/ijbs.8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathan S, Ankeny CJ, Arjunon S, Ferdous Z, Kumar S, Fernandez Esmerats J, Heath JM, Nerem RM, Yoganathan AP, Jo H. Identification of side- and shear-dependent microRNAs regulating porcine aortic valve pathogenesis. Sci Rep. 2016;6:25397. doi: 10.1038/srep25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao C, Sun W, Zhang P, Ling S, Li Y, Zhao D, Peng J, Wang A, Li Q, Song J, Wang C, Xu X, Xu Z, Zhong G, Han B, Chang YZ, Li Y. miR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA Biol. 2015;12:343–353. doi: 10.1080/15476286.2015.1017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Guo B, Li Q, Peng J, Yang Z, Wang A, Li D, Hou Z, Lv K, Kan G, Cao H, Wu H, Song J, Pan X, Sun Q, Ling S, Li Y, Zhu M, Zhang P, Peng S, Xie X, Tang T, Hong A, Bian Z, Bai Y, Lu A, Li Y, He F, Zhang G, Li Y. miR-214 targets ATF4 to inhibit bone formation. Nat Med. 2013;19:93–100. doi: 10.1038/nm.3026. [DOI] [PubMed] [Google Scholar]
- 15.Shi K, Lu J, Zhao Y, Wang L, Li J, Qi B, Li H, Ma C. MicroRNA-214 suppresses osteogenic differentiation of C2C12 myoblast cells by targeting Osterix. Bone. 2013;55:487–494. doi: 10.1016/j.bone.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Richards J, El-Hamamsy I, Chen S, Sarang Z, Sarathchandra P, Yacoub MH, Chester AH, Butcher JT. Side-specific endothelial-dependent regulation of aortic valve calcification: interplay of hemodynamics and nitric oxide signaling. Am J Pathol. 2013;182:1922–1931. doi: 10.1016/j.ajpath.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weissleder R, Nahrendorf M, Pittet MJ. Imaging macrophages with nanoparticles. Nat Mater. 2014;13:125–138. doi: 10.1038/nmat3780. [DOI] [PubMed] [Google Scholar]
- 18.New SE, Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res. 2011;108:1381–1391. doi: 10.1161/CIRCRESAHA.110.234146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SH, Choi JH. Involvement of Immune Cell Network in Aortic Valve Stenosis: Communication between Valvular Interstitial Cells and Immune Cells. Immune Netw. 2016;16:26–32. doi: 10.4110/in.2016.16.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng XF, Hong YX, Feng GJ, Zhang GF, Rogers H, Lewis MA, Williams DW, Xia ZF, Song B, Wei XQ. Lipopolysaccharide-induced M2 to M1 macrophage transformation for IL-12p70 production is blocked by Candida albicans mediated up-regulation of EBI3 expression. PLoS One. 2013;8:e63967. doi: 10.1371/journal.pone.0063967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monzack EL, Masters KS. Can valvular interstitial cells become true osteoblasts? A side-by-side comparison. J Heart Valve Dis. 2011;20:449–463. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Shi L, Mei H, Zhang J, Zhu Y, Han X, Zhu D. Inflamed macrophage microvesicles induce insulin resistance in human adipocytes. Nutr Metab (Lond) 2015;12:21. doi: 10.1186/s12986-015-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Chen R, Kemper S, Charrier A, Brigstock DR. Suppression of fibrogenic signaling in hepatic stellate cells by Twist1-dependent microRNA-214 expression: Role of exosomes in horizontal transfer of Twist1. Am J Physiol Gastrointest Liver Physiol. 2015;309:G491–499. doi: 10.1152/ajpgi.00140.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Han Q, Zhu Y, Yu Y, Wang J, Jiang X. Down-regulation of miR-214 contributes to intrahepatic cholangiocarcinoma metastasis by targeting Twist. FEBS J. 2012;279:2393–2398. doi: 10.1111/j.1742-4658.2012.08618.x. [DOI] [PubMed] [Google Scholar]
- 25.Chakraborty S, Wirrig EE, Hinton RB, Merrill WH, Spicer DB, Yutzey KE. Twist1 promotes heart valve cell proliferation and extracellular matrix gene expression during development in vivo and is expressed in human diseased aortic valves. Dev Biol. 2010;347:167–179. doi: 10.1016/j.ydbio.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mudry JM, Massart J, Szekeres FL, Krook A. TWIST1 and TWIST2 regulate glycogen storage and inflammatory genes in skeletal muscle. J Endocrinol. 2015;224:303–313. doi: 10.1530/JOE-14-0474. [DOI] [PubMed] [Google Scholar]
- 27.Zhang XW, Zhang BY, Wang SW, Gong DJ, Han L, Xu ZY, Liu XH. Twist-related protein 1 negatively regulated osteoblastic transdifferentiation of human aortic valve interstitial cells by directly inhibiting runt-related transcription factor 2. J Thorac Cardiovasc Surg. 2014;148:1700–1708. e1701. doi: 10.1016/j.jtcvs.2014.02.084. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Chen S, Deng C, Li F, Wang Y, Hu X, Shi F, Dong N. MicroRNA-204 Targets Runx2 to Attenuate BMP-2-induced Osteoblast Differentiation of Human Aortic Valve Interstitial Cells. J Cardiovasc Pharmacol. 2015;66:63–71. doi: 10.1097/FJC.0000000000000244. [DOI] [PubMed] [Google Scholar]


