Kilohertz-frequency spinal cord stimulation (KHF-SCS) is a new mode of SCS that may offer better pain relief than conventional SCS. However, the mechanism of action is poorly characterized, especially the effects of stimulation on dorsal column (DC) axons, which are the primary target of stimulation. This study provides the first recordings of single DC axons during KHF-SCS to quantify DC activity that has the potential to mediate the analgesic effects of KHF-SCS.
Keywords: kilohertz frequency, spinal cord stimulation, dorsal column, pain, axon
Abstract
Kilohertz-frequency spinal cord stimulation (KHF-SCS) is a potential paresthesia-free treatment for chronic pain. However, the effects of KHF-SCS on spinal dorsal column (DC) axons and its mechanisms of action remain unknown. The objectives of this study were to quantify activation and conduction block of DC axons by KHF-SCS across a range of frequencies (1, 5, 10, or 20 kHz) and waveforms (biphasic pulses or sinusoids). Custom platinum electrodes delivered SCS to the T10/T11 dorsal columns of anesthetized male Sprague-Dawley rats. Single DC axons and compound action potentials were recorded during KHF-SCS to evaluate SCS-evoked activity. Responses to KHF-SCS in DC axons included brief onset firing, slowly accommodating asynchronous firing, and conduction block. The effects of KHF-SCS mostly occurred well above motor thresholds, but isolated units were activated at amplitudes shown to reduce behavioral sensitivity in rats. Activity evoked by SCS was similar across a range of frequencies (5–20 kHz) and waveforms (biphasic and sinusoidal). Stimulation at 1-kHz SCS evoked more axonal firing that was also more phase-synchronized to the SCS waveform, but only at amplitudes above motor threshold. These data quantitatively characterize the central nervous system activity that may modulate pain perception and paresthesia, and thereby provide a foundation for continued investigation of the mechanisms of KHF-SCS and its optimization as a therapy for chronic pain. Given the asynchronous and transient nature of DC activity, it is unlikely that the same mechanisms underlying conventional SCS (i.e., persistent, periodic DC activation) apply to KHF-SCS.
NEW & NOTEWORTHY Kilohertz-frequency spinal cord stimulation (KHF-SCS) is a new mode of SCS that may offer better pain relief than conventional SCS. However, the mechanism of action is poorly characterized, especially the effects of stimulation on dorsal column (DC) axons, which are the primary target of stimulation. This study provides the first recordings of single DC axons during KHF-SCS to quantify DC activity that has the potential to mediate the analgesic effects of KHF-SCS.
although multiple factors related to the development of chronic pain have been identified, treatment of chronic pain remains a significant clinical challenge. Spinal cord stimulation (SCS) is a rapidly growing therapy for chronic pain, but despite substantial advances in stimulation hardware, clinical success rates and SCS efficacy have improved little since early clinical studies (Zhang et al. 2014). As a result, new stimulation modes, including kilohertz-frequency SCS (KHF-SCS) and burst SCS, have emerged with the potential to improve efficacy.
KHF-SCS involves the epidural application of pulse trains of up to 10 kHz over the dorsal columns (DCs), compared with frequencies of 50–150 Hz for conventional SCS. Early clinical results suggest that KHF-SCS may be more effective than conventional SCS and produce analgesia without the characteristic paresthesias associated with conventional SCS (Al-Kaisy et al. 2015; Kapural et al. 2015; Tiede et al. 2013; Van Buyten et al. 2013). Understanding the mechanisms underlying pain relief from KHF-SCS is required to optimize this promising therapy, but the mechanisms, and how they compare to those of conventional SCS, are largely unknown.
KHF stimulation is best known for its ability to block axonal conduction, with applications in both peripheral nerve and the central nervous system (Bhadra and Kilgore 2005; Cuellar et al. 2013; Fisher et al. 2015; Soin et al. 2015). However, computational modeling suggests that conduction block does not occur during KHF-SCS (Lempka et al. 2015). In vivo rat experiments suggested that conduction block is unlikely during KHF-SCS at amplitudes that reduce behavioral sensitivity, but these studies did not directly measure the effects of KHF-SCS on DC axons (Shechter et al. 2013; Song et al. 2014). In addition to conduction block, KHF stimulation can also evoke firing in axons. Stimulation between 1 and 20 kHz evoked highly irregular and asynchronous firing in frog and cat sciatic and tibial nerves (Bowman and McNeal 1986; Woo and Campbell 1964), but it is unclear whether such firing occurs in DC axons during KHF-SCS.
Conventional SCS modulates pain sensation, at least in part, by engaging the classic gate-control mechanism through antidromic activation of inhibitory circuitry in the dorsal horn (Linderoth and Foreman 1999). Although modeling suggests that superficial, large-diameter DC axons are activated by conventional SCS (Holsheimer 2002), until recently this had not been directly confirmed experimentally, and activation of DC axons during SCS was only inferred from antidromic compound action potentials measured in the periphery or from synaptic activation of dorsal horn neurons (Baba et al. 1994; Bantli et al. 1975; Hunter and Ashby 1994). Parker et al. (2012) recorded compound action potentials from the dorsal columns in human patients during SCS and were the first to demonstrate activation of large-diameter DC axons evoked by SCS. However, no study has recorded from single DC axons during SCS to quantify the firing patterns produced by SCS, nor have any direct recordings of DC activity been performed during KHF-SCS. Since KHF stimulation may evoke asynchronous firing (Bowman and McNeal 1986; Woo and Campbell 1964), prior techniques using compound action potentials or integrated neural recordings distant from the dorsal columns are insufficient to determine activity in DC axons during KHF-SCS.
We present the first direct recordings from single DC axons during epidural SCS at kilohertz frequencies and during conventional 50-Hz SCS, both in naive rats and in rats with persistent neuropathic pain from a spared nerve injury (SNI). Clinically, KHF-SCS is applied in patients at frequencies ranging from 1 to 10 kHz (Kapural et al. 2015; Perruchoud et al. 2013; Smith et al. 2015), but there is no consensus or rationale for the choice of kilohertz frequency. Therefore, we measured the effects of KHF-SCS on DC axons for different kilohertz frequencies as well as waveform shapes. The results quantify the nervous system inputs, to both the dorsal horn and the dorsal column nuclei, generated by SCS as necessary to understand the mechanisms that mediate the changes in pain perception and paresthesia produced by SCS.
MATERIALS AND METHODS
Animals.
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Duke University and were in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council 2011). Adult male Sprague-Dawley rats (n = 52, 300–520 g; Charles River Laboratories) were housed in pairs under USDA- and AAALAC-compliant conditions, with a 12:12-h light-dark cycle, free access to food and water, and environmental enrichment.
Dorsal column axon recordings.
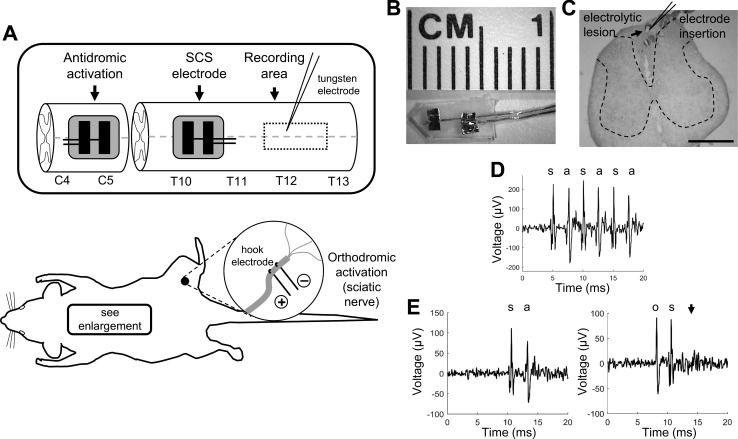
Rats were anesthetized with urethane (1.2 g/kg initial sc, 0.4 g/kg supplemental ip), and a tracheotomy was performed for intubation. Hair was clipped from the region over the cervical spine and the lower thoracic spine. A single incision was made from the C2-T1 vertebrae, and the muscle was separated to expose the mid-cervical vertebrae. A bilateral laminectomy was performed at C3/C4 and a custom bipolar platinum paddle lead was placed centrally over the dorsal columns under the C5 lamina (Fig. 1, A and B). A second incision was made from T10 to L2, and the muscle and connective tissue were separated to expose the vertebrae. A bilateral laminectomy was performed at T12/T13, and a second bipolar platinum paddle lead was inserted rostrally under the T10/T11 lamina (Fig. 1A). Rats were immobilized with a stereotaxic frame (David Kopf Instruments, Tujunga, CA), and the spinal cord was bathed in 37°C mineral oil. Core temperature was maintained at 35–37°C using heating pads and a rectal temperature probe. Respiration, heart rate, and oxygen saturation were also monitored throughout the procedure (PhysioSuite; Kent Scientific, Torrington, CT).
Fig. 1.
Experimental setup and dorsal column axon characterization. A: rats were instrumented with 2 custom bipolar platinum electrodes (see B) for antidromic activation of dorsal column axons at the C5 spinal cord and for kilohertz-frequency spinal cord stimulation (KHF-SCS) at T10/T11. A bipolar hook electrode was used for sciatic nerve stimulation. A recording microelectrode was placed in the dorsal columns at T12/T13 to record from single dorsal column axons. C: representative recording location marked by an electrolytic lesion with Prussian blue staining. Electrodes were inserted at a 65–70° angle relative to the sagittal plane. Scale bar, 1 mm. Axons were characterized by demonstrating invariant latency and faithful response to 200-Hz antidromic activation (D) and orthodromic/antidromic collision (E). s, Stimulation artifact; a, antidromic spike; o, orthodromic spike. Arrowhead indicates no antidromic spike following collision between the orthodromic and antidromic action potentials.
Evoked compound action potentials (eCAPs) were recorded using a low-impedance (250 Ω at 1 kHz) stainless steel wire electrode placed over the dorsal columns on the dural surface of the recording area at T12/T13 (Fig. 1A) to confirm that the cervical and thoracic stimulating electrodes were each placed correctly and could activate DC axons. Motor threshold (MT) was identified in each experiment separately for each SCS waveform and frequency by slowly increasing the stimulation amplitude until persistent twitches or tetanic contractions were observed in the thoracic or lumbar musculature. After MTs were identified, rats were paralyzed with gallamine triethiodide (1 ml/h at 0.2 g/ml ip). The dura was resected over the recording area at T12/T13 (Fig. 1A), and the pia was removed with the use of fine forceps from small areas of the dorsal columns such that the recording electrode could be inserted without resistance. A bipolar tungsten microelectrode (impedance = 8–10 MΩ, tip spacing = 190 μm; FHC, Bowdoin, ME) was used to record activity of single DC axons. Signals were bandpass filtered from 500 Hz to 5 kHz, amplified by 100 (XCell3; FHC), and then bandstop filtered specifically to remove artifact from the KHF stimulation (SR650; Stanford Research Systems, Sunnyvale, CA). Signals were further amplified to a total gain of 10,000 (SR560; Stanford Research Systems), and sampled at 20 kHz (PowerLab; ADInstruments; Dunedin, New Zealand). Because the large posterior spinal vasculature covered much of the dorsal columns along the midline, the recording electrode was inserted mediolaterally at an angle of 65–70° relative to the sagittal plane to pass under the blood vessel (Fig. 1C). The recording electrode was advanced slowly through the dorsal columns using a micropositioner (model 2660; David Kopf Instruments) while a search stimulus (biphasic pulse, 300 μs per phase, 1.3 Hz) was delivered at the cervical spinal cord to evoke antidromic action potentials.
Once a single unit was acquired, it was confirmed to be a continuously projecting axon from the thoracolumbar spinal cord by using Lipski's criteria for antidromic identification of neurons (Lipski 1981), including 1) short, invariant latency (generally <7 ms for the conduction distances in this study, with <0.05-ms variance), 2) faithful response to a short train of high-frequency stimuli (3 pulses at 200 Hz), and 3) demonstration of orthodromic/antidromic collision, if the cutaneous receptive field could be identified by brushing the hindlimb and tail (Fig. 1). For rats with spared nerve injury, only units that originated in the injured sciatic nerve were included; their origination was confirmed by activating the sciatic nerve (biphasic pulse, 300 μs per phase, 1.3 Hz) and observing orthodromic action potentials, rather than searching for a cutaneous receptive field.
Each qualifying unit underwent SCS using the bipolar epidural electrode at T10/T11. SCS was produced by a waveform generator (Keithley 3390; Tektronix, Beaverton, OR) and was converted from voltage- to current-controlled stimulation by using one to three stimulus isolators in parallel (model 2200; A-M Systems; Carlsborg, WA). Many laboratory and clinical waveform generators exhibited small direct current (dc) leakage when delivering KHF stimulation (Franke et al. 2014). Small dc can influence the activity of nerve fibers and even damage neural tissue in the long term (Bhadra and Kilgore 2004; Hurlbert et al. 1993). Therefore, a modified alternating current coupler was used to eliminate dc leakage and isolate the effects of KHF-SCS without the potential confound of small dc offsets. The alternating current (ac) coupler, described by Franke et al. (2014), consisted of capacitors (1 μF) in series with both the anode and cathode of the SCS electrode and dc-shunting high-value inductors (1.3 H at 1 kHz) placed in parallel before and after the series capacitors. The capacitive and inductive properties of the filter were chosen to optimize delivery of 10-kHz SCS, and the filter was tested in vitro and in vivo to confirm that delivered 10-kHz SCS waveforms were free of dc offset.
SCS was delivered as a biphasic pulse train at 1, 5, 10, or 20 kHz. The pulse width (24 μs per phase) was chosen to enable comparison with previous KHF-SCS studies in rats (Shechter et al. 2013; Song et al. 2014). Sinusoidal 10-kHz SCS and a 50-Hz biphasic pulse train (200 μs per phase) were used for comparison in subsets of experiments. A set of 11 SCS amplitudes (0.2, 0.4, 0.6, 0.8, 1, 1.2, 1.5, 2, 3, 4, and 6 mA) was applied in random order during a series of stimulation trials, each trial consisting of a 10-s baseline period, 30 s of SCS, and a 10-s recovery period. The ac coupler partially attenuated SCS amplitudes due to imperfect frequency selectivity of the filter, so actual applied currents for each trial were measured across a 1-kΩ series resistor and binned into nine different amplitude groups, ranging from <0.2 to 3–5 mA. Stimulation trials were separated by 60 s, or until axonal conduction recovered from conduction block. Cervical antidromic activation (biphasic pulse, 300 μs per phase, 3.2 Hz) was used to test axonal conduction continuously throughout each trial. Antidromic activation was not included for 50-Hz SCS trials because 50-Hz SCS is not known to block axonal conduction. Some KHF-SCS trials were repeated without antidromic activation to test for any effects of the antidromic spikes.
The placement of the recording electrode was confirmed in a subset of rats by inserting a stainless steel microelectrode in the same fashion as the tungsten electrode to the stereotaxic coordinates where a dorsal column axon was identified. An electrolytic lesion was created by passing 25 μA of direct current through the electrode. Rats were perfusion-fixed and spinal cord samples postfixed in 10% formalin with 1% potassium ferrocyanide. Samples were frozen at −80°C and then cryosectioned at 50-μm thick and mounted on slides. The lesion was identified with Prussian blue staining, counterstained with nuclear red dye, and then imaged using a light microscope (Fig. 1C). Rats were euthanized at the termination of experiments with Euthasol (0.5 ml ip; Virbac, Fort Worth, TX).
Evoked compound action potential recording.
In a subset of rats, eCAPs were recorded from the dorsal columns to measure population-level responses during KHF-SCS. Potentials were initiated in the sciatic nerve because orthodromic eCAPs were more robust than antidromic eCAPs from the cervical spinal cord. The sciatic nerve was exposed by incising the skin on the right hindlimb and separating the muscle overlying the nerve. A bipolar hook electrode was attached to the nerve using silicone elastomer (Kwik-Cast; World Precision Instruments, Sarasota, FL) (Fig. 1A). The thoracic spinal cord was exposed as described above, but the SCS electrode was inserted caudally under the L1 lamina, rather than rostrally under T11, to deliver SCS to axons at a location between the recording electrode and the site of activation in the sciatic nerve. The rat was immobilized on the stereotaxic frame and paralyzed with gallamine as described above. The sciatic nerve was stimulated using biphasic pulses (100 μs per phase) at amplitudes sufficient to generate motor activation of the hind paw. The eCAPs were recorded using a low-impedance (250 Ω at 1 kHz) stainless steel wire electrode placed on the T12 dorsal column, ipsilateral to the stimulated nerve. SCS trials again consisted of a 10-s baseline period, 30 s of 10-kHz biphasic SCS using the parameters and amplitudes described above, and a 10-s recovery period, with continuous sciatic nerve activation at 3.2 Hz. Stimulation trials were separated by a 60-s rest period.
Spared nerve injury and mechanical allodynia.
The spared nerve injury (SNI) model was used to produce a neuropathic pain state (Decosterd and Woolf 2000). Under isoflurane anesthesia (3% induction, 1.5% maintenance), the right hindlimb was shaved and sterilized using alcohol wipes and iodine solution. Using aseptic technique, a small incision was made over the biceps femoris, and the muscle was bluntly dissected to expose the branch point of the sciatic nerve. The tibial and common peroneal branches of the nerve were tightly ligated with 5-0 silk suture and then transected distal to the ligation to remove a 1- to 2-mm segment of each branch. The sural branch was left intact, and care was taken to avoid mechanical insult to the intact nerve. The muscle and skin were closed in two layers using resorbable 4-0 polyglycolic acid suture, and rats recovered in room air.
Mechanical allodynia was evaluated by measuring the paw withdrawal threshold (PWT; Chaplan et al. 1994) before SNI (baseline) and on days 1, 3, 7, and 14 after SNI. On each testing day, PWT was acquired for the ipsilateral and contralateral hind paws by applying a logarithmic series of Von Frey filaments in ascending order (2, 4, 6, 10, 15, and 26 g) to the lateral plantar region of each hind paw innervated by the intact sural nerve. If the rat responded to a given stimulus by withdrawing, licking, or shaking the hind paw, then a positive response was recorded and the next smallest filament was applied. If no withdrawal was observed, then a negative response was recorded and the next largest filament was applied. Five total responses were recorded beginning with the first positive response, and then the 50% PWT was calculated as previously described (Chaplan et al. 1994), with a minimum of 2 g and a maximum of 26 g. PWT was averaged across three rounds of testing for each hind paw on each day and compared between paws and with the preinjury baseline using a two-way repeated-measures ANOVA with post hoc Tukey's honestly significant difference (HSD) test.
Data analysis.
Recordings of single-unit activity were digitally bandpass filtered before spike sorting to remove residual KHF stimulation artifact, using filter settings that varied with stimulation frequency. Spikes from the unit of interest were identified using principal component-based sorting (Plexon Offline Sorter; Dallas, TX). Conduction velocity was calculated for each unit on the basis of the latency of antidromic action potentials and the measured conduction distance from the cervical spinal cord to the recording electrode. Fiber diameter was estimated on the basis of the relationship between conduction velocity and diameter for Aβ-fibers (Boyd and Kalu 1979). The depth of each unit was also estimated on the basis of the micropositioner penetration distance and insertion angle of the electrode.
In each stimulation trial, spikes that occurred at the known latency of antidromic action potentials from the cervical spine were identified as such, and the remaining spikes were classified as evoked by KHF-SCS. In this study, activation was defined as the presence of spikes evoked by KHF-SCS at the site of SCS. Activation thresholds were identified as the lowest amplitude at which at least one spike was evoked at the onset of KHF-SCS. To quantify the activation of axons by KHF-SCS, curves of the firing rate of SCS-evoked spikes were plotted as a function of time using a fixed-width sliding window (1-s width, 0.1-s step). In addition, averaged peristimulus time histograms (PSTHs) for the 30-s SCS period were calculated for each of the nine SCS amplitudes; PSTHs were smoothed with a Gaussian kernel (σ = 0.5 s). Spikes were sparse in many stimulation trials, so averaged PSTH curves were compared on a bin-by-bin basis using a binomial model applicable to PSTHs with low spike counts (Dorrscheidt 1981).
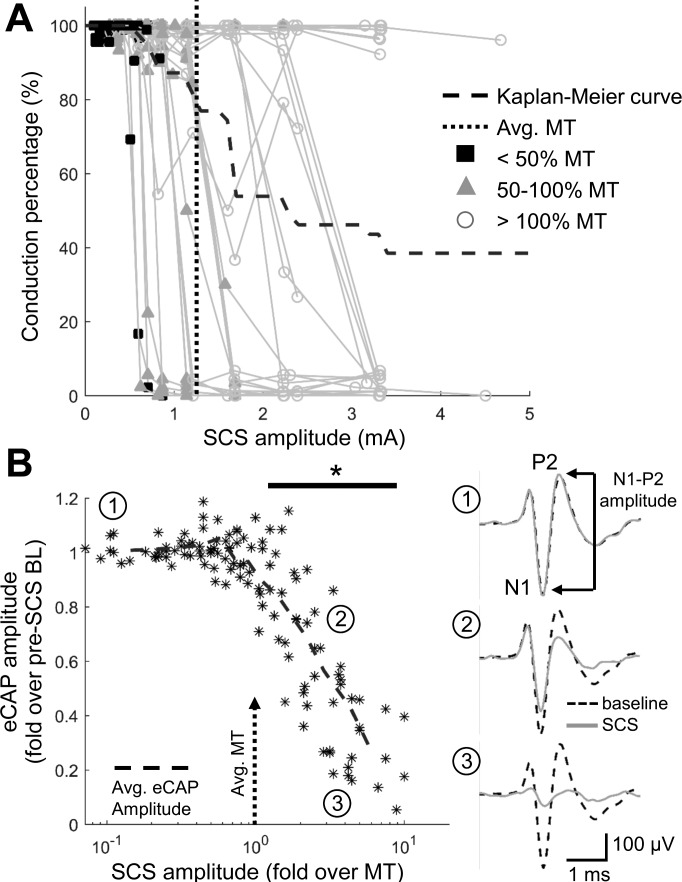
Conduction block was evaluated separately from activation using the antidromic action potentials initiated in the cervical spinal cord. Conduction block thresholds were determined on the basis of the SCS amplitude at which antidromic potentials failed to pass through the site of SCS to the recording electrode. Note that the lack of action potential propagation from the cervical spinal cord to the recording electrode did not preclude the presence of SCS-evoked spikes, and some recorded axons displayed both SCS-evoked activation and conduction block in the same stimulation trial. Conduction percentage was calculated as the total percentage of antidromic action potentials that were recorded at the expected latency following conduction through the site of SCS. Kaplan-Meier survival curves were generated to show the progression of conduction block in the population of recorded axons as SCS amplitude increased. Units were considered blocked when their conduction percentage fell below 50%, and units were censored if they were not yet blocked at the highest applied SCS amplitude. Survival curves were compared using the nonparametric log-rank test with post hoc Dunn-Sidak-corrected multiple pairwise comparisons.
Vector strength was used to measure the phase synchrony of SCS-evoked spikes to the SCS waveform. Vector strength (R) was calculated on the basis of the average time between each spike and the nearest pulse in the SCS waveform, and was normalized on a scale from 0 (asynchronous) to 1 (perfectly phase-locked) (Goldberg and Brown 1969):
where θi is the phase angle that defines the relationship between the i-th spike and the nearest stimulation pulse. Because the sampling rate for spike recordings matched the frequency of the 20-kHz SCS, spikes always appeared perfectly synchronized with the 20-kHz SCS waveform. Therefore, vector strength was calculated for all stimulation trials with 50-Hz and 1- to 10-kHz SCS, but not 20-kHz SCS, and compared using a one-way ANOVA with post hoc Tukey's HSD test.
The amplitudes of eCAPs were quantified to show population-level changes in dorsal column conduction during KHF-SCS. Averages triggered on the sciatic stimulation pulses were calculated for eCAPs during the baseline period and the last 3 s of the SCS period for each stimulation trial. The first negative peak (N1) and second positive peak (P2) were the most consistent features in the sciatic-evoked CAP, so the absolute magnitude of the eCAP from the N1 peak to the P2 peak was calculated as the amplitude of each potential (see Fig. 5B1). Amplitudes of eCAPs during SCS were normalized to the amplitudes of pre-SCS baseline eCAPs and compared with the lowest SCS amplitude using a one-way ANOVA with post hoc Tukey's HSD.
Fig. 5.
Conduction block during 10-kHz biphasic SCS. A: conduction percentage (percentage of antidromic action potentials conducted through the site of SCS) is shown for each unit, with symbols representing the SCS amplitude relative to motor threshold (MT). A Kaplan-Meier survival curve is overlaid with the percentage of axons still conducting (>50% conduction percentage) at a given SCS amplitude. B: evoked compound action potential (eCAP) amplitudes for individual SCS trials normalized to baseline (BL) eCAP amplitude, with mean eCAP amplitude shown as dashed line. Normalized eCAP amplitudes declined significantly from baseline beginning at SCS amplitudes over MT (*P < 0.0001 vs. lowest SCS amplitude; n = 7 trials per amplitude; 1-way ANOVA with post hoc Dunn-Sidak-corrected multiple pairwise comparisons). Representative eCAPs at baseline and during the last 3 s of the 30-s SCS trial are shown for no attenuation (1), moderate attenuation (2), and complete attenuation (3) of the eCAP at increasing SCS amplitudes. N1, first negative peak of the eCAP; P2, second positive peak of the eCAP.
Throughout the analyses, SCS amplitudes were normalized to MT on a rat-by-rat basis. Amplitudes at or below 50% MT were noted, because those amplitudes were shown to be sufficient to attenuate neuropathic pain in rats during KHF-SCS, and were believed to be subthreshold to produce paresthesia (Shechter et al. 2013; Song et al. 2014). All data are means ± SD.
RESULTS
Activation and conduction block of DC axons during 10-kHz biphasic SCS.
Single DC axons were recorded across a wide range of fiber diameters and depths in the spinal cord. The mean, 95% confidence interval, and range of fiber diameters and recording depths were largely the same across groups of axons that were subjected to different SCS waveforms (Table 1). Note that the groups of axons were not independent, because some axons were subjected to multiple waveforms and therefore appear in multiple groups. Average motor threshold (MT) was the same for all SCS waveforms at 5, 10, or 20 kHz; 1-kHz and 50-Hz SCS waveforms each had lower MTs (P < 0.0001; Table 1).
Table 1.
Properties of dorsal column axons recorded during epidural kilohertz-frequency or 50-Hz SCS
| Fiber Diameter, μm |
Recording Depth, μm |
Motor Threshold, mA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SCS Waveform | n | Mean | 95% CI | Range | Mean | 95% CI | Range | Mean | 95% CI |
| Biphasic 10 kHz | 39 | 3.3 | 3.0–3.7 | 1.8–5.6 | 221 | 179–264 | 12–531 | 1.2 | 1.0–1.4 |
| SNI 10 kHz | 21 | 3.4 | 3.1–3.7 | 2.1–4.7 | 127† | 106–148 | 59–223 | 0.95 | 0.9–1.0 |
| Sinusoid 10 kHz | 21 | 3.6 | 3.1–4.1 | 1.8–5.6 | 206 | 155–257 | 13–385 | 1.2 | 0.9–1.5 |
| Biphasic 1 kHz | 18 | 3.8 | 3.1–4.5 | 2.0–6.6 | 258 | 189–328 | 29–591 | 0.6‡ | 0.3–0.8 |
| Biphasic 5 kHz | 18 | 3.8 | 3.2–4.5 | 2.0–6.6 | 179 | 128–230 | 29–408 | 1.25 | 1.0–1.5 |
| Biphasic 20 kHz | 21 | 3.6 | 3.1–4.1 | 2.0–5.8 | 185 | 136–234 | 29–408 | 1.3 | 1.1–1.6 |
| Biphasic 50 Hz | 13 | 4.3* | 3.4–5.3 | 1.8–7.6 | 171 | 110–231 | 55–369 | 0.082‡ | 0.07–0.09 |
P = 0.01; †P = 0.006; ‡P <0.0001 vs. biphasic 10 kHz (1-way ANOVA with Tukey's HSD). CI, confidence interval; SCS, spinal cord stimulation; SNI, spared nerve injury.
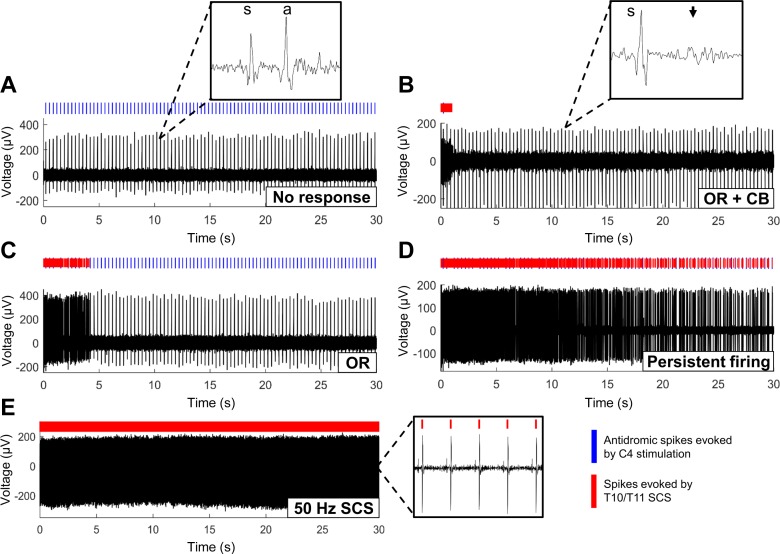
The response of axons (n = 39) to 10-kHz biphasic pulse trains was heterogeneous across the population (Fig. 2). Conduction of antidromic action potentials from the cervical spinal cord was either unaffected by SCS (Fig. 2A) or was partially or fully blocked at the site of SCS (Fig. 2B). KHF-SCS also evoked additional action potentials, independently of the presence (Fig. 2B) or absence (Fig. 2C) of conduction block. The most common feature was an onset response that began when SCS was switched on and ranged from a single spike to several hundred spikes over the first 5–10 s of SCS (Fig. 2C). Some axons exhibited persistent KHF-SCS-evoked firing that outlasted the 30-s period of SCS (Fig. 2D). The firing patterns evoked by KHF-SCS were notably different from the periodic, phase-synchronized activation of axons by conventional 50-Hz stimulation (Fig. 2E).
Fig. 2.
Dorsal column axons exhibited a range of firing patterns in response to biphasic 10-kHz SCS, including no SCS-evoked spikes or change in axonal conduction (A); transient onset firing response (OR) followed by conduction block (CB), indicated by a lack of antidromic spikes (B), transient onset firing response (OR) without conduction block (C), or persistent SCS-evoked firing (D). Periodic, phase-synchronized activation of axons by 50-Hz SCS is shown for comparison (E). s, Stimulation artifact; a, antidromic spike. Arrowhead indicates conduction block, as shown by no conduction of antidromic spike.
Because antidromic activation might influence the response of an axon to KHF-SCS, the responses of 8 units to 10-kHz SCS were tested with and without antidromic activation. Firing rate curves (i.e., see Fig 3, D and E) were compared during the 30-s period of SCS with and without antidromic activation. For all stimulation trials in which SCS evoked axonal activity, the firing rate curves during stimulation with and without antidromic activation had an average normalized correlation of 0.83 ± 0.28, indicating that the presence or absence of antidromic spikes did not alter the response of DC axons to SCS.
Fig. 3.
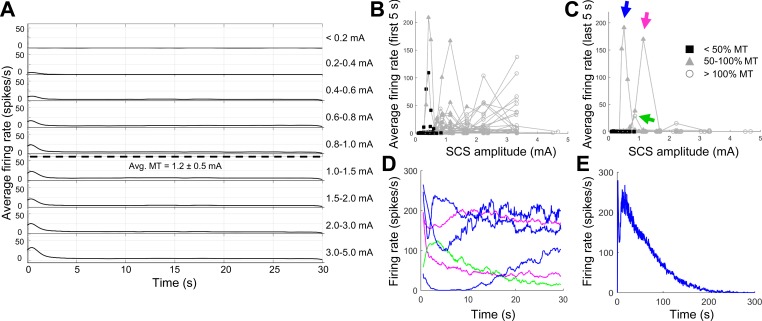
Activation of dorsal column axons during 10-kHz biphasic SCS. A: averaged peristimulus time histograms show the development of onset firing as SCS amplitude increases, but little to no persistent firing. Avg. MT, average motor threshold of rats from which axons were recorded. B: average firing rates of axons during the first 5 s of SCS. C: average firing rates of axons during the last 5 s of a 30-s epoch of SCS. Symbols in B and C indicate SCS amplitude for each stimulation trial with respect to MT of each rat. Firing rate curves for 3 highly active units (arrows in C) are shown in D, where each line corresponds to an SCS trial with persistent activity (i.e., 3 blue lines correspond to 3 different SCS amplitudes at which the unit marked by the blue arrow was highly active). E: firing rate of the unit marked by the blue arrow during a longer stimulation trial (5 min), demonstrating accommodation of activity in a unit with “persistent” firing.
SCS-evoked firing was most frequently observed at the onset of stimulation. Some amount of onset firing was observed at one or more SCS amplitudes in 36 of 39 axons (92%). Furthermore, the onset response during the first 5 s of stimulation was the only notable feature when the activity of all 39 axons was combined in an average PSTH (Fig. 3A). Onset firing began as SCS amplitudes approached 50% of the average MT, and the peak average firing rate continued to increase at supra-MT amplitudes as more axons exhibited an onset response (Fig. 3, A and B). However, the firing rates of individual axons indicated that few axons were activated by KHF-SCS at amplitudes below 50% MT (Fig. 3B), which is the amplitude at which rats show significant reductions in behavioral hypersensitivity during KHF-SCS (Shechter et al. 2013; Song et al. 2014).
A small number of units (6 of 39 axons, 15%) had average firing rates >1 spike/s during the last 5 s of SCS, but only 3 of those units (7.5%) were highly active during that time and were classified as “persistently firing” (Fig. 3C). For each of those units, persistent activity occurred in a very narrow range of SCS amplitudes and appeared in most cases to have slowly accommodating firing rates over the course of stimulation (see green and magenta curves, Fig. 3D). For the one unit whose firing did not appear to slow during 30 s of SCS (see blue curves, Fig. 3D), firing did indeed slow and eventually cease when the stimulation period was extended to 5 min (Fig. 3E). When the SCS period was extended to 5 min, no units exhibited persistent SCS-evoked firing without accommodation.
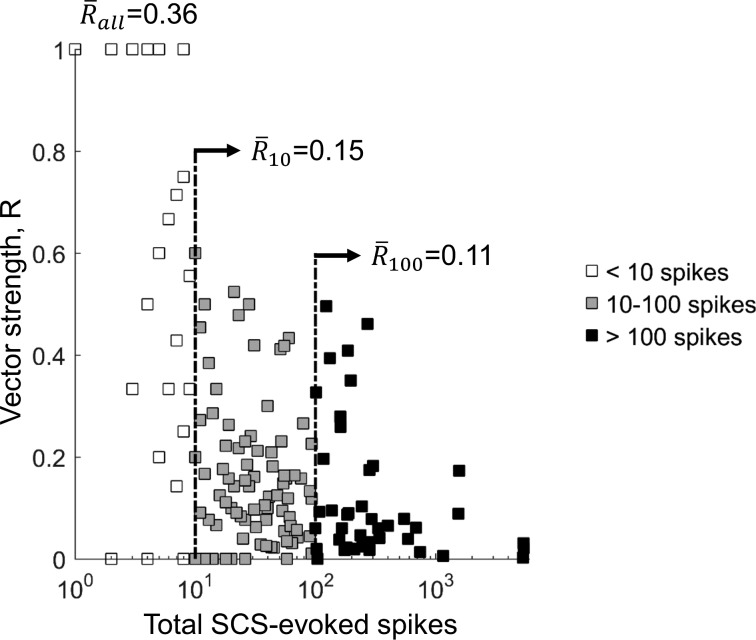
Phase synchrony of SCS-evoked firing with the stimulation waveform was quantified using vector strength (Fig. 4). Of the 429 stimulation trials (39 axons × 11 SCS amplitudes for the 10-kHz biphasic waveform), SCS generated at least 1 spike in 268 trials (62%), and the average vector strength for those trials was 0.36. However, vector strength can be highly influenced by spike trains with very few spikes that by chance are well-synchronized with the SCS waveform. Average vector strengths (R̄) were lower among trials with at least 10 SCS-evoked spikes (R̄10 = 0.15) or at least 100 SCS-evoked spikes (R̄100 = 0.11), indicating that SCS-evoked firing patterns were highly asynchronous with the 10-kHz biphasic pulse train. For comparison, the average vector strength of spike trains evoked by 50-Hz SCS was 0.99, indicating near perfect phase synchrony between the 50-Hz pulses and the evoked spikes. This is an important confirmation of the effects of conventional SCS on DC axons and also highlights the very different effects of KHF-SCS compared with conventional SCS.
Fig. 4.
Vector strength (R) of SCS-evoked firing for all 10-kHz biphasic SCS trials. Averages are shown separately for all trials (white + gray + black squares, R̄all), trials in which 10 or more spikes were evoked (gray + black squares, R̄10), and trials in which 100 or more spikes were evoked (black squares, R̄100). Low average vector strengths, especially in SCS trials with at least 10 spikes, indicate that dorsal column axon firing evoked by 10-kHz SCS was poorly synchronized with the 10-kHz pulse train.
Within the 30-s period of SCS, conduction block (<50% conduction percentage) was observed in 24 of 39 axons (62%) at or below the maximum SCS amplitude (Fig. 5A). SCS amplitudes expressed with respect to MT for each individual axon confirmed that, much like SCS-evoked activity, conduction block rarely occurred at amplitudes below 50% MT (Fig. 5A). Nearly 80% (31 of 39) of axons were not yet blocked when the SCS amplitude reached MT, and the majority of units that exhibited conduction block did not do so until SCS amplitudes exceeded MT.
Quantification of eCAP amplitudes demonstrated the progression of SCS-induced conduction block of the population of axons in the dorsal columns. When the SCS electrode was placed between the sciatic nerve (the origin of the eCAP) and the recording electrode on the dorsal columns, the amplitude of eCAPs decreased gradually as the KHF-SCS amplitude was increased (Fig. 5B). However, the average eCAP amplitude, normalized to the pre-SCS baseline eCAP amplitude, did not significantly decrease until SCS amplitudes surpassed MT (P < 0.0001 compared with eCAP amplitude during the lowest SCS amplitude; Fig. 5B). Thus both single-axon recordings and population-level recordings support recent in vivo and computational findings that KHF-SCS is highly unlikely to block DC axons at therapeutic amplitudes (Lempka et al. 2015; Song et al. 2014), and conduction block is therefore unlikely to contribute to KHF-SCS-induced analgesia.
Effects of KHF-SCS on DC axons in naive rats are representative of those in the chronic neuropathic state.
The central nervous system undergoes significant plasticity in the setting of chronic pain, including a host of molecular and electrophysiological changes that augment and disinhibit pain signals (Latremoliere and Woolf 2009; Sivilotti and Woolf 1994). Peripherally, injured Aβ-fibers, the same myelinated afferents that constitute the dorsal columns, may also develop ectopic activity that drives neuropathic pain (Devor 2009). Because SCS is implemented in cases of chronic pain, we recorded the responses of DC axons to KHF-SCS in rats with persistent neuropathic pain following SNI.
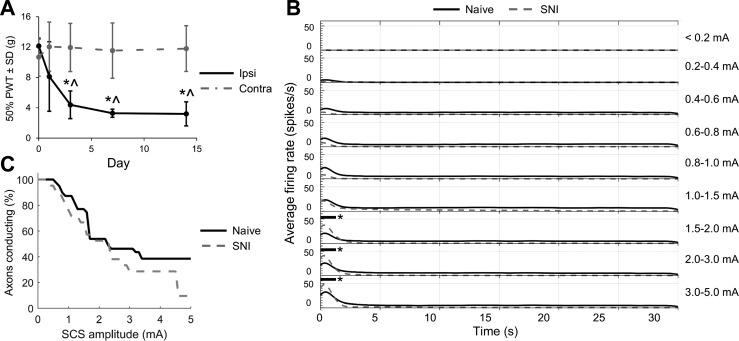
Rats developed mechanical allodynia within the first 1–3 days after SNI. In the ipsilateral hind paw, the PWT decreased significantly on days 3, 7, and 14 compared with pre-SNI baseline (P < 0.007) and compared with the uninjured contralateral hind paw (P < 0.006, 2-way ANOVA with Tukey's HSD; Fig. 6A). SCS-evoked activity in DC axons during 10-kHz biphasic SCS was strikingly similar to activity in axons from naive rats (Fig. 6B). The averaged PSTHs were not significantly different, with the exception of a brief period at the onset of stimulation for the three highest SCS amplitudes, during which axons were more active in the SNI group (P < 0.05; Fig. 6B). Conduction block also developed similarly in axons recorded from naive and SNI rats; the Kaplan-Meier survival curves for axonal conduction were not significantly different (P = 0.08, log-rank test; Fig. 6C), suggesting that conduction block thresholds, on average, were not changed by SNI. Additionally, recorded axons showed very little spontaneous activity in naive rats (average firing rate = 0.0008 ± 0.0022 Hz) and SNI rats (average firing rate = 0.0004 ± 0.0014 Hz; P = 0.44 vs. naive, Student's t-test), suggesting that after nerve injury there was no increase in ectopic activity in recorded afferents.
Fig. 6.
Responses of dorsal column axons to KHF-SCS in naive and spared nerve injury (SNI) rats. A: 50% paw withdrawal threshold (PWT) decreased in the ipsilateral (ipsi) hind paw after SNI compared with baseline (*P < 0.007; n = 4 per group; 2-way repeated-measures ANOVA with Tukey's HSD) and the contralateral (contra) hind paw (^P < 0.006). B: average peristimulus time histograms show firing rates for dorsal column axons recorded in naive rats (solid black lines) and SNI rats (dashed gray lines). Black horizontal bars with asterisk indicate P < 0.05. C: Kaplan-Meier survival curve for axonal conduction. Percentages of axons conducting in naive and SNI rats were not different (P = 0.08; log-rank test).
Although extensive differences in pain processing can exist between normal subjects and those with chronic neuropathic pain, the similarities between DC axon responses to KHF-SCS in naive and SNI rats suggest that, for the scope of this work, DC axon recordings from naive rats can be considered as representative of the neuropathic pain state.
Frequency-dependent effects of KHF-SCS on DC axons.
KHF-SCS is used clinically at frequencies from 1 to 10 kHz (Kapural et al. 2015; Perruchoud et al. 2013; Smith et al. 2015), and kilohertz stimulation is applied to peripheral nerves at even higher frequencies (Bhadra and Kilgore 2005; Bhadra et al. 2006; Patel and Butera 2015), but little rationale exists for the selection of KHF-SCS frequency. Therefore, we recorded the responses of DC axons during KHF-SCS at 1, 5, 10, and 20 kHz.
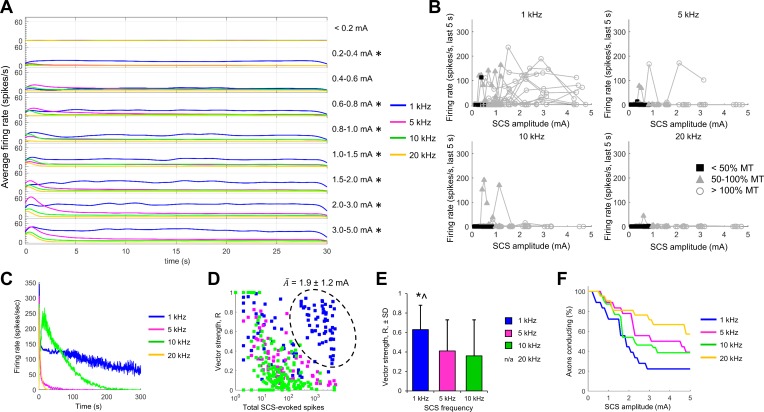
SCS-evoked firing in DC axons was largely similar during 5-, 10-, and 20-kHz SCS. Onset firing was greater during 5-kHz stimulation compared with that during stimulation at 10 and 20 kHz, especially at the highest SCS amplitudes, but after the onset response there was very little persistent firing during SCS at any of the three frequencies (Fig. 7A). Axonal activity was significantly greater during 1-kHz SCS compared with that during stimulation at 5, 10, and 20 kHz (P < 0.05) at all SCS amplitudes >0.6 mA (Fig. 7A). The increase in mean firing rate during 1-kHz SCS resulted from a greater number of axons with persistent activity, as indicated by the incidence of high firing rates during the last 5 s of stimulation (Fig. 7B). However, only one axon was persistently active during 1-kHz SCS at amplitudes below 50% MT (Fig. 7B), suggesting that SCS-evoked activity in the therapeutically relevant amplitude range was not markedly different across the tested kilohertz frequencies. Furthermore, one persistently firing unit was recorded during 5 min of SCS at each of the four frequencies. Firing slowed and eventually ceased in all four trials (Fig. 7C), demonstrating that accommodation of activity occurred if stimulation was continued for longer periods, regardless of the KHF-SCS frequency.
Fig. 7.
Responses of dorsal column axons to different frequencies of KHF-SCS. A: average peristimulus time histograms for dorsal column axons during 1-kHz (blue), 5- kHz (magenta), 10- kHz (green), and 20-kHz SCS (gold). Firing rates were similar with 5- to 20-kHz stimulation, but 1 kHz evoked more persistent firing, especially at higher SCS amplitudes (overall *P < 0.05, 1 kHz vs. each of 5, 10, and 20 kHz). B: average firing rates for axons during the last 5 s of SCS at 1, 5, 10, or 20 kHz, with symbols representing amplitudes relative to motor threshold (MT). Very few axons exhibited persistent firing at amplitudes <50% MT for any SCS frequency. C: SCS-evoked firing rates during 5 min of SCS for 1 persistently active unit that was stimulated with each of the 4 frequencies. Accommodation was observed at all frequencies. D: vector strength for all SCS trials at 1, 5, and 10 kHz as a function of the total number of SCS-evoked spikes. A subset of trials is circled for which high spike totals and above-average vector strength was observed in response to 1-kHz stimulation, with the average SCS amplitude for the encircled trials (Ā; mean ± SD). E: average vector strength for all trials in which at least 1 spike was evoked by SCS. Data are means ± SD (*P < 0.0001 vs. 5 kHz; ^P < 0.0001 vs. 10 kHz; 1-way ANOVA with Tukey's HSD). Vector strength calculations were not possible for 20-kHz SCS. F: Kaplan-Meier survival curves for axonal conduction. Percentages of axons conducting during 1-, 5-, 10-, and 20-kHz SCS were not different (overall P = 0.07, log-rank test, no significant difference; Dunn-Sidak-corrected multiple pairwise comparisons).
Vector strength was used to quantify the phase synchrony of firing evoked by 1-, 5-, and 10-kHz SCS (Fig. 7D; vector strengths for 10 kHz repeated from Fig. 4). As with 10-kHz SCS, vector strength decreased sharply with increased numbers of evoked spikes during 5-kHz SCS, and the average vector strength in all trials with at least one SCS-evoked spike was not different between 5 and 10 kHz. Average vector strength was significantly higher for 1-kHz SCS-evoked spikes (P < 0.0001, 1-way ANOVA; Fig. 7E), especially in trials in which the spike total was high (see circled cluster in Fig. 7D). Although spikes were, on average, more phase-synchronized with the 1-kHz pulse train, the SCS amplitude in the circled subset of trials was 1.9 ± 1.2 mA (Fig. 7D), which was more than three times greater than the average MT (P < 0.0001, one-sample t-test; Table 1), so the increased phase synchrony at 1 kHz may not be relevant at therapeutic amplitudes in the rat.
Conduction block developed similarly for all four KHF-SCS frequencies, because the Kaplan-Meier survival curves were not significantly different overall (P = 0.07, log-rank test; Fig. 7F). Only the comparison between the 1- and 20-kHz Kaplan-Meier curves approached significance (P = 0.051, Sidak-corrected pairwise comparison). Together, these observations of activation and conduction block demonstrated that although some minor differences in thresholds and intensity of activation and block occurred, the same general response behaviors were observed during KHF-SCS ranging from 1 to 20 kHz. Note that 1-kHz SCS evoked more spikes despite blocking slightly higher percentages of axons; this was possible because the two measurements were independent: SCS-evoked firing could still occur after conduction was blocked in the axon, and a larger percentage of units overall had high rates of persistent firing during the 30-s SCS period (Fig. 7B). This phenomenon did highlight one limitation of the measurement of conduction block in highly active units. Antidromic action potentials could collide with SCS-evoked spikes, giving the false impression of conduction block; similarly, SCS-evoked spikes could, by chance, occur at the expected latency of an antidromic potential, suggesting conduction when, in fact, block may have occurred. However, the measurement of eCAPs during 10-kHz SCS, which was not subject to the same limitations, validated our findings of conduction block in single axons (Fig. 5).
Biphasic and sinusoidal KHF-SCS similarly activate and block DC axons.
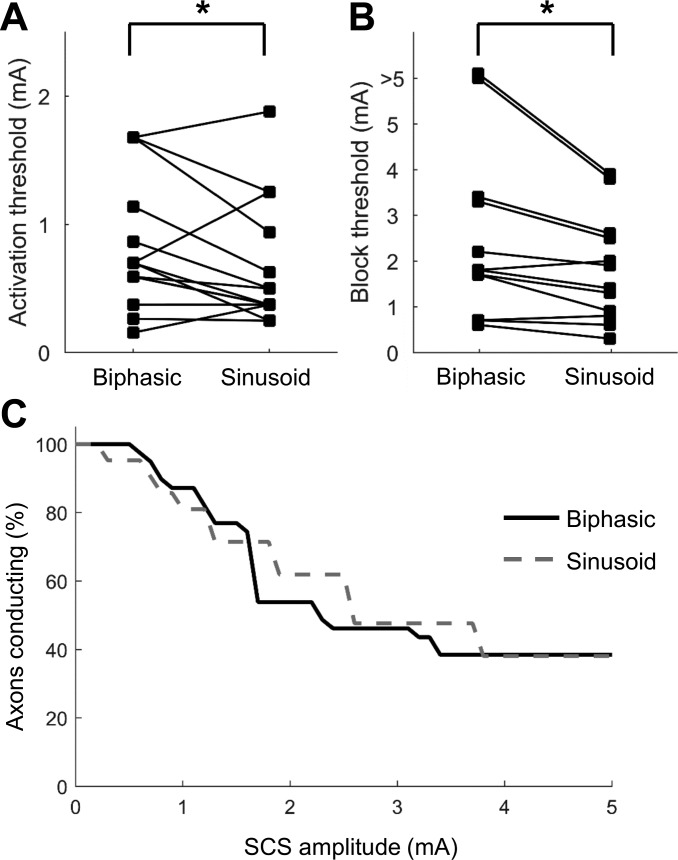
SCS typically employs biphasic pulse trains (Song et al. 2015), but KHF stimulation, especially of peripheral nerves, has also been studied using sinusoidal waveforms (Bhadra and Kilgore 2005; Bhadra et al. 2006; Patel and Butera 2015). To compare the effects of the two waveforms, DC axons were recorded during stimulation with 10-kHz biphasic pulse trains and 10-kHz sinusoidal SCS. Thresholds for activation were 1.42 ± 0.57 times higher (P = 0.01, paired Student's t-test) and thresholds for conduction block were 1.29 ± 0.41-times higher (P = 0.03) during biphasic SCS compared with sinusoidal SCS (Fig. 8). Those ratios were consistent with the 1.3-fold difference in charge per phase between the 10-kHz sinusoid and the 24-μs rectangular pulse of equal amplitude, suggesting that if the amplitude of biphasic SCS was increased to match the charge delivery of the sinusoid, there would be no differences in the activation and block thresholds between waveform shapes. Furthermore, the Kaplan-Meier survival curves were not different, indicating that the development of conduction block was independent of the SCS waveform shape. The similarities between the effects of biphasic pulse trains and sinusoidal KHF-SCS provide a bridge between data acquired using the two different waveforms and facilitate a comparison of axon responses based on the modeling and in vivo experiments that used sinusoidal KHF stimulation.
Fig. 8.
Responses of dorsal column axons to 10-kHz biphasic and 10-kHz sinusoidal SCS. Activation thresholds (A; *P = 0.01; Student's t-test) and conduction block thresholds (B; *P = 0.032; Student's t-test) were significantly higher for biphasic SCS. C: Kaplan-Meier survival curves for axonal conduction. Percentages of axons conducting during 10-kHz biphasic and 10-kHz sinusoidal SCS were not different (P = 0.83; log-rank test).
DISCUSSION
This study presents the first recording and quantification of the responses of dorsal column (DC) axons to epidural kilohertz-frequency spinal cord stimulation (KHF-SCS). KHF-SCS activated and/or blocked conduction in DC axons, especially at supra-motor threshold (MT) amplitudes. However, KHF-SCS was unlikely to evoke widespread firing or conduction block at SCS amplitudes that reduce pain in rats (typically 40–50% MT; Shechter et al. 2013; Song et al. 2014). Because of the primary role that DC axons play in modulating pain signals during conventional SCS, establishing the patterns of DC activity during KHF-SCS is critical to understand the mechanisms and enable optimization of this promising therapy.
Recordings were made from axons with a wide range of diameters and depths in the dorsal columns. The mean diameter of recorded axons at T12/T13 (3.6 μm) was comparable to that of fibers in the T6 (3.08 μm) and L3 (3.83 μm) rat dorsal columns (Yamamoto and Ohnishi 1996). A small percentage of thoracic rat DC axons are larger than the maximum-diameter (6.6 μm) axons recorded in this study (Yamamoto and Ohnishi 1996), so a small group of large-diameter axons were likely not sampled because of their sparseness. Very little activity occurred at SCS amplitudes below 50% MT (Fig. 3), but because activation thresholds are inversely related to axon diameter (Bement and Ranck 1969), it is possible that the largest diameter fibers would be more likely to fire at amplitudes near 50% MT. That pattern would be consistent with evidence from conventional SCS suggesting that superficial, large-diameter fibers are the first neural elements activated during SCS and are most responsible for SCS-induced analgesia (Holsheimer 2002).
Activity generated in DC axons by KHF-SCS was highly asynchronous with the SCS waveform, especially during 5- and 10-kHz SCS (Figs. 4 and 7), but firing was mostly limited to an onset response lasting less than 5–10 s (Fig. 3). Asynchronous onset firing has been noted during KHF stimulation of peripheral nerves (Bhadra and Kilgore 2005; Gerges et al. 2010; Patel and Butera 2015). However, those studies sought to abolish the onset response and achieve rapid conduction block, and therefore viewed onset firing as an “undesirable” precursor to conduction block (Gerges et al. 2010). In the present study, onset firing developed independently of conduction block and also occurred at sub-block amplitudes (Fig. 2C), similar to the findings of Woo and Campbell (1964) and raising the possibility of KHF-SCS producing DC activity while maintaining normal sensation and proprioception.
The activation of DC axons by epidural stimulation is canonical as part of the mechanism of analgesia for conventional SCS (Linderoth and Foreman 1999). Large-diameter DC axons drive subpopulations of inhibitory interneurons in the superficial dorsal horn, where they are well-situated to suppress activation of projection neurons (Daniele and MacDermott 2009; Dubuisson 1989). Asynchronous firing in DC axons during KHF-SCS may be sufficient to modulate the output of pain-transmitting projection neurons. However, unlike conventional SCS, KHF-SCS evoked only transient activity, and even axons that were persistently active during 30 s of KHF-SCS eventually slowed and ceased firing without exception (Figs. 3 and 7). That accommodation was consistent with reports of diminishing activity during KHF stimulation of peripheral nerves at 2–20 kHz (Bowman and McNeal 1986; Woo and Campbell 1964). Therefore, it seems unlikely that continuous KHF-SCS would evoke asynchronous DC activity sufficient to modulate dorsal horn pain processing in the long term.
Activation of DC axons by KHF-SCS likely also produces orthodromic spikes, which would project rostrally to the dorsal column nuclei (DCN). In conventional SCS, orthodromic spikes are believed to cause paresthesias (Linderoth and Foreman 1999), and activity in a single myelinated afferent can be sufficient to elicit conscious paresthetic sensation (Ochoa and Torebjörk 1983; Vallbo et al. 1984). KHF-SCS reportedly provides paresthesia-free analgesia (Tiede et al. 2013), implying that orthodromic DC activation does not occur. Recordings from the DCN in rats showed no activation at any time during 10-kHz SCS (Song et al. 2014), suggesting that no DC axonal activity reached the DCN. The lack of onset firing in the DCN is surprising given the prevalence of onset firing in DC axons (Fig. 3); however, Song et al. recorded activity in only 11 DCN units. More comprehensive recordings from the DCN may reveal sparse activity, especially at the onset of KHF-SCS, which corresponds with the firing observed in a small group of axons at amplitudes below 50% MT (Fig. 3). Nonetheless, the reported lack of activity in the DCN in the post-onset period (>10 s after SCS began) is generally supported by the finding that SCS-evoked activity in DC axons diminished rapidly (Figs. 3 and 7) to a state where no orthodromic activity, and therefore no paresthesia, was likely to occur.
The lack of paresthesia during KHF-SCS in humans and the absence of persistent DC and DCN activity in the rat bring into question whether the traditional gate-control mechanism is activated by KHF-SCS. Arle et al. (2016) suggested that “differential block” of DC axons could activate the gating mechanism without producing paresthesia. In a computational model, large-diameter DC fibers that were reportedly most likely to cause paresthesia were not activated, whereas smaller fibers with the potential to engage the gate-control mechanism were highly active. Although the present study did not record the largest subset of axons in the dorsal column during KHF-SCS, there was no indication of differential block in the groups of fibers that were recorded. In particular, there was a lack of diameter-dependent activation with increasing KHF-SCS amplitude, because fibers of similar diameter were distributed in the dorsal columns at different distances from the stimulating electrode and therefore had nonuniform activation and block thresholds despite their similar diameters. Furthermore, the model simulated only 1 s of stimulation; the accommodation of firing on a longer timescale (Fig. 3) suggests that 1 s is insufficient to fully capture the in vivo dynamics of DC axons during KHF-SCS. Nonetheless, the theory presented by Arle et al. (2016) further emphasizes the importance of quantifying the in vivo responses of DC axons to KHF-SCS.
The responses of DC axons to KHF-SCS were highly similar across 5–20 kHz, but-1 kHz SCS evoked significantly more firing during the 30-s stimulation period (Fig. 7). Stimulation at 1 kHz potentially approached a frequency in which axons were better able to follow the pulse train or a subharmonic of the stimulation, as indicated by the increased vector strength compared with higher frequencies (Fig. 7). Indeed, prior recordings in peripheral nerves demonstrated the ability of myelinated axons to fire synchronously or at subharmonics of the stimulation at frequencies up to 1 kHz (Bowman and McNeal 1986). Note, however, that the increased firing evoked by 1-kHz SCS occurred at amplitudes near and above MT (Fig. 7); as with 5- to 20-kHz SCS, a vast majority of axons were inactive when 1-kHz SCS was applied at or below 50% MT.
Conduction block thresholds in mammalian peripheral nerves increase with stimulation frequency from 1 kHz to at least 30 kHz (Bhadra et al. 2006; Bhadra and Kilgore 2005; Joseph and Butera 2011). Those studies used sinusoidal stimulation, but the similarities between the effects of sinusoidal and biphasic KHF-SCS (Fig. 8) suggest that conduction block thresholds will trend similarly for biphasic KHF-SCS. Even though the overall percentages of blocked axons were not different among SCS frequencies, the Kaplan-Meier curves show that the likelihood of conduction block at a given SCS amplitude was slightly higher at lower SCS frequencies (Fig. 7F). However, regardless of the frequency-dependent changes in block thresholds, axons were not consistently blocked until SCS amplitudes far exceeded those that produce analgesia in rats, indicating that conduction block is unlikely to contribute to the mechanism of KHF-SCS (Lempka et al. 2015; Song et al. 2013).
Increased rate of battery use is a significant consideration for KHF-SCS. Patients are required to recharge stimulators every 1–2 days, compared with an average of 5 times per month with conventional SCS (Kapural et al. 2015). Some patients have even reported better pain relief from KHF-SCS but opted to receive conventional SCS because of its lower recharge burden (Smith et al. 2015). Frequency could be an important consideration in the parameterization of KHF-SCS, because axons responded similarly to stimulation across a wide range of frequencies, at least within the relevant therapeutic amplitude range. The potential to produce similar pain relief at lower frequencies and realize significant energy savings emphasizes the need to compare directly the effects of different kilohertz frequencies in well-controlled clinical studies.
Patterns of activation and block were similar in DC axons from naive rats and SNI rats (Fig. 6), suggesting that the findings of this study are relevant to the use of KHF-SCS in the setting of chronic pain. The SNI model was used to demonstrate the effects of KHF-SCS on DC axons in the context of neuropathic pain, but animal models of pain, especially those that rely on evoked pain measurements such as mechanical allodynia, may not adequately represent the human chronic pain condition (Blackburn-Munro 2004; Mogil and Crager 2004). By directly recording neural activity in afferent fibers as the primary outcome, this study measured the effects of KHF-SCS in a way that minimized the contributions of the emotional, psychological, social, and behavioral factors that make clinical correlates of pain in animal models so challenging. Nonetheless, there may be factors not represented in the SNI model that influence the way KHF-SCS affects DC axons in humans with chronic pain. For example, pain in humans usually must present for a minimum of 3 mo before being considered chronic (Blackburn-Munro 2004), but this study only extended 2 wk beyond the time of SNI. It is unclear whether further pathophysiological changes may occur over longer time periods and thereby alter the responses of DC axons to KHF-SCS. Furthermore, SCS is used to treat pain from a variety of etiologies, most commonly failed back surgery syndrome, and a rat partial nerve injury model may not best represent all of those different etiologies.
This study determined patterns of dorsal column activity and thresholds for activation and conduction block of individual axons during KHF-SCS. It is highly unlikely that the effects of KHF-SCS are mediated by conduction block. KHF-SCS may evoke activity, especially in a few superficial, large-diameter axons, much like conventional 50-Hz SCS. However, unlike 50-Hz SCS, KHF-SCS-evoked firing accommodated rapidly and was highly asynchronous with the stimulus waveform. These data provide a quantitative characterization of the central nervous system activity that may reach antidromically to the dorsal horn and orthodromically to the DCN to modulate pain perception and paresthesia, and thereby provide a foundation for investigation of the mechanisms of KHF-SCS and its optimization as a therapy for chronic pain.
GRANTS
Funding for this research was provided by Stryker Corporation.
DISCLOSURES
J. J. Janik is an employee of Stryker Corporation who has traveled to conferences on behalf of Stryker Corporation and who owns Stryker Corporation stock.
AUTHOR CONTRIBUTIONS
N.D.C., J.J.J., and W.M.G. conception and design of research; N.D.C. performed experiments; N.D.C. and W.M.G. analyzed data; N.D.C., J.J.J., and W.M.G. interpreted results of experiments; N.D.C. prepared figures; N.D.C. drafted manuscript; N.D.C., J.J.J., and W.M.G. edited and revised manuscript; W.M.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Brandon Thio for assistance with histology and Gilda Mills for assistance with animal procedures.
REFERENCES
- Al-Kaisy A, Palmisani S, Smith T, Harris S, Pang D. The use of 10-kilohertz spinal cord stimulation in a cohort of patients with chronic neuropathic limb pain refractory to medical management. Neuromodulation 18: 18–23, 2015. [DOI] [PubMed] [Google Scholar]
- Arle JE, Mei L, Carlson KW, Shils JL. High-frequency stimulation of dorsal column axons: potential underlying mechanism of paresthesia-free neuropathic pain relief. Neuromodulation 19: 385–397, 2016. [DOI] [PubMed] [Google Scholar]
- Baba H, Yoshimura M, Nishi S, Shimoji K. Synaptic responses of substantia gelatinosa neurones to dorsal column stimulation in rat spinal cord in vitro. J Physiol 478: 87–99, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantli H, Bloedel JR, Long DM, Thienprasit P. The distribution of activity in spinal pathways evoked by dorsal column stimulation. J Neurosurg 42: 290–295, 1975. [DOI] [PubMed] [Google Scholar]
- Bement SL, Ranck JB. A quantitative study of electrical stimulation of central myelinated fibers. Exp Neurol 24: 147–170, 1969. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Bhadra N, Kilgore KL, Gustafson KJ. High frequency electrical conduction block of the pudendal nerve. J Neural Eng 3: 180–187, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra N, Kilgore KL. Direct current electrical conduction block of peripheral nerve. IEEE Trans Neural Syst Rehabil Eng 12: 313–324, 2004. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Kilgore KL. High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle Nerve 32: 782–790, 2005. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G. Pain-like behaviors in animals–how human are they? Trends Pharmacol Sci 25: 299–305, 2004. [DOI] [PubMed] [Google Scholar]
- Bowman BR, McNeal DR. Response of single alpha motoneurons to high-frequency pulse trains. Firing behavior and conduction block phenomenon. Appl Neurophysiol 49: 121–138, 1986. [DOI] [PubMed] [Google Scholar]
- Boyd IA, Kalu KU. Scaling factor relating conduction velocity and diameter for myelinated afferent nerve fibres in the cat hind limb. J Physiol 289: 277–297, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53: 55–63, 1994. [DOI] [PubMed] [Google Scholar]
- Cuellar JM, Alataris K, Walker A, Yeomans DC, Antognini JF. Effect of high-frequency alternating current on spinal afferent nociceptive transmission. Neuromodulation 16: 318–327, 2013. [DOI] [PubMed] [Google Scholar]
- Daniele CA, MacDermott AB. Low-threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse. J Neurosci 29: 686–695, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87: 149–158, 2000. [DOI] [PubMed] [Google Scholar]
- Devor M. Ectopic discharge in Aβ afferents as a source of neuropathic pain. Exp Brain Res 196: 115–128, 2009. [DOI] [PubMed] [Google Scholar]
- Dorrscheidt GH. The statistical significance of the peristimulus histogram (PSTH). Brain Res 220: 397–401, 1981. [DOI] [PubMed] [Google Scholar]
- Dubuisson D. Effect of dorsal-column stimulation on gelatinosa and marginal neurons of cat spinal cord. J Neurosurg 70: 257–265, 1989. [DOI] [PubMed] [Google Scholar]
- Fisher KM, Jillani NE, Oluoch GO, Baker SN. Blocking central pathways in the primate motor system using high-frequency sinusoidal current. J Neurophysiol 113: 1670–1680, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke M, Bhadra N, Bhadra N, Kilgore K. Direct current contamination of kilohertz frequency alternating current waveforms. J Neurosci Methods 232: 74–83, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerges M, Foldes EL, Ackermann DM, Bhadra N, Bhadra N, Kilgore KL. Frequency- and amplitude-transitioned waveforms mitigate the onset response in high-frequency nerve block. J Neural Eng 7: 066003, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol 32: 613–636, 1969. [DOI] [PubMed] [Google Scholar]
- Holsheimer J. Which neuronal elements are activated directly by spinal cord stimulation? Neuromodulation 5: 25–31, 2002. [DOI] [PubMed] [Google Scholar]
- Hunter JP, Ashby P. Segmental effects of epidural spinal cord stimulation in humans. J Physiol 474: 407–419, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbert RJ, Tator CH, Theriault E. Dose-response study of the pathological effects of chronically applied direct current stimulation on the normal rat spinal cord. J Neurosurg 79: 905–916, 1993. [DOI] [PubMed] [Google Scholar]
- Joseph L, Butera RJ. High-frequency stimulation selectively blocks different types of fibers in frog sciatic nerve. IEEE Trans Neural Syst Rehabil Eng 19: 550–557, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapural L, Yu C, Doust MW, Gliner BE, Morgan DM, Brown LL, Yearwood TL, Bundschu R, Burton AW, Yang T, Benyamin R, Burgher AH. Novel 10-kHz high-frequency therapy (HF10 Therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain. Anesthesiology 123: 851–860, 2015. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10: 895–926, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempka SF, McIntyre CC, Kilgore KL, Machado AG. Computational analysis of kilohertz frequency spinal cord stimulation for chronic pain management. Anesthesiology 122: 1–15, 2015. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Foreman RD. Physiology of spinal cord stimulation: review and update. Neuromodulation 2: 150–164, 1999. [DOI] [PubMed] [Google Scholar]
- Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods 4: 1–32, 1981. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain 112: 12–15, 2004. [DOI] [PubMed] [Google Scholar]
- National Research Council Committee. Guide for the Care and Use of Laboratory Animals (8th ed). Washington, DC: National Academies, 2011. [Google Scholar]
- Ochoa J, Torebjörk E. Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. J Physiol 342: 633–654, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JL, Karantonis DM, Single PS, Obradovic M, Cousins MJ. Compound action potentials recorded in the human spinal cord during neurostimulation for pain relief. Pain 153: 593–601, 2012. [DOI] [PubMed] [Google Scholar]
- Patel Y, Butera RJ. Differential fiber-specific block of nerve conduction in mammalian peripheral nerves using kilohertz electrical stimulation. J Neurophysiol 113: 3923–3929, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruchoud C, Eldabe S, Batterham AM, Madzinga G, Brookes M, Durrer A, Rosato M, Bovet N, West S, Bovy M, Rutschmann B, Gulve A, Garner F, Buchser E. Analgesic efficacy of high-frequency spinal cord stimulation: a randomized double-blind placebo-controlled study. Neuromodulation 16: 363–369, 2013. [DOI] [PubMed] [Google Scholar]
- Shechter R, Yang F, Wacnik PW, Guan Y, Dong X, Meyer RA, Raja SN. Conventional and kilohertz-frequency spinal cord stimulation produces intensity- and frequency-dependent inhibition of mechanical hypersensitivity in a rat model of neuropathic pain. Anesthesiology 119: 422–432, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol 72: 169–179, 1994. [DOI] [PubMed] [Google Scholar]
- Smith H, Youn Y, Pilitsis JG. Successful use of high-frequency spinal cord stimulation following traditional treatment failure. Stereotact Funct Neurosurg 93: 190–193, 2015. [DOI] [PubMed] [Google Scholar]
- Soin A, Syed Shah N, Fang ZP. High-frequency electrical nerve block for postamputation pain: a pilot study. Neuromodulation 18: 197–206, 2015. [DOI] [PubMed] [Google Scholar]
- Song Z, Meyerson BA, Linderoth B. High-frequency (1 kHz) spinal cord stimulation–is pulse shape crucial for the efficacy? A pilot study. Neuromodulation 18: 714–719, 2015. [DOI] [PubMed] [Google Scholar]
- Song Z, Viisanen H, Meyerson BA, Pertovaara A, Linderoth B. Efficacy of kilohertz-frequency and conventional spinal cord stimulation in rat models of different pain conditions. Neuromodulation 17: 226–234, 2014. [DOI] [PubMed] [Google Scholar]
- Tiede J, Brown L, Gekht G, Vallejo R, Yearwood T, Morgan D. Novel spinal cord stimulation parameters in patients with predominant back pain. Neuromodulation 16: 370–375, 2013. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Olsson KA, Westberg KG, Clark FJ. Microstimulation of single tactile afferents from the human hand: sensory attributes related to unit type and properties of receptive fields. Brain 107: 727–749, 1984. [DOI] [PubMed] [Google Scholar]
- Van Buyten JP, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation 16: 59–65, 2013. [DOI] [PubMed] [Google Scholar]
- Woo M, Campbell B. Asynchronous firing and block of peripheral nerve conduction by 20 KC alternating current. Bull Los Angel Neuro Soc 29: 87–94, 1964. [PubMed] [Google Scholar]
- Yamamoto K, Ohnishi A. Ascending myelinated axons of primary sensory neurons of the sciatic nerve in the posterior column of the rat spinal cord. Acta Neuropathol 92: 27–34, 1996. [DOI] [PubMed] [Google Scholar]
- Zhang TC, Janik JJ, Grill WM. Mechanisms and models of spinal cord stimulation for the treatment of neuropathic pain. Brain Res 1569: 19–31, 2014. [DOI] [PubMed] [Google Scholar]