This work is unique since it examines the contribution of combined, bilateral hip and knee proprioceptive sense on the recovery of skilled walking function, in addition to characterizing gaze behavior during a skilled walking task in people with motor-incomplete spinal cord injury.
Keywords: proprioception, gaze behavior, obstacle crossing, spinal cord injury, functional mobility
Abstract
Skilled walking, such as obstacle crossing, is an essential component of functional mobility. Sensorimotor integration of visual and proprioceptive inputs is important for successful obstacle crossing. The objective of this study was to understand how proprioceptive deficits affect obstacle-crossing strategies when controlling for variations in motor deficits in ambulatory individuals with spinal cord injury (SCI). Fifteen ambulatory individuals with SCI and 15 able-bodied controls were asked to step over an obstacle scaled to their motor abilities under full and obstructed vision conditions. An eye tracker was used to determine gaze behaviour and motion capture analysis was used to determine toe kinematics relative to the obstacle. Combined, bilateral hip and knee proprioceptive sense (joint position sense and movement detection sense) was assessed using the Lokomat and customized software controls. Combined, bilateral hip and knee proprioceptive sense in subjects with SCI varied and was significantly different from able-bodied subjects. Subjects with greater proprioceptive deficits stepped higher over the obstacle with their lead and trail limbs in the obstructed vision condition compared with full vision. Subjects with SCI also glanced at the obstacle more frequently and with longer fixation times compared with controls, but this was not related to proprioceptive sense. This study indicates that ambulatory individuals with SCI rely more heavily on vision to cross obstacles and show impairments in key gait parameters required for successful obstacle crossing. Our data suggest that proprioceptive deficits need to be considered in rehabilitation programs aimed at improving functional mobility in ambulatory individuals with SCI.
NEW & NOTEWORTHY This work is unique since it examines the contribution of combined, bilateral hip and knee proprioceptive sense on the recovery of skilled walking function, in addition to characterizing gaze behavior during a skilled walking task in people with motor-incomplete spinal cord injury.
controlling the end point of a limb (e.g., our hand during reaching or our feet during walking) is a critical component of our daily lives. Whether we are reaching for a glass or stepping over an obstacle, the precise control of the end point is required. This control requires intricate coordination across multiple joints. Humans encounter such precision goal-directed movements everyday as they adapt their walking patterns to maneuver and cross obstacles of different dimensions. Obstacle crossing is a key component of community ambulation and has been identified as an important goal for gait recovery following stroke (Said et al. 1999, 2001), Parkinson's disease (Martens and Almeida 2011), and spinal cord injury (SCI) (Pramodhyakul et al. 2013).
Sensorimotor integration of visual and proprioceptive inputs is critical for producing the appropriate locomotor modifications for safe and successful obstacle crossing (Marigold et al. 2011). Vision is primarily used in a feedforward manner to identify an obstacle's attributes (e.g., height, width), as well as its location relative to body position (Gibson 1958; Patla 1997, 1998; Rhea and Rietdyk 2007). Proprioceptive input is critical for the maintenance and production of the initial motor plan based on the attributes identified by visual input (Blouin et al. 1993; Bard et al. 1995; Sober and Sabes 2003, 2005). When approaching an obstacle, vision is used to intermittently scan the obstacle and the surrounding area, and during the step over the obstacle, vision is used to sample the upcoming landing area rather than the obstacle itself (Patla and Vickers 1997).
When peripheral vision of the lower limbs and obstacle are obstructed, participants are still able to step over the obstacle but adopt certain safety strategies. One such strategy is to step further away from the obstacle (increase foot placement) (Mohagheghi et al. 2004; Rietdyk and Rhea 2006). Introducing environmental cues during visually obstructed obstacle crossing can minimize the changes in foot placement. For example, if the lower visual field is obstructed and a spatial cue regarding obstacle location (e.g., a door frame) is introduced into the environment, foot placement becomes comparable to full vision conditions (Rietdyk and Rhea 2006). Although vision of the spatial cue provides information about head position relative to the obstacle, previous authors have speculated that this visual exproprioception of head position relative to the obstacle needs to be integrated with lower limb proprioceptive sense to drive accurate foot placement in such conditions (Rietdyk and Rhea 2006).
Another safety strategy that is adopted when the lower visual field is obstructed is to step higher over the obstacle (Mohagheghi et al. 2004; Rietdyk and Rhea 2006). However, subjects can be trained to minimize foot clearance if given auditory feedback of performance. The ability to modify foot clearance to an “optimal height” is likely due to the integration of the auditory feedback with lower limb proprioceptive sense. Thus the ability to adjust foot clearance and foot placement, both critical components of skilled walking, without the need for on-going direct visual monitoring, is possible due to the integration of feedback (e.g., visual or auditory) with lower limb proprioceptive sense.
Better understanding of the sensorimotor integration required for the control of complex locomotor tasks, such as obstacle crossing, will be important for improving therapeutic strategies to enhance functional ambulation after injury. For instance, the loss of proprioception at even one joint, such as may occur in people with diabetes mellitus (Gutierrez et al. 2001), may lead to alterations in toe clearance height during obstacle crossing (Liu et al. 2010). In these individuals, the risk of tripping and falling during walking is of concern as they display reduced lead limb toe clearance over the obstacle. Similarly, in people with central nervous system injuries, disruptions in sensorimotor processing can lead to altered obstacle-crossing strategies. One report demonstrated that people with stroke tend to adopt a safety strategy by increasing toe clearance height over the obstacle (Said et al. 2001). The reasons for this were not clear since the clinical presentation of the stroke subjects was widely variable in that study and could have included visual deficits as well as somatosensory deficits given their lesion sites. Increased toe clearance height during obstacle crossing has also been reported in individuals with visual impairments (Patla et al. 1995; Timmis and Pardhan 2012) and those with pathologies of the cerebellum (Morton et al. 2004).
Few studies have investigated obstacle-crossing strategies in ambulatory individuals with SCI. One report demonstrated that people with SCI display decreased range of motion and adopted compensatory strategies, such as hip hiking, when crossing an obstacle (Ladouceur et al. 2003). Another report showed that a lower visual field obstruction during a precision obstacle-crossing task on a treadmill led to more obstacle hits in ambulatory individuals with SCI compared with able-bodied participants (van Hedel et al. 2005). Interestingly, of the three participants in that study who hit the obstacle most often, two of them were reported to have abnormal somatosensory evoked potentials. These previous reports suggest that ambulatory individuals with SCI present with abnormalities when crossing obstacles that could, in part, be due to somatosensory deficits.
Obstacle crossing is dependent on multiple factors, including dynamic balance, muscle strength, proprioceptive sense, and visual acuity. Given that the major motor and sensory pathways traverse the spinal cord, an individual with SCI may present with varied motor and sensory deficits depending on the location of the injury. Previous studies have mainly considered the effect of motor deficits on obstacle-crossing strategies, but little is known of the impact of proprioceptive deficits, probably due to difficulties in quantifying proprioceptive sense in people with partial paralysis. Using a robotic exoskeleton, we can now accurately measure hip and knee proprioceptive deficits [joint position sense (Domingo and Lam 2014) and movement detection sense (Chisholm et al. 2016)] in people with SCI. These recent advancements provide us with the unique opportunity to investigate the effects of hip and knee proprioceptive deficits on skilled walking function.
The objective of this study was to understand how proprioceptive deficits affect obstacle-crossing strategies using a paradigm that allowed us to control for the variations in motor deficits in ambulatory individuals with SCI. We hypothesized that impairments in hip and knee proprioceptive sense in people with SCI will be associated with greater reliance on gaze during full-vision obstacle-crossing trials. We also hypothesized that hip and knee proprioceptive impairments will be associated with greater distance between foot placement and the obstacle and with higher foot clearance over the obstacle (both indicative of a safety strategy) when vision is obstructed compared with full vision trials.
METHODS
Subjects
Fifteen individuals with motor-incomplete SCI and 15 able-bodied (AB) controls were recruited. The inclusion criteria for the subjects with SCI were as follows: 1) SCI at least 9 mo ago; 2) ability to walk at least 5 meters with or without an aid; and 3) normal or corrected to normal (with contact lenses or glasses) visual acuity of 20/40 (confirmed by the Snellen chart examination). The exclusion criteria for the subjects with SCI were as follows: 1) presence of any musculoskeletal injury or other neurological condition affecting mobility or sensory function; 2) severe spasticity interfering with walking function; and 3) weight >136 kg or height >1.85 m (limits of the Lokomat). The inclusion and exclusion criteria for the AB subjects were the same, except for the presence of SCI. The University of British Columbia and Vancouver Coastal Health Research Institute ethics committees approved all procedures.
Protocol
Session 1: baseline functional assessments.
PROPRIOCEPTIVE SENSE (SCI AND AB).
Proprioceptive sense (static joint position sense and movement detection sense) at the hip and knee of the lower limbs was measured using custom-written software of the Lokomat (Hocoma AG, Volketswil, Switzerland) and adapted from procedures described in detail elsewhere (Domingo and Lam 2014; Chisholm et al. 2016). For all tests of proprioceptive sense, participants were suspended above the treadmill belt by an overhead harness system and their legs were strapped into the Lokomat. The test limb was suspended in the air and the untested limb was supported by a short platform placed on the treadmill belt. A large drape covered the subjects' legs to block vision of the lower limbs. To test joint position sense, the test joint was moved at a speed of 7°/s to a target angle (25° flexion or 25° extension) and the subject was asked to memorize this position (target angle). The Lokomat subsequently moved the joint away from the memorized position to a distractor position. Subjects were then asked to use a joystick (which controlled the Lokomat's legs at a speed of either 3 or 6°/s, depending on the angle of the joystick) to position their test joint back to the memorized position (actual position). Six trials were performed at each of the hip and knee joints, bilaterally. To test movement detection sense, subjects were instructed to press a button on the joystick as soon as they detected movement in the test joint. The test limb moved from its starting position (10° at the hip and 30° at the knee) through 10° of motion or until the joystick button was pressed. The Lokomat initiated movement after a random delay at the start of each trial. Three different speeds were tested (0.5, 1, and 2°/s), all in random order. Catch trials, where the Lokomat did not move the joint at all, were also randomly presented. The test joint was moved into either flexion or extension. Subjects were also asked to identify the direction of movement at the end of each trial.
CLINICAL MEASURES (SCI ONLY).
Lower extremity strength was measured by manual muscle testing using the Lower Extremity Motor Score (LEMS) (Maynard et al. 1997). Ambulatory capacity was assessed by the self-selected 10-m walk test (10MWT) (Jackson et al. 2008). Balance was assessed using the Berg Balance Scale (Blum and Korner-Bitensky 2008; Wirz et al. 2010).
DETERMINING OBSTACLE HEIGHT (SCI AND AB).
Obstacle height was scaled to the motor abilities of each person's lead limb. For the subjects with SCI, the lead limb was their stronger limb, determined by the LEMS. For the AB subjects, the lead limb was their dominant limb, determined by asking which limb they would use to kick a soccer ball. Subjects walked on a treadmill (subjects with SCI used the handrails while AB subjects did not) at their self-selected comfortable speed. Two trials of ∼20 steps each were collected. In the first trial, subjects were instructed to walk normally (usual stepping). In the second trial, they were instructed to walk while stepping as high as possible (maximum stepping). The average peak toe height during the swing phase was calculated from each condition. Obstacle height for each subject was set at a level corresponding to average peak toe height during usual stepping plus 25% of the difference in peak toe height between maximum and usual stepping (Fig. 1). We chose to determine stepping ability on the treadmill rather than over-ground because treadmill stepping isolates the lead limb in the absence of balance control and it also allowed for more steps to be recorded.
Fig. 1.
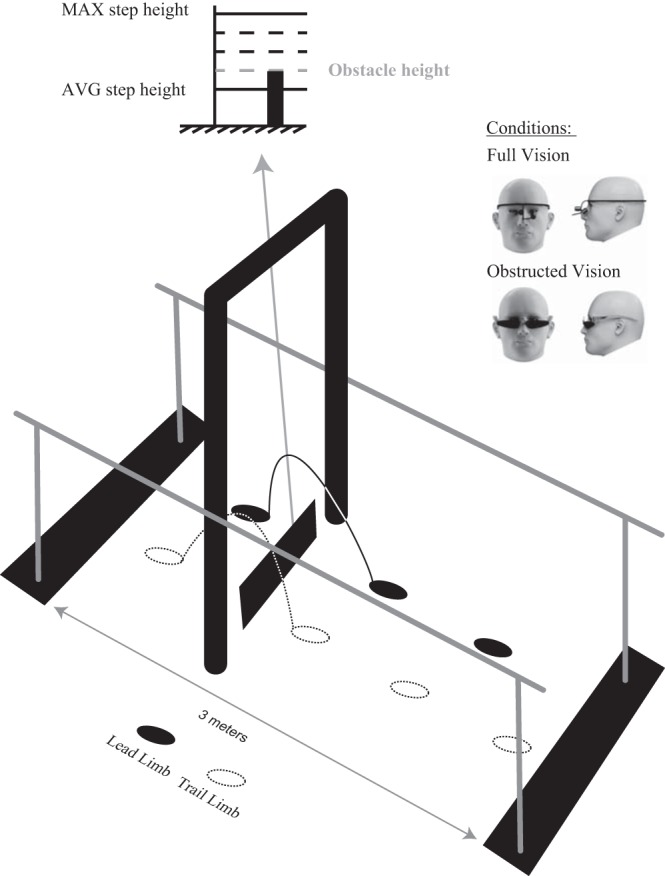
Experimental setup. Participants walked between parallel bars and were asked to step over an obstacle positioned between a doorframe (indicating its spatial location) under full and obstructed vision conditions. Obstacle height for each subject was set at a level corresponding to average peak toe height during usual stepping plus 25% of the difference in peak toe height between maximum and usual stepping of the stronger limb [subjects with spinal cord inujry (SCI)] or dominant limb [ able-bodied (AB) subjects].
Session 2: obstacle crossing experiment.
The experimental setup is depicted in Fig. 1. Subjects walked along a 3-m walkway between parallel bars. All subjects with SCI used the parallel bars to avoid the potential confounding effects of the reliance on different hand-held gait aids. AB subjects did not use the parallel bars. A “door frame” made of PVC tubing was positioned around the obstacle to indicate its spatial location (Rietdyk and Rhea 2006; Graci et al. 2010). The obstacle was 47.5-cm wide and 1 cm in depth and made of a wooden dowel supported between two brackets and covered in black cloth draped to the ground. The vertical position of the obstacle could be adjusted by changing the height of the support bracket. The obstacle was very light and easily fell off the support bracket with light foot contact, reducing the risk of tripping.
Before data collection, the appropriate start position for each individual was determined such that they took two full strides with the lead leg before stepping over the obstacle. Two conditions (full vision and obstructed vision) were then presented, each consisting of 8–10 trials of obstacle crossing. In the full vision (FV) condition, subjects walked along the walkway at their self-selected walking speed and were instructed to step over the obstacle. In the obstructed vision (OV) condition, participants donned dribble goggles to occlude the lower visual field immediately in front of them (∼3.0 meters) and were asked to maintain a straight-ahead gaze throughout the approach phase and while stepping over the obstacle. The dribble goggles prevented the participants from relying on peripheral visual feedback of the lower limbs and obstacle, while the doorframe provided an exteroceptive cue about the spatial location of the obstacle along the walkway (Rietdyk and Rhea 2006; Graci et al. 2010). During the OV condition, a researcher monitored the head position of the subject to ensure that they did not look down at their lower limbs or obstacle. Trials were discarded and repeated if this was observed.
Gaze behavior was recorded at 25 Hz using a head-mounted monocular eye tracking system (Dikablis; Ergoneers) during the FV condition only. Toe trajectory was recorded by infrared-emitting diodes (Optotrak; NDI, Waterloo, Canada) affixed bilaterally at the tips of the shoes. Two additional diodes were placed on the obstacle to mark its height and spatial location. Marker data were collected at a sampling frequency of 100 Hz. A common square-wave pulse was sent to both systems for off-line synchronization of the data.
Data Analysis
Joint position sense (JPS) was evaluated by calculating the absolute average difference between the actual and target position across six trials for each joint (Domingo and Lam 2014). An overall JPS score was then determined by summing the scores from both right and left hip and knee joints; smaller differences correspond to better JPS (Domingo and Lam 2014). Movement detection sense (MDS) was calculated for each joint by the sum of 1) joint excursion before the button was pressed, divided by 10 [the maximum joint excursion (10°) during a trial]; and 2) whether the subject correctly identified the movement direction (0 = correct response; 1 = incorrect response), giving a maximum possible score (worst performance) of 2 (Chisholm et al. 2016). Movement detection scores from the three different test speeds were summed, and then an overall MDS score was determined by summing the scores from both right and left hip and knee joints; lower scores indicate better MDS out of a maximum possible score of 24.
Gaze data were processed with D-Lab 3.5 software (Dikablis, Ergoneers) and custom-written routines in MATLAB (MathWorks, Natick, MA). Two parameters were extracted to define gaze behavior with respect to the steps before obstacle crossing: 1) number of saccades, defined as the number of pupil displacements towards the obstacle; and 2) fixation duration, defined as the duration of each saccade to the obstacle. Data from AB10 and SCI14 had to be excluded from this analysis due to equipment error.
Kinematic data were analyzed offline using MATLAB. Toe kinematic data were low-pass filtered with a fourth-order dual-pass Butterworth filter at 6 Hz. Data were divided into individual steps by defining heel contact and toe off times based on the horizontal (anterior-posterior) excursions of the toe marker. Individual trials were discarded if the subject made contact with the obstacle. If the subject made contact with the lead limb, both lead and trail toe clearance data were discarded for that trial. If the subject hit the obstacle or was unable to cross the obstacle with the trail limb, only trail toe clearance data for that trial were discarded. Trail toe clearance analysis could not be completed from SCI03, 08, 10, 11, 14, and 16 because they could never cross the obstacle with their trail limb. Trail horizontal distance analysis also could not be completed for SCI03 and trail toe clearance analysis could not be completed for AB06, 07, and 10 due to technical error.
Two parameters were extracted to define the kinematic trajectories of both the lead (L) and trail (T) limbs relative to the obstacle: 1) horizontal distance (HORZ-L, HORZ-T), defined as the distance between the toe marker and the front edge of the obstacle during the stance phase of the step over the obstacle, normalized to stride length; and 2) vertical toe clearance (VERT-L, VERT-T), defined as the vertical distance between the toe marker and the top edge of the obstacle at the point of crossing, normalized to obstacle height. To determine the effect of obstructed vision on toe kinematics, the change in horizontal distance (ΔHORZ-L, ΔHORZ-T) and vertical toe clearance (ΔVERT-L, ΔVERT-T) between conditions was defined by dividing the values measured during the OV condition by those from the FV condition. A value of 1 represents no change between conditions. Values greater than 1 represent a greater horizontal distance and higher toe clearance in the OV condition compared with the FV condition. Values less than 1 represent a shorter horizontal distance and lower toe clearance in the OV condition compared with the FV condition.
Statistical Analysis
SPSS v.20 statistics (IBM, Armonk, NY) was used to conduct all statistical analysis. The critical value for significance of all statistical tests was set at a α value of 0.05. Descriptive statistics were used to describe baseline demographic characteristics of the subject groups. Independent samples t-tests were used to confirm that the SCI and AB were comparable for age, height, and weight. Independent samples t-tests were also used to compare overall JPS and overall MDS between SCI and AB subjects.
Nonparametric statistical methods were used to analyze kinematic and gaze variables since these data did not satisfy the assumptions of normality (Shapiro-Wilk tests P < 0.05). The Mann-Whitney U-test was used to compare the number of saccades and fixation durations in the two steps before obstacle crossing between SCI and AB subjects during the FV condition with a Bonferroni-adjusted α value of 0.025. The change in toe kinematics from FV to OV conditions was compared between SCI and AB subjects using a Mann-Whitney U-test. Spearman's rho (ρ) was calculated to explore the relationships among overall JPS and overall MDS, gaze behavior, and the change in toe kinematics in the SCI group.
RESULTS
Subject Characteristics
Individual subject characteristics of the SCI group are summarized in Table 1.There were no significant differences between the SCI and AB group in terms of age (t28 = −0.96, P = 0.34; SCI, M: 52 yr, range: 32–63 yr; AB, M: 48 yr, range: 28–65 yr), height (t28 = 0.72, P = 0.47; SCI, M: 171 cm, range: 144–190 cm; AB, M: 174 cm, range: 152–188 cm), or weight (t28 = −0.94, P = 0.35; SCI, M: 79 kg, range: 58–115 kg; AB, M: 77 kg, range: 52–100 kg).
Table 1.
Subject characteristics
| ID | Age, yr | Sex | Level of Injury | Chronicity, yr | BBS | LEMS | 10MWT, m/s | MDS | JPS, ° | Usual Gait Aids |
|---|---|---|---|---|---|---|---|---|---|---|
| SCI01 | 62 | M | C4/5 | 2 | 49 | 35 | 0.83 | 1.04 | 9.17 | None |
| SCI03 | 37 | M | C7 | 21 | 30 | 24 | 0.57 | 2.18 | 10.34 | Walker |
| SCI04 | 58 | F | L4/5 | 34 | 54 | 42 | 0.76 | 3.52 | 16.01 | Walker |
| SCI05 | 58 | F | C5 | 3 | 56 | 42 | 0.95 | 2.76 | 13.89 | None |
| SCI08 | 32 | M | T5/6 | 12 | 10 | 12 | 0.17 | 4.31 | 14.57 | Walker +1 AFO |
| SCI09 | 63 | M | T11 | 1 | 55 | 49 | 0.90 | 2.23 | 6.6 | Walking sticks |
| SCI10 | 33 | M | C5/6 | 11 | 25 | 14 | 0.11 | 1.98 | 6.14 | Walker + AFO |
| SCI11 | 58 | F | T3 | 7 | 44 | 27 | 0.41 | 8.46 | 18.04 | Walker |
| SCI12 | 56 | F | C4/5 | 10 | 56 | 41 | 1.26 | 0.67 | 7.27 | None |
| SCI13 | 54 | M | L4/5 | 15 | 45 | 25 | 1.01 | 0.67 | 5.82 | Cane +2 AFOs |
| SCI14 | 57 | M | C4 | 4 | 24 | 30 | 0.05 | 7.00 | 20.99 | Walker |
| SCI15 | 44 | M | L1 | 8 | 54 | 30 | 0.53 | 0.88 | 6.73 | Forearm crutches +2 AFOs |
| SCI16 | 55 | M | C2-4 | 1 | 33 | 29 | 0.18 | 6.89 | 15.83 | Walker |
| SCI17 | 58 | M | C2-4 | 5 | 52 | 34 | 0.23 | 2.53 | 7.78 | 2 Forearm crutches |
| SCI18 | 57 | M | C3-5 | 39 | 54 | 37 | 0.77 | 3.66 | 13.13 | None |
| Mean | 52 | 11M, 4F | – | 12 | 43 | 31 | 0.58 | 3.25 | 11.49 | – |
M, male; F, female; BBS, Berg Balance Scale; LEMS, lower extremity motor score; 10MWT, 10-meter walk test; JPS, joint position sense; MDS, movement detection sense; AFO, ankle foot orthosis.
The subjects with SCI had significant impairments in overall JPS (M: 11.5°, range: 5.8–21°, t27 = −4.24, P < 0.01) and overall MDS scores (M: 3.3, range: 0.7–8.5, t28 = −3.79, P < 0.01) compared with the AB group (JPS, M: 5.94°, range: 3.79–8.57°; MDS, M: 0.79, range: 0.38–2.69).
The average obstacle heights used for the subjects with SCI was 11.3 cm with a range of 6.3 to 16.7 cm, and for the AB subjects the average obstacle height was 16.5 cm with a range of 10.7 to 19.7 cm. The subjects with SCI hit the obstacle more often than the AB subjects with both the lead and trail limbs. For the lead limb, there were seven incidences of obstacle hits in the SCI group (across 4 subjects: SCI03, 10, 11, and 14) and four hits in the AB group (across 4 subjects: AB05, 07, 08, and 10) in the FV condition. In the OV condition there were 40 obstacle hits in the SCI group (across 9 subjects: SCI03, 08, 10, 11, 13, 14, 16, 17, and 18) and 5 hits in the AB group (across 4 subjects: AB07, 09, 11, and 14) for the lead limb. For the trail limb, there were 51 obstacle hits in the SCI group (across 6 subjects: SCI03, 08, 10, 11, 14, and 16) and 4 hits in the AB group (across 4 subjects: AB07, 08, 10 and 14) in the FV condition. In the OV condition there were 65 obstacle in the SCI group (across 11 subjects: SCI03, 04, 05, 08, 10, 11, 13, 14, 15, 16, and 17) and 21 hits in the AB group (across 7 subjects: AB08, 09, 10, 11, 13, 14, and 17) for the trail limb. Visual inspection of the data indicated that an occurrence of obstacle hits did not appear to affect foot clearance patterns in subsequent obstacle crossings.
We were successful in scaling the height of the obstacle to lead limb motor ability, as evidenced by the fact that the SCI participants never needed to elevate their foot to their maximum step height when stepping over the obstacle. There was also no relationship between the LEMS and lead toe clearance height in either the FV (ρ = −0.28, P = 0.32) or OV (ρ = 0.34, P = 0.22) conditions.
Gaze Behavior
Figure 2 illustrates the average number of saccades and fixation duration in the two steps before crossing the obstacle. Subjects with SCI gazed towards the obstacle more often (U = 51.5, P = 0.03; Fig. 2A) and for a longer duration (U = 53.0, P = 0.03; Fig. 2B) compared with the AB controls in the second step before crossing the obstacle but just missed significance (Bonferroni-adjusted α = 0.025). Although the SCI group tended to have greater reliance on vision during obstacle crossing, there were no significant relationships between overall JPS or MDS and gaze behavior (Table 2).
Fig. 2.
Gaze behavior. Total number of saccades (A) and total fixation time (B) in the second (Step-2) and (Step-1) step before crossing the obstacle are plotted for both able-bodied (white boxes) and spinal cord injured (black boxes) subjects.
Table 2.
Spearman's correlation coefficients (ρ) between proprioceptive sense and gaze behavior
| Number of Saccades |
Fixation Time |
|||
|---|---|---|---|---|
| Step-2 | Step-1 | Step-2 | Step-1 | |
| Overall JPS | 0.26 (0.37) | −0.04 (0.90) | 0.02 (0.94) | −0.03 (0.93) |
| Overall MDS | 0.19 (0.88) | 0.04 (0.88) | 0.06 (0.85) | 0.06 (0.84) |
Values in parentheses indicate P values.
Toe Kinematic Trajectories
Figure 3 illustrates the median ΔHORZ-L and ΔHORZ-T, as well as ΔVERT-L and ΔVERT-T between FV and OV conditions in the AB and SCI groups. There were no significant differences between the SCI and AB groups for either ΔHORZ-L (U = 105.0, P = 0.76; Fig. 3A) or ΔHORZ-T (U = 71.5, P = 0.14; Fig. 3B). There were no significant relationships between overall JPS or MDS and ΔHORZ-L or ΔHORZ-T in the SCI group (Table 3).
Fig. 3.
Toe kinematics. Changes in lead (L) and trail (T) horizontal distance [ΔHORZ-L (A); ΔHORZ-T (B)] and vertical toe clearance [ΔVERT-L (C); ΔVERT-T (D)] between obstructed and full vision conditions are plotted for both able-bodied (white boxes) and spinal cord injured (black boxes) subjects. Horizontal line at 1 represents no change between conditions. Values greater than 1 represent a greater horizontal distance and higher toe clearance in the obstructed vision (OV) condition compared with the full vision (FV) condition. Values less than 1 represent a shorter horizontal distance and lower toe clearance in the OV condition compared with the FV condition.
Table 3.
Spearman's correlation coefficients (ρ) between proprioceptive sense and the changes in lead and trail horizontal distance and vertical toe clearance between obstructed and full vision conditions
| ΔHORZ-L | ΔHORZ-T | ΔVERT-L | ΔVERT-T | |
|---|---|---|---|---|
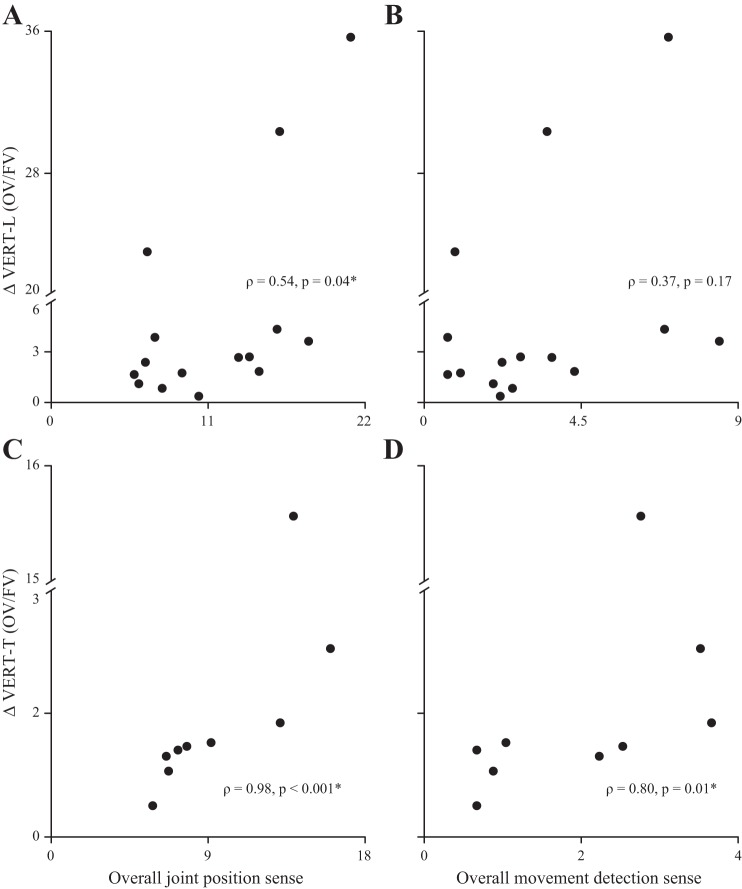
| Overall JPS | 0.15 (0.61) | 0.06 (0.85) | 0.54 (0.04)* | 0.97 (<0.001)* |
| Overall MDS | 0.14 (0.61) | 0.17 (0.56) | 0.37 (0.17) | 0.80 (0.01)* |
Values in parentheses indicate P values. L, lead; T, trail; ΔHORZ-L, ΔHORZ-T, change in lead and trail horizontal distance; ΔVERT-L, ΔVERT-T, change in lead and trail vertical toe clearance.
Significant values.
ΔVERT-L was significantly higher in the SCI compared with the AB group (U = 54.0, P = 0.02; Fig. 3C), but there was no significant difference in ΔVERT-T between the SCI and AB group (U = 47.0, P = 0.62; Fig. 3D). In the SCI group, overall JPS was correlated to ΔVERT-L and ΔVERT-T, and overall MDS was correlated to ΔVERT-T (Table 3 and Fig. 4).
Fig. 4.
Relationship between proprioceptive sense and the changes in vertical toe clearance between conditions. Changes in lead (L) and trail (T) vertical toe clearance (ΔVERT-L; A and B; ΔVERT-T, C and D) between obstructed and full vision conditions are plotted against overall joint position sense (A and C) and overall movement detection sense (B and D) for subjects with SCI.
DISCUSSION
In this study, we sought to analyze the impact of combined, bilateral hip and knee proprioceptive impairments on obstacle crossing strategies in ambulatory individuals with SCI under full and obstructed vision conditions. During full vision conditions, we found that subjects with SCI relied more heavily on vision; however, this increased reliance on vision was not related to proprioceptive sense. The change in foot placement in obstructed compared with full vision conditions was not different between groups and was not related to overall JPS nor overall MDS in the SCI group, contrary to our initial hypothesis. This suggests that visual exproprioception of head position relative to the obstacle (via the doorframe), independent of combined, bilateral hip and knee proprioceptive sense, is enough to drive appropriate foot placement. For foot clearance, the change in the lead limb in obstructed compared with full vision conditions was significantly higher in the SCI compared with the AB group, but the change in trail limb foot clearance between conditions was not different between groups. In agreement with our hypothesis, impairments in combined, bilateral hip and knee proprioceptive sense in the SCI group were related to changes in lead and trail limb foot clearance between full and obstructed vision conditions.
Methodological Considerations
We were only able to measure hip and knee, but not ankle proprioceptive sense, using the Lokomat. Although the ankle contributes to foot trajectory during walking, the predominant contribution appears to originate at the hip and knee, especially for locomotor modifications to step over obstacles (Winter 1992; Patla and Prentice 1995; McFadyen and Carnahan 1997). Previous research has shown that swing limb elevation during obstacle crossing is mainly achieved by hip translational energy of the stance limb and knee rotational energy of the swing limb (Winter 1992; Patla and Prentice 1995; McFadyen and Carnahan 1997). This is in accordance with sensitivity analysis, which showed that toe trajectory height during walking is most influenced by the stance hip and swing knee joint angle (Winter 1992). Angular changes of as little as 0.86° in the stance hip or 1.25° in the swing knee significantly affect foot clearance (Winter 1992).
Although previous studies have shown that our measure of proprioceptive sense is reliable and valid in people with SCI (Domingo and Lam 2014; Chisholm et al. 2016), the passive nature of the task precludes feedback from fusimotor drive or an efference copy. This could have reduced the sensitivity of our proprioceptive measure and possibly contributed to our inability to detect significant relationships between proprioceptive sense (measured passively) and measures that were determined during a dynamic task (stepping over an obstacle). Neuronal recordings encoding proprioceptive information from the primary sensory cortex and dorsal cerebellar spinal tracts show that neurons firing during both passive and active movements display similar directional tuning but active movements lead to greater firing rates (Bosco et al. 2006; London and Miller 2013). This is also consistent with observations in human subjects that joint position sense following an active movement is correlated to joint position sense during passive movement (Djupsjöbacka and Domkin 2005). However, recordings of somatosensory area 2 neurons in macaques have also shown that there are also neurons that only fire during active movements (London and Miller 2013). These changes in neuronal activity reflect the additional feedback available regarding limb position and movement through fusimotor drive, from the muscle spindles, and an efference copy, a copy of the motor commands (Gandevia et al. 2006), during active movements. This additional feedback allows for greater accuracy/sensitivity when determining joint position sense (Paillard and Brouchon 1968; Laufer et al. 2001; Farrer et al. 2003; Fuentes and Bastian 2010) and movement detection sense (Colebatch and McCloskey 1987; Gandevia et al. 1992).
We provided the same walking aid for the SCI group (i.e., parallel bars) to minimize the effects of different walking aids. However, we were unable to quantify the amount of force applied to the parallel bars by each subject, which could have altered load-related proprioceptive feedback from the lower limb (Dietz 1998; Dietz and Duysens 2000; Bosco et al. 2006; Proske and Gandevia 2012), and therefore, we were unable to account for potential variations in load-related proprioceptive feedback from the lower limb during the obstacle-crossing task.
Dynamic balance control is also an important component of obstacle crossing; however, we feel it is unlikely that deficits in balance significantly altered the results of this study. The majority of our participants with SCI were at low to medium risk for experience a fall, as indicated by their Berg Balance scores (Table 1). Moreover, all of the subjects with SCI in our study used the parallel bars during the obstacle-crossing task. Previous studies have shown that feedback from even light touch can improve balance in individuals with Parkinson's disease (Rabin et al. 2013), multiple sclerosis (Kanekar et al. 2013), and stroke (Boonsinsukh et al. 2009). Thus the use of parallel bars by the subjects with SCI likely minimized any changes in obstacle crossing behavior due to balance deficits.
Ambulatory Individuals with SCI Rely More Heavily on Vision During Obstacle Crossing
During obstacle crossing, vision is used in a feedforward manner to plan the locomotor trajectory (Patla and Greig 2006), providing critical information for identifying potential hazards (i.e., obstacles) in the environment. Humans generally fixate on the obstacle two steps before crossing and rarely glance towards the obstacle while stepping over it (Patla and Vickers 1997). This type of gaze behavior was consistently observed in the AB subjects in our study. In contrast, the subjects with SCI relied more heavily on gaze in the second step before obstacle crossing, glancing more often and for longer durations at the obstacle compared with the AB group. However, contrary to our initial hypothesis, an increased reliance on vision was not linked to the degree of hip and knee proprioceptive impairment. This was surprising given prior evidence from studies of deafferented individuals. These studies demonstrated that the absence of proprioceptive sense results in a greater reliance on continuous online visual regulation of the limbs to complete a task (Rothwell et al. 1982; Sanes et al. 1984; Sainburg et al. 1993, 1995), indicating that the ability to extract relevant visual information and maintain a spatial representation of the environment in a feed-forward manner is strongly dependent on an intact proprioceptive system. Previous literature has also shown that elderly individuals at high risk of falling tend to have a greater dependency on vision (Chapman and Hollands 2006; Greany and Di Fabio 2008), which may account for their increased likelihood of experiencing a fall (Greany and Di Fabio 2008). For example, when high- and low-risk fallers are asked to traverse through an environment stepping on specific targets, participants categorized as high-risk fallers fixate on the targets for significantly longer than low-risk fallers (Greany and Di Fabio 2008). Other work has also shown that there are many clinical features that distinguish high-risk from low-risk fallers. For example, high-risk fallers tend to have lower self-efficacy in activities of daily living (Lajoie et al. 1996; Schepens et al. 2010), lower limb muscle weakness (Rubenstein 2006; Kwan et al. 2011; Benavent-Caballer et al. 2015), lower functional balance (Lajoie and Gallagher 2004; Rubenstein 2006; Kwan et al. 2011; Benavent-Caballer et al. 2015), and increased levels of pain (Stubbs et al. 2014) Thus it is plausible that the increased reliance on vision seen in the subjects with SCI in our study could be attributed to multiple clinical features.
Combined, Bilateral Hip, and Knee Proprioceptive Sense Is Related to Obstacle Clearance Height
Our findings demonstrate that those individuals who stepped higher with their lead and trail limb over the obstacle when vision was obstructed were those with greater impairments in combined, bilateral hip and knee proprioceptive sense. These findings are consistent with Takeoka and colleagues (2014) who have shown that mutant mice lacking muscle spindles, the primary organ for proprioceptive feedback, show difficulties in safely completing a skilled locomotor task (Takeoka et al. 2014). For example, when walking across a horizontal ladder, wild type mice (who have no impairments) can complete this task with ease and only slipped on or missed a ladder rung ∼3% of the time, whereas mutant mice show severe deficits in paw placement, slipping on or missing a rung ∼67% of the time.
Proprioception is a critical sensory modality for accurately controlling end-point trajectory by regulating both intra- and interlimb coordination during multijoint movements. In humans, complete loss of proprioception below the neck, as seen in deafferented individuals, causes severe impairments in intralimb coordination. These individuals are unable to appropriately control for the intersegmental dynamics present during multijoint movements. In fact, as the requirement to control for intersegmental dynamics increases, their movements become more impaired (Sainburg et al. 1995). Proprioceptive sense also affects interlimb coordination. During swimming, wild-type mice with no impairments display well coordinated, bilateral out of phase hindlimb movements, whereas mutant mice lacking muscle spindles display uncoordinated, in-phase hindlimb movements (Takeoka et al. 2014).
During walking, the end-point trajectory of the limb is under the control of a seven-segment chain consisting of the stance limb, pelvis, and the swing limb (Winter 1992). Small angular changes at any segment, which can be affected by impairments in intra- and/or interlimb coordination, can lead to significant changes in end-point trajectory (i.e., toe clearance height) (Winter 1992). Thus it is plausible that the higher stepping strategy adopted by the subjects with SCI when vision was obstructed could be due proprioceptive-related impairments in the control of intra- and/or interlimb coordination and not just a safety strategy.
Given the intricate coordination of the lead and trail limbs, it is plausible that lead limb foot clearance over the obstacle influenced trail limb foot clearance. Research in both cats (McVea and Pearson 2007) and humans (Lajoie et al. 2012) has shown that movement of the lead/forelimbs over an obstacle establishes motor memories that can be maintained for minutes (tested up to 2 min for humans and 10 min in cats). These motor memories of the lead/forelimbs crossing the obstacle are subsequently used to guide trail/hindlimb foot clearance. Active movement of the lead/forelimbs over the obstacle or vision of the lead limb going over the obstacle is necessary to establish these long-lasting motor memories. Neurobiological research in cats shows that long-lasting memories are mediated in area 5 of the posterior parietal cortex, a sensory integration area, where visual and proprioceptive inputs may communicate (Lajoie et al. 2010).
During obstacle crossing, a person does not need to remember the location of an obstacle for long periods of time, but the mechanisms for controlling trail limb foot clearance over an obstacle are likely similar. As previously mentioned, proprioception plays a role in the maintenance and production of the motor plan, and it is possible that proprioceptive sense also plays a role in updating the spatial representation of the body relative to the obstacle (i.e., environment) to guide trail limb foot clearance over the obstacle. Although this is an interesting concept, we did not design our study to specifically test the control of trail limb clearance. Future studies could be designed to understand how afferent signals from the lead limb influence trail limb foot clearance in situations where there is asymmetrical involvement of motor and sensory systems, as in many individuals with SCI as well as poststroke.
Clinical Implications
In this study we have shown that ambulatory individuals with SCI, regardless of their motor impairments, can have deficits in proprioceptive sense and that these deficits could affect locomotor strategies underlying skilled locomotion. Although the results of this study may be limited to those individuals with partial paralysis who can walk over-ground, consideration of the factors contributing to skilled walking recovery are likely to be most relevant to this subset of individuals with SCI who have already recovered basic ambulatory function. It is known that proprioception plays a vital role in coordinating multijoint movements and walking (especially skilled walking) requires intricate coordination across multiple joints. Most gait rehabilitation studies to date have relied on simple measures of walking speed or distance, such as the 10-m walk test or 6-min walk test (Wessels et al. 2010; Morawietz and Moffat 2013), which are primarily related to motor capacity, i.e., strength (Kim and Eng 2003; Kim et al. 2004). Future studies are required to gain a deeper understanding of the multiple factors contributing to the recovery of functional skilled walking in people with SCI, including the effects of proprioceptive impairment as well as other clinical features that could impact obstacle-crossing strategies (e.g., balance, self-efficacy). Lastly, our ability to quantify combined, bilateral hip and knee proprioceptive sense in people with SCI may make this population a useful model to further understand the role of proprioception in locomotor control and recovery.
Conclusions
In this study, we showed that individuals with SCI with greater impairments in hip and knee joint position sense and movement detection sense had greater impairments in their control of toe trajectory during obstacle crossing with obstructed vision. We also found that during full vision conditions individuals with SCI rely more heavily on vision in the second step before crossing an obstacle, but surprisingly, this increased reliance on vision was not related to combined, bilateral hip and knee proprioceptive sense. These findings emphasize the importance of taking into account both motor and sensory impairments when evaluating movement outcomes and designing rehabilitation strategies for people with SCI.
GRANTS
This work was supported by the International Foundation for Research in Paraplegia. T. Lam was supported by a Canadian Institutes of Health Research New Investigator Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.N.M. and T.L. conception and design of research; R.N.M. and R.C. performed experiments; R.N.M. and R.C. analyzed data; R.N.M. and T.L. interpreted results of experiments; R.N.M. prepared figures; R.N.M. drafted manuscript; R.N.M. and T.L. edited and revised manuscript; R.N.M., R.C., and T.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants for volunteering time and effort to this study, Dr. Bradford J. McFadyen for valuable suggestions on our experimental set-up, Franco Chan, George Shipley, Jerry Hurn, and Ron Bailey for technical contributions, Andrea Lynn for her contributions to data collection and analysis, and Raed Alamro for conducting the clinical assessments. We are also grateful to Dr. Janice J. Eng and Dr. J. Timothy Inglis for valuable input on this project.
REFERENCES
- Bard C, Fleury M, Teasdale N, Paillard J, Nougier V. Contribution of proprioception for calibrating and updating the motor space. Can J Physiol Pharmacol 73: 246–254, 1995. [DOI] [PubMed] [Google Scholar]
- Benavent-Caballer V, Sendín-Magdalena A, Lisón JF, Rosado-Calatayud P, Amer-Cuenca JJ, Salvador-Coloma P, Segura-Ortí E. Physical factors underlying the Timed “Up and Go” test in older adults. Geriatr Nurs 37: 122–127, 2016. [DOI] [PubMed] [Google Scholar]
- Blouin J, Bard C, Teasdale N, Paillard J, Fleury M, Forget R, Lamarre Y. Reference systems for coding spatial information in normal subjects and a deafferented patient. Exp Brain Res 93: 324–331, 1993. [DOI] [PubMed] [Google Scholar]
- Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther 88: 559–566, 2008. [DOI] [PubMed] [Google Scholar]
- Boonsinsukh R, Panichareon L, Phansuwan-Pujito P. Light touch cue through a cane improves pelvic stability during walking in stroke. Arch Phys Med Rehabil 90: 919–926, 2009. [DOI] [PubMed] [Google Scholar]
- Bosco G, Eian J, Poppele RE. Phase-specific sensory representations in spinocerebellar activity during stepping: evidence for a hybrid kinematic/kinetic framework. Exp Brain Res 175: 83–96, 2006. [DOI] [PubMed] [Google Scholar]
- Chapman GJ, Hollands MA. Evidence for a link between changes to gaze behavior and risk of falling in older adults during adaptive locomotion. Gait Posture 24: 288–294, 2006. [DOI] [PubMed] [Google Scholar]
- Chisholm AE, Domingo A, Jeyasurya J, Lam T. Quantification of lower extremity kinesthesia deficits using a robotic exoskeleton in people with a spinal cord injury. Neurorehabil Neural Repair 30: 199–208, 2016. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, McCloskey DI. Maintenance of constant arm position or force: reflex and volitional components in man. J Physiol 386: 247–261, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture 11: 102–110, 2000. [DOI] [PubMed] [Google Scholar]
- Dietz V. Evidence for a load receptor contribution to the control of posture and locomotion. Neurosci Biobehav Rev 22: 495–499, 1998. [DOI] [PubMed] [Google Scholar]
- Djupsjöbacka M, Domkin D. Correlation analysis of proprioceptive acuity in ipsilateral position-matching and velocity-discrimination. Somatosens Mot Res 22: 85–93, 2005. [DOI] [PubMed] [Google Scholar]
- Domingo A, Lam T. Reliability and validity of using the Lokomat to assess lower limb joint position sense in people with incomplete spinal cord injury. J Neuroeng Rehabil 11: 167, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer C, Franck N, Paillard J, Jeannerod M. The role of proprioception in action recognition. Consciousness Cogn 12: 609–619, 2003. [DOI] [PubMed] [Google Scholar]
- Fuentes CT, Bastian AJ. Where is your arm? Variations in proprioception across space and tasks. J Neurophysiol 103: 164–171, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI, Burke D. Kinaesthetic signals and muscle contraction. Trends Neurosci 15: 62–65, 1992. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Smith JL, Crawford M, Proske U, Taylor JL. Motor commands contribute to human position sense. J Physiol 571: 703–710, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JJ. Visually controlled locomotion and visual orientation in animals. Br J Psychol 49: 182–194, 1958. [DOI] [PubMed] [Google Scholar]
- Graci V, Elliott DB, Buckley JG. Utility of peripheral visual cues in planning and controlling adaptive gait. Optom Vis Sci 87: 21–27, 2010. [DOI] [PubMed] [Google Scholar]
- Greany JF, Di Fabio RP. Saccade to stepping delays in elders at high risk for falling. Aging Clin Exp Res 20: 428–433, 2008. [DOI] [PubMed] [Google Scholar]
- Gutierrez EM, Helber MD, Dealva D, Ashton-Miller JA, Richardson JK. Mild diabetic neuropathy affects ankle motor function. Clin Biomech (Bristol, Avon) 16: 522–528, 2001. [DOI] [PubMed] [Google Scholar]
- Jackson AB, Carnel CT, Ditunno JF, Read MS, Boninger ML, Schmeler MR, Williams SR, Donovan WH; Gait and Ambulation Subcommittee. Outcome measures for gait and ambulation in the spinal cord injury population. J Spinal Cord Med 31: 487–499, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekar N, Lee YJ, Aruin AS. Effect of light finger touch in balance control of individuals with multiple sclerosis. Gait Posture 38: 643–647, 2013. [DOI] [PubMed] [Google Scholar]
- Kim CM, Eng JJ, Whittaker MW. Level walking and ambulatory capacity in persons with incomplete spinal cord injury: relationship with muscle strength. Spinal Cord 42: 156–162, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CM, Eng JJ. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys Ther 83: 49–57, 2003. [PubMed] [Google Scholar]
- Kwan MM, Lin SI, Chen CH, Close JC, Lord SR. Sensorimotor function, balance abilities and pain influence Timed Up and Go performance in older community-living people. Aging Clin Exp Res 23: 196–201, 2011. [DOI] [PubMed] [Google Scholar]
- Ladouceur M, Barbeau H, McFadyen BJ. Kinematic adaptations of spinal cord-injured subjects during obstructed walking. Neurorehabil Neural Repair 17: 25–31, 2003. [DOI] [PubMed] [Google Scholar]
- Lajoie K, Andujar JE, Pearson K, Drew T. Neurons in area 5 of the posterior parietal cortex in the cat contribute to interlimb coordination during visually guided locomotion: a role in working memory. J Neurophysiol 103: 2234–2254, 2010. [DOI] [PubMed] [Google Scholar]
- Lajoie K, Bloomfield LW, Nelson FJ, Suh JJ, Marigold DS. The contribution of vision, proprioception, and efference copy in storing a neural representation for guiding trail leg trajectory over an obstacle. J Neurophysiol 107: 2283–2293, 2012. [DOI] [PubMed] [Google Scholar]
- Lajoie Y, Gallagher SP. Predicting falls within the elderly community: comparison of postural sway, reaction time, the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers. Arch Gerontol Geriatr 38: 11–26, 2004. [DOI] [PubMed] [Google Scholar]
- Lajoie Y, Teasdale N, Cole JD, Burnett M, Bard C, Fleury M, Forget R, Paillard J, Lamarre Y. Gait of a deafferented subject without large myelinated sensory fibers below the neck. Neurology 47: 109–115, 1996. [DOI] [PubMed] [Google Scholar]
- Laufer Y, Hocherman S, Dickstein R. Accuracy of reproducing hand position when using active compared with passive movement. Physiother Res Int 6: 65–75, 2001. [DOI] [PubMed] [Google Scholar]
- Liu MW, Hsu WC, Lu TW, Chen HL, Liu HC. Patients with type II diabetes mellitus display reduced toe-obstacle clearance with altered gait patterns during obstacle-crossing. Gait Posture 31: 93–99, 2010. [DOI] [PubMed] [Google Scholar]
- London BM, Miller LE. Responses of somatosensory area 2 neurons to actively and passively generated limb movements. J Neurophysiol 109: 1505–1513, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigold DS, Andujar JÉ, Lajoie K, Drew T. Motor Planning of Locomotor Adaptations on the Basis of Vision: the Role of the Posterior Parietal Cortex (1st ed). New York: Elsevier, 2011. [DOI] [PubMed] [Google Scholar]
- Martens KA, Almeida QJ. Dissociating between sensory and perceptual deficits in PD: More than simply a motor deficit. Mov Disord 27: 387–392, 2011. [DOI] [PubMed] [Google Scholar]
- Maynard FM, Bracken MB, Creasey G, Ditunno JF, Donovan WH, Ducker TB, Garber SL, Marino RJ, Stover SL, Tator CH, Waters RL, Wilberger JE, Young W. International standards for neurological and functional classification of spinal cord injury. Am Spinal Injury Assoc Spinal Cord 35: 266–274, 1997. [DOI] [PubMed] [Google Scholar]
- McFadyen BJ, Carnahan H. Anticipatory locomotor adjustments for accommodating versus avoiding level changes in humans. Exp Brain Res 114: 500–506, 1997. [DOI] [PubMed] [Google Scholar]
- McVea DA, Pearson KG. Stepping of the forelegs over obstacles establishes long-lasting memories in cats. Curr Biol 17: R621–R623, 2007. [DOI] [PubMed] [Google Scholar]
- Mohagheghi AA, Moraes R, Patla AE. The effects of distant and on-line visual information on the control of approach phase and step over an obstacle during locomotion. Exp Brain Res 155: 459–468, 2004. [DOI] [PubMed] [Google Scholar]
- Morawietz C, Moffat F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil 94: 2297–2308, 2013. [DOI] [PubMed] [Google Scholar]
- Morton SM, Dordevic GS, Bastian AJ. Cerebellar damage produces context-dependent deficits in control of leg dynamics during obstacle avoidance. Exp Brain Res 156: 149–163, 2004. [DOI] [PubMed] [Google Scholar]
- Paillard J, Brouchon M. Active and passive movements in the calibration of position sense. In: The Neuropsychology of Spatially Oriented Behavior. Homewood, IL: Dorsey, 1968, p. 37–55. [Google Scholar]
- Patla AE, Ellion DB, Flanagan J, Rietdyk S. Effects of age-related maculopathy on strategies for going over obstacles of different heights and contrast. Gait Posture 3: 106, 1995. [Google Scholar]
- Patla AE, Greig M. Any way you look at it, successful obstacle negotiation needs visually guided on-line foot placement regulation during the approach phase. Neurosci Lett 397: 110–114, 2006. [DOI] [PubMed] [Google Scholar]
- Patla AE, Prentice SD. The role of active forces and intersegmental dynamics in the control of limb trajectory over obstacles during locomotion in humans. Exp Brain Res 106: 499–504, 1995. [DOI] [PubMed] [Google Scholar]
- Patla AE, Vickers JN. Where and when do we look as we approach and step over an obstacle in the travel path? Neuroreport 8: 3661–3665, 1997. [DOI] [PubMed] [Google Scholar]
- Patla AE. Understanding the roles of vision in the control of human locomotion. Gait Posture 5: 54–69, 1997. [Google Scholar]
- Patla AE. How is human gait controlled by vision. Ecol Psychol 10: 287–302, 1998. [Google Scholar]
- Pramodhyakul W, Wattanapan P, Siritaratiwat W, Eungpinichpong W, Amatachaya S. Immediate effects of obstacle crossing training in independent ambulatory patients with spinal cord injury. Spinal Cord 51: 379–383, 2013. [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92: 1651–1697, 2012. [DOI] [PubMed] [Google Scholar]
- Rabin E, Chen J, Muratori L, DiFrancisco-Donoghue J, Werner WG. Haptic feedback from manual contact improves balance control in people with Parkinson's disease. Gait Posture 38: 373–379, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea CK, Rietdyk S. Visual exteroceptive information provided during obstacle crossing did not modify the lower limb trajectory. Neurosci Lett 418: 60–65, 2007. [DOI] [PubMed] [Google Scholar]
- Rietdyk S, Rhea CK. Control of adaptive locomotion: effect of visual obstruction and visual cues in the environment. Exp Brain Res 169: 272–278, 2006. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Day BL, Obeso JA, Thomas PK, Marsden CD. Manual motor performance in a deafferented man. Brain 105: 515–542, 1982. [DOI] [PubMed] [Google Scholar]
- Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing 35: ii37–ii41, 2006. [DOI] [PubMed] [Google Scholar]
- Said CM, Goldie PA, Patla AE, Sparrow WA, Martin KE. Obstacle crossing in subjects with stroke. Arch Phys Med Rehabil 80: 1054–1059, 1999. [DOI] [PubMed] [Google Scholar]
- Said CM, Goldie PA, Patla AE, Sparrow WA. Effect of stroke on step characteristics of obstacle crossing. Arch Phys Med Rehabil 82: 1712–1719, 2001. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Ghilardi MF, Poizner H, Ghez C. Control of limb dynamics in normal subjects and patients without proprioception. J Neurophysiol 73: 820–835, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Poizner H, Ghez C. Loss of proprioception produces deficits in interjoint coordination. J Neurophysiol 70: 2136–2147, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Mauritz KH, Evarts EV, Dalakas MC, Chu A. Motor deficits in patients with large-fiber sensory neuropathy. Proc Natl Acad Sci USA 81: 979–982, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens S, Goldberg A, Wallace M. The short version of the Activities-specific Balance Confidence (ABC) scale: Its validity, reliability, and relationship to balance impairment and falls in older adults. Arch Gerontol Geriatr 51: 9–12, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sober SJ, Sabes PN. Multisensory integration during motor planning. J Neurosci 23: 6982–6992, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sober SJ, Sabes PN. Flexible strategies for sensory integration during motor planning. Nat Neurosci 8: 490–497, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs B, Schofield P, Binnekade T, Patchay S, Sepehry A, Eggermont L. Pain is associated with recurrent falls in community-dwelling older adults: evidence from a systematic review and meta-analysis. Pain Med 15: 1115–1128, 2014. [DOI] [PubMed] [Google Scholar]
- Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell 159: 1626–1639, 2014. [DOI] [PubMed] [Google Scholar]
- Timmis MA, Pardhan S. Patients with central visual field loss adopt a cautious gait strategy during tasks that present a high risk of falling. Invest Ophthalmol Vis Sci 53: 4120–4129, 2012. [DOI] [PubMed] [Google Scholar]
- van Hedel HJ, Wirth B, Dietz V. Limits of locomotor ability in subjects with a spinal cord injury. Spinal Cord 43: 593–603, 2005. [DOI] [PubMed] [Google Scholar]
- Wessels M, Lucas C, Eriks I, de Groot S. Body weight-supported gait training for restoration of walking in people with an incomplete spinal cord injury: a systematic review. J Rehabil Med 42: 513–519, 2010. [DOI] [PubMed] [Google Scholar]
- Winter DA. Foot trajectory in human gait: a precise and multifactorial motor control task. Phys Ther 72: 45–53, 1992. [DOI] [PubMed] [Google Scholar]
- Wirz M, Muller R, Bastiaenen C. Falls in persons with spinal cord injury: validity and reliability of the berg balance scale. Neurorehabil Neural Repair 24: 70–77, 2010. [DOI] [PubMed] [Google Scholar]