In the lamprey following spinal lesion-mediated interruption of long axonal projections of reticulospinal (RS) neurons, sensory stimulation still elicited relatively normal locomotor muscle burst activity, but with some coordination deficits. Computer models incorporating the spinal lesions could mimic many aspects of the experimental results. Thus, after disruption of long-axon projections from RS neurons in the lamprey, descending propriospinal (PS) neurons appear to be a viable compensatory mechanism for indirect activation of spinal locomotor networks.
Keywords: spinal cord injury, reticulospinal, locomotion, CPGs, coordination, swimming
Abstract
Following spinal cord injury (SCI) in the lamprey, there is virtually complete recovery of locomotion within a few weeks, but interestingly, axonal regeneration of reticulospinal (RS) neurons is mostly limited to short distances caudal to the injury site. To explain this situation, we hypothesize that descending propriospinal (PS) neurons relay descending drive from RS neurons to indirectly activate spinal central pattern generators (CPGs). In the present study, the contributions of PS neurons to locomotor recovery were tested in the lamprey following SCI. First, long RS neuron projections were interrupted by staggered spinal hemitransections on the right side at 10% body length (BL; normalized from the tip of the oral hood) and on the left side at 30% BL. For acute recovery conditions (≤1 wk) and before axonal regeneration, swimming muscle burst activity was relatively normal, but with some deficits in coordination. Second, lampreys received two spaced complete spinal transections, one at 10% BL and one at 30% BL, to interrupt long-axon RS neuron projections. At short recovery times (3–5 wk), RS and PS neurons will have regenerated their axons for short distances and potentially established a polysynaptic descending command pathway. At these short recovery times, swimming muscle burst activity had only minor coordination deficits. A computer model that incorporated either of the two spinal lesions could mimic many aspects of the experimental data. In conclusion, descending PS neurons are a viable mechanism for indirect activation of spinal locomotor CPGs, although there can be coordination deficits of locomotor activity.
NEW & NOTEWORTHY In the lamprey following spinal lesion-mediated interruption of long axonal projections of reticulospinal (RS) neurons, sensory stimulation still elicited relatively normal locomotor muscle burst activity, but with some coordination deficits. Computer models incorporating the spinal lesions could mimic many aspects of the experimental results. Thus, after disruption of long-axon projections from RS neurons in the lamprey, descending propriospinal (PS) neurons appear to be a viable compensatory mechanism for indirect activation of spinal locomotor networks.
in vertebrate animals, reticulospinal (RS) neurons in the brain project descending axons to the spinal cord to activate central pattern generators (CPGs) and initiate locomotor behavior. These RS neurons have both short and long descending axons and directly activate the CPG networks distributed along the entire spinal cord (reviewed in Jordan 1986; McClellan 1996; Orlovsky et al. 1998). Once activated, the spinal CPGs generate the coordinated pattern of rhythmic motor output that causes muscles to contract in the proper temporal sequence to produce locomotion.
Following severe rostral spinal cord injury (SCI), the descending axons of RS neurons are disrupted, resulting in loss of locomotor function caudal to the injury site. In higher vertebrates, including birds and mammals, the injured descending axons of RS neurons normally do not regenerate, and as a result paralysis and loss of other functions below a complete SCI usually are permanent (reviewed in Schwab and Bartholdi 1996). Certain experimental manipulations can induce a very modest degree of short-distance axonal regeneration following complete SCI (reviewed in Bradbury and McMahon 2006; David and Lacroix 2003), but in general these manipulations result in little or no functional recovery.
Propriospinal (PS) neurons, which are distributed throughout the spinal cord and project outside their own segment, contribute in a number of ways to motor functions (reviewed in Conta and Stelzner 2009; Flynn et al. 2011; Jordan and Schmidt 2002; Orlovsky et al. 1998), including coordinating locomotor activity generated by different regions of the spinal cord (Juvin et al. 2005; Reed et al. 2006) and relaying descending motor commands to more caudal areas of the spinal cord (Alstermark et al. 1987; Shik 1983, 1997). In addition, PS neurons are involved in transmitting motor feedback signals from the spinal cord to the brain (Tracey 2003) as well as conducting sensory feedback (proprioceptive) information between different regions of the spinal cord (Skinner et al. 1980; Tracey 2003).
Descending PS neurons have been shown to activate spinal motor and locomotor networks in normal uninjured animals as well as in animals following SCI (reviewed in Flynn et al. 2011; Jordan and Schmidt 2002; Schomburg 1990; Shik 1983). First, in cats and turtles, sensory stimulation of the body or stimulation of the rostral spinal cord activates descending PS neurons, which in turn activate CPGs in the lumbar cord for rhythmic scratching or wiping movements (Berkinblit et al. 1978; Berkowitz and Stein 1994). In several vertebrates, stimulation of the brain or spinal cord elicits locomotor activity, in part via activation of a descending PS system (Arshavsky et al. 1985; Cowley et al. 2008, 2015; Gerasimenko et al. 2002, 2009; Kazennikov et al. 1979, 1983a, 1983b; Yakovenko et al. 2007). In addition, brain-initiated lumbar locomotor activity can be blocked when synaptic transmission is abolished at mid-spinal levels (Cowley et al. 2010; Zaporozhets et al. 2006, 2011). In cats, rats, and turtle, stimulation of the spinal dorsolateral funiculus (DLF), which contains a descending PS system, can initiate spinal locomotor activity (Iwahara et al. 1991a, 1991b; Kazennikov et al. 1985; Lennard and Stein 1977; Stein, 1978; Yamaguchi 1986, 1987). In some cases, blocking activity in, or acute lesions of, the DLF can abolish or compromise brain-initiated locomotor activity (Jiang and Drew 1996; Loy et al. 2002; Muir et al. 2007; Noga et al. 1991). Finally, excitotoxic lesions of upper lumbar gray matter result in significant locomotor deficits in rats even though the major descending pathways remain intact (Magnuson et al. 1999).
Second, in rodents, following staggered right-left spinal hemitransections (HTs) that interrupt long-axon descending projections from the brain, stimulation in the brain stem can still elicit hindlimb locomotor activity in the lumbar cord below the HTs (Cowley et al. 2008; also see Courtine et al. 2008; Stelzner and Cullen 1991; however, see Lassek and Anderson 1961 for contradictory results from monkeys). Following single spinal HTs in rats, RS neurons (Ballermann and Fouad 2006) or PS neurons (Filli et al. 2014) can sprout around the injury site to mediate recovery of locomotor function below the lesion. Finally, although acute lesions of the ventrolateral funiculus interrupt reticulospinal pathways and usually block initiation of locomotion (Eidelberg et al. 1981; Steeves and Jordan 1980; Windle et al. 1958), improvement in locomotor function can occur at longer recovery times (Brustein and Rossignol 1998; Webb and Muir 2004), suggesting that other descending systems, including perhaps PS neurons, compensate and are sufficient to transmit descending drive to spinal CPGs.
In lower vertebrates, such as lampreys, fish, and certain amphibians, the axons of RS neurons display robust spontaneous regeneration following complete SCI and make functional synapses in the spinal cord caudal to the lesion (reviewed in Clarke and Ferretti 1998; McClellan 1994b, 1998, 2013; Shifman et al. 2007). For example, following rostral SCI in the lamprey, swimming behavior and muscle burst activity recover almost completely within about 8 wk (reviewed in McClellan 1994b). However, at intermediate recovery times following rostral SCI (∼8–12 wk), soon after restoration of locomotor function, axonal regeneration of RS neurons is incomplete and restricted mostly to short distances just below the injury site (reviewed in McClellan 2013). Even at relatively long recovery times (24–32 wk), when a few RS neurons have projected into the caudal spinal cord, axonal regeneration of RS neurons typically is limited to moderately short distances past the injury site. In contrast, in normal lampreys, about 50–60% of RS neurons have relatively long axons that project into the caudal half of the spinal cord (Davis and McClellan 1994; Zhang et al. 2002). In addition, results from in vitro brain-spinal cord preparations suggest that RS neurons can directly activate locomotor CPG networks at each level of the spinal cord (Fig. 1A) (McClellan 1994a).
Fig. 1.
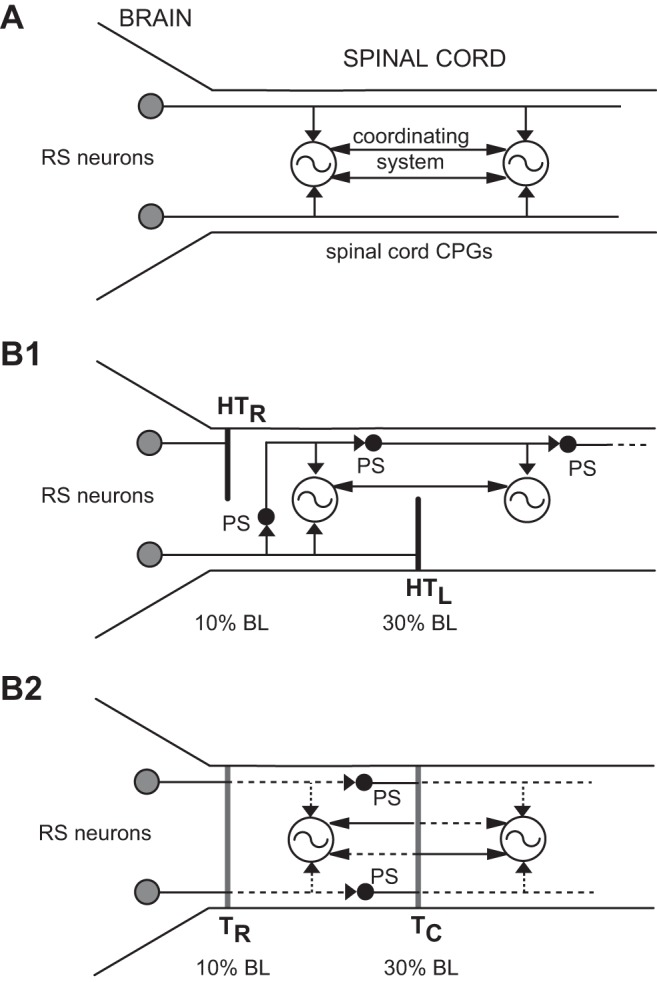
A: simplified diagram of locomotor system in normal lampreys. In the brain locomotor command system, reticulospinal (RS) neurons, which in the lamprey have mostly ipsilateral descending axons (Shaw et al. 2010), directly activate central pattern generators (CPGs) along the entire spinal cord. A coordinating system couples rostral and more caudal CPG networks. B1: proposed organization of lamprey locomotor system following acute staggered right-left spinal hemitransections (HTs) at 10% (HTR) and 30% (HTL) body length (BL; normalized distance from anterior head). The CPG networks in the rostral cord receive direct inputs from RS neurons and possibly indirect inputs from propriospinal (PS) neurons, but more caudal CPG networks receive only indirect descending drive via PS neurons. B2: proposed organization of lamprey locomotor system after relatively short recovery times (3–5 wk) following 2 spaced complete spinal transections, one rostral (10% BL, TR) and one caudal (30% BL, TC). Coordinating neurons will have regenerated their axons for short distances (dotted lines) (Armstrong et al. 2003). Both RS and PS neurons will have regenerated their axons for relatively short distances (dotted lines) (Armstrong et al. 2003; Davis and McClellan 1994) and potentially form a polysynaptic descending command pathway. Thus the CPG networks in the rostral cord will receive direct inputs from RS neurons, and possibly indirect inputs from PS neurons (not shown), but more caudal CPG networks will receive only indirect descending drive via PS neurons.
The above results from the lamprey suggest that at most recovery times following behavioral recovery from rostral SCI, the rostral spinal CPGs are directly activated by RS neurons, whereas more caudal CPGs receive indirect descending command drive from the brain via descending PS relay neurons (reviewed in McClellan 2013). Thus PS neurons are hypothesized to compensate for the lack of or diminished direct descending command drive mediated by RS neurons. In normal lampreys, descending PS neurons are distributed along much of the spinal cord, and following SCI, the descending axons of these neurons appear to sprout (Rouse and McClellan 1997), which would be expected to enhance their compensatory function.
For the present study, spinal cord-injured lampreys were used to test the role of descending PS neurons in relaying locomotor command signals to spinal CPG networks. Two different spinal cord lesion paradigms were used to interrupt long-axon projections of RS neurons to spinal CPGs. Under these conditions, sensory stimulation of the head elicited relatively normal locomotor movements and muscle burst activity, but in some cases with modest deficits in locomotor coordination (Benthall et al. 2013). In addition, a computer model that incorporated the spinal cord lesions could mimic many of the experimental results. The present results are discussed with regard to the roles of descending PS systems in contributing to recovery of locomotor function following SCI.
METHODS
Animal Care
Larval sea lampreys (Petromyzon marinus) were collected from streams in Michigan and Massachusetts and during the study were maintained in ∼10-liter aquaria at ∼23°C. The procedures used in this study have been approved by the Animal Care and Use Committee at the University of Missouri.
Spinal Cord Injury
Staggered right-left spinal HTs.
Staggered spinal HTs were one method used in the present study to interrupt long-axon projections of RS neurons to test the contributions of PS neurons in activating spinal CPGs and initiating locomotion. Animals (102–152 mm) were anesthetized in ∼200 mg/l tricaine methanosulfonate (MS-222; Sigma Chemical, St. Louis, MO) and pinned dorsal side up in a preparation dish. Short incisions were made on the dorsal surface of animals to expose the spinal cord at 10% body length (BL; normalized from the rostral tip of the head) and 30% BL. With the use of iridectomy scissors and fine forceps, a right HT was made at 10% BL and a left HT was made at 30% BL (see Figs. 1B1 and 2A). Along the midline of the spinal cord, a blood vessel and sometimes the central canal were used as landmarks for making the HTs. The completeness of the HTs was confirmed by passing the tips of the forceps through the spinal gaps. In some animals, staggered right-left-right spinal HTs were made at 10%, 30%, and 50% BL.
Following right-left spinal HTs, animals were returned to their home aquaria and recovered for various times: ≤1 wk (acute), 2 wk, 4 wk, 6 wk, and 12 wk. The lengths of experimental animals in the various spinal HT groups were not significantly different from those for normal animals (ANOVA with Dunnett's posttest). At acute recovery times after spinal HTs (≤1 wk), before axonal regeneration (Davis and McClellan 1994), long-axon projections of RS neurons are expected to be interrupted (Fig. 1B1). With increasing recovery times, coordinating neurons regenerate their axons across the injury site for relatively short distances and restore coupling between CPG networks (Armstrong et al. 2003; McClellan 1990a). In addition, RS neurons and descending PS relay neurons will regenerate their axons for short distances across the HTs (Armstrong et al. 2003; Davis and McClellan 1994) and partially restore direct descending locomotor command pathways to spinal CPGs.
It should be noted that in larval lampreys, staggered HTs that are substantially closer than ∼20% BL (∼20 segments) dramatically reduce or block descending drive such that locomotor patterns usually are incomplete or abolished (Shaw et al. 2010). Thus the descending PS neuron system in the lamprey does not appear to be as extensive as that in rodents, in which brain-initiated locomotor activity can still be elicited below staggered spinal HTs that are separated by only a few segments (Cowley et al. 2008).
Two spaced complete spinal transections.
Spaced spinal transections were a second method used in the present study to interrupt long-axon projections of RS neurons to test the contributions of PS neurons in activating spinal CPGs and initiating locomotion. Animals (94–127 mm) were anesthetized, and the spinal cord was exposed at 10% and 30% BL and completely transected with iridectomy scissors (Fig. 1B2; see Fig. 7A). The completeness of the two transections was confirmed by using fine forceps to gently lift the meninges, exposing the ends of the severed cord. Animals were returned to their home aquariums and recovered for 3–5 wk (short recovery) or 12 wk (long recovery). The lengths of experimental animals with two spaced spinal transection groups were not significantly different from those for normal animals (ANOVA with Dunnett's posttest). In some animals, spinal transections were made at 10%, 30%, and 50% BL.
Fig. 7.
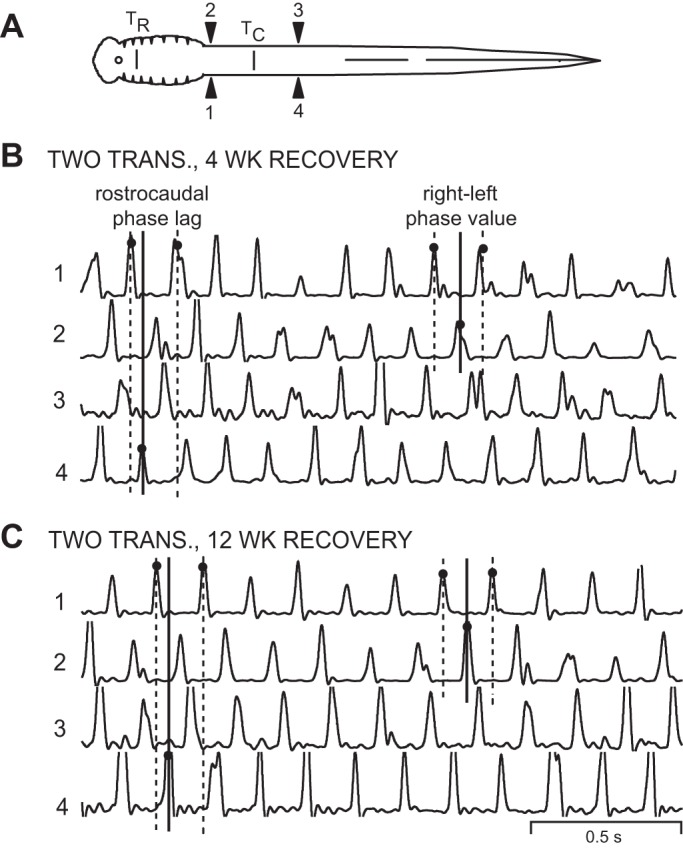
Muscle burst activity in animals that recovered following 2 spaced spinal transections. A: diagram of lamprey showing muscle recording electrodes at 20% BL (1, 2) and 40% BL (3, 4), and rostral (10% BL; TR) and more caudal (30% BL; TC) spinal transections (vertical bars; see methods). B: integrated muscle burst activity in an animal 4 wk after it received 2 spaced spinal transections. The 3 vertical lines at left indicate the rostrocaudal phase lag on the left (Φ1→4), whereas the 3 vertical lines at right indicate the rostral right-left phase value (Φ2-1; see legend for Fig. 2). C: integrated muscle burst activity from an animal 12 wk after it received 2 spaced spinal transections. The 3 vertical lines at left and right of each recording indicate that the left rostrocaudal phase lag (Φ1→4) and the rostral right-left phase value (Φ2-1), respectively, did not change appreciably with increasing recovery times.
At 3–5 wk, coordinating neurons will have regenerated their axons for short distances across the injury site at 30% BL (Armstrong et al. 2003; McClellan 1990a) and partially restored coupling between rostral and caudal CPG networks. In addition, RS neurons and descending PS relay neurons will have regenerated their axons for relatively short distances across the rostral and caudal healed spinal transection sites, respectively (Armstrong et al. 2003; Davis and McClellan 1994), possibly creating a polysynaptic descending activation pathway to spinal CPGs (Fig. 1B2), but long-axon projections of RS neurons will be absent. At 12 wk, RS neurons and descending PS relay neurons will have regenerated their axons across the injury sites in greater numbers and for greater distances, thus more fully restoring direct descending locomotor command pathways to spinal CPGs.
Locomotor Movements and Muscle Burst Activity
Following spinal lesions, locomotor movements and muscle burst activity were used to assess locomotor capabilities. Animals with staggered spinal HTs recovered for various times: ≤1 wk (acute, n = 9), 2 wk (n = 5), 4 wk (n = 5), 6 wk (n = 6), and 12 wk (n = 7). Animals with two spaced spinal transections recovered for 3–5 wk (short recovery, n = 8) or 12 wk (long recovery, n = 8). Animals with staggered spinal HTs, animals with two spaced spinal transections, or normal animals (n = 12 total) were placed in a tank (24 × 44 cm) with ∼3–5 cm of aquarium water to examine the general features of swimming movements. Electrical stimulation (0.1- to 2.0-mA pulses at 100 Hz for 50 ms) or mechanical stimulation (forceps) was applied to the oral hood (anterior head) and is known to reliably elicit swimming via descending pathways in normal larval lampreys (Davis et al. 1993; Shaw et al. 2010). However, spinal-lesioned animals also could swim spontaneously or in response to tail stimulation. Swimming movements were videotaped using an S-VHS camera (Panasonic PVS 770; Yokohama, Japan; 30 frames/s, 8-ms shutter speed) mounted ∼133 cm above the animals.
Kinematic analyses were performed for normal animals, animals with staggered HTs (≤1 wk), and animals with two spaced spinal transections (3–5 wk). Video recordings were transferred to DVD, and still images during swimming were imported into an image analysis program and displayed on a computer screen. Eleven points were marked along the body and used to calculate wavelength (λ; in body length, bl), as previously described in detail (McClellan et al. 2016).
For muscle recording experiments, animals were anesthetized and pinned dorsal side up in a chamber containing ∼3–5 cm of aquarium water. Pairs of fine copper wires (∼56-μm diameter), insulated except at the tips, were inserted into musculature on both right and left sides of the body at 20% and 40% BL (see Figs. 2A and 7A), as previously described (Davis et al. 1993). The bundle of wires was sutured to the dorsal surface of the animals at ∼10% BL and attached to a swivel arm over the swim chamber, allowing the animals to move freely within the tank without appreciable loading from the EMG wires. After recovery from anesthesia, sensory stimulation of the oral hood was used to elicit swimming muscle burst activity, which was recorded (model 1700; A-M Systems, Carlsborg, WA), amplified (initial gain of 1,000×), filtered (0.1–5 kHz), and stored on VHS tape (NeuroData DR886; Cygnus Technologies, Delaware Water Gap, PA; 11-kHz sampling rate per channel). Simultaneously, swimming movements were videotaped using the S-VHS camera described above, and a custom video frame counter was used to synchronize the video recordings and muscle activity recordings, as previously described (Davis et al. 1993). After muscle recordings, animals were reanesthetized, the body lengths were measured, and the numbers of segments between ipsilateral recording electrodes were counted (1→4, 2→3; see Fig. 2A).
Fig. 2.
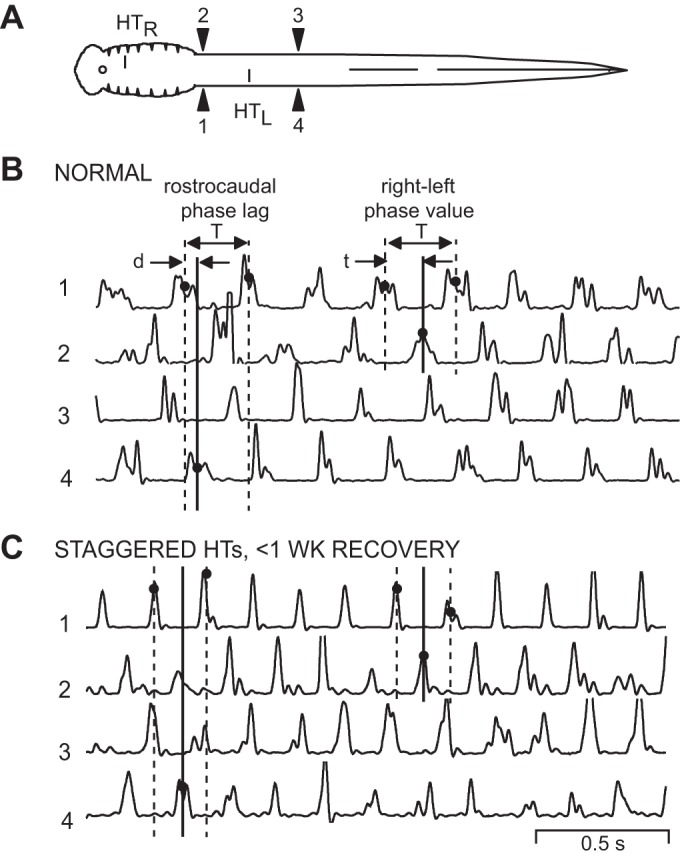
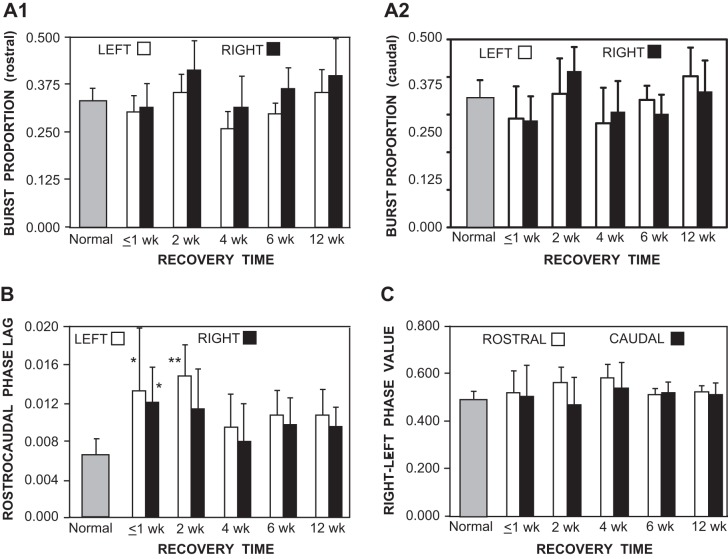
Muscle burst activity in normal animals and in animals following acute staggered right-left spinal HTs. A: diagram of a lamprey showing muscle recording electrodes at 20% BL (1, 2) and 40% BL (3, 4). Staggered right-left spinal HTs at 10% and 30% BL (HTR, HTL) are applicable for the recording in C only (see methods). B: in a normal animal, sensory stimulation of the oral hood elicited swimming muscle burst activity (integrated; see methods) consisting of left-right alternation (1↔2, 3↔4) and rostrocaudal phase lags (1→4, 2→3). The vertical lines at left indicate the cycle time (T) and the delay (d) between ipsilateral burst activity (solid line; 1→4). This delay and cycle time were used to calculate rostrocaudal phase lags on the left side (Φ1→4; see methods). The vertical lines at right indicate the cycle time (T) and the delay (t) between contralateral burst activity (solid line; 1→2). This delay divided by cycle time (t/T) was used to calculate rostral right-left phase values (Φ2-1; see methods). C: in an animal 3 days after it received staggered right-left spinal HTs, relatively normal muscle burst activity occurred during swimming. The 3 vertical lines at left indicate a larger rostrocaudal phase lag compared with those in normal animals (see B), whereas the 3 vertical lines at right indicate that right-left phase values were similar to those in normal animals.
Additional background information.
First, for in vitro brain-spinal cord preparations from larval lampreys, locomotor activity can be initiated by focal brain stem stimulation to test the function of locomotor networks in the absence of sensory feedback (Hagevik and McClellan 1994; McClellan 1994a). However, these preparations appear to have lower excitability than intact animals, and PS systems do not seem to be particularly active (reviewed in McClellan 2013). Attempts to test the contributions of PS systems to locomotor initiation following staggered HTs or two spaced spinal transections using in vitro brain-spinal cord preparations were not successful. Second, it should be emphasized that in lampreys following an acute, mid-body spinal transection, S-wave-type undulations travel caudally along the entire body during swimming, but muscle burst activity only is present rostrally above the lesion site (McClellan 1990b). Active undulatory movements generated in the rostral body are passively transmitted to the caudal body. Thus CPGs below an acute spinal lesion are not activated by either mechanosensory feedback or electric field effects transmitted across a lesion site. Third, for lamprey in vitro spinal cord preparations, focal pharmacological activation of locomotor patterns in the rostral spinal cord does not result in initiation of locomotor activity in more caudal CPG networks. Thus descending transmission via small, local interneurons (e.g., metachronal transmission; Cazalets 2005) does not appear to be a significant mechanism for activation of spinal CPGs in the lamprey.
Analysis of Muscle Burst Activity
Recorded raw muscle burst activity during swimming was played back, rectified, integrated (τ = 12.5 ms), and then acquired using a custom data acquisition/analysis system (DT-3016 board; Data Translations, Marlboro, MA; within each 2-ms sampling interval, the minimum and maximum voltage values of muscle burst activity were captured and displayed). The onsets and offsets of integrated muscle bursts were manually marked with the data acquisition/analysis system, and these time points were imported into a spreadsheet to calculate the following parameters of locomotor activity (Davis et al. 1993; McClellan and Hagevik 1997): cycle time (T), the interval between the onsets of consecutive bursts; burst proportions (BP1–BP4), the burst duration (onset to offset) for a given recording channel (1–4; see Fig. 2A) divided by cycle time; rostrocaudal phase lags (Φ2→3 or Φ1→4), the ratio of the delay (d) between the midpoints of ipsilateral bursts (2→3 or 1→4; see Fig. 2A) and cycle time divided by the numbers of intervening segments (N), [(d/T)N; see Fig. 2B]; and right-left phase values (Φ2-1 or Φ3–4), the phase of the midpoint of a right burst relative to the cycle time defined by the midpoints of bursts on the left side (t/T; see Fig. 2B).
For normal animals, BP1 vs. BP2, BP3 vs. BP4, Φ2→3 vs. Φ1→4, and Φ2-1 vs. Φ3–4 were not significantly different (t-tests), and these pairs of values were averaged together for each animal to calculate BProstral, BPcaudal, Φrostrocaudal, and ΦRt-Lt, respectively, for comparison with values for experimental animals. For animals with two spaced spinal transections, BP1 vs. BP2, BP3 vs. BP4, and Φ2→3 vs. Φ1→4 were not significantly different (t-tests), and BProstral, BPcaudal, and Φrostrocaudal were calculated. For each parameter of locomotor activity (see Figs. 3 and 8), the individual values for normal animals, animals with staggered right-left spinal HTs, and animals with two spaced spinal transections (10% and 30% BL) were entered into a statistical software package (InStat; GraphPad Software, La Jolla, CA) and used to run a single one-way ANOVA or Kruskal-Wallis analysis. With the use of the appropriate posttest, locomotor parameter values for normal animals were compared with those for different recovery times following staggered right-left HTs as well as those for different recovery times following two spaced complete spinal transections. In addition, for animals with a particular recovery time following spinal HTs, right and left burst proportions and right and left rostrocaudal phase lags were compared (e.g., BP1 vs. BP2, Φ2–3 vs. Φ1–4). Finally, for particular recovery times for both experimental groups of animals, rostral and caudal right-left phase values were compared. Graphs were plotted for locomotor parameters (means ± SD; see Figs. 3 and 8), and statistical significance was assumed for P ≤ 0.05.
Fig. 3.
Graphs of locomotor parameters (bars indicate means; vertical lines indicate SD) for normal animals (shaded bars; see methods) and for animals at various recovery times following staggered right-left spinal HTs (open and solid bars; see Fig. 2A). A: burst proportions for left and right locomotor muscle activity in the rostral body (A1; 20% BL) and caudal body (A2; 40% BL) were not significantly different in animals with spinal HTs compared with those for normal animals (ANOVA with Tukey-Kramer posttests). Also, at each recovery time, burst proportions for left muscle activity were not significantly different from those on the right. B: at relatively short recovery times following staggered spinal HTs (≤1, 2 wk), rostrocaudal phase lags were, for the most part, significantly larger than those in normal animals (*P ≤ 0.05; **P ≤ 0.01; Kruskal-Wallis test with Dunn's posttests). At longer recovery times (4, 6, 12 wk), mean phase lags were larger than, but not significantly different from, those in normal animals. C: right-left phase values for muscle burst activity in the rostral and more caudal body were not significantly different in animals with spinal HTs compared with those for normal animals (Kruskal-Wallis test with Dunn's posttests).
Fig. 8.
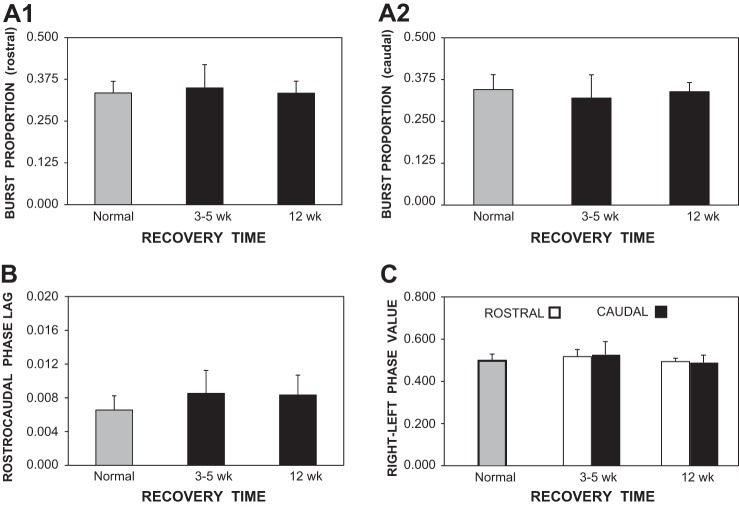
Graphs of locomotor parameters (bars indicate means; vertical lines indicate SD) for normal animals (shaded bars) and for animals at various recovery times following 2 spaced spinal transections (solid bars; see Fig. 7A). A: burst proportions for locomotor muscle burst activity in the rostral body (A1) and caudal body (A2) were not significantly different in animals that recovered for 3–5 wk or 12 wk following 2 spaced spinal transections compared with those for normal animals. B: at short or long recovery times following spinal transections, rostrocaudal phase lags of locomotor burst activity were ∼30% larger than, but not significantly different from, those in normal animals. C: right-left phase values for muscle burst activity in the rostral body (20% BL) and more caudal body (40% BL) were not significantly different in animals that recovered following spinal transections compared with those for normal animals.
Retrograde Labeling of Descending Brain Neurons and PS Neurons
Retrograde labeling was used to determine if long-axon projections of RS neurons were interrupted at various recovery times following staggered spinal HTs: ≤1 wk (acute, n = 8), 2 wk (n = 3), 4 wk (n = 7), 6 wk (n = 4), and 12 wk (n = 4). Similar procedures were used for animals that had recovered following two spaced spinal transections: 3–5 wk (n = 7) and 12 wk (n = 3). Normal (n = 7) and spinal-lesioned animals were anesthetized, and a short dorsal incision was made at 40% BL. The spinal cord was exposed and transected, and a small Gelfoam pledget (Upjohn, Kalamazoo, MI; ∼1 mm3) soaked in 40% horseradish peroxidase type VI (HRP; Sigma Chemical) and 1% dimethyl sulfoxide (DMSO) was applied between the cut ends of the spinal cord, as previously described (Davis and McClellan 1994; Zhang et al. 2002). The incision was closed and sealed with cyanoacrylate (Super Glue Gel; Loctite, Hartford, CT), and animals were returned to their home aquariums. After a period of 14 days to allow for retrograde transport, animals were reanesthetized, and the brains and spinal cords (up to 40% BL) were removed in cold Ringer's solution. The isolated tissue was reacted for HRP using a modified Hanker-Yates protocol (Davis and McClellan 1994; Zhang et al. 2002), dehydrated in an ethanol series, and cleared in methyl salicylate. Histologically processed whole mount tissue was placed on slides and coverslipped with Permount (Fisher Scientific, Fair Lawn, NJ).
The nervous system tissue was viewed using a research microscope, and the outlines of the brain and spinal cord were traced using a custom computer-based marking/tracing system (Armstrong et al. 2003; Davis and McClellan 1994). Subsequently, HRP-labeled descending brain neurons were marked, as previously described (Davis and McClellan 1994; Zhang et al. 2002), and counted in several reticular nuclei (see Fig. 4A): mesencephalic reticular nucleus and the anterior, middle, and posterior rhombencephalic reticular nuclei. In addition, descending brain neurons were counted in nonreticular cell groups: diencephalic group, anteriolateral vagal group, dorsolateral vagal group, and posteriolateral vagal group. Also, labeled PS neurons were marked and counted along the spinal cord between ∼8% BL (brain-spinal cord border) and 30% BL (caudal spinal lesion site).
Fig. 4.
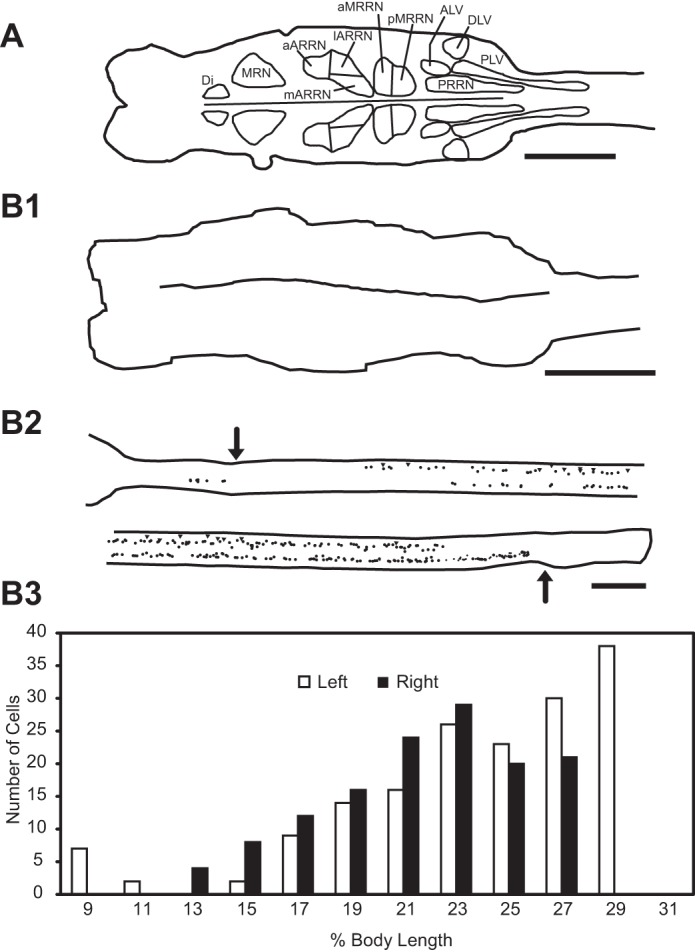
A: diagram of larval lamprey brain (left) and rostral spinal cord (right) showing contours around different groups of descending brain neurons (Davis and McClellan 1994): reticular nuclei, including mesencephalic reticular nucleus (MRN) and anterior (ARRN), middle (MRRN), and posterior rhombencephalic reticular nuclei (PRRN); and nonreticular cell groups, including diencephalic (Di), anteriolateral vagal (ALV), dorsolateral vagal (DLV), and posteriolateral vagal (PLV) groups. B: experimental animal in which HRP was applied to the spinal cord at 40% BL at 7 days (acute animal group) following staggered right-left spinal HTs. B1: brain showing no labeling of descending brain neurons. B2: spinal cord (∼8–32% BL; rostral is upper left) showing labeled descending PS neurons (dots) and sites of staggered HTs (arrows). B3: histogram showing distribution of these neurons vs. percent body length (histogram bins = 2% BL ≈ 2 mm). Scale bars, 1 mm.
Computer Modeling
An iterative computer model of locomotor CPGs in the spinal cord was used to simulate locomotor output in normal animals and in animals with spinal lesions. In normal lampreys, spinal CPGs consisted of rostral (1, 2) and caudal (3, 4) left-right pairs of phase oscillators (see Figs. 5A1 and 10A1), each representing ∼20 segment regions of spinal cord, as previously described (Hagevik and McClellan 1994; McClellan and Hagevik 1999). The left and right oscillators (1–2 or 3–4) were coupled by net symmetrical reciprocal inhibition (strength = −0.3), as previously described (Hagevik and McClellan 1994; McClellan and Hagevik 1997, 1999). Ipsilateral oscillators (1–4 and 2–3) were coupled by asymmetrical reciprocal excitation, in which the descending connections (DE = 1.0) were stronger than the ascending connections (AE = 0.25), as previously described (Hagevik and McClellan 1994; McClellan and Hagevik 1999). The contralateral and ipsilateral synaptic inputs to each phase oscillator were summed, and this net synaptic input (+, excitatory; −, inhibitory) was applied to an excitatory (PRCE) or inhibitory (PRCI) phase-response curve (PRC), respectively (see Fig. 1B in Hagevik and McClellan 1994), to determine the degree to which the phase of a given oscillator would be advanced or delayed. These PRC functions were idealized versions derived from resetting experiments conducted on tetrodotoxin-resistant membrane potential oscillations of lamprey spinal neurons, some of which are thought to be members of the spinal CPGs for locomotion (Wallén and Grillner 1985). The phase of oscillator j at the i + 1 point in time is given as
where θi is the phase at the previous point in time, Δt is the iterative time step (= 1 ms), Tj was the intrinsic cycle time of the j oscillator, Δt/Tj is the increment in phase with each time step, and ΔθPRC is the phase shift resulting from synaptic inputs to the oscillator, calculated from the appropriate PRC function (PRCE for excitation, PRCI for inhibition). The output waveform for this oscillator j at the i + 1 point in time is given by
where Aj is the peak amplitude of the oscillator output waveforms and was set to 1.0. Thus the model calculated the voltage waveforms for each of the oscillators at a given point in time based on the voltages at the previous point in time (Hagevik and McClellan 1994; McClellan and Hagevik 1999). For the model representing “normal” animals (see Figs. 5A1 and 10A1), the intrinsic cycle time of each oscillator was 0.85 s, which resulted in an overall cycle time for the entire CPG network of 1.0 s (see Fig. 5B).
Fig. 5.
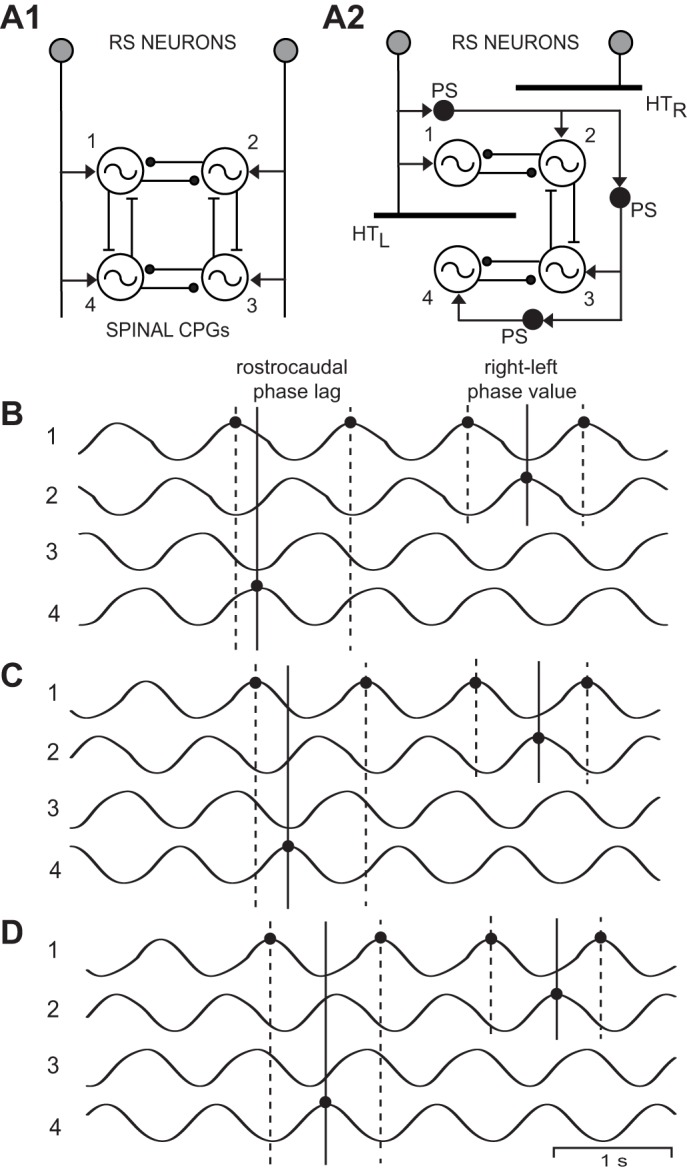
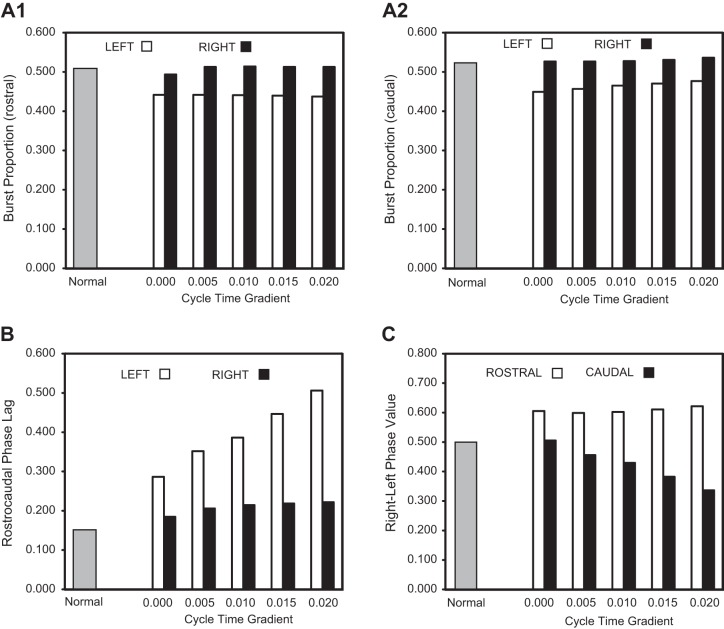
A1: diagram of computer model representing “normal” animals and showing RS neurons directly activating rostral (1, 2) and caudal (3, 4) left-right pairs of spinal oscillators that were connected by reciprocal inhibition (solid line ending in filled circle). Ipsilateral oscillators were connected by asymmetrical reciprocal excitation (solid line ending in crossbar) that was stronger in the descending direction than the ascending direction (Hagevik and McClellan 1994; McClellan and Hagevik 1999). A2: model incorporating acute staggered right-left spinal HTs (HTR and HTL, thick horizontal bars) in which coupling between the 1 and 4 oscillators was removed (for waveforms in C and D only). B: rhythmic “locomotor” output waveforms generated by a model (A1) representing “normal” animals (T1–T4 = 0.85 s) consisting of left-right alternation (1↔2, 3↔4) and rostrocaudal phase lags (1→4, 2→3). C: “locomotor” output waveforms from a model (A2) incorporating staggered right-left spinal HTs in which 1–4 coupling was removed, but without a cycle time gradient (T1–T4 = 0.85 s; see methods). D: “locomotor” output waveforms from a model (A2) incorporating staggered right-left spinal HTs in the absence of 1–4 coupling and with a cycle time gradient (T1 = 0.85 s; T2 = 0.87 s; T3 = 0.89 s; and T4 = 0.91 s; see methods). In B–D, the 3 vertical lines at left indicate the rostrocaudal phase lag on the left side (Φ1→4), whereas the 3 vertical lines at right indicate the rostral right-left phase value (Φ2-1; see legend for Fig. 2). Note that in D, the left rostrocaudal phase lags and, to a lesser extent, rostral right-left phase values were further increased compared with those in C.
Staggered right-left spinal HTs (see Fig. 5A2) created two possible conditions that were explored in the model: 1) Because of the HTs, the coupling between oscillators #1 and #4 was eliminated (see Fig. 5A2). 2) A lower (higher) drive to an oscillator will result in a longer (shorter) cycle time. Thus spinal oscillators receiving relatively strong, direct RS neuron input (i.e., oscillator #1) might have relatively short cycle times, whereas oscillators farther away from direct RS input (i.e., oscillator #4) might be expected to exhibit longer cycle times. Thus a cycle time gradient was tested in the model such that T1 < T2 < T3 < T4, where the numbers refer to the different oscillators in Fig. 5A.
At relatively short recovery times (3–5 wk) following two spaced spinal transections, one at 10% and one at 30% BL (see Fig. 10A2), the axons of RS neurons, PS neurons, and coordinating neurons will have regenerated, but only for relatively short distances (Armstrong et al. 2003; Davis and McClellan 1994). Accordingly, this created three potential conditions that were explored in the model: 1) Rostral oscillators (1 and 2) were expected to receive direct, but possibly weaker than normal, input from the brain command system due to short-distance axonal regeneration of RS neurons (see Fig. 10A2). Thus increased cycle times for the rostral oscillators were explored such that T1 = T2 > 0.85 s. 2) Caudal oscillators (3 and 4) would be expected to receive indirect, possibly weaker than normal, descending drive via regenerated axons of PS neurons (see Fig. 10A2). Thus increased cycle times for the caudal oscillators were explored such that T3 = T4 > 0.85 s. 3) The restored coupling between ipsilateral oscillators (1–4 and 2–3) due to axonal regeneration of coordinating neurons (see Fig. 10A2) might be weaker than normal. In the present study, reduced drive to rostral or caudal oscillators and reduced coupling between rostral and caudal oscillators were investigated to determine which conditions might result in oscillator output waveforms that mimicked the experimental results.
Fig. 10.
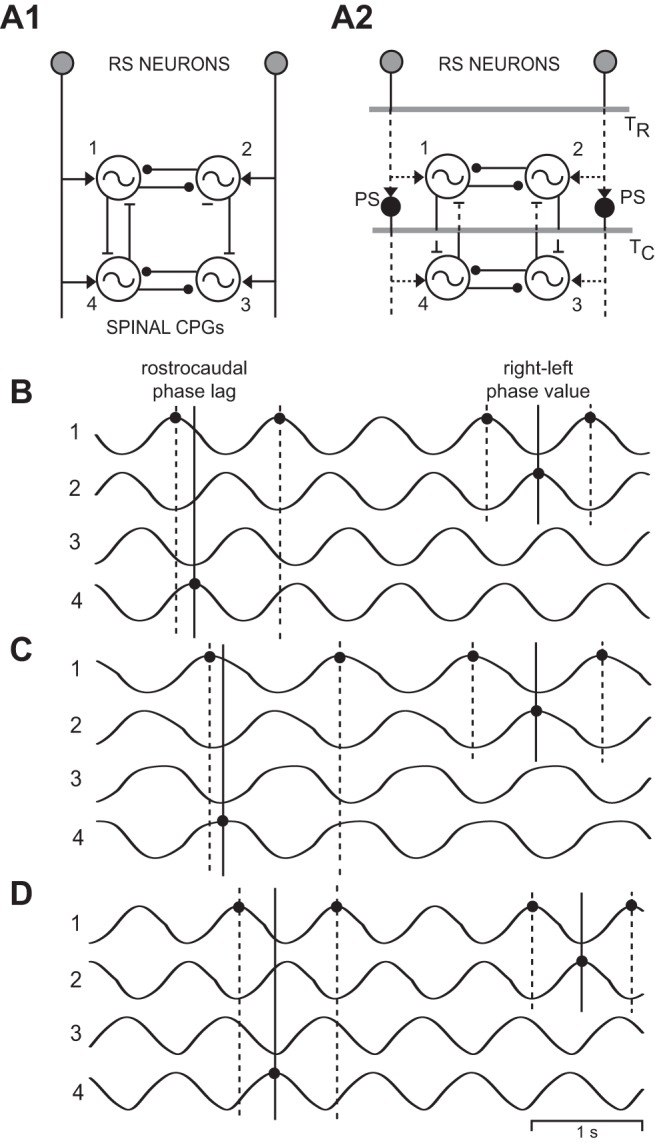
A: diagrams of computer models representing “normal” animals (A1; see Fig. 5A1) and animals that had recovered following spinal transections (A2) at 10% BL (TR) and 30% BL (TC; horizontal shaded lines; see Fig. 7A and methods). In A2, dotted lines indicate axonal regeneration of RS neurons, PS neurons, and coordinating neurons. B: “locomotor” output waveforms from a model incorporating spinal transections at 10% and 30% BL, with a 50% reduction in coupling between ipsilateral oscillators (see methods). C: “locomotor” output waveforms from a model with reduced drive to rostral oscillators (T1 = T2 = 0.95 s, T3 = T4 = 0.85 s), but without a reduction in coupling between ipsilateral oscillators. D: “locomotor” output waveforms from a model with reduced drive to caudal oscillators (T3 = T4 = 0.95 s, T1 = T2 = 0.85 s), but without a reduction in coupling between ipsilateral oscillators. In B–D, the 3 vertical lines at left indicate the rostrocaudal phase lag on the left (Φ1→4), whereas the 3 vertical lines at right indicate the rostral right-left phase value (Φ2-1; see legend for Fig. 2).
RESULTS
Animals with Staggered Right-Left Spinal HTs
Locomotor muscle burst activity.
Staggered spinal HTs were one method used in the present study to interrupt long-axon projections of RS neurons to test the contributions of PS neurons in activating spinal CPGs and initiating locomotion. In normal animals and animals with staggered right-left spinal HTs (Fig. 2A), locomotor muscle activity was recorded in response to sensory stimulation of the oral hood (anterior head). For normal animals (n = 12), sensory stimulation elicited well-coordinated swimming muscle burst activity consisting of left-right alternation (1↔2 and 3↔4) and rostocaudal phase lags (1→4 and 2→3) (Fig. 2B), as previously described (Davis et al. 1993). At acute recovery times (≤1 wk) following staggered right-left spinal HTs, sensory stimulation elicited locomotor muscle burst activity at all four recording locations (Fig. 2A) in a total of 18 of 23 animals, and in 9 of the 18 animals there were sufficient numbers of cycles (>10) to obtain meaningful averages (see discussion).
In experimental animals that did produce complete swimming motor patterns, undulatory swimming movements were transmitted caudally along the body, but the wavelengths (λ = 0.522 ± 0.125 bl) were significantly shorter than those for normal animals (0.787 ± 0.141 bl; P < 0.01, ANOVA with Dunnett's posttest). Thus, during swimming, there were more S waves along the body for experimental animals compared with normal animals. For experimental animals, locomotor muscle burst activity was relatively normal (Fig. 2C), but there appeared to be some deficits in rostrocaudal coordination (compare timing of solid and dotted vertical lines in Fig. 2, B and C, left).
A detailed analysis indicated that at all recovery times following staggered right-left HTs, cycle times for swimming were not significantly different than those in normal animals (ANOVA with Dunnett's posttest). For each recovery time in experimental animals, burst proportions for rostral or caudal muscle burst activity were not significantly different from the corresponding values in normal animals. Also, at all recovery times in experimental animals, burst proportions for muscle activity in the rostral body (20% BL) tended to be larger on the right side below a right HT than those on the left side, but the differences were not significant (ANOVA with Tukey-Kramer posttests; Fig. 3A1). For muscle burst activity in the more caudal body (40% BL) at intermediate recovery times (2 and 4 wk), burst proportions tended to be larger on the right side of the body, but the differences were not significant (ANOVA with Tukey-Kramer posttests; Fig. 3A2).
At relatively short recovery times following staggered spinal HTs (≤1 and 2 wk), rostrocaudal phase lags for muscle burst activity were, for the most part, significantly larger than those in normal animals (Kruskal-Wallis test with Dunn's posttest; Fig. 3B). These larger phase lags would result in shorter wavelengths, as described above. Phase lags for muscle burst activity on the left side of the body tended to be larger relative to those on the right side, but the differences were not significant (Kruskal-Wallis test with Dunn's posttest). At longer recovery times (4, 6, and 12 wk), both right and left phase lags tended to be larger than those in normal animals, although the differences were no longer significant. For all recovery times following spinal HTs, right-left phase values for muscle burst activity in the rostral (20% BL) and more caudal body (40% BL) were not significantly different from those in normal animals (Kruskal-Wallis test with Dunn's posttest; Fig. 3C; compare three vertical lines in Fig. 2, B and C, right). At relatively short recovery times (≤1, 2, and 4 wk), right-left phase values for burst activity in the rostral body tended to be larger than those in the more caudal body, but the differences were not significant. Similar coordination deficits in locomotor muscle burst activity were observed in animals with acute staggered right-left-right HTs at 10%, 30%, and 50% BL (data not shown).
Anatomical labeling following staggered right-left spinal HTs.
To confirm that long-axon descending projections from RS neurons were interrupted following staggered right-left spinal HTs, HRP was applied to the spinal cord at 40% BL. In contrast to normal animals, in which application of HRP results in relatively large numbers of labeled descending brain neurons (data not shown), as previously described (Davis and McClellan 1994), at relatively short recovery times (≤1, 2, and 4 wk) following staggered spinal HTs, no descending brain neurons were labeled in any of the animals (Fig. 4B1). Thus, at these recovery times, the staggered right-left spinal HTs interrupted long-distance descending axons of RS neurons. In the ≤1-wk recovery group, 182 ± 61 labeled descending PS neurons were distributed along the spinal cord between ∼8% and 30% BL (8% BL = brain-spinal cord border; 30% BL = left HT; Fig. 4, B2 and B3).
At relatively long recovery times (6 and 12 wk), an average of 23 ± 25 and 67 ± 64 descending brain neurons, respectively, were labeled, including RS neurons. Most of the descending brain neurons that were labeled are known to project their axons on the left side of the spinal cord (Shaw et al. 2010). Thus the labeling of descending brain neurons described above was mostly due to regeneration of axons through the left HT at 30% BL to the HRP application site at 40% BL, whereas most of the descending axons severed by right HTs at 10% BL had not regenerated far enough to reach the tracer application site. At these longer recovery times, more than 300 labeled descending PS neurons were distributed along the spinal cord between ∼8% and 30% BL (data not shown).
Computer model: staggered right-left spinal HTs.
An iterative computer model of locomotor CPGs in the spinal cord was used to simulate locomotor output for normal animals and for animals with staggered spinal HTs. To represent “normal” animals, a computer model was used consisting of rostral and more caudal left-right pairs of oscillators that were directly activated by RS neurons (Fig. 5A1). For this model, the “locomotor” output waveforms were characterized by burst proportions of ∼0.50, left-right alternation (1↔2 and 3↔4; ΦRt-Lt = 0.5), and a rostrocaudal phase lag (1→4 and 2→3; Φrostrocaudal = 0.15; Figs. 5B and 6), as previously described (Hagevik and McClellan 1994; McClellan and Hagevik 1999). Because the rostral and more caudal left-right pairs of oscillators corresponded to regions of spinal cord that were separated by ∼20 segments, a rostrocaudal phase lag of 0.15 from the model is equivalent to an intersegmental phase lag of ∼0.075 (Hagevik and McClellan 1994), which was similar to that obtained from muscle recordings (see shaded bar in Fig. 3B).
Fig. 6.
Parameters of rhythmic “locomotor” output waveforms generated by the computer models in Fig. 5. Burst proportions (rostral and caudal; A), rostrocaudal phase lags (left and right; B), and right-left phase values (rostral and caudal; C) for the models representing “normal” animals (shaded bars; see Fig. 5B) and animals with staggered spinal HTs (open and solid bars) in which 1–4 coupling was omitted. A cycle time gradient (T1 < T2 < T3 < T4; see methods) was tested such that T(n + 1) = T(n) + cycle time gradient, where T1 = 0.85 s, and the x-axis values indicate the difference between the cycle times of the different successive oscillators: (e.g., for Fig. 5, C and D, the gradients were 0.00 and 0.02 s, respectively).
To incorporate staggered right-left spinal HTs in the model, two types of conditions were explored (see methods). First, the coupling between oscillators #1 and #4 was eliminated (Fig. 5A2). For this condition, the model still generated relatively normal “locomotor” output waveforms (Fig. 5C), but with a few exceptions: 1) rostral and caudal burst proportions for left “locomotor” output waveforms were reduced and were less than those for the right side (Fig. 6, A1 and A2; cycle time gradient = 0.000); 2) rostrocaudal phase lags for right and left “locomotor” waveforms both increased, but the phase lag on the left side was larger than that on the right side (Fig. 6B; cycle time gradient = 0.000); 3) the right-left phase value produced by the rostral oscillator pair was >0.5 and larger than the caudal phase value (Fig. 6C; cycle time gradient = 0.000; compare vertical lines in Fig. 5, B and C, right); and 4) the overall cycle time of the waveforms decreased by ∼6% (not shown).
Second, in addition to eliminating coupling between oscillators #1 and #4, an oscillator cycle time gradient was introduced to incorporate reduced drive to oscillators further away from direct RS neuron input (T1 = 0.85 s, T2 = 0.87 s, T3 = 0.89 s, T4 = 0.91 s; Fig. 5A2; see methods). Under these conditions, the disparity increased between left and right rostrocaudal phase lags (Fig. 5D, compare three vertical lines at left with those in Fig. 5, B and C). Also, the difference increased between rostral and more caudal right-left phase values (Fig. 5D, compare three vertical lines at right with those in Fig. 5, B and C). A systematic increase in cycle time gradient [i.e., T(n + 1) = T(n) + gradient value, where T1 = 0.85 s] resulted in an increase in the difference between right and left rostrocaudal phase lags (Fig. 6B) and an increase in the difference between rostral and caudal right-left phase values (Fig. 6C). Thus the model (Fig. 6) was able to mimic several general aspects of the experimental results (Fig. 3), although the differences in the parameters for locomotor muscle burst activity between experimental and normal animals were not always statistically significant (see discussion).
Animals with Two Spaced Spinal Transections (10% and 30% BL)
Locomotor muscle burst activity.
Spaced spinal transections were a second method used in the present study to interrupt long-axon projections of RS neurons to test the contributions of PS neurons in activating spinal CPGs and initiating locomotion. In normal animals and animals that had recovered for various times following spinal transections at 10% and 30% BL, locomotor muscle activity was recorded in response to sensory stimulation of the oral hood (Fig. 7A). In normal animals, sensory stimulation elicited well-coordinated swimming movements and muscle burst activity, as described for Fig. 2B. In animals that had recovered for 3–5 wk following two spaced spinal transections (10% and 30% BL), RS neurons, PS neurons, and spinal coordinating neurons will have regenerated their axons for relatively short distances (Armstrong et al. 2003; Davis and McClellan 1994). At this early recovery time following two spaced spinal transections, sensory stimulation elicited locomotor muscle burst activity at all four recording locations (Fig. 7A) in a total of 14 of 29 animals, and in 8 of the 14 animals there were sufficient numbers of cycles (>10) to obtain meaningful averages (see discussion).
In experimental animals that did produce complete swimming motor patterns, undulatory swimming movements were transmitted caudally along the body, but the wavelengths (λ = 0.572 ± 0.161 bl) were significantly shorter than those for normal animals (0.787 ± 0.141 bl; P < 0.01, ANOVA with Dunnett's posttest). Thus, during swimming, there were more S waves along the body for experimental animals compared with normal animals. In the experimental animals, locomotor activity was relatively normal (Fig. 7B) and included burst proportions and right-left phase values that were not significantly different than those in normal animals (one-way ANOVA with Tukey-Kramer posttests, and Kruskal-Wallis test with Dunn's posttests, respectively; Fig. 8, A1, A2, and C). Rostrocaudal phase lags were ∼30% larger than those in normal animals (Fig. 8B), but the differences were not significant (Kruskal-Wallis test with Dunn's posttests). Larger phase lags would be expected to result in shorter wavelengths, as described above. Animals with spinal transections at 10%, 30%, and 50% BL that recovered for short times (3–5 wk) also exhibited relatively normal locomotor muscle burst activity (data not shown).
In experimental animals that had recovered for 12 wk, locomotor activity also was relatively normal (Fig. 7C). Again, rostrocaudal phase lags were ∼30% larger than those in normal animals (Fig. 8B), but the differences were not significant. At all recovery times following two spaced spinal transections, cycle times for swimming were not significantly different from those in normal animals (ANOVA with Dunnett's posttest).
Anatomical labeling following spinal transections at 10% and 30% BL.
To confirm that following two spaced spinal transections and 3–5 wk recovery times, long descending pathways from RS neurons were interrupted, HRP was applied to the spinal cord at 40% BL. In contrast to normal animals, in which relatively large numbers of descending brain neurons were labeled, at short recovery times following two spaced spinal transections (3–5 wk), no brain neurons were retrogradely labeled in seven animals (Fig. 9A1). In addition, between ∼8% and 30% BL (∼8% BL = brain-spinal cord border; 30% BL = left HT site), only a few descending PS neurons were labeled (Fig. 9A2). Because these animals recovered locomotor behavior, it is very likely that additional descending PS neurons had extended their axons for short distances past the spinal transection site at 30% BL but not to the HRP application site at 40% BL.
Fig. 9.
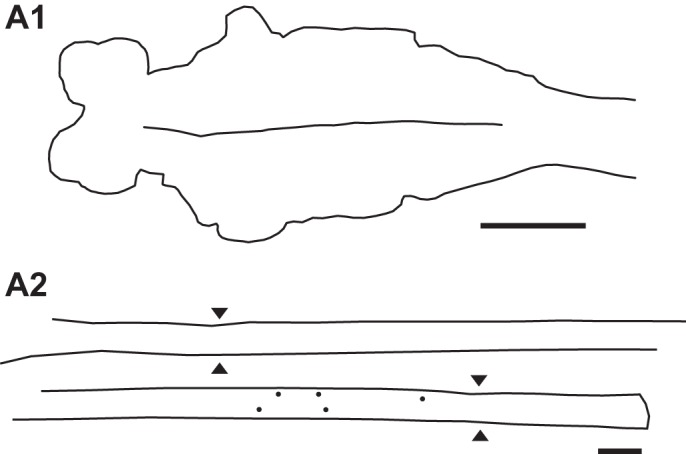
Experimental animal in which HRP was applied to the spinal cord at 40% BL at 4 wk following 2 spaced spinal transections, one at 10% BL and one at 30% BL. A1: in the brain there was no labeling of descending brain neurons in reticular or nonreticular cell groups (see Fig. 4A). A2: spinal cord (∼8–33% BL; rostral is upper left) showing labeling of a few descending PS neurons just rostral to the transection site at 30% BL that had extended their axons to the HRP application site. Sites of healed spinal transections are indicated by double arrowheads. Scale bars, 1 mm.
At relatively long recovery times (12 wk; n = 3), only an average of 6 ± 10 descending brain neurons, including some RS neurons, were retrogradely labeled, indicating that these neurons had extended their axons through both healed spinal transection sites to the tracer application site at 40% BL. In addition, at this recovery time ∼200–300 labeled descending PS neurons were distributed along the spinal cord between ∼8% and 30% BL (data not shown).
Computer model: two spaced spinal transections.
An iterative computer model of locomotor CPGs in the spinal cord was used to simulate locomotor output for normal animals and for animals with two spaced spinal transections. For the computer model representing “normal” animals (Fig. 10A1), the “locomotor” output waveforms were characterized by left-right alternation and rostrocaudal phase lags, as described for Fig. 5B. At relatively short recovery times following rostral (TR; 10% BL) and more caudal (TC; 30% BL) spinal transections (Fig. 10A2), coupling between rostral and caudal oscillators might be reduced, drive to rostral oscillators might be reduced, and/or drive to caudal oscillators might be reduced (see methods). Variations in these three parameters were examined in the computer model. First, when the coupling between ipsilateral oscillators (1–4 and 2–3) was reduced by 50%, the “locomotor” output waveforms were relatively normal (Fig. 10B) with only moderate changes in the pattern compared with that for the “normal” model parameters (Fig. 5B). In particular, when ipsilateral coupling was systematically reduced from 100% to 50%, burst proportions decreased by ∼6%, rostrocaudal phase lags increased by ∼5% (Fig. 10B, three vertical lines at left), overall cycle times decreased by ∼5%, and right-left phase values did not change (Fig. 10B, three vertical lines at right).
Second, when the drive from RS neurons to rostral oscillators (Fig. 10A2, 1 and 2) was reduced such that the intrinsic cycle times of these oscillators were increased (T1 = T2 = 0.95 s, T3 = T4 = 0.85 s), the “locomotor” output waveforms were relatively normal but with a decrease in rostrocaudal phase lags (Fig. 10C, compare three vertical lines at left with those in Fig. 10B). Specifically, a systematic increase in the cycle times of the rostral oscillators from 0.85 to 0.90 s resulted in a ∼30% reduction in rostrocaudal phase lags (Fig. 11A) and an ∼10% increase in overall cycle times (see Fig. 10C), but very few changes in other aspects of the “locomotor” output waveforms.
Fig. 11.
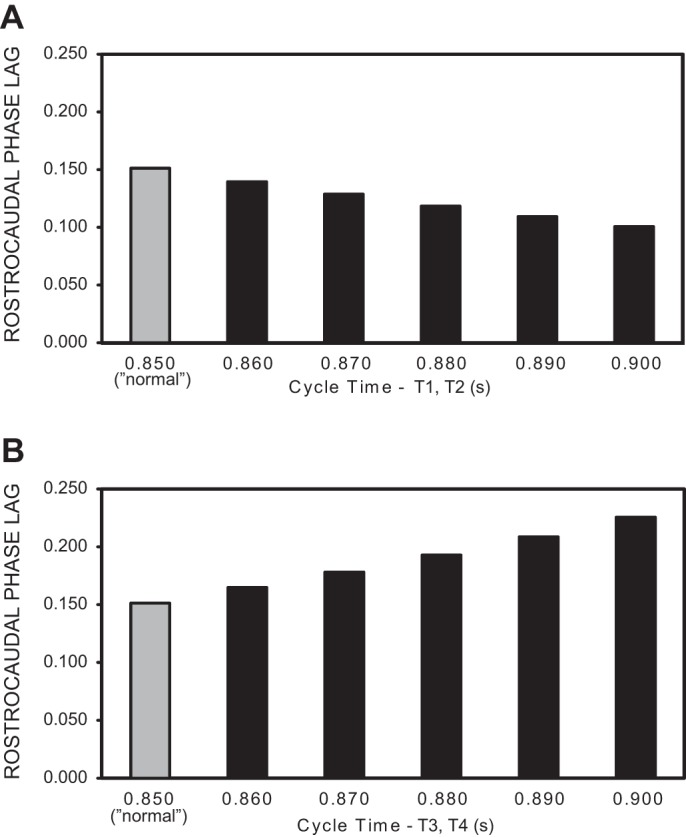
Rostrocaudal phase lags of rhythmic “locomotor” output waveforms generated by the computer models in Fig. 10. Shaded bars are phase lags for model representing “normal” animals, with the same drive to rostral and caudal oscillators (T1–T4 = 0.85 s; see Fig. 5B and methods). A: rostrocaudal phase lags vs. drive to rostral oscillators from RS neurons (see Fig. 10A2), with normal coupling between ipsilateral oscillators (see Fig. 10C). The intrinsic cycle times of the caudal oscillators were T3 = T4 = 0.85 s, and the x-axis values indicate the intrinsic cycle times of rostral oscillators (T1, T2). B: rostrocaudal phase lags vs. drive to caudal oscillators from PS neurons (see Fig. 10A2), with normal coupling between ipsilateral oscillators (see Fig. 10D). The intrinsic cycle times of the rostral oscillators were T1 = T2 = 0.85 s, and the x-axis values indicate the intrinsic cycle times of caudal oscillators (T3, T4).
Third, when the drive from PS neurons to the more caudal oscillators (Fig. 10A2, 3 and 4) was reduced such that the intrinsic cycle times of these oscillators were increased (T3 = T4 = 0.95 s, T1 = T2 = 0.85 s), the “locomotor” output waveforms were relatively normal but with an increase in rostrocaudal phase lags (Fig. 10D, compare three vertical lines at left with those in Fig. 10B). Specifically, a systematic increase in the cycle times of the more caudal oscillators from 0.85 to 0.90 s resulted in a ∼50% increase in rostrocaudal phase lags (Fig. 11B) and an ∼4% decrease in overall cycle times (see Fig. 10D), but very few changes in other aspects of the output pattern.
Following spaced spinal transections, the locomotor muscle burst activity was mainly characterized by mean intersegmental phase lags that were ∼30% larger in lesioned animals compared with normal animals (Fig. 8B), although the differences were not significant (see discussion). Similar changes in the “locomotor” output waveforms generated by the model could be achieved with reduced drive to the more caudal oscillators (Figs. 10D and 11B). In addition, burst proportions and right-left phase values for muscle burst activity were similar in normal and spinal-lesioned animals (Fig. 8, A and C), and in the model these features of the “locomotor” output waveforms were relatively insensitive to changes in model parameters.
DISCUSSION
Following rostral SCI and interruption of long-axon projections of RS neurons, recovery of locomotor function potentially could occur as a result of long-axon regeneration of these neurons. However, long-axon regeneration usually has been very difficult to achieve in the mammalian central nervous system (CNS; Bradbury and McMahon 2006), and it occurs only to a limited extent even in lower vertebrates, which have a permissive CNS for neural regeneration (reviewed in McClellan 2013). Alternatively, following SCI and short-distance axonal regeneration of RS neurons, a system of descending PS neurons potentially could relay descending locomotor command signals to more caudal spinal CPG networks (Bonner and Steward 2015).
Initiation of Locomotion in the Absence of Long-Axon RS Neuron Pathways in Lampreys
First, at acute recovery times (≤1 wk) following staggered right-left HTs, sensory stimulation of the oral hood (anterior head) elicited relatively normal swimming muscle burst activity, but with some deficits in intersegmental phase lags. A computer model of spinal CPG networks that incorporated the staggered right-left HTs could generate motor patterns with similar coordination defects. Second, at relatively short recovery times following two spaced spinal transections (3–5 wk), sensory stimulation of the oral hood elicited relatively normal swimming muscle burst activity, with minor coordination deficits. A computer model of spinal CPG networks incorporating the two spinal transections could mimic the experimental data. Previous studies in the lamprey have shown that mechanosensory feedback, electrical field effects across spinal lesions, or transmission via small, local interneurons do not appear to be capable of activating (turning on) spinal CPGs to initiate locomotor burst activity (McClellan 1990b; see methods). However, although mechanosensory feedback alone does not activate lamprey spinal CPGs, in the present study these sensory inputs might have maintained a certain level of excitability in the more caudal CPGs to allow them to respond to inputs from descending PS neurons. Therefore, following SCI in the lamprey and disruption of long-axon projections from RS neurons, descending PS neurons appear to be a viable mechanism for indirect activation of spinal locomotor CPGs. Because descending PS neurons can initiate locomotor activity in the absence of long brain-spinal cord projections, the results from the present study suggest that these neurons very likely contribute significantly to activation of spinal CPGs in normal animals. Nevertheless, depending on the nature of the SCI, there can be coordination deficits of locomotor activity, as shown in the present study (Figs. 3 and 8). In humans, deficits in intralimb coordination following SCI are considered to be a sensitive indicator of the degree of motor control impairment (Awai and Curt 2014).
Incorporation of right-left HTs or two spaced spinal transections in the computer model resulted in several differences in the “locomotor” output waveforms compared with the output waveforms corresponding to “normal” animals (Figs. 6 and 11). Similar differences were observed between muscle burst activity recorded from experimental animals and those from normal animals (Figs. 3 and 8). However, except for differences in rostrocaudal phase lags at short recovery times following HTs (Fig. 3B), several of these differences were not statistically significant. This lack of significance might have been due to experimental variability of muscle burst activity, which compromised statistical comparisons for real biological data but which was not present in the models. In addition, following spinal lesions, some of the changes in CPG output might have been compensated for by mechanosensory feedback, which was not incorporated into the computer model.
The same basic phase-oscillator computer model used in the present study has been employed successfully to simulate the biological results from four other lamprey locomotor studies: resetting and entrainment of spinal CPG activity (McClellan and Jang 1993), short-distance coupling between CPG oscillators (Hagevik and McClellan 1994), long-distance coupling (McClellan and Hagevik 1999), and turning maneuvers (McClellan and Hagevik 1997). Although the model is relatively simple compared with biophysical models, this phase oscillator model appears to capture many, but not all, aspects of the lamprey spinal CPG networks.
Efficacy of Descending PS Neuron Activation Systems
In the present study, some of the experimental animals with spinal lesions failed to generate enough locomotor cycles for meaningful analysis, generated incomplete locomotor patterns (i.e., some muscle recording channels were weak or absent), or failed to produce locomotor activity. This may have been due to several factors: 1) lower excitability (responsiveness) than usual of spinal CPGs to descending PS neuron inputs and 2) RS neuron projections pre-lesion that might have played a more significant role in some animals than in those that did produce complete swimming motor patterns post-lesion. Also, for lamprey in vitro brain-spinal cord preparations, which appear to have lower excitability than intact animals, the descending PS neuron system does not seem to be particularly active and does not activate spinal CPGs (reviewed in McClellan 2013). Thus, in the lamprey, the descending PS neuron system by itself does not reliably activate spinal CPGs and initiate a complete pattern of locomotor activity. Similar results have been obtained in mammals (Cowley et al. 2008). In contrast, experiments using lamprey in vitro brain-spinal cord preparations suggest that by itself, the RS neuron system consistently and reliably activates CPGs along virtually the entire spinal cord (McClellan 1990b, 1994a).
Comparison with Other Studies
In normal as well as spinal cord-injured animals, descending PS neurons have been shown to relay locomotor drive to more caudal spinal motor networks (Flynn et al. 2011; Jordan and Schmidt 2002; Schomburg 1990; Shik 1983). First, in mammals, brain-initiated lumbar locomotor activity can be blocked when synaptic transmission is abolished in mid-spinal levels, suggesting the involvement of descending PS relay neurons (Cowley et al. 2010; Zaporozhets et al. 2006, 2011). Second, excitotoxic lesions of upper lumbar gray matter result in significant locomotor deficits in rats even though the major supraspinal descending pathways are intact (Magnuson et al. 1999). Finally, in cats, rats, and turtle, stimulation of the spinal dorsolateral funiculus (DLF), which contains a descending PS system, can initiate spinal locomotor activity (Iwahara et al. 1991a, 1991b; Kazennikov et al. 1985; Lennard and Stein 1977; Stein 1978; Yamaguchi 1986, 1987).
Second, for in vitro CNS preparations from neonatal rats in which staggered right-left spinal HTs were made in the cervical and thoracic cord to interrupt long-axon descending projections, stimulation in the brain stem could still elicit hindlimb locomotor-like activity below the HTs (Cowley et al. 2008). However, this was effective in only ∼25% of preparations, and incomplete rhythmic patterns (e.g., flexor-extensor alternation was required on only one side) were counted as locomotor-like activity (Cowley et al. 2008). Also, in this study it was assumed but not shown that long-axon descending projections were interrupted by the HTs. In young weanling rats, following staggered right-left HTs separated by one to three midthoracic spinal segments, little behavioral recovery was observed except when some descending axons were spared from brain neurons projecting in the ventrolateral tracts (Stelzner and Cullen 1991). In contrast, much better behavioral recovery, including restoration of some locomotor function, was observed in neonatal animals. In adult mice with staggered HTs at lower and middle thoracic levels that were performed 10 wk apart, hindlimb stepping was initially compromised after the second HT but then improved over the course of 4 wk (Courtine et al. 2008).
Plasticity of PS Neuron Systems
Following rostral SCI in the lamprey, RS neurons do regenerate their axons, but mostly for relatively short distances beyond the lesion site (Davis and McClellan 1994). It is very likely that descending PS neurons compensate for this incomplete axonal regeneration of RS neurons and relay descending drive to CPG networks in the middle and caudal spinal cord (reviewed in McClellan 2013). In addition, following SCI, the axons of these PS neurons appear to undergo plasticity and sprout to extend their axons for greater distances caudally in the spinal cord (Rouse and McClellan 1997), and this would be expected to enhance their compensatory functions.
In spinal cord-injured mammals, possible therapeutic approaches that might induce axonal regeneration of RS neurons very likely will do so for relatively short distances (Bradbury and McMahon 2006). Therefore, treatments that target descending PS systems may be a viable method for inducing neuroplasticity and enhancing the compensatory functions of these neurons to restore locomotor activity below a SCI (Dunlop 2008; Filli and Schwab 2015; Flynn et al. 2011). However, the enhancement of neuroplasticity and improvement of motor functions need to be balanced against possible significant negative side effects, such as neuropathic pain and autonomic dysfunction (Brown and Weaver 2012). Finally, it may be important to focus therapeutic approaches on rescuing PS neurons from cell death, because certain groups of these neurons appear to be vulnerable following SCI (reviewed in Conta and Stelzner 2009).
Conclusions
In the lamprey following staggered right-left spinal HTs or two spaced spinal transections to interrupt long-axonal projections of RS neurons, sensory stimulation still elicited relatively normal locomotor muscle burst activity. However, depending on the nature of the SCI, there could be rostral-caudal and/or left-right coordination deficits of locomotor activity. Computer models incorporating the right-left spinal HTs or two spaced spinal transections could mimic many aspects of the experimental results. Thus, following SCI in the lamprey and disruption of long axon projections from RS neurons, descending PS neurons appear to be a viable compensatory mechanism for indirect activation of spinal locomotor CPGs. Similar mechanisms may be important for neurological improvement following SCI in higher vertebrates, including perhaps humans.
GRANTS
K. N. Benthall was supported by a fellowship from the University of Missouri Life Sciences Undergraduate Research Opportunity Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.N.B. and R.A.H. performed experiments; K.N.B., R.A.H., and A.D.M. analyzed data; K.N.B., R.A.H., and A.D.M. interpreted results of experiments; K.N.B., R.A.H., and A.D.M. prepared figures; K.N.B., R.A.H., and A.D.M. edited and revised manuscript; K.N.B., R.A.H., and A.D.M. approved final version of manuscript; A.D.M. drafted manuscript.
ACKNOWLEDGMENTS
We thank Lené Holland for very useful comments on an earlier version of this manuscript. Also, we thank Dylan Lacewell for writing the data acquisition/analysis program and Carl Groat for expert machining. We are grateful to Lamprey Services, Northwoods Custom Products & Services, and Acme Lamprey for lamprey collection.
REFERENCES
- Alstermark B, Lundberg A, Pinter M, Sasaki S. Subpopulations and functions of long C3–C5 propriospinal neurons. Brain Res 404: 395–400, 1987. [DOI] [PubMed] [Google Scholar]
- Armstrong J, Zhang L, McClellan AD. Axonal regeneration of descending and ascending spinal projection neurons in larval lamprey. Exp Neurol 180: 156–166, 2003. [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Meizerov ES, Orlovsky GN, Pavlova GA, Popova LB. Activity of propriospinal neurons in C3 and C4 during fictitious locomotion in cats. Neurophysiology 17: 226–231, 1985. [Google Scholar]
- Awai L, Curt A. Intralimb coordination as a sensitive indicator of motor-control impairment after spinal cord injury. Front Hum Neurosci 8: 1–8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballermann M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci 23: 1988–1996, 2006. [DOI] [PubMed] [Google Scholar]
- Benthall KN, Hough RA, McClellan AD Propriospinal neurons can contribute to behavioral recovery following spinal cord injury in the lamprey. Program No. 830.13. 2013 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2013. Online. [Google Scholar]
- Berkinblit MB, Deliagina TG, Feldman AG, Gelfand IM, Orlovsky GN. Generation of scratching. I. Activity of spinal interneurons during scratching. J Neurophysiol 41: 1040–1057, 1978. [DOI] [PubMed] [Google Scholar]
- Berkowitz A, Stein PS. Activity of descending propriospinal axons in the turtle hindlimb enlargement during two forms of fictive scratching: broad tuning to regions of the body surface. J Neurosci 14: 5089–5104, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, Steward O. Repair of spinal cord injury with neuronal relays: from fetal grafts to neural stem cells. Brain Res 1619: 115–123, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci 7: 644–653, 2006. [DOI] [PubMed] [Google Scholar]
- Brown A, Weaver LC. The dark side of neuroplasticity. Exp Neurol 235: 133–141, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustein E, Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. I. Deficits and adaptive mechanisms. J Neurophysiol 80: 1245–1267, 1998. [DOI] [PubMed] [Google Scholar]
- Cazalets JR. Metachronal propagation of motoneurone burst activation in isolated spinal cord of newborn rat. J Physiol 568.2: 583–597, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Ferretti P. CNS regeneration in lower vertebrates. In: Cellular and Molecular Basis of Regeneration: From Invertebrates to Humans, edited by Ferretti P, Geraudie J. Chichester, UK: Wiley, 1998, p. 255–269. [Google Scholar]
- Conta AC, Stelzner DJ. The propriospinal system. In: The Spinal Cord, edited by Watson C, Paxinos G, and Kayalioglu G. New York: Academic, 2009, p. 180–190. [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 14: 69–74, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley KC, MacNeil BJ, Chopek JW, Sutherland S, Schmidt BJ. Neurochemical excitation of thoracic propriospinal neurons improves hindlimb stepping in adult rats with spinal cord lesions. Exp Neurol 264: 174–187, 2015. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Zaporazhets E, Schmidt BJ. Propriospinal neurons are sufficient for bulbospinal transmission of the locomotor command signal in the neonatal rat spinal cord. J Physiol 586: 1623–1635, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley KC, Zaporazhets E, Schmidt BJ. Propriospinal transmission of the locomotor command signal in the neonatal rat. Ann NY Acad Sci 1198: 42–53, 2010. [DOI] [PubMed] [Google Scholar]
- David S, Lacroix S. Molecular approaches to spinal cord repair. Annu Rev Neurosci 26: 411–440, 2003. [DOI] [PubMed] [Google Scholar]
- Davis GR, McClellan AD. Extent and time course of restoration of descending brainstem projections in spinal-transected lamprey. J Comp Neurol 344: 65–82, 1994. [DOI] [PubMed] [Google Scholar]
- Davis GR, Troxel M, Kohler VJ, Grossmann EM, McClellan AD. Time course of locomotor recovery and functional regeneration in spinal-transected lamprey: kinematics and electromyography. Exp Brain Res 97: 83–95, 1993. [DOI] [PubMed] [Google Scholar]
- Dunlop SA. Activity dependent plasticity: Implications for recovery after spinal cord injury. Trends Neurosci 31: 410–418, 2008. [DOI] [PubMed] [Google Scholar]
- Eidelberg E, Story JL, Walden JG, Meyer BL. Anatomical correlates of return of locomotor function after partial spinal cord lesions in cats. Exp Brain Res 42: 81–88, 1981. [DOI] [PubMed] [Google Scholar]
- Filli L, Engmann AK, Zörner B, Winmann O, Moraitis T, Gullo M, Kasper H, Schneider R, Schwab ME. Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J Neurosci 34: 13399–13410, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filli L, Schwab ME. Structural and functional reorganization of propriospinal connections promotes functional recovery after spinal cord injury. Neural Regen Res 10: 509–513, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JR, Graham BA, Galea MP, Callister RJ. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology 60: 809–822, 2011. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Makarovskii AN, Nikitin OA. Control of locomotor activity in humans and animals in the absence of supraspinal influences. Neurosci Behav Physiol 32: 417–423, 2002. [DOI] [PubMed] [Google Scholar]
- Gerasimenko Y, Musienko P, Bogacheva I, Moshonkina T, Savochin A, Lavrov I, Roy VR, Edgerton VR. Propriospinal bypass of the serotonergic system that can facilitate stepping. J Neurosci 29: 5681–5689, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagevik A, McClellan AD. Coupling of spinal locomotor networks in larval lamprey revealed by receptor blockers for inhibitory amino acids: neurophysiology and computer modeling. J Neurophysiol 72: 1810–1829, 1994. [DOI] [PubMed] [Google Scholar]
- Iwahara T, Atsuta Y, Garcia-Rill E, Skinner RD. Locomotion induced by spinal cord stimulation in the neonate rat in vitro. Somatosens Mot Res 8: 281–287, 1991a. [DOI] [PubMed] [Google Scholar]
- Iwahara T, Atsuta Y, Garcia-Rill E, Skinner RD. Spinal cord stimulation-induced locomotion in the adult cat. Brain Res Bull 28: 99–105, 1991b. [DOI] [PubMed] [Google Scholar]
- Jiang W, Drew T. Effects of bilateral lesions of the dorsolateral funiculi and dorsal columns at the level of the low thoracic spinal cord on the control of locomotion in the adult cat. I. Treadmill walking. J Neurophysiol 76: 849–866, 1996. [DOI] [PubMed] [Google Scholar]
- Jordan LM. Initiation of locomotion from the mammalian brainstem. In: Neurobiology of Vertebrate Locomotion (Wenner-Gren Centre International Symposium Series), edited by Grillner S, Stein PS, Stuart DG, Forssberg H, and Herman RM. London: Macmillan, 1986, vol. 45, p. 21–37. [Google Scholar]
- Jordan LM, Schmidt BJ. Propriospinal neurons involved in the control of locomotion: potential targets for repair strategies? Prog Brain Res 137: 125–139, 2002. [DOI] [PubMed] [Google Scholar]
- Juvin L, Simmers J, Morin D. Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J Neurosci 25: 6025–6035, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazennikov OV, Selionov VA, Shik ML, Iakovleva GV. Neurons of upper cervical segments responding to stimulation of the bulbar locomotor strip. Neurophysiology 11: 179–185, 1979. [PubMed] [Google Scholar]
- Kazennikov OV, Shik ML, Iakovleva GV. Stepping movements induced in cats by stimulation of the dorsolateral funiculus of the spinal cord. Bull Exp Biol Med 96: 1036–1039, 1983a. [PubMed] [Google Scholar]
- Kazennikov OV, Shik ML, Iakovleva GV. Responses of upper cervical spinal neurons in cats to stimulation of the brain-stem locomotor region at different frequencies. Neurophysiology 15: 256–261, 1983b. [PubMed] [Google Scholar]
- Kazennikov OV, Shik ML, Iakovleva GV. Synaptic responses of propriospinal neurons to stimulation of the stepping strip of the dorsolateral funiculus in cats. Neurophysiology 17: 195–202, 1985. [PubMed] [Google Scholar]
- Lassek AM, Anderson PA. Motor function after spaced contralateral hemisections in the spinal cord. Neurology 11: 362–365, 1961. [DOI] [PubMed] [Google Scholar]
- Lennard PR, Stein PS. Swimming movements elicited by electrical stimulation of turtle spinal cord. I. Low-spinal and intact preparations. J Neurophysiol 40: 768–778, 1977. [DOI] [PubMed] [Google Scholar]
- Loy DN, Talbott JF, Onifer SM, Mills MD, Burke DA, Dennison JB, Fajardo LC, Magnuson DS, Whittemore SR. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp Neurol 177: 575–580, 2002. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Trinder TC, Zhang YP, Burke D, Morassutti DJ, Shields CB. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp Neurol 156: 191–204, 1999. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Locomotor recovery in spinal-transected lampreys. Regenerated coordinating neurons and mechanosensory inputs couple locomotor activity across a spinal lesion. Neuroscience 35: 675–685, 1990a. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Locomotor recovery in spinal-transected lampreys. Role of functional regeneration of descending axons from brainstem locomotor command neurons. Neuroscience 37: 781–798, 1990b. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Time course of locomotor recovery and functional regeneration in spinal cord-transected lamprey: in vitro preparations. J Neurophysiol 72: 847–860, 1994a. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Functional regeneration and restoration of locomotor activity following spinal cord transection in the lamprey. Prog Brain Res 103: 203–217, 1994b. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Organization of spinal locomotor networks: contributions from model systems. Comments Theo Biol 4: 63–91, 1996. [Google Scholar]
- McClellan AD. Spinal cord injury: lessons from locomotor recovery and axonal regeneration in lower vertebrates. Neuroscientist 4: 250–263, 1998. [Google Scholar]
- McClellan AD. Spinal cord injury–the lamprey model. In: Animal Models of Spinal Cord Repair, Neuromethods, edited by Aldskogius H. New York: Humana, 2013, vol. 76, chapt. 4, p. 63–108. [Google Scholar]
- McClellan AD, Hagevik A. Descending control of turning locomotor activity in larval lamprey: neurophysiology and computer modeling. J Neurophysiol 78: 214–228, 1997. [DOI] [PubMed] [Google Scholar]
- McClellan AD, Hagevik A. Coordination of spinal locomotor activity in the lamprey: long distance coupling of spinal oscillators. Exp Brain Res 126: 93–108, 1999. [DOI] [PubMed] [Google Scholar]
- McClellan AD, Jang W. Mechanosensory inputs to the central pattern generators for locomotion in the lamprey spinal cord: resetting, entrainment, and computer modeling. J Neurophysiol 70: 2442–2454, 1993. [DOI] [PubMed] [Google Scholar]
- McClellan AD, Pale T, Messina A, Buso S, Shebib A. Similarities and differences for swimming in larval and adult lampreys. Physiol Biochem Zool 89: 294–312, 2016. [DOI] [PubMed] [Google Scholar]
- Muir GD, Webb AA, Kanagal S, Taylor L. Dorsolateral cervical spinal injury differentially affects forelimb and hindlimb action in rats. Eur J Neurosci 25: 1501–1510, 2007. [DOI] [PubMed] [Google Scholar]
- Noga BR, Kriellaars DJ, Jordan LM. The effect of selective brainstem or spinal cord lesions on treadmill locomotion evoked by stimulation of the mesencephalic or pontomedullary locomotor regions. J Neurosci 11: 1691–1700, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion. From Mollusc to Man. New York: Oxford University Press, 1998. [Google Scholar]
- Reed WR, Shum-Siu A, Onifer SM, Magnuson DS. Inter-enlargement pathways in the ventrolateral funiculus of the adult rat spinal cord. Neuroscience 142: 1195–1207, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse DT, McClellan AD. Descending propriospinal neurons in normal and spinal cord-transected lamprey. Exp Neurol 146: 13–124, 1997. [DOI] [PubMed] [Google Scholar]
- Schomburg ED. Spinal sensorimotor systems and their supraspinal control. Neurosci Res 7: 265–340, 1990. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 76: 319–370, 1996. [DOI] [PubMed] [Google Scholar]
- Shaw AC, Jackson AW, Holmes T, Thurman S, Davis GR, McClellan AD. Descending brain neurons in larval lamprey: spinal projection patterns and initiation of locomotion. Exp Neurol 224: 527–541, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman MI, Jin LQ, Selzer ME. Regeneration in the lamprey spinal cord. In: Model Organisms in Spinal Cord Regeneration, edited by Becker CG, Becker T. New York: Wiley, 2017, p. 229–262. [Google Scholar]
- Shik ML. Action of the brainstem locomotor region on spinal stepping generators via propriospinal pathways. In: Spinal Cord Reconstruction, edited by Kao CC, Bunge RP, and Reier RP. New York: Raven, 1983, p. 421–434. [Google Scholar]
- Shik ML. Recognizing propriospinal and reticulospinal systems of initiation of stepping. Motor Control 1: 310–313, 1997. [Google Scholar]
- Skinner RD, Adams RJ, Remmel RS. Responses of long descending propriospinal neurons to natural and electrical types of stimuli in cat. Brain Res 196: 387–403, 1980. [DOI] [PubMed] [Google Scholar]
- Steeves JD, Jordan LM. Localization of a descending pathway in the spinal cord which is necessary for controlled treadmill locomotion. Neurosci Lett 20: 283–288, 1980. [DOI] [PubMed] [Google Scholar]
- Stein PS. Swimming movements elicited by electrical stimulation of the turtle spinal cord: the high spinal preparation. J Comp Physiol 124: 203–210, 1978. [Google Scholar]
- Stelzner DJ, Cullen JM. Do propriospinal projections contribute to hindlimb recovery when all long tracts are cut in neonatal or weanling rats? Exp Neurol 114: 193–205, 1991. [DOI] [PubMed] [Google Scholar]
- Tracey DJ. Ascending and descending pathways in the spinal cord. In: The Rat Nervous System (3rd ed), edited by Paxinos G. New York: Academic, 2004, p. 149–164. [Google Scholar]
- Wallén P, Grillner s. The effect of current passage on N-methyl-d-aspartate-induced, tetrodotoxin-resistant membrane potential oscillations in lamprey neurons active during locomotion. Neurosci Lett 56: 87–93, 1985. [DOI] [PubMed] [Google Scholar]
- Webb AA, Muir GD. Course of motor recovery following ventrolateral spinal cord injury in the rat. Behav Brain Res 155: 55–65, 2004. [DOI] [PubMed] [Google Scholar]
- Windle WF, Smart JO, Beers JJ. Residual function after subtotal spinal cord transection in adult cats. Neurology 8: 518–521, 1958. [DOI] [PubMed] [Google Scholar]
- Yakovenko S, Kowalczewski J, Prochazka A. Intraspinal stimulation caudal to spinal cord transections in rats. Testing the propriospinal hypothesis. J Neurophysiol 97: 2570–2574, 2007. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T. Descending pathways eliciting forelimb stepping in the lateral funiculus: experimental studies with stimulation and lesions of the cervical cord in decerebrate cats. Brain Res 379: 125–136, 1986. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T. Monopodal fictive locomotion evoked by cervical cord stimulation in decerebrate cats. Neurosci Lett 74: 69–74, 1987. [DOI] [PubMed] [Google Scholar]
- Zaporozhets E, Cowley KC, Schmidt BJ. Propriospinal neurons contribute to bulbospinal transmission of the locomotor command signal in the neonatal rat spinal cord. J Physiol 572: 443–458, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaporozhets E, Cowley KC, Schmidt BJ. Neurochemical excitation of propriospinal neurons facilitates locomotor command signal transmission in the lesioned spinal cord. J Neurophysiol 105: 2818–2829, 2011. [DOI] [PubMed] [Google Scholar]
- Zhang L, Palmer R, McClellan AD. Increase in descending brain-spinal cord projections with age in larval lamprey: implications for spinal cord injury. J Comp Neurol 447: 128–137, 2002. [DOI] [PubMed] [Google Scholar]