Clinical administration of the macrolide antibiotic clarithromycin has been associated with side effects such as mania, agitation, and delirium. Here, we investigated the adverse effects of this antibiotic on CA3 pyramidal cell excitability. Clarithromycin induces hyperexcitability in single neurons and is related to a reduction in GABAergic signaling. Our results support a potentially new application of clarithromycin as a stimulant to facilitate emergence from anesthesia or to normalize vigilance.
Keywords: clarithromycin, hippocampus, neuronal excitability, GABAA receptor
Abstract
Antibiotics are used in the treatment and prevention of bacterial infections, but effects on neuron excitability have been documented. A recent study demonstrated that clarithromycin alleviates daytime sleepiness in hypersomnia patients (Trotti LM, Saini P, Freeman AA, Bliwise DL, García PS, Jenkins A, Rye DB. J Psychopharmacol 28: 697–702, 2014). To explore the potential application of clarithromycin as a stimulant, we performed whole cell patch-clamp recordings in rat pyramidal cells from the CA3 region of hippocampus. In the presence of the antibiotic, rheobase current was reduced by 50%, F-I relationship (number of action potentials as a function of injected current) was shifted to the left, and the resting membrane potential was more depolarized. Clarithromycin-induced hyperexcitability was dose dependent; doses of 30 and 300 μM clarithromycin significantly increased the firing frequency and membrane potential compared with controls (P = 0.003, P < 0.0001). We hypothesized that clarithromycin enhanced excitability by reducing GABAA receptor activation. Clarithromycin at 30 μM significantly reduced (P = 0.001) the amplitude of spontaneous miniature inhibitory GABAergic currents and at 300 μM had a minor effect on action potential width. Additionally, we tested the effect of clarithromycin in an ex vivo seizure model by evaluating its effect on spontaneous local field potentials. Bath application of 300 μM clarithromycin enhanced burst frequency twofold compared with controls (P = 0.0006). Taken together, these results suggest that blocking GABAergic signaling with clarithromycin increases cellular excitability and potentially serves as a stimulant, facilitating emergence from anesthesia or normalizing vigilance in hypersomnia and narcolepsy. However, the administration of clarithromycin should be carefully considered in patients with seizure disorders.
NEW & NOTEWORTHY Clinical administration of the macrolide antibiotic clarithromycin has been associated with side effects such as mania, agitation, and delirium. Here, we investigated the adverse effects of this antibiotic on CA3 pyramidal cell excitability. Clarithromycin induces hyperexcitability in single neurons and is related to a reduction in GABAergic signaling. Our results support a potentially new application of clarithromycin as a stimulant to facilitate emergence from anesthesia or to normalize vigilance.
γ-aminobutyric acid (GABA) is the most abundant inhibitory neurotransmitter in the brain and plays a principal role in reducing neuronal excitability throughout the nervous system. Electrophysiological evidence of GABA-mediated postsynaptic inhibitory currents exists for most types of subcortical, hippocampal, and cortical neurons (Galarreta and Hestrin 2002; Henderson et al. 2011; MacIver et al. 2011; Nishikawa and MacIver 2000). GABAergic neurons are involved in the regulation of arousal and transitions between conscious states such as sleep and coma (Kretschmannova et al. 2013; Rye et al. 2012; Schwartz et al. 2010). In addition to a role in sleep disorders, modulation of the GABA neurotransmitter system is involved in neurological disorders such as Alzheimer's disease, traumatic brain injury, and stroke (Clarkson et al. 2010; Lake et al. 2015; Limon et al. 2012; Wright et al. 2014).
Rye and collaborators (2012) demonstrated that an endogenous substance present in the cerebrospinal fluid from hypersomnolent patients augments inhibitory GABAA receptor currents in heterologous expression systems. These patients' symptoms could be mitigated with administration of the GABAA receptor antagonist flumazenil. This work was followed by Trotti et al. (2014) describing effective treatment of GABA-related hypersomnia with the antibiotic clarithromycin, a semisynthetic macrolide derived from erythromycin and mainly prescribed for respiratory infections. Clinically, administration of clarithromycin has been associated with the development of mania, febrile euphoria, agitation and paranoid delusions (Abouesh et al. 2002; Geiderman 1999), and delirium due to nonconvulsive status epilepticus (di Poggio et al. 2011; Mermelstein 1998; Özsoylar et al. 2007; Steinman and Steinman 1996); however, its effects on GABA signaling remain largely uncharacterized.
The GABAA receptor has multiple binding sites for exogenous and endogenous modulatory agents. Anesthetic and anticonvulsant drugs enhance Cl− influx through the channel in response to GABA binding (Garcia et al. 2010). In general, GABAA antagonists result in central nervous system excitation and convulsions. Many clinical therapeutics have off-target effects on GABAA receptors, although at commonly administered doses these effects are rarely consequential. Furosemide (Korpi and Lüddens 1997), fluoroquinolones (Sarro and Sarro 2001), and sulfonylureas (Grenhoff and Johnson 1996) negatively allosterically modulate inhibitory Cl− currents through the GABA channels. Penicillin-induced convulsions have been attributed to the interaction of this drug with specific sites on the GABA protein (Wallace 1997).
In light of the clinical evidence demonstrating a mitigating effect of clarithromycin on hypersomnic patients, we hypothesized that clarithromycin antagonizes postsynaptic GABAA receptors in neurons and could potentially cause an increase of neuron excitability that resembles seizure activity. We tested this hypothesis by pharmacological investigation of the neurophysiology of the CA3 region of the rodent hippocampus, a well-established ex vivo model often used to characterize hyperexcitability and seizure generation (Leschinger et al. 1993; Moddel et al. 2003).
METHODS
Slice preparation, solutions, and electrophysiology.
All animal procedures were carried out according to protocols reviewed and approved by the Atlanta VA Medical Center Institutional Animal Care and Use Committee and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA), and postnatal pups (15–28 days) were used in the study. On each experimental day, pups were deeply anesthetized with isoflurane (Piramal Healthcare, Digwal, India) and transcardial perfusion was performed with ice-cold sucrose solution containing (in mM) 200 sucrose, 2.5 KCl, 1.2 NaH2PO4, 25 NaHCO3, 20 dextrose, 0.5 CaCl2, 7 MgCl2, 2.4 sodium pyruvate, and 1.3 l-ascorbic acid and oxygenated with 95% O2-5% CO2. Rat decapitation was performed by guillotine, and the brain was quickly removed, immersed in cold sucrose solution, and mounted on the flat surface of the tray of a Leica VT-1000 S microtome (Leica Microsystems, Bannockburn, IL). Horizontal slices (350 μm thick) were put to recover in a holding chamber in regular artificial cerebrospinal fluid (ACSF) at 34°C. The ACSF contained (in mM) 125 NaCl, 2.8 KCl, 1 NaH2PO4, 26 NaHCO3, 10 glucose, 2 CaCl2, and 1.5 MgSO4.
Chemicals were purchased from Sigma (St. Louis, MO) or Abcam (Cambridge, MA). Individual slices were transferred to the recording chamber and continuously superfused with oxygenated ACSF at 32°C and a flow rate of 1 ml/min. The hippocampus was visually identified by video microscopy (Olympus model BX51WI outfitted with differential interface contrast and an IR-sensitive digital CCD camera, Orca-05G, Hamamatsu, Tokyo, Japan). The CA3 region of hippocampus was identified under ×4 magnification; ×40 magnification was used to identify individual cells for patch-clamp recording in the whole cell configuration (Wu et al. 2015).
Whole cell patch-clamp recordings were done on the soma of CA3 pyramidal neurons with micropipettes made from thin-walled capillaries (WPI, Sarasota, FL). Electrodes were fashioned at resistances in the range 4–8 MΩ with a P-1000 Flaming/Brown puller (Sutter Instrument, Novato, CA). Pipettes were filled with a K-gluconate solution with a low concentration of Cl− for current-clamp recordings containing (in mM) 130 K-gluconate, 10 NaCl, 10 KCl, 10 HEPES, 1 MgCl2, 0.5 Na-GTP, and 1 Mg-ATP, titrated to pH 7.2 with KOH. All voltages were corrected for liquid junction potential (∼30 mV) before a gigaohm seal was formed. Once whole cell configuration was established, each neuron was not stimulated for at least 5 min. Cells were included in the experiments if they met the following criteria: the resting membrane potential (VRest) was at least −55 mV, and series resistance (Rs) was lower than 25 MΩ. During the course of the experiments input resistance (Rin) and Rs were periodically monitored, and experiments were rejected if Rs or Rin deviated by >30% of their respective initial values.
The outward potassium currents were recorded in voltage-clamp mode with a pipette solution containing (in mM) 120 KCl, 10 EGTA, 10 HEPES, 2 MgCl2, 0.5 Na-GTP, and 1 Mg-ATP, titrated to pH 7.2 with KOH. Junction potential (approximately −7 mV) was corrected for all voltages. Rs was compensated 30–60%. Linear leak and residual capacity currents were subtracted with a P/4 subtraction protocol.
Miniature inhibitory postsynaptic currents (mIPSCs) were recorded in voltage-clamp mode with high-Cl− concentration pipette solution containing (in mM) 94 K-gluconate, 10 NaCl, 36 KCl, 1.1 EGTA, 10 HEPES, 1 MgCl2, 0.1 CaCl2, 0.5 Na-GTP, and 1 Mg-ATP, titrated to pH 7.2 with KOH. Spontaneous miniature inhibitory GABAergic currents (GABA-mPSCs) were recorded at a holding potential set up to VRest of individual cells, on average −65.1 ± 0.8 mV (mean ± SE, n = 30). GABA-mPSCs were isolated by using glutamate synaptic antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μM), d-(−)-2-amino-5-phosphonopentanoic acid (d-AP5; 50 μM), and the Na+ channel blocker TTX (1 μM). They were analyzed with the Mini Analysis Program (Synaptosoft, Fort Lee, NJ). During recordings Rs was monitored, and when it exceeded 20 MΩ the experiment was finalized.
Data acquisition was performed with MultiClamp Amplifier 700B in conjunction with pCLAMP 10.3 software and Digidata 1322A AD/DA interface (Molecular Devices, Sunnyvale, CA). Whole cell patch-clamp recordings were low-pass filtered at 10 kHz and digitized at 20–40 kHz. GABA-mPSCs were low-pass filtered at 6 kHz. All analysis of electrophysiological data was performed with Clampfit 10.3 (Molecular Devices).
Drug application.
Two methods of delivery of clarithromycin were employed for the study: acute application of study drug and preincubation. Clarithromycin powder (manufactured by Sigma) was dissolved in dimethyl sulfoxide (DMSO) to 100 mM stock solution. With this stock solution, clarithromycin was diluted to 3, 30, and 300 μM with ACSF. The final DMSO concentration was ≤0.3%, well below toxic dose ranges (1–10%) as observed by Galvao et al. (2014). Control data were recorded in equivalent DMSO concentrations.
For experiments using acute application of study drug, hippocampal slices were transferred into holding chambers with ACSF solution immediately after cutting and were first exposed to study drug after whole cell patch-clamp configuration was achieved. The effect of clarithromycin was evident 3–5 min after the solution change. To determine whether saturation of the slice with clarithromycin affected the results, several of these experiments were repeated in slices that were exposed to clarithromycin for 2–4 h (preincubation) before transfer to the recording chamber. To measure the excitability characteristics of CA3 neurons in the absence of spontaneous network firing, synaptic blockers of glutamatergic signaling CNQX (10 μM) and d-AP5 (50 μM) were included in the patch-clamp superfusion but not in the holding chambers or chambers for clarithromycin preincubation.
Quantitative measurement of biophysical parameters.
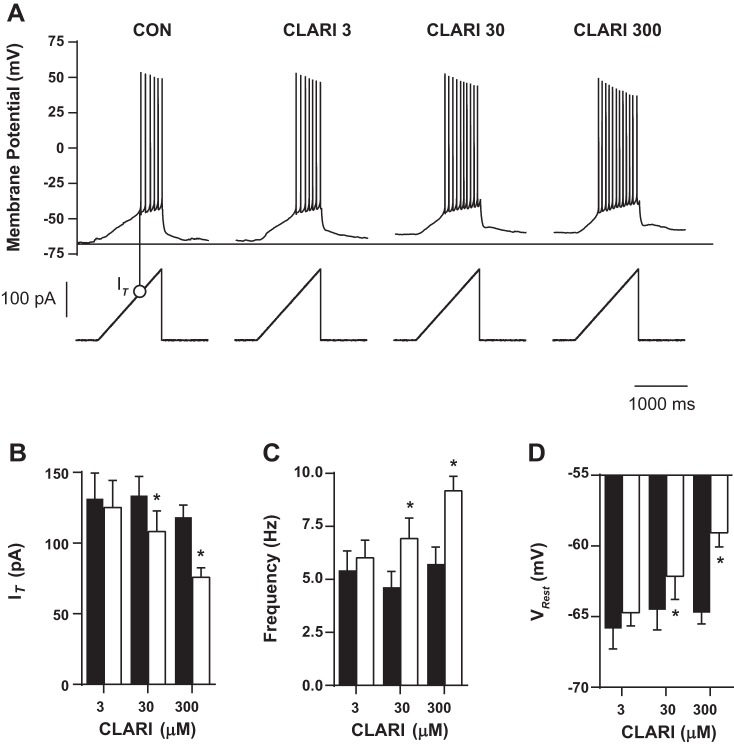
Neuronal excitability and passive properties such as whole cell membrane capacitance and membrane resistance were periodically monitored with the automated membrane test function in Clampex 10.3 (Molecular Devices). Custom protocols were used to quantify intrinsic and synaptic parameters among CA3 pyramidal cells in the study; further details are presented below. To evaluate intrinsic excitability, neurons were injected with depolarizing current pulses (ranging from 20 to 220 pA; 800 ms in duration). Action potential firing frequency was calculated for each pulse. Only action potentials that occurred at 50 ms or longer after the onset of the step current injection were included in the analysis (Dougherty et al. 2012). F-I curves (frequency of action potential firing as a function of injected current) were constructed. To measure Rin, a hyperpolarizing 20-pA pulse current of 800 ms was applied and voltage response was measured at steady state. Rheobase (the minimal depolarizing current amplitude injected at the soma to make the cell fire an action potential) was measured with 1-pA current steps at duration 800 ms. Action potential voltage threshold (VT) was determined as the measured voltage where the change of voltage with time exceeded 20 mV/ms in response to a current step lasting 800 ms (Dougherty et al. 2012). Action potential current threshold (IT) was measured as minimum injected ramp current initializing action potential burst (see Fig. 2A). Action potential half-width was measured as the duration of the spike at the half-maximum of the action potential amplitude.
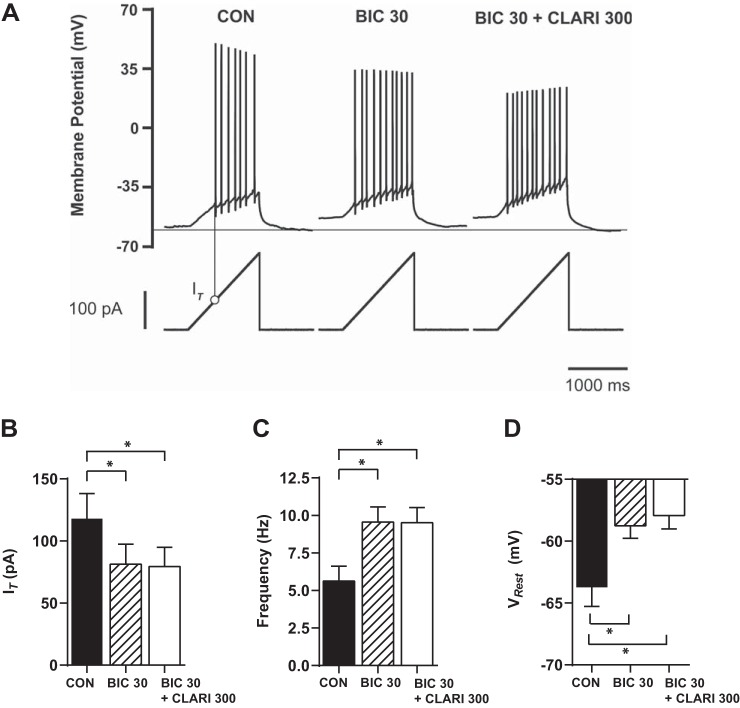
Fig. 2.
Clarithromycin-induced hyperexcitability is dose dependent. A: characteristic voltage responses (top) to an injected current ramp (bottom). Minimum injected ramp current initializing action potentials was measured. Action potential threshold current (IT) is designated for the control experiment by a thin vertical line with an open circle and summarized in B. Horizontal line indicates level of VRest in control conditions (CON). B–D: neurons were exposed to different clarithromycin concentrations. Each neuron was also exposed to control conditions and acts as its own control. Bar graph pairs represent average values ± SE in controls (filled bars) and in clarithromycin (open bars). Doses of 30 and 300 μM clarithromycin significantly affected neuronal firing expressed as IT (CON vs. CLARI 3: n = 7, CON vs. CLARI 30: n = 12, CON vs. CLARI 300: n = 20; paired t-test, P = 0.2, *P = 0.002, *P = 0.0001, respectively), frequency (n = 7, n = 12, n = 20; paired t-test, P = 0.1, *P = 0.003, *P < 0.0001), and depolarized VRest (n = 7, n = 13, n = 21; paired t-test, P = 0.3, *P = 0.04, *P < 0.0001).
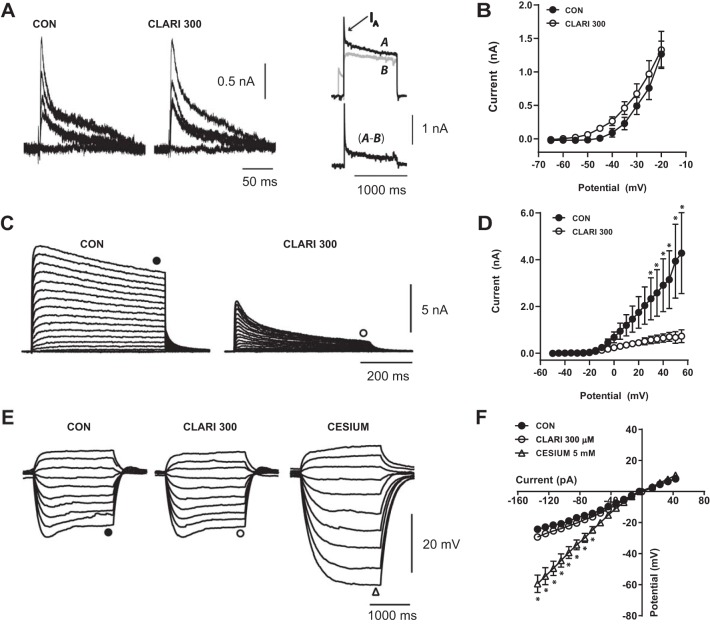
To obtain fast transient outward K+ currents (IA), 5-mV voltage steps (ranging from −65 to −20 mV; 1,000 ms in duration) were applied from a holding potential of −80 mV, eliciting total K+ currents including IA. The same voltage step was preceded by brief (100 ms) prepulse to −40 mV, which inactivates IA. To isolate IA, a subtraction of these two traces was applied (see Fig. 5A, right) (Wilhelm et al. 2009; Zbicz and Weight 1985).
Fig. 5.
Acute 300 μM clarithromycin affects sustained K+ currents during repolarization. A: representative isolated IA current traces activated by 1-s steps to potentials −65, −35, −30, −25, −20 mV from a holding potential of −80 mV in controls (left) and after 5 min of clarithromycin treatment (center). Top right: representative K+ currents (A, black trace) elicited by depolarizing step to −10 mV from a holding potential of −80 mV. To isolate IA from total K+ current the same voltage step was preceded by a brief (100 ms) prepulse to −40 mV (B, gray trace). Bottom right: difference current (A − B) reveals a fast-inactivating K+ current IA. B: peak current-voltage relationships remain the same after 5-min exposure to clarithromycin (n = 7, 2-way repeated-measures ANOVA, P > 0.9). C: representative sustained K+ current evoked by depolarizing 5-mV steps (ranging from −55 to 50 mV) from a −40-mV holding potential obtained in control (left) and in the presence of clarithromycin ACSF (right) in the same neuron. D: clarithromycin reduces sustained K+ current compared with controls (n = 5, 2-way repeated-measures ANOVA, *P < 0.01). E and F: characteristic membrane potential responses to hyperpolarizing and depolarizing 20-pA current steps (ranged from −130 to 50 pA of resting membrane potential −65.4 mV) in the representative neuron. E: voltage “sag” resulting from activation of Ih in controls (left) was still present in 300 μM of clarithromycin (center). F: I-V relationship between steady-state amplitude and injected current did not change after antibiotic exposure (CON vs. CLARI 300; n = 7; 2-way repeated-measures ANOVA, P ≥ 0.26). To distinguish Ih currents, external Cs+ (Ih antagonist) was added, resulting in blockade of characteristic “sag” current (E, right) and a linear voltage-current relationship below −15 mV (cesium vs. CON, cesium vs. CLARI 300; n = 7; 2-way repeated-measures ANOVA, *P ≤ 0.025; F). Each symbol represents mean ± SE.
Sustained outward K+ currents were achieved by applying depolarizing steps ranging from −50 to 55 mV (in 5-mV increments, 500-ms duration) from a holding potential of −40 mV (to inactivate IA). The peak of IA and the steady-state amplitude of the sustained currents were calculated for each pulse to construct an I-V plot (current as a function of depolarizing voltage step). To evaluate hyperpolarization-activated cationic currents (Ih), hyperpolarizing and depolarizing 5-pA current steps (range −130 to 50 pA; 1,000-ms duration) of VRest (approximately −62.4 mV) were applied in current-clamp mode (see Fig. 5E).
Local field potential recordings.
Experiments were performed with a Kerr Tissue Recording System and amplifier (Kerr Scientific Instruments, Auckland, New Zealand). Signals were processed with a Digidata 1440A digitizer and acquired with pCLAMP version 10.3 software (Molecular Devices). After incubation in normal ACSF, slices were transferred to the recording chamber. Inflow to the recording chamber of perfusion solution at temperatures above 26°C was gravity controlled at 1–2 ml/min. An extracellular metal wire recording electrode was placed in contact with the stratum pyramidale in the CA3 region of the hippocampus to capture spontaneous local field potentials. Spontaneous activity in normal ACSF was recorded for at least 10 min before wash in of epileptogenic solution. Seizure activity (seizure model) was induced with high K+-low Mg2+ ACSF (8.5 mM KCl and 0 mM MgSO4) and recorded for at least 30 min. All field potential data were analyzed off-line with Clampfit 10.3 software (Molecular Devices). Trace files were 250-Hz low-pass filtered (sampled at 20 Hz) to decrease background noise, and baseline variability was adjusted manually. Seizurelike activity, defined as short bursts of synchronized multiunit firing, was identified via amplitude threshold detection. Artifact detection was performed through visual inspection of burst shape, duration, and amplitude. The following parameters were derived from the spontaneous seizure activity: latency of onset following wash in of epileptogenic solution, average frequency of seizurelike events, and peak frequency. Because of variability in the length of seizure episodes, a 2-min region of interest was used to calculate the average burst frequency from an area of high activity in the burst envelope consistent among preparations.
Statistics.
Statistical analysis was performed with Prism 6 (GraphPad Software, La Jolla, CA). Statistical significance between comparisons of two groups was analyzed by paired or unpaired t-test for data that were normally distributed (as measured by either Pearson or Shapiro-Wilk normality test). When data were not normally distributed, Wilcoxon matched-pairs signed-rank test or unpaired Mann-Whitney U-test was administered. Comparisons between F-I curve relationships were performed with two-way repeated-measures ANOVA test followed by Bonferroni test for multiple comparisons. Normally distributed data are presented as means ± SE in the text, whereas skewed data are presented as medians with quartiles.
RESULTS
Acute exposure to clarithromycin enhances CA3 pyramidal neuron excitability via increase in rheobase current.
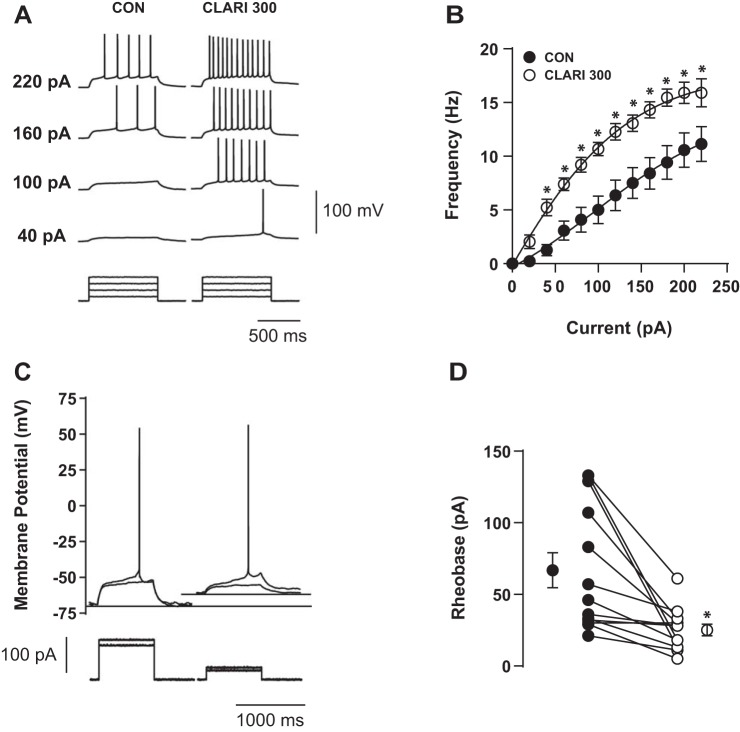
Figure 1A shows characteristic voltage responses to increasing current injections recorded in a representative neuron in control conditions (CON; Fig. 1A, left) and in the presence of 300 μM clarithromycin (CLARI 300; Fig. 1A, right). To prevent the influence of spontaneous excitatory synaptic input on action potential generation, glutamatergic synapses were pharmacologically blocked via application of antagonists CNQX (10 μM) and d-AP5 (50 μM). At each tested (nonzero) amplitude of injected current, 300 μM clarithromycin exposure enhanced the firing frequency for all injected current ≥40 pA (n = 11, 2-way repeated-measures ANOVA, P < 0.0001; Fig. 1B). Three hundred micromolar clarithromycin reduced the minimal applied current necessary to induce an action potential (rheobase current) (Fig. 1C). Every recorded cell demonstrated a decrease in rheobase current (n = 13, paired t-test, P = 0.003; Fig. 1D), suggesting a change in excitability due to clarithromycin exposure. Bath addition of 300 μM clarithromycin depolarized VRest (CON: −64.8 ± 3.5 mV, CLARI 300: −58.3 ± 1.1 mV; n = 13; paired t-test, P = 0.0003). Changes in Rin (CON: 276.5 ± 22.9 MΩ, CLARI 300: 304.9 ± 22.3 MΩ; n = 18; paired t-test, P = 0.3) and in VT (CON: −40.8 ± 0.6 mV, CLARI 300: −40.0 ± 0.5 mV; n = 12; paired t-test, P = 0.08) did not reach statistical significance.
Fig. 1.
Clarithromycin induced hyperexcitability in CA3 region of hippocampus. A: characteristic voltage responses to increasing current injections (bottom) recorded in a representative neuron in control bath solution (CON; left) and after 5-min acute application of 300 μM clarithromycin (CLARI 300; right). To block glutamatergic synaptic inputs, AMPA/kainate and NMDA receptor antagonists CNQX (10 μM) and d-AP5 (50 μM) were applied in both situations. B: short-term bath exposure to 300 μM clarithromycin significantly enhanced firing frequency and shifted the F-I curve to the left compared with controls (CON) (n = 11, 2-way repeated-measures ANOVA, *P < 0.0001). Each symbol represents mean ± SE. C: characteristic single action potential response (top) to minimum current injection (bottom) from the neuron in A. Rheobase current was reduced by half in the presence of 300 μM clarithromycin (right). Thin horizontal lines illustrate levels of resting membrane potential (VRest). In this example, clarithromycin administration depolarized VRest ∼5 mV compared with baseline (−65 mV). D: lines indicate paired measurements in the same neuron in controls (●) and washed in clarithromycin ACSF (○). In 13 tested cells, 5- to 10-min exposure to antibiotic+ACSF significantly decreased rheobase current vs. controls (CON: 66.8 ± 12.2 pA, CLARI 300: 25.1 ± 4.1 pA; paired t-test, *P = 0.003). Symbols on left and right represent average values ± SE.
Concentration of clarithromycin affects CA3 pyramidal neuron excitability.
Representative examples of voltage responses to ramp stimulation at different concentrations of clarithromycin application are shown in Fig. 2A. In the presence of physiologically relevant concentrations of clarithromycin (3–300 μM) burst firing occurred at lower IT (CON vs. CLARI 3: 131.5 ± 18.0 pA vs. 125.2 ± 19.1 pA, n = 7; CON vs. CLARI 30: 133.7 ± 13.4 pA vs. 108.1 ± 14.6 pA, n = 12; CON vs. CLARI 300: 118.3 ± 8.7 pA vs. 75.8 ± 6.8 pA, n = 20; paired t-test, P = 0.2, P = 0.002, P = 0.0001, respectively; Fig. 2B), and firing frequency progressively increased with increasing concentrations of clarithromycin (3, 30, 300 μM; CON vs. CLARI 3: 5.4 ± 0.9 Hz vs. 6.02 ± 0.8 Hz, n = 7; CON vs. CLARI 30: 4.6 ± 0.7 Hz vs. 6.9 ± 1.0 Hz, n = 12; CON vs. CLARI 300: 5.7 ± 0.8 Hz vs. 9.2 ± 0.7 Hz; n = 20; paired t-test, P = 0.1, P = 0.003, P < 0.0001, respectively; Fig. 2C). Our lowest tested concentration of clarithromycin (3 μM) did not result in significant changes in CA3 response to ramp stimulation.
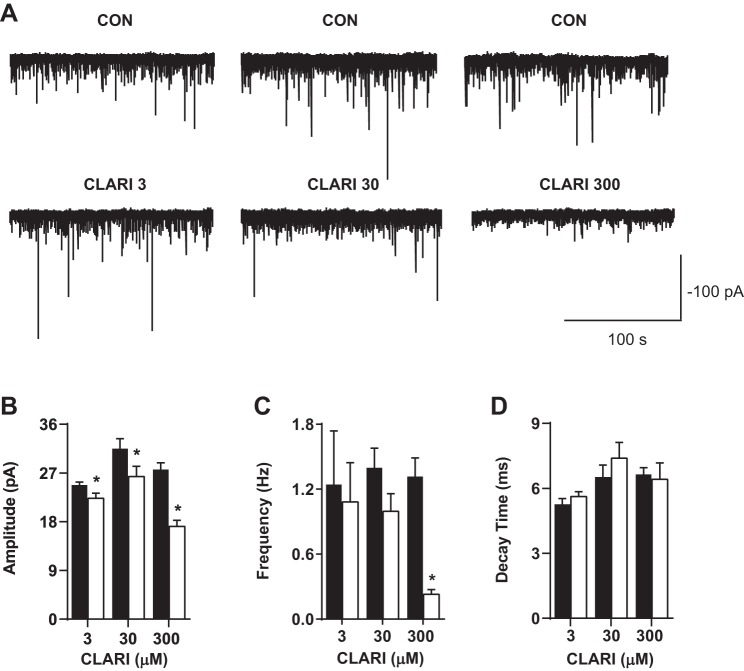
Clarithromycin inhibits GABA-mPSCs.
Isolation of mIPSCs resulting from quantal release of GABA was accomplished by applying TTX (1 μM), CNQX (10 μM), and d-AP5 (50 μM) to the superfused solution. Figure 3A depicts representative traces of GABA-mPSCs in control and clarithromycin conditions. Clarithromycin at all concentrations reduced the amplitude of miniature currents, suggesting antagonism of postsynaptic GABA receptors by this antibiotic (CON vs. CLARI 3: 24.8 ± 0.6 pA vs. 22.3 ± 0.9 pA, n = 15; CON vs. CLARI 30: 31.5 ± 1.9 pA vs. 26.4 ± 1.9 pA, n = 12; CON vs. CLARI 300: 27.6 ± 1.2 pA vs. 17.1 ± 1.1 pA; n = 9; paired t-test, P = 0.02, P = 0.001, P = 0.0001, respectively; Fig. 3B). At the highest tested concentration (300 μM) clarithromycin also revealed a decrease in the frequency of mIPSCs (CON vs. CLARI 300: 1.3 ± 0.5 Hz vs. 0.23 ± 0.14 Hz; n = 9; paired t-test, P = 0.0001; Fig. 3C). The kinetics of miniature currents were not affected at any tested clarithromycin concentration (Fig. 3D). Furthermore, application of TTX did not change the passive properties (e.g., membrane capacitance and resistance) (data not shown). At the end of the clarithromycin treatment, we applied a washout procedure. One hour after washout of clarithromycin from the bath, the GABA-mPSCs returned to normal activity (data not shown), confirming that blocking effect of the antibiotic is reversible.
Fig. 3.
Clarithromycin inhibits miniature GABAergic currents. A: representative traces of mIPSC recordings from 3 representative neurons bathed in ACSF containing CNQX, d-AP5, and TTX before (top) and after (bottom) application of clarithromycin. B–D: bar graph pairs represent average values ± SE in controls (filled bars) and in 3, 30, and 300 μM clarithromycin (open bars). B: mIPSC amplitude was significantly reduced in the presence of all tested concentrations of clarithromycin (CON vs. CLARI 3: 24.8 ± 0.6 pA vs. 22.3 ± 0.9 pA, n = 15; CON vs. CLARI 30: 31.5 ± 1.9 pA vs. 26.4 ± 1.9 pA, n = 12; CON vs. CLARI 300: 27.6 ± 1.2 pA vs. 17.1 ± 1.1 pA, n = 9; paired t-test, *P = 0.02, *P = 0.001, *P = 0.0001, respectively). C: the frequency of current events was almost completely abolished at high dose (CON vs. CLARI 300: 1.3 ± 0.5 Hz vs. 0.23 ± 0.14 Hz; n = 9; paired t-test, *P = 0.0001). D: decay time was not changed with clarithromycin application (P > 0.1).
Bicuculline occludes clarithromycin-induced hyperexcitability in CA3 neurons.
To verify that the effect of clarithromycin on CA3 pyramidal cell hyperexcitability was mediated through alterations of GABA signaling, the GABAA receptor antagonist bicuculline (30 μM) was preapplied to the superfusate. Representative examples of voltage responses to ramp stimulation with application of bicuculline alone and in combination with clarithromycin are shown in Fig. 4A. As expected, bicuculline alone significantly increases neuronal firing (CON vs. BIC 30: IT, n = 11; frequency, n = 14; VRest, n = 14; 2-way repeated-measures ANOVA, P = 0.02, P < 0.001, P < 0.0001, respectively; Fig. 4, B–D), similar to changes observed in CLARI 300 alone (CON vs. CLARI 300; see Fig. 2, B–D). We observed no further increase in hyperexcitability during coapplication in IT (BIC 30 vs. BIC 30 + CLARI 300: 81.3 ± 16.1 pA vs. 79.4 ± 15.5 pA; n = 11; 2-way repeated-measures ANOVA, P > 0.9), frequency (BIC 30 vs. BIC 30 + CLARI 300: 9.5 ± 1.0 Hz vs. 9.5 ± 1.0 Hz; n = 14; 2-way repeated-measures ANOVA, P > 0.9), or depolarization of VRest (BIC 30 vs. BIC 30 + CLARI 300: −58.7 ± 1.0 mV vs. −57.9 ± 1.1 mV; n = 14; 2-way repeated-measures ANOVA, P > 0.9) (Fig. 4, B–D). In three additional neurons we reversed the order of drug application, and the same trend was observed in IT (CLARI 300 vs. CLARI 300 + BIC 30: 86.8 ± 13.7 pA vs. 84.4 ± 2.1 pA; n = 3), frequency (CLARI 300 vs. CLARI 300 + BIC 30: 9.1 ± 2.3 Hz vs. 9.8 ± 1.2 Hz; n = 3), and VRest (CLARI 300 vs. CLARI 300 + BIC 30: −60.1 ± 1.3 mV vs. −60.2 ± 1.3 mV; n = 3) (data not shown).
Fig. 4.
GABAA receptor antagonism occludes the effect of 300 μM clarithromycin. A: characteristic voltage responses (top) to an injected current ramp (bottom) for a representative neuron in control ACSF (left), 5 min after acute bath application of 30 μM bicuculline (BIC 30; center) and in ACSF containing both 300 μM clarithromycin and 30 μM bicuculline (BIC 30+CLARI 300; right). B–D: bar graphs represents average values ± SE in control conditions (filled bars), in the presence of bicuculline (hatched bars), and in the presence of bicuculline and clarithromycin combined (open bars). Bicuculline alone significantly affected neuronal firing expressed as reduction in IT (B; CON vs. BIC 30: n = 11; 2-way repeated-measures ANOVA, *P = 0.02), increased firing frequency (C; n = 14; 2-way repeated-measures ANOVA, *P < 0.001), and depolarized VRest (D; n = 14; 2-way repeated-measures ANOVA, *P < 0.0001). Application of clarithromycin to neurons previously exposed to bicuculline did not induce additional effects on IT (BIC 30 vs. BIC 30+CLARI 300: 81.3 ± 16.1 pA vs. 79.4 ± 15.5 pA; n = 11; 2-way repeated-measures ANOVA, P > 0.9), frequency (BIC 30 vs. BIC 30+CLARI 300: 9.5 ± 1.0 Hz vs. 9.5 ± 1.0 Hz; n = 14; 2-way repeated-measures ANOVA, P > 0.9), and VRest (BIC 30 vs. BIC 30+CLARI 300: −58.7 ± 1.0 mV vs. −57.9 ± 1.1 mV; n = 14; 2-way repeated-measures ANOVA, P > 0.9).
Acute 300 μM clarithromycin affects sustained potassium currents during repolarization.
In CA3 pyramidal neurons, action potential VT did not change in the presence of clarithromycin (CON: −40.8 ± 0.6 mV, CLARI 300: −40.0 ± 0.5 mV; n = 12; paired t-test, P = 0.08), but action potential half-width time was slightly lengthened (CON: 1.3 ± 0.02 ms, CLARI 300: 1.5 ± 0.04 ms; n = 14; Wilcoxon matched-pairs test, P = 0.0001), suggesting a possible involvement of potassium channels contributing to repolarization. Furthermore, investigation of the effect of clarithromycin on K+ conductances was performed in the presence of TTX (1 μM), CNQX (10 μM) and d-AP5 (50 μM). Figure 5A shows representative traces of fast transient IA currents that contribute to repolarization (see methods for an explanation of stimulation paradigms required for isolating this current). Threshold activation for IA currents remains at approximately −50 mV and the voltage relationship of peak IA currents does not change after exposure to clarithromycin (n = 7, 2-way repeated-measures ANOVA, P > 0.9; Fig. 5B), thus suggesting that the antibiotic did not affect early spike repolarization.
Other K+ currents capable of a more sustained outward current [e.g., the delayed rectifier current, IDR, and the calcium-activated K+ current, IK(Ca)] show a sensitivity to clarithromycin exposure. Figure 5C displays characteristic responses to depolarizing command potentials in control and clarithromycin conditions recorded in the same neuron. Steady-state amplitude was significantly reduced at membrane potential greater or equal to +30 mV (n = 5, 2-way repeated-measures ANOVA, P < 0.01; Fig. 5D). The reduction in outward sustained K+ currents can explain the measured delay in spike repolarization caused by clarithromycin but is likely a noncontributing factor to the enhanced hyperexcitability of CA3 neuron firing by clarithromycin (see discussion). In two slices bathed in Ca2+-free ACSF [eliminating IK(Ca)] clarithromycin also reduced remaining current (more likely IDR) (n = 2, data not shown), suggesting that the calcium-independent K+ conductances may be most sensitive to clarithromycin.
To investigate the involvement of the hyperpolarization-activated cationic channels (Ih) on clarithromycin-induced firing, membrane potential was recorded in current-clamp mode in the presence of TTX (1 μM), CNQX (10 μM), and d-AP5 (50 μM). Figure 5E shows representative voltage traces in response to injected currents depicting the characteristic voltage “sag” from activation of Ih at hyperpolarizing potentials, both in control experiments (Fig. 5E, left) and after exposure to 300 μM clarithromycin (Fig. 5E, center). These voltage responses change with application of the Ih antagonist Cs+ (Fig. 5E, right). Steady-state measures of voltage are represented in Fig. 5F (CON vs. CLARI 300, cesium vs. CON, cesium vs. CLARI 300; n = 7, 2-way repeated-measures ANOVA, P ≥ 0.26, P ≤ 0.025, P ≤ 0.025, respectively).
Hyperexcitability of CA3 pyramidal neurons is maintained after preincubation in clarithromycin.
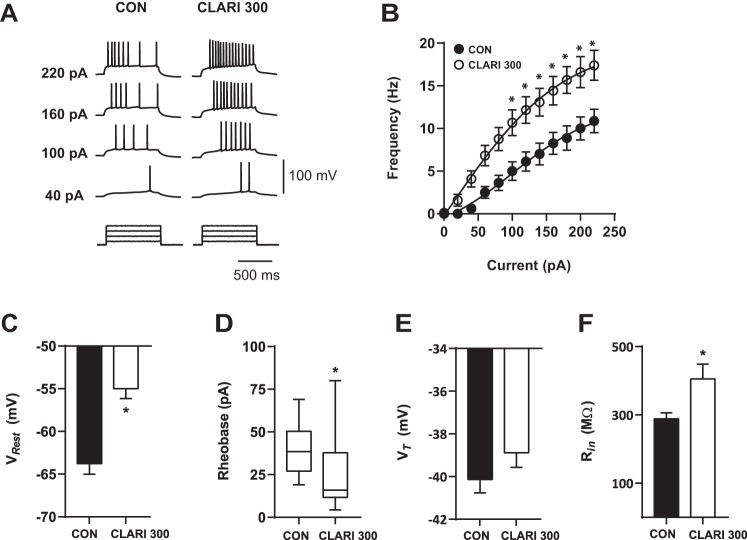
Clarithromycin is a bulky macrocyclic molecule with molar mass 747.953 g/mol. To investigate whether drug penetration into deeper layers of the slice would affect the observed hyperexcitability caused by clarithromycin, key experiments were repeated after preincubation of the slices for 60–180 min in 300 μM clarithromycin. Figure 6A shows traces of voltage responses used to test currents in a representative slice preincubated with ACSF (Fig. 6A, left) and with 300 μM clarithromycin for 2 h (Fig. 6A, right). As observed in the experiments using acute clarithromycin exposure, the F-I relationship was shifted to the left in the presence of clarithromycin. For all injected current ≥100 pA the firing frequency was higher in the presence of 300 μM clarithromycin (CON n = 10, CLARI 300 n = 11; 2-way repeated-measures ANOVA, P ≤ 0.018; Fig. 6B). Similarly, preincubation depolarized VRest (CON: −63.8 ± 1.2 mV, n = 10; CLARI 300: −55.0 ± 1.1 mV, n = 11; unpaired t-test, P = 0.00001; Fig. 6C) and decreased rheobase current (CON: 38.5 pA [27.1, 50.3], n = 10; CLARI 300: 15.9 pA, [11.6, 37.8], n = 11; Mann-Whitney test, P = 0.035; Fig. 6D). Consistent with the acute application, preincubation with 300 μM clarithromycin did not change action potential VT [CON vs. CLARI 300: −40.1 ± 0.6 (n = 10) vs. −38.9 ± 0.7 mV (n = 11); unpaired t-test, P = 0.2; Fig. 6E]. However, significant differences were noted in Rin compared with controls [CON vs. CLARI 300: 288.7 ± 17.4 MΩ (n = 10) vs. 405.2 ± 43.5 MΩ (n = 11); unpaired t-test, P = 0.03; Fig. 6F]. A similar but not statistically significant trend is noted with the acute application.
Fig. 6.
Preincubation of clarithromycin maintains hyperexcitability in CA3 neurons. A: characteristic voltage responses to increasing current injections (bottom) recorded in a representative neuron in control bath solution (left) and after 2–4 h of incubation in ACSF containing 300 μM clarithromycin (right). To block glutamatergic synaptic inputs, AMPA/kainate and NMDA receptor antagonists CNQX (10 μM) and d-AP5 (50 μM) were applied in both situations. Neurons recorded after preincubation in clarithromycin solution showed an increase in firing frequency compared with matched controls. B: enhanced firing frequency is demonstrated after preincubation in clarithromycin compared with controls (CON: n = 10; CLARI 300: n = 11; 2-way repeated-measures ANOVA, *P ≤ 0.018). Each circle represents mean ± SE. C: differences in VRest appeared in neurons after preincubation in the antibiotic (CON vs. CLARI 300; unpaired t-test, *P = 0.00001). D: clarithromycin induced a decrease in rheobase current compared with controls (CON vs. CLARI 300; Mann-Whitney test, *P = 0.035). Box and whisker plots represent medians, quartiles, and 5–95% percentiles. E: voltage threshold (VT) showed no changes in clarithromycin-preincubated neurons compared with controls (unpaired t-test, P = 0.2). F: input resistance (Rin) after preincubation of 300 μM clarithromycin changed significantly (unpaired t-test, *P = 0.03).
Clarithromycin enhanced seizure activity in an ex vivo model of epilepsy.
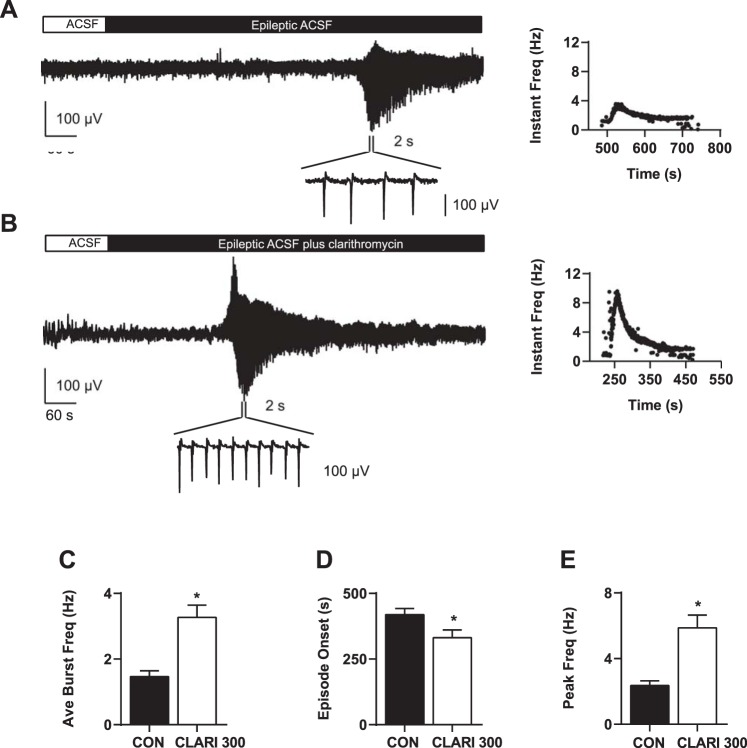
As a clinically relevant extension of our investigation of the effects of clarithromycin on burst firing via ramp currents, we applied 300 μM clarithromycin to slices in the context of an ex vivo model of epileptiform activity. We recorded local field potentials from slices prepared in the same way to determine the effect of clarithromycin on synchronized network activity. In control conditions (i.e., superfusion with normal ACSF) hippocampal slices did not demonstrate synchronized spontaneous discharges or seizurelike events (n = 27, 8 rats). However, upon exposure to high K+-low Mg2+ ACSF (model of epilepsy), all slices exhibited organized spontaneous bursting activity within the CA3 region. Figure 7A shows the characteristic time course and burst pattern of high K+-low Mg2+ ACSF-induced spontaneous activity. Slices (n = 11) perfused with 300 μM clarithromycin in combination with high K+-low Mg2+ ACSF demonstrated enhanced neuronal hyperexcitability in the seizure model, with a representative trace shown in Fig. 7B. The differences in peak and distribution of instantaneous burst frequencies in a representative case from each experimental condition are shown in Fig. 7, A and B, right.
Fig. 7.
Clarithromycin-enhanced seizurelike activity in an ex vivo epilepsy model. A and B: examples of spontaneous local field potential recordings from representative slices perfused with epileptogenic solution (A) and with epileptogenic solution + 300 μM clarithromycin in ACSF (B). Relative distributions of instantaneous burst frequencies are shown on right. C–E: epileptogenic ACSF + 300 μM clarithromycin elicited greater average burst frequencies (C) and higher peak frequency (E) compared with control conditions (burst frequency CON vs. CLARI 300: 1.5 ± 0.2 Hz vs. 3.3 ± 0.4 Hz; n = 11 each; unpaired t-test, *P = 0.0006; peak frequency CON vs. CLARI 300: 2.4 ± 0.3 Hz vs. 5.9 ± 0.8 Hz; unpaired t-test, *P = 0.001). D: onset of seizure burst activity in the clarithromycin/epileptic condition occurred significantly earlier compared with the control group (CON vs. CLARI 300: 419.3 ± 23.0 s, n = 10 vs. 331 ± 29.8 s, n = 11; unpaired t-test, *P = 0.03). All bars represent means ± SE.
Wash in of clarithromycin + epileptogenic ACSF elicited significantly greater average burst frequencies compared with control slices [CON vs. CLARI 300: 1.5 ± 0.2 Hz (n = 11) vs. 3.3 ± 0.4 Hz (n = 11); unpaired t-test, P = 0.0006; Fig. 7C]. The peak frequency in clarithromycin + epileptogenic ACSF was significantly higher, reaching an average peak of 5.9 ± 0.8 Hz compared with controls (2.4 ± 0.3 Hz) (Fig. 7E; unpaired t-test, P = 0.001).
Onset of seizure bursts activity in the clarithromycin-high K+ condition occurred significantly earlier compared with the control group [CON vs. CLARI 300: 419.3 ± 23.0 s (n = 10) vs. 331 ± 29.8 s (n = 11); unpaired t-test, P = 0.03; Fig. 7D].
A subset of slices was perfused with 300 μM clarithromycin in normal ACSF after a 10-min baseline recording period in normal ACSF. No cases exhibited spontaneous seizurelike activity upon exposure to clarithromycin in the absence of epileptogenic solution (data not shown, n = 5).
DISCUSSION
In this study we report an increase in CA3 pyramidal cell excitability as measured by increased firing frequency, decreased rheobase current, and a more depolarized VRest due to exogenous clarithromycin administration. Pharmacological testing revealed that these effects are mostly mediated by dose-dependent blockade of GABAergic activity. These results confirm the reported clinical effects of clarithromycin in inducing hyperactivity and delirium (di Poggio et al. 2011; Steinman and Steinman 1996) and increased arousal for those suffering from idiopathic hypersomnia (Trotti et al. 2014, 2015). Clarithromycin is not the only antibiotic with off-target effects that increase neuronal excitability. For decades, administration of the β-lactam antibiotic penicillin has been recognized as a potential cause of convulsions (Humphries 1963). Hippocampal slices superfused with high concentrations of penicillin have demonstrated synchronized epileptic-like event bursting associated with GABA inhibition in the CA3 region (Schwartzkroin and Prince 1977). Levofloxacin and other fluoroquinolones induce excitability through GABA inhibition (Imanishi et al. 1995). Clinically significant adverse effects of these antibiotics are relatively rare and mostly common in situations in which the permeability of the blood-brain barrier is compromised or metabolic clearance of the drug is impaired.
To test the minimum dose of acutely administered clarithromycin that generates significant hyperexcitability in single CA3 neurons, 3, 30, and 300 μM clarithromycin were applied in the presence of AMPA/kainate (CNQX) and NMDA (d-AP5) receptor antagonists. Average firing frequency and VRest were significantly increased by 30 and 300 μM clarithromycin compared with controls, whereas significant reduction in IT for action potential firing was noted. Miniature postsynaptic GABAergic currents were recorded in the presence of CNQX, d-AP5, and TTX to determine whether GABAergic neurotransmission was effected by 3, 30, and 300 μM concentrations of clarithromycin. At as low as 3 μM clarithromycin, the average of mIPSC amplitude shows significant reduction while the frequency remains unaffected, although the frequency of miniature postsynaptic currents was decreased with the application of 300 μM clarithromycin. Furthermore, the GABAA receptor blocker bicuculline occluded the stimulatory effect of clarithromycin, supporting the hypothesis that clarithromycin application depolarizes the membrane potential of CA3 pyramidal cells in hippocampal slices via GABA antagonism. In addition, bicuculline alone depolarized VRest similarly to clarithromycin alone (Fig. 4D and Fig. 2D).
Several factors contribute to excitability and firing in CA3 pyramidal cells besides reduction in GABAergic signaling. Potassium currents known to be present in these cells [e.g., IK(Ca), IDR, Ih, IA] can also control an individual cell's approach to its next action potential; therefore, an investigation of the possible contribution of potassium currents to hyperexcitability induced by clarithromycin was undertaken. Neither Ih nor IA appeared to be affected by clarithromycin at the highest tested dose. However, clarithromycin sensitivity was shown for sustained outward potassium currents. These are important for repolarization after action potential upstroke and are likely contributing to the observation of a slightly wider action potential with clarithromycin exposure. These potassium currents are initially activated by depolarization beyond ∼30 mV and given their relatively slow kinetics appear to mainly contribute to the refractory period (Rudy 1988; Storm 1990). Because increases in action potential width can lead to an increase in the absolute refractory period, blocking these currents may influence neuronal firing. In addition, Mitterdorfer and Bean (2002) reported that in CA3 rat neurons the contribution of sustained K+ currents to action potential shape was minimal (2%) and likely a noncontributing factor to the enhanced firing frequency observed in clarithromycin.
Chu et al. (1992) studied the absolute bioavailability of clarithromycin in humans after a single 250-mg oral dose. Two hours after administration, an average plasma concentration of 0.6 μg/ml (equivalent to 2.6 μM) was measured. Gustavson et al. (1995) reported an average maximum concentration of 3.8 μg/ml (16.34 μM) in the plasma of patients receiving an oral clarithromycin dose of 500 mg in 8-h intervals for 5 days; an even higher concentration of 16.5 μg/g was detected in the gastric fundus. Similarly, Chu et al. (1991) reported a maximum plasma concentration of 2.4 μg/ml (10.3 μM) in adult men who received twice-daily 500-mg oral doses of clarithromycin; similar levels have been detected in cerebrospinal fluid (Maniu et al. 2001). The terminal plasma half-life was ∼4 h. Typical dosing of clarithromycin is 250–500 mg three or four times daily. Clarithromycin at these concentrations has been associated with mania, delirium, hyperexcitability, and agitation in hospitalized patients (Bhattacharyya et al. 2016).
Acute exposure to 300 μM clarithromycin induced an increasing trend in Rin (CON vs. CLARI 300: 276.5 ± 22.9 MΩ vs. 304.9 ± 22.3 MΩ; P = 0.3). Preincubation for 2–4 h evoked a significant rise in Rin (CON vs. CLARI 300: 288.7 ± 17.4 MΩ vs. 405.2 ± 43.5 MΩ; P = 0.03; Fig. 6F), suggesting that the changes in cellular excitability are due to the influence of clarithromycin on inhibitory synaptic inputs and/or the blocking of tonic inhibition generated from extrasynaptic GABA receptors. The contribution of non-GABAergic currents can be determined by future experiments conducted with different compositions of intracellular solutions. One limitation of our study is the lack of a complete characterization of the specific GABA receptor subtypes associated with subcellular localization (somatic vs. dendritic, extra- vs. intrasynaptic); these features remain unexplored as possible area for future studies.
Using an ex vivo model of epileptiform activity, we evaluated the effects of clarithromycin in seizure generation. Bathing normal hippocampal slices with the high dose of clarithromycin (300 μM) did not generate spontaneous epileptic activity in populations of CA3 neurons captured via local field potential recordings. Enhancement of spontaneous seizurelike bursting in susceptible slices bathed in high K+-low Mg2+ ACSF suggests that clarithromycin can potentially exert a neurotoxic effect in vulnerable patients. Our network model (local field potential) exhibits wide heterogeneity in excitability and health of slices especially after continued exposure to clarithromycin. Our main goal was to test the capacity of clarithromycin to exacerbate epileptiform activity, as a clinically relevant extension. However, the causative effects of clarithromycin-induced seizures have not been characterized in depth; the failure to establish a stationary baseline in network excitability is another limitation of our study. Drugs designed to enhance GABAA receptor function have a long history of clinical use in anesthesia and promotion of sleep (Bianchi 2010; Franks and Zecharia 2011). Most anesthetics currently in clinical use act primarily through GABAA receptors (Franks and Lieb 1994; Garcia et al. 2010; MacIver 2014; Solt and Forman 2007). Rye et al. (2012) studied cerebrospinal fluid from a hypersomnolent subject with habitually long sleepiness and proposed a GABA-related mechanism for idiopathic hypersomnia. Furthermore, Trotti et all (2014, 2015) reported reduction in daytime sleepiness through clarithromycin administration in patients with symptoms of hypersomnia. Taken together, this suggests that clarithromycin may be useful in the treatment of hypersomnia associated with enhancement of GABAA receptor function. Recent interest in hastening arousal from anesthesia via excitation of the brain stem arousal network (Kenny et al. 2015; Schiff 2008; Solt et al. 2011, 2014) proposes other novel applications of the proexcitatory effects of clarithromycin. Our work on the influence of clarithromycin on neuronal excitability could have important implications in clinical scenarios encountered during the treatment of sleep disorders and epilepsy or during anesthesia care.
GRANTS
This research was supported by departmental funds and VA Career Development Award BX001677 (P. S. García). P. S. García's research efforts are partially supported by a grant (220020346) from the James S. McDonnell Foundation (www.jsmf.org).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.K.B. and P.S.G. conceived and designed research; E.K.B. and C.C.E. performed experiments; E.K.B. and C.C.E. analyzed data; E.K.B. and P.S.G. interpreted results of experiments; E.K.B. prepared figures; E.K.B. and P.S.G. drafted manuscript; E.K.B. and P.S.G. edited and revised manuscript; E.K.B. and P.S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jonathan Fidler and Dr. Matthias Kreuzer for critical comments on the manuscript. In addition, we are grateful to Dr. Carlos Gonzales-Islas for helpful discussions during preparation of the manuscript and to Dr. September Hesse for comments on statistical analysis.
REFERENCES
- Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol 22: 71–81, 2002. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Darby RR, Raibagkar P, Castro LN, Berkowitz AL. Antibiotic-associated encephalopathy. Neurology 86: 963–971, 2016. [DOI] [PubMed] [Google Scholar]
- Bianchi MT. Context dependent benzodiazepine modulation of GABAA receptor opening frequency. Curr Neuropharmacol 8: 10–17, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Deaton R, Cavanaugh J. Absolute bioavailability of clarithromycin after oral administration in humans. Antimicrob Agents Chemother 36: 1147–1150, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SY, Sennello LT, Sonders RC. Simultaneous determination of clarithromycin and 14(R)-hydroxyclarithromycin in plasma and urine using high-performance liquid chromatography with electrochemical detection. J Chromatogr 571: 199–208, 1991. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, MacIsaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 468: 305–309, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Poggio MB, Anfosso S, Audenino D, Primavera A. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 18: 313–318, 2011. [DOI] [PubMed] [Google Scholar]
- Dougherty KA, Islam T, Johnston D. Intrinsic excitability of CA1 pyramidal neurones from the rat dorsal and ventral hippocampus. J Physiol 590: 5707–5722, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N, Lieb W. Molecular and cellular mechanisms of general anaesthesia. Nature 367: 607–614, 1994. [DOI] [PubMed] [Google Scholar]
- Franks NP, Zecharia AY. Sleep and general anesthesia. Can J Anaesth 58: 139–148, 2011. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci USA 99: 12438–12443, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao J, Davis B, Tilley M, Normando E, Duchen MR, Cordeiro MF. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J 28: 1317–1330, 2014. [DOI] [PubMed] [Google Scholar]
- Garcia PS, Kolesky SE, Jenkins A. General anesthetic actions on GABAA receptors. Curr Neuropharmacol 8: 2–9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiderman JM. Central nervous system disturbances following clarithromycin ingestion. Clin Infect Dis 29: 464–465, 1999. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Johnson S. Sulfonylureas enhance GABAA synaptic potentials in rat midbrain dopamine neurones. Acta Physiol Scand 156: 147–148, 1996. [DOI] [PubMed] [Google Scholar]
- Gustavson LE, Kaiser JF, Edmonds AL, Locke CS, DeBartolo ML, Schneck DW. Effect of omeprazole on concentrations of clarithromycin in plasma and gastric tissue at steady state. Antimicrob Agents Chemother 39: 2078–2083, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JM, Jaffe RA, Brock-Utne JG. Human subthalamic neuron spiking exhibits subtle responses to sedatives. Anesthesiology 115: 254–264, 2011. [DOI] [PubMed] [Google Scholar]
- Humphries S. Convulsant effect of penicillin on the cerebral cortex. Lancet 281: 115–116, 1963. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Akahane K, Akaike N. Attenuated inhibition by levofloxacin, l-isomer of ofloxacin, on GABA response in the dissociated rat hippocampal neurons. Neurosci Lett 193: 81–84, 1995. [DOI] [PubMed] [Google Scholar]
- Kenny JD, Taylor NE, Brown EN, Solt K. Dextroamphetamine (but not atomoxetine) induces reanimation from general anesthesia: implications for the roles of dopamine and norepinephrine in active emergence. PLoS One 10: e0131914, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Lüddens H. Furosemide interactions with brain GABAA receptors. Br J Pharmacol 120: 741–748, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmannova K, Hines RM, Revilla-Sanchez R, Terunuma M, Tretter V, Jurd R, Kelz MB, Moss SJ, Davies PA. Enhanced tonic inhibition influences the hypnotic and amnestic actions of the intravenous anesthetics etomidate and propofol. J Neurosci 33: 7264–7273, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake EM, Chaudhuri J, Thomason L, Janik R, Ganguly M, Brown M, McLaurin J, Corbett D, Stanisz GJ, Stefanovic B. The effects of delayed reduction of tonic inhibition on ischemic lesion and sensorimotor function. J Cereb Blood Flow Metab 35: 1601–1609, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschinger A, Stabel J, Igelmund P, Heinemann U. Pharmacological and electrographic properties of epileptiform activity induced by elevated K2+ and lowered Ca2+ and Mg2+ concentration in rat hippocampal slices. Exp Brain Res 96: 230–240, 1993. [DOI] [PubMed] [Google Scholar]
- Limon A, Reyes-Ruiz JM, Miledi R. Loss of functional GABAA receptors in the Alzheimer diseased brain. Proc Natl Acad Sci USA 109: 10071–10076, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver MB. Anesthetic agent-specific effects on synaptic inhibition. Anesth Analg 119: 558–569, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver MB, Bronte-Stewart HM, Henderson JM, Jaffe RA, Brock-Utne JG. Human subthalamic neuron spiking exhibits subtle responses to sedatives. Anesthesiology 115: 254–264, 2011. [DOI] [PubMed] [Google Scholar]
- Maniu CV, Hellinger WC, Chu SY, Palmer R, Elcoro SA. Failure of treatment for chronic Mycobacterium abscessus meningitis despite adequate clarithromycin levels in cerebrospinal fluid. Clin Infect Dis 33: 745–748, 2001. [DOI] [PubMed] [Google Scholar]
- Mermelstein HT. Clarithromycin-induced delirium in a general hospital. Psychosomatics 39: 540–542, 1998. [DOI] [PubMed] [Google Scholar]
- Mitterdorfer J, Bean BP. Potassium currents during the action potential of hippocampal CA3 neurons. J Neurosci 22: 10106–10115, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moddel G, Gorji A, Speckmann EJ. Background potassium concentrations and epileptiform discharges. I. Electrophysiological characteristics of neuronal activity. Brain Res 959: 135–148, 2003. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, MacIver MB. Membrane and synaptic actions of halothane on rat hippocampal pyramidal neurons and inhibitory interneurons. J Neurosci 20: 5915–5923, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özsoylar G, Sayın A, Bolay H. Clarithromycin monotherapy-induced delirium. J Antimicrob Chemother 59: 331–331, 2007. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience 25: 729–749, 1988. [DOI] [PubMed] [Google Scholar]
- Rye DB, Bliwise DL, Parker K, Trotti LM, Saini P, Fairley J, Freeman A, Garcia PS, Owens MJ, Ritchie JC. Modulation of vigilance in the primary hypersomnias by endogenous enhancement of GABAA receptors. Sci Transl Med 4: 161ra151, 2012. [DOI] [PubMed] [Google Scholar]
- Sarro A, Sarro G. Adverse reactions to fluoroquinolones. An overview on mechanistic aspects. Curr Med Chem 8: 371–384, 2001. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann NY Acad Sci 1129: 105–118, 2008. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, coma. N Engl J Med 363: 2638–2650, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA, Prince DA. Penicillin−induced epileptiform activity in the hippocampal in vitro preparation. Ann Neurol 1: 463–469, 1977. [DOI] [PubMed] [Google Scholar]
- Solt K, Cotten JF, Cimenser A, Wong KF, Chemali JJ, Brown EN. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology 115: 791–803, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt K, Forman SA. Correlating the clinical actions and molecular mechanisms of general anesthetics. Curr Opin Anesthesiol 20: 300–306, 2007. [DOI] [PubMed] [Google Scholar]
- Solt K, Van Dort CJ, Chemali JJ, Taylor NE, Kenny JD, Brown EN. Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology 121: 311–319, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MA, Steinman TI. Clarithromycin-associated visual hallucinations in a patient with chronic renal failure on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 27: 143–146, 1996. [DOI] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res 83: 161–187, 1990. [DOI] [PubMed] [Google Scholar]
- Trotti LM, Saini P, Bliwise DL, Freeman AA, Jenkins A, Rye DB. Clarithromycin in γ−aminobutyric acid-related hypersomnolence: a randomized, crossover trial. Ann Neurol 78: 454–465, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti LM, Saini P, Freeman AA, Bliwise DL, García PS, Jenkins A, Rye DB. Improvement in daytime sleepiness with clarithromycin in patients with GABA-related hypersomnia: clinical experience. J Psychopharmacol 28: 697–702, 2014. [DOI] [PubMed] [Google Scholar]
- Wallace KL. Antibiotic-induced convulsions. Crit Care Clin 13: 741–762, 1997. [DOI] [PubMed] [Google Scholar]
- Wilhelm JC, Rich MM, Wenner P. Compensatory changes in cellular excitability, not synaptic scaling, contribute to homeostatic recovery of embryonic network activity. Proc Natl Acad Sci 106: 6760–6765, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DW, Yeatts SD, Silbergleit R, Palesch YY, Hertzberg VS, Frankel M, Goldstein FC, Caveney AF, Howlett-Smith H, Bengelink EM. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med 371: 2457–2466, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Torre E, Cuellar-Giraldo D, Cheng L, Yi H, Bichler EK, García PS, Yepes M. Tissue-type plasminogen activator triggers the synaptic vesicle cycle in cerebral cortical neurons. J Cereb Blood Flow Metab 35: 1966–1976, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbicz KL, Weight FF. Transient voltage and calcium-dependent outward currents in hippocampal CA3 pyramidal neurons. J Neurophysiol 53: 1038–1058, 1985. [DOI] [PubMed] [Google Scholar]