We explored the functional contributions of striatal fast-spiking interneurons (FSIs), presumed GABAergic interneurons, to distinct steps of movement generation in monkeys performing a reaching task. The activity of individual FSIs was modulated before and during the movement, consisting mostly of increased in firing rates. Changes in activity also occurred during movement preparation. We interpret this variety of modulation types at different moments of task performance as reflecting differential FSI control over distinct phases of movement.
Keywords: basal ganglia, movement initiation, motor preparation, nonhuman primate
Abstract
Recent works highlight the importance of local inhibitory interneurons in regulating the function of the striatum. In particular, fast-spiking interneurons (FSIs), which likely correspond to a subgroup of GABAergic interneurons, have been involved in the control of movement by exerting strong inhibition on striatal output pathways. However, little is known about the exact contribution of these presumed interneurons in movement preparation, initiation, and execution. We recorded the activity of FSIs in the striatum of monkeys as they performed reaching movements to a visual target under two task conditions: one in which the movement target was presented at unsignaled left or right locations, and another in which advance information about target location was available, thus allowing monkeys to react faster. Modulations of FSI activity around the initiation of movement (53% of 55 neurons) consisted mostly of increases reaching maximal firing immediately before or, less frequently, after movement onset. Another subset of FSIs showed decreases in activity during movement execution. Rarely did movement-related changes in FSI firing depend on response direction and movement speed. Modulations of FSI activity occurring relatively early in relation to movement initiation were more influenced by the preparation for movement, compared with those occurring later. Conversely, FSI activity remained unaffected, as monkeys were preparing a movement toward a specific location and instead moved to the opposite direction when the trigger occurred. These results provide evidence that changes in activity of presumed GABAergic interneurons of the primate striatum could make distinct contributions to processes involved in movement generation.
NEW & NOTEWORTHY We explored the functional contributions of striatal fast-spiking interneurons (FSIs), presumed GABAergic interneurons, to distinct steps of movement generation in monkeys performing a reaching task. The activity of individual FSIs was modulated before and during the movement, consisting mostly of increased in firing rates. Changes in activity also occurred during movement preparation. We interpret this variety of modulation types at different moments of task performance as reflecting differential FSI control over distinct phases of movement.
the striatum is composed of a vast majority of projection neurons and several types of interneurons, among which GABAergic interneurons constitute a diverse group of cells that can be characterized by expression of different neurochemical markers and basic electrophysiological properties (Silberberg and Bolam 2015; Tepper et al. 2010). These interneurons have been implicated in regulating the information processing within the striatal network. Among them, the fast-spiking inhibitory interneurons (FSIs), that correspond to the parvalbumin-containing group of GABAergic interneurons (Kawaguchi 1993; Mallet et al. 2005; Sharott et al. 2012), have received the most interest. This electrophysiologically identifiable cell type is regarded as the main source of inhibition onto neighboring output neurons, thus shaping the output of the striatum to the downstream nuclei of the basal ganglia (Koós and Tepper 1999; Mallet et al. 2005). Given that these presumed interneurons exert a strong inhibitory effect on the activity of output neurons, it has been suggested that disruption of FSI function can result in the inability to control or inhibit inappropriate motor responses. Experimental studies in animals provide evidence for specific involvement of these interneurons in striatal-based movement disorders, including dystonia (Gernert et al. 2000) and dyskinesia (Gittis et al. 2011). In addition, neuropathological studies in humans have demonstrated reduced density of striatal GABAergic interneurons containing parvalbumin in Tourette syndrome (Kalanithi et al. 2005; Kataoka et al. 2010) and Huntington's disease (Reiner et al. 2013). It is, therefore, crucial to understand how FSIs contribute to normal behavioral states to gain an insight into their role in the pathophysiology of movement disorders.
Single-neuron recording studies in behaving rats have investigated how striatal FSIs modulate their firing rate during performance in a variety of tasks. The results have shown different patterns of task-related changes in FSI activity occurring at specific times, such as reaching a goal in the context of spatial navigation tasks (Berke 2008; Berke et al. 2004; Schmitzer-Torbert and Redish 2008) and selecting a particular action in the presence of a choice between movement options (Gage et al. 2010). FSIs have also been shown to be sensitive to prediction errors linked to changes in an action's expected value (Stalnaker et al. 2012) and to expectation and receipt of rewards (Lansink et al. 2010). It, therefore, appears that FSIs might make contributions to different aspects of task performance and information processing. In addition, regional differences in the striatum are likely to be important for interpreting task-related changes in FSI activity, with modulations related to actions resulting in rewarded outcomes preferentially occurring in the dorsolateral striatum, assumed to subserve motor functions (Berke 2008; Gage et al. 2010; Schmitzer-Torbert and Redish 2008), whereas changes in FSI activity related to reward outcomes are assumed to be found in dorsomedial (Stalnaker et al. 2012) and ventromedial portions of the striatum (Lansink et al. 2010), which have been proposed to be more involved in cognitive and motivational functions, respectively.
In the primate striatum, electrophysiologically identifiable cell types generally fall into two categories, called phasically active neurons (PANs), assumed to be projection neurons, and tonically active neurons (TANs), thought to be cholinergic interneurons. Recently, recording studies have pointed to the presence of a rarely encountered category displaying spike waveforms and firing rates similar to those described for striatal FSIs in rodents (Adler et al. 2013; Yamada et al. 2016). Knowledge about the functional contributions of FSIs in the striatum of behaving monkeys is, however, still limited. In a Pavlovian conditioning procedure, in which monkeys were passively exposed to pairings of visual stimuli with different outcomes, striatal FSIs have been reported to display changes in activity consisting of increases in firing rate after presentation of conditioned stimuli and occurrence of outcome (Adler et al. 2013). More recently, a study has emphasized that FSIs are sensitive both to the actions resulting in rewarded outcomes, as well as to the outcomes themselves in monkeys performing arm or eye movement to obtain rewards (Yamada et al. 2016). However, the exact nature of their contribution to behavioral processes carried out in the striatum remains obscure, and we still lack an understanding of how this population of presumed GABAergic interneurons participate in distinct steps of movement generation.
The purpose of this study was to further characterize the functional properties of striatal FSIs by examining their capacity to carry signals related to the preparation, initiation, and execution of target-directed arm movements. Our results indicate that individual FSIs that were not restricted to the motor part of the striatum changed their firing rate around the time of movement initiation, with distinct groups that either increase or decrease their activity, suggesting that they may exert differential control over distinct phases of movement.
MATERIALS AND METHODS
Behavioral conditions.
The experimental protocol was in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Comité d'Éthique en Neurosciences INT-Marseille (protocol A2–10–12). Two adult male rhesus monkeys (Macaca mulatta), F and T, were trained to make visually guided arm-reaching movements to obtain a liquid reward. The behavioral apparatus was similar to that previously described (Deffains et al. 2010). Each monkey was seated in a specially designed restraining box, facing a panel 30 cm from its head. The panel contained two metal knobs (10 × 10 mm) separated by 20 cm horizontally, as well as two light-emitting diodes (two-color green/red LEDs), one above each knob, at eye level of the animal. An unmovable metal bar was mounted at the center of the panel at waist level. The temporal sequence of task events is schematically presented in Fig. 1A. The animals kept the hand on the bar until the illumination of a red LED either to the left or to the right of the panel, with the location of the stimulus varying pseudorandomly across trials. In response to this stimulus, monkeys had to reach the target positioned just below the illuminated LED, and the movements were rewarded by a small amount of fruit juice (0.3 ml) delivered immediately after touching the target. The monkey then brought the hand back on the bar and waited for the total duration of the trial (6 s) to elapse before the next trial started.
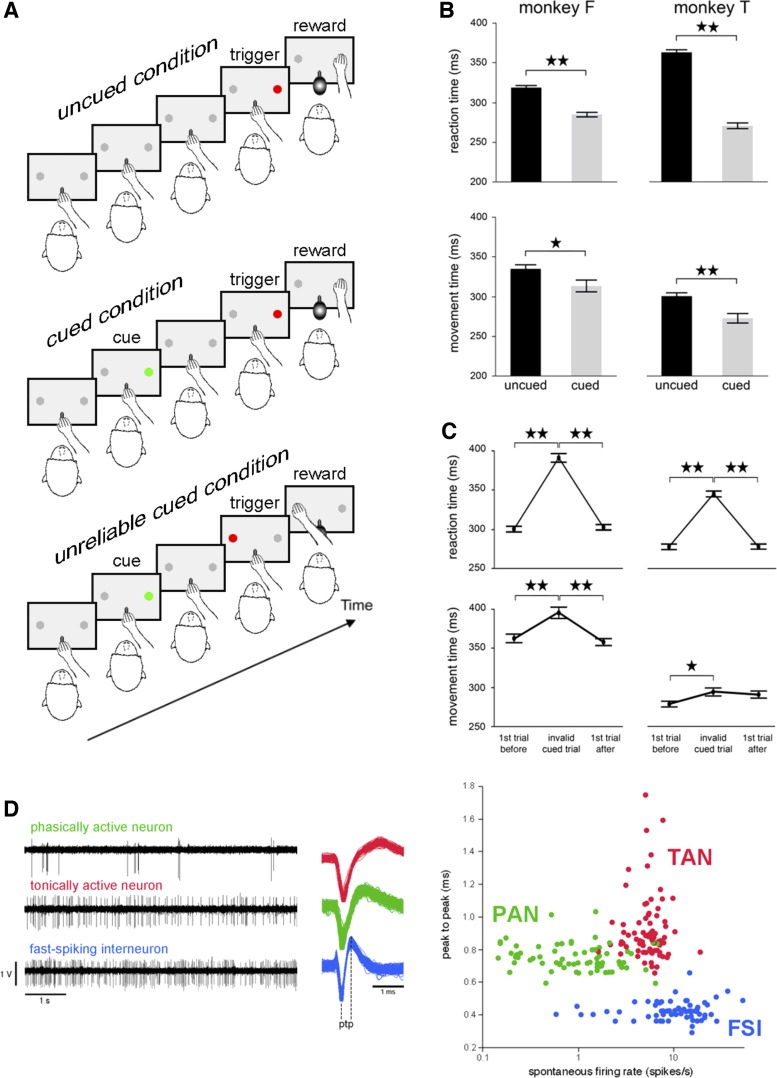
Fig. 1.
Behavioral task conditions, reaching performance, and classification of striatal neurons. A: the monkey kept its hand on a bar while waiting for the presentation of a visual stimulus (red light) on either the right or the left side of a panel in front of it. In response to this signal, the animal was required to release the bar and reach for the target located below the light, which it had to touch to receive a liquid reward. There were three task conditions with the same basic structure: 1) an uncued condition, in which the trigger stimulus occurred at irregular time intervals and in the absence of any predictive cues; 2) a cued condition, in which a visual stimulus (green light) gave advance information regarding the location of the forthcoming trigger presented 1.5 s later; 3) an unreliable cued condition in which the specification of trigger location was invalid on a small percentage of trials (invalid cued trials). In the example shown, a rightward movement is cued, and the trigger stimulus is presented on the opposite side, thereby eliciting a leftward movement. The three conditions were tested in separate blocks of trials. B: reaction times and movement times for the uncued and cued conditions. Values are means ± SE (numbers of trials: 283–317 for monkey F, 238–403 for monkey T). ⋆★P < 0.01, ⋆P < 0.05. C: reaction times and movement times from the invalid cued trials and the valid cued trials immediately before and after the invalid ones. Each value was obtained by calculating the mean ± SE (199 and 235 trials of each type for monkey F and monkey T, respectively). ★⋆P < 0.01, ★P < 0.05. D: comparison of the electrophysiological characteristics of the three main types of neurons found in the striatum of the awake macaque monkey. Left: sample spike trains from each category of neuron. Middle: mean waveforms for one representative striatal neuron of each category. ptp, Peak to peak, i.e., time elapsed between the first negative peak and the following positive peak. Right: scatterplot of mean spike waveform durations [x-axis, firing rate (logarithmic scale); y-axis, peak-to-peak time]. Each dot represents a single neuron colored according to its category.
In the standard reaching task condition, called the uncued condition, the trigger stimulus occurred at irregular time intervals and in the absence of any predictive cues, so that the monkey could not predict the location of the target on each trial. To address the question whether the state of preparation for movement influences neuronal activity, a cued condition was also employed in which the monkeys received advance information about the location of the trigger stimulus. In this case, the onset of a green light (duration: 500 ms) presented either to the left or to the right served as a cue for the trigger stimulus, which came on 1.5 s later at the same location. In this condition, the cue allowed the animal to prepare arm movement toward a specific location. In another version of the cued condition, called the unreliable cued condition, advance information about target location was valid in 80% and invalid in 20% of trials. On valid cued trials, the trigger stimulus was presented at the same location as that indicated previously by the cue, whereas, on invalid cued trials, the trigger stimulus was presented on the side opposite to that indicated by the cue. In the latter case, a motor reprogramming process is needed after trigger onset before the correct movement can be executed. This condition was designed to test the monkeys' ability to inhibit the prepared response and replace it with the other one. Task performance was assessed in terms of reaction time (RT) and movement time (MT), which were the time taken to release the bar in response to trigger onset and to move from the bar to a target, respectively. Both monkeys performed the task with their right arm, and the different conditions were presented to the animals in successive blocks of 30–50 trials, except for the unreliable cued condition, which was run in blocks long enough (about 80 trials) to ensure at least 15 invalid cued trials.
Neuronal recordings.
Data were collected after monkeys were well trained on the three task conditions. After completion of training, surgery was carried out under gas anesthesia (isoflurane 2.5%) and sterile conditions to implant a head-holding device and a recording chamber aimed to the left striatum. We recorded the extracellular activity of single neurons with movable glass-insulated tungsten microelectrodes over a wide area of the anterior and posterior regions of the striatum, mostly in the dorsal part of the putamen, with some penetrations extending to the ventral most part of this nucleus. The electrode was driven by a hydraulic microdrive (MO-95; Narishige, Tokyo, Japan) through a stainless steel guide tube, which was used to penetrate the dura. The electrode was manually advanced until the spikes generated by a single neuron were isolated. Neuronal signals were amplified (×5,000), filtered with a band pass of 0.3 to 1.5 kHz, and monitored with oscilloscopes. Single-neuron activity was detected online by threshold crossing using a time-amplitude window discriminator (Neurolog; Digitimer), with the waveform of each discriminated spike being monitored continuously during recording. The resulting standard digital pulses were stored on a computer, together with markers of task events. Neuronal signals were also digitized and stored on computer (Spike2; Cambridge Electronic Design, Cambridge, UK) for offline analysis of spike duration. We classified each unit as belonging to one of three major classes of neurons in the striatum, identified by spike waveform and firing rate criteria, as described previously (Adler et al. 2013). The relationship between neuronal activity and behavioral performance was assessed online in the form of rasters aligned on the task events using a custom-designed computer software (LabVIEW, National Instruments, Dallas, TX). The same software was used to present visual stimuli, to deliver liquid reward, to measure behavioral parameters, and to collect neuronal data for offline analyses. Once a neuron was isolated, we usually first examined its activity in the uncued condition. If the isolation could be maintained satisfactorily, the neuron was then tested in another condition, either the cued or unreliable cued condition, with the order of the conditions being counterbalanced across trial blocks.
Data analysis.
The Student's t-test and one-way ANOVAs with Tukey's honestly significant difference post hoc tests were conducted to test for any significant differences in RT and MT between task conditions (uncued, cued) and trial types (valid cued, invalid cued). As regards neuronal activity changes, rasters and perievent time histograms of single-neuron activity aligned to each of the three task events (trigger onset, movement onset, and target contact) were first examined visually and then statistically analyzed. We determined whether neurons showed significant changes in activity occurring at any particular moment during the performance of the reaching task. The baseline firing rate was calculated during the 500 ms preceding the presentation of the first visual stimulus (the trigger stimulus in the uncued condition and the cue in the cued condition), defined as the control period. A test window of 100-ms duration was moved in steps of 10 ms, starting at the presentation of the trigger stimulus, from 300 ms before movement onset, or from 300 ms before target contact. The mean discharge rate in the 100-ms window was compared, at each step, with that in the control period. The onset of a modulation was taken to be the beginning of the first of at least five consecutive steps showing a significant difference (P < 0.05, Dunn's test for multiple nonparametric comparisons) as against the baseline activity. The offset of a modulation was defined in the same manner by the first of at least five consecutive steps with activity back to control. We then determined the onset and offset of the modulation in the firing of the neuron centered on each task events with 10-ms resolution. In addition, task-related increases in neuronal activity were evaluated in terms of the latency of the peak of activation. To do so, we divided the trial into successive bins of 10 ms around each of the three task events, and we calculated the spike count in each bin. Peak latency was estimated, for each neuron, as the 10-ms interval showing the highest level of activity in the perievent time histogram referenced to each task event. Based on these measures, we classified FSIs into distinct categories, according to the timing of peak activations relative to movement onset.
For each neuron, we also used a 300-ms window immediately before or after movement onset to test for dependence of neuronal activity on target location (left, right), condition (uncued, cued), and trial type (valid cued, invalid cued), on the basis of mean firing rate measured in these windows. The assessment of statistical differences between conditions was performed with the use of multivariate analysis of variance (MANOVA). Further details on data analysis are given at the appropriate place in the results section. To examine the temporal relation between changes in FSI activity and behavioral indexes of task performance (i.e., RT and MT), we made a correlation analysis on a trial-by-trial basis (Pearson's correlation, P < 0.05).
In addition to the quantitative assessment of task-related activities of individual FSIs, we pooled activities across samples of neurons modulated during particular task conditions and trial types and made population activity curves. For each neuron, a normalized perievent time histogram was obtained by dividing the content of each bin by the number of trials, and the population activity was obtained by averaging all normalized histograms referenced to a particular task event. Data were analyzed using custom-written MATLAB (version R2015b, Mathworks, Natick, MA) scripts and statistical analyses were performed using JMP software (version 11, Chicago, IL).
Reconstruction of recording sites.
Histological confirmation of recording sites is available in monkey T, but not in monkey F, which is still participating in another study. Consequently, for this latter animal, approximated recording sites were determined from the neuronal activity characteristic to the striatum and electrophysiological features of neighboring structures, including the external and internal segments of the globus pallidus, the internal capsule, and the anterior commissure. In monkey T, the block of tissue, including striatal recording sites, was cut into frontal sections of 40-μm thickness on a freezing microtome, and every section was stained with cresyl violet. Based on the location of recording sites identified histologically in this animal, FSIs were recorded between 4 mm anterior and 8 mm posterior to the anterior commissure, over the entire lateral to medial extent of the nucleus, including some penetrations in the most ventral portion of the precommissural putamen. Based on the known topographic organization of cortical projections, the region of the striatum explored in the present study is divided into a “motor” region, which predominantly includes the postcommissural part of the dorsal putamen, and an “associative” region, which includes mainly the precommissural part of the dorsal caudate nucleus and putamen. The ventral region of the precommissural part of the caudate nucleus and putamen is usually referred to the “limbic” striatum (Haber and McFarland 1999).
RESULTS
Behavior.
As shown in Fig. 1B, both monkeys had significantly shorter RTs in the cued condition than in the uncued condition (Student's t-test, P < 0.01), suggesting that the presence of prior information on location of the trigger stimulus prepared the animals for directing their movement at a specific target, leading, therefore, to a shortening of RT. There was also a significant effect of condition on MTs in monkey T (P < 0.01) and monkey F (P < 0.05) with the MT in cued trials being shorter than the MT in uncued trials. Both monkeys continued to perform well the task in the unreliable cued condition (above 90% correct) when unexpected invalid cued trials occurred in the course of a block; namely they reached the target accurately, regardless of whether the cue was a valid predictor or not of target location. Figure 1C shows the mean RTs and MTs for invalid cued trials and for trials immediately preceding and immediately following them. In both monkeys, RTs for invalid cued trials were significantly longer than for trials with a valid cue [one-way ANOVA, monkey F: F(2,594) = 143.59, P < 0.01; monkey T: F(2,702) = 124.45, P < 0.01], reflecting a slowness of movement initiation when responding to the trigger stimulus which did not match the cued location. There was also a significant effect of trial type on MT in monkey F [F(2,594) = 12.19, P < 0.01] who took longer to reach the target in invalid than in valid cued trials, whereas such a difference was less evident in monkey T [F(2,702) = 3.33, P < 0.05].
Neuronal data.
We recorded from 701 neurons in two monkeys (413 and 288 neurons requiring 19 and 11 recording months in animals F and T, respectively). Of the 701 neurons, 83 (12%) were judged to be FSIs, and we report here on 64 of them (32 in each monkey) that were recorded in at least one of the three task conditions employed in this study. Table 1 gives an overview of the number of recorded FSIs in the different task conditions and the mean number of trials per recording. Consistent with prior studies (Adler et al. 2013; Yamada et al. 2016), FSIs were rarely encountered in awake monkeys compared with PANs and TANs. Despite their relative scarcity, FSIs were clearly distinguishable from the other neuron types (i.e., PANs and TANs) usually recorded in the monkey striatum. The patterns of neuronal discharge in the striatum of our monkeys (Fig. 1D) were similar to those described previously (Adler et al. 2013; Yamada et al. 2016). The FSIs had a mean baseline firing rate of 12.8 ± 8.9 spikes/s (n = 64), which is higher than that of the TANs (5.7 ± 2.2, n = 85) or PANs (1.4 ± 1.8, n = 87). The duration of spikes of the FSIs, defined as the time interval between the first negative and second positive peaks of the spike, was generally shorter (424 ± 57 μs, n = 63) than that of the TANs (919 ± 198 μs, n = 80) and PANs (754 ± 80 μs, n = 80) (Wilcoxon's rank-sum test, P < 0.01). These spike waveform and firing rate characteristics are very similar to those of FSIs recorded in the rodent striatum, which have been closely associated with parvalbumin-containing GABAergic interneurons.
Table 1.
Number of recorded neurons and recorded trials in the different task conditions
| Increase |
||||||
|---|---|---|---|---|---|---|
| Task Conditions | N | Early | Late | Decrease | Recorded Trials | Range of Recorded Trials |
| Uncued | 55 | 14 | 6 | 9 | 33 ± 6 | 21-49 |
| Cued | 32 | 11 | 5 | 4 | 34 ± 8 | 15–54 |
| Unreliable cued | 58 | 17 | 12 | 16 | 43 ± 11 | 22–70 |
Values are means ± SD of no. of recorded trials per condition.
N, total no. of neurons recorded in each task condition.
Increase and decrease denote neurons that are activated and depressed around the time of movement, respectively. Early and late denote neurons whose activations peaked before and after movement onset, respectively.
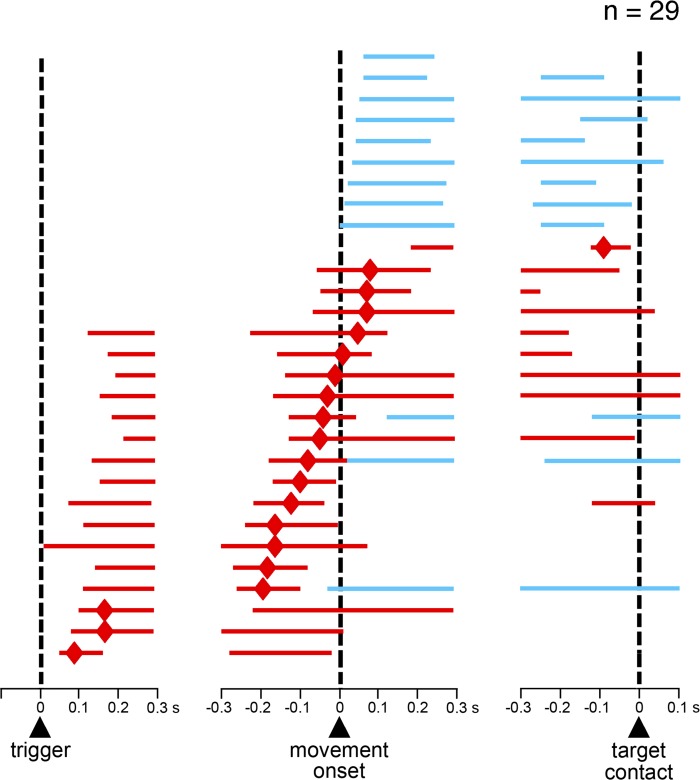
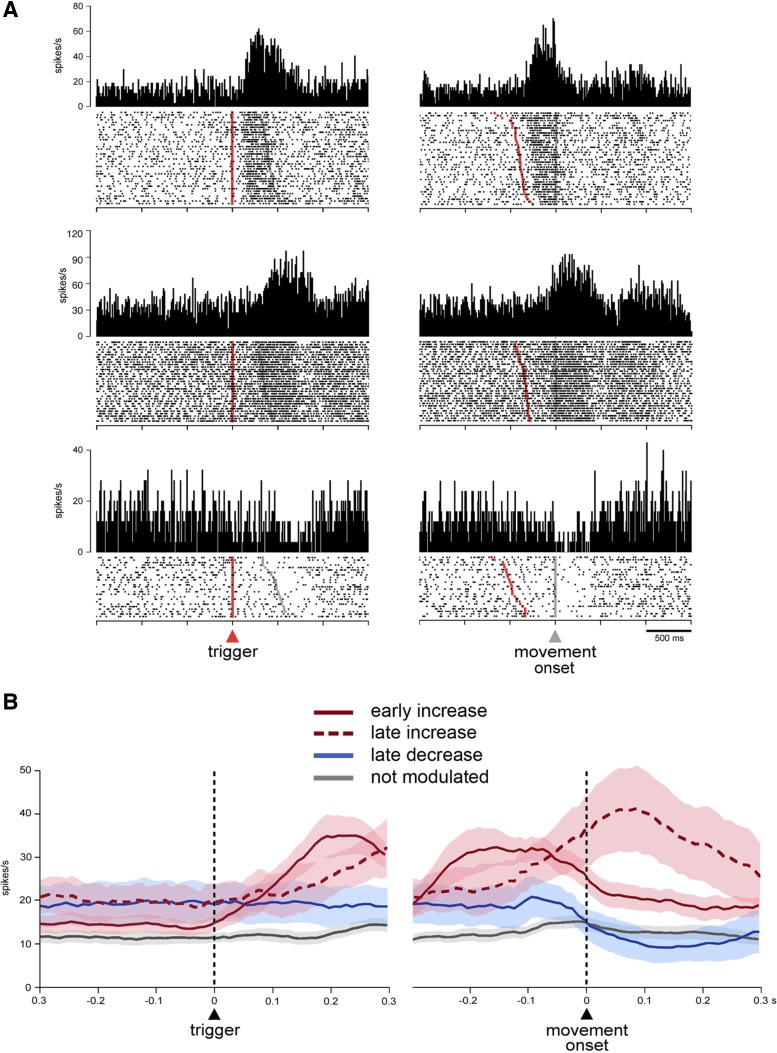
We first examined temporal profiles of activity changes for each neuron recorded in the uncued condition (n = 55) using the sliding time window procedure (see materials and methods), starting at the onset of the trigger stimulus and at 300 ms before the movement onset and the target contact. Here we focused on FSI activity just before and during movement for analysis, with the modulations at a later stage of the task (i.e., reward period) being addressed in another report. We found that 53% (29 of 55) of neurons (11 and 18 in monkeys F and T, respectively) showed statistically significant changes in activity in relation to movement initiation (Dunn's test, P < 0.05). Among these, 17 neurons showed increases in activity, 9 neurons showed decreases in activity, and 3 neurons had increases followed by decreases. Figure 2 shows the activity profile for each of the 29 modulated FSIs, sorted according to the latency of the peak of activation for neurons that showed increased activity and to the earliest time of significant modulation for neurons that showed decreased activity only. As it can be seen, increases in activity were more prominent before the onset of movement, with neurons reaching their peak activation just after the presentation of the trigger stimulus (3 neurons) or within a 300-ms window immediately preceding movement onset (11 neurons). Other neurons reached their peak activation within a 300-ms window immediately following movement onset (6 neurons). We classified the neurons that showed increases in activity into two groups according to which of the two 300-ms windows was the one in which the peak activation occurred. Neurons having peak activation time prior to or following the initiation of arm movement were classified as early increase type and late increase type, respectively. All neurons displaying decreased activity only were modulated after movement onset and were included in a decrease-type category. Figure 3A shows example neurons from each category. To give an overview of the modulations at the population level, averaged activities of all FSIs of each of the three categories are illustrated in Fig. 3B. Neurons with no modulation in relation to movement initiation are also shown for comparison.
Fig. 2.
Time courses of change in FSI activity around the initiation of reaching movement. The scheme shows the temporal profile of activity changes for all FSIs tested in the uncued condition (n = 29), separately referenced to trigger onset (left), movement onset (middle) and target contact (right). Each horizontal line represents the duration of statistically significant changes in activity for a single neuron. Red and blue indicate neuronal activity above and below the baseline firing rate, respectively. The small diamonds on red lines indicate the onset time of the peak activation. Lines are ordered according to the timing of the peak for neurons with increased activity and to the timing of the onset of modulation for neurons with decreased activity only.
Fig. 3.
FSI activity during the initiation and execution of reaching movements. A: activity of three example neurons with significant changes in activity around the initiation of movements. Data are shown as corresponding raster displays and perievent histograms aligned on the onsets of the trigger stimulus (left) and the movement (right). Each dot in the raster displays represents a single spike, and each line of dots corresponds to the activity during one trial. Trials were ordered offline according to the latency of movement. Trigger and movement onsets are indicated by red and gray markers in raster displays, respectively. Bin width for histograms, 20 ms. B: population activities for each of the three categories of movement-related FSIs. Colored curves depict mean activity (±SE) for the three categories of FSIs: early increase type (n = 14), late increase type (n = 6), and late decrease type (n = 9). FSIs that were not activated during movement initiation are indicated for comparison (n = 26). Shading indicates SE. Activity is aligned to the onset of the trigger (left) and movement (right), which are marked by vertical dashed lines.
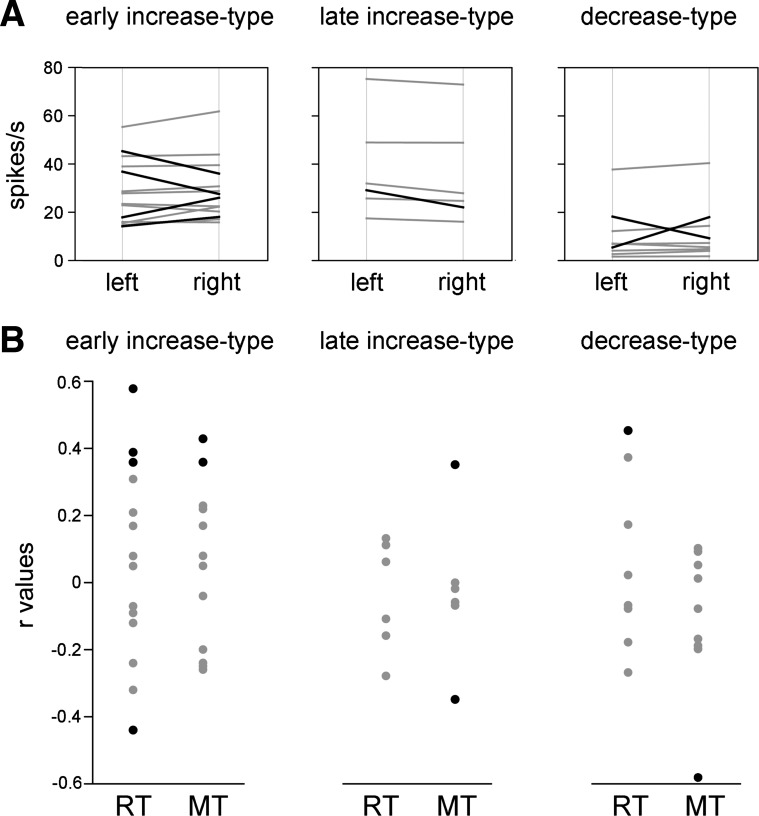
We next examined whether FSIs were sensitive to the target location and/or the direction of the arm movement. We performed this analysis by comparing the spike counts in the 300-ms window before (early-increase-type neurons) or after the movement onset (late-increase-type and decrease-type neurons) between leftward and rightward movements, for each modulated neuron in our sample. Of the 29 modulated FSIs, 7 (24%) showed activity that differed significantly between the two directions of movement (Wilcoxon's test, P < 0.05), of which one-half showed stronger activity associated with movements directed to the side ipsilateral to the moving arm and the other one-half with movements directed to the contralateral side (Fig. 4A). Although FSI modulations dependent on the direction of movement were found in all three categories of neurons, they seem to be most frequent in early-increase-type neurons, but the sample sizes were too small to allow for adequate statistical analysis. We also examined whether FSI activity was concerned with the fluctuations of reaching performance. For each modulated neuron, we calculated the trial-by-trial correlation (Pearson's r) between movement parameters (RT and MT) and FSI activity, using the same time windows defined before. Across the sample of studied neurons, 17% (5 of 29) were sensitive to the RT and 17% (5 of 29) to the MT, and there was no trend toward a more pronounced relationship between FSI modulation and motor variables in one category of neuron than another (Fig. 4B), with significant correlations being either in the same or opposite direction (i.e., the FSI activity increased or decreased as RT or MT increased). In sum, we have provided evidence for FSIs increasing their firing rates around the initiation of movements, with most of them having their peak activations that preceded movement onset, whereas a comparatively lower number of FSIs had peak activations that followed movement onset. We also found neurons that had opposite changes in activity (i.e., decreases in firing rate) after movement onset. It, therefore, appears that striatal FSI can display a variety of patterns of modulation as the monkeys initiated reaching movements in response to the trigger stimulus.
Fig. 4.
FSI activity in relation to the location of the target stimulus and motor variables. A: changes in FSI activity as a function of target location (left or right). Significant differences (P < 0.05) are indicated by black lines. B: correlation of movement latency and duration with changes in FSI activity. Black dots indicate the data with a significant r value (P < 0.05). RT, reaction time; MT, movement time.
Effect of motor preparation on FSI activity.
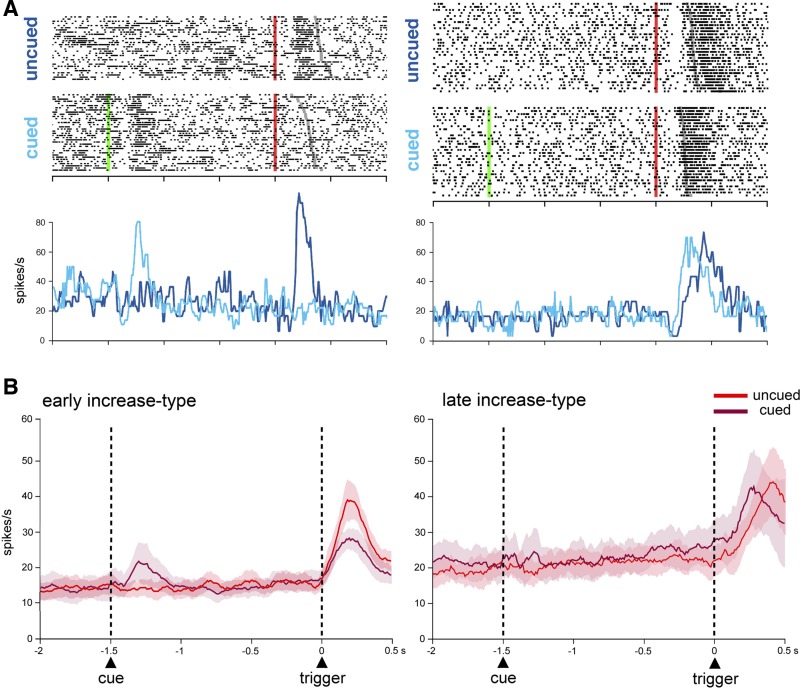
To determine whether the activity of FSIs was influenced by the presence of spatial cues that allowed the monkeys to prepare themselves to react more quickly to the trigger stimulus, the activity of 32 neurons was tested in the cued condition (14 and 18 in monkeys F and T, respectively). In this condition, short-lasting increases in FSI activity in response to the cue onset were seen in four neurons, whereas two other neurons exhibited increases in activity which began at 200–410 ms before the trigger and terminated in close temporal relation to its presentation, with one neuron being activated both after cue onset and before trigger. Among FSIs that were tested, none showed sustained changes in activity throughout the whole delay period (i.e., the 1.5-s interval between cue and trigger presentations). Figure 5A (left) shows an example of early-increase-type neuron displaying a phasic increase in activity in response to the trigger stimulus in the uncued condition. When tested in the cued condition, the same neuron responded to the cue, while the trigger stimulus became ineffective for eliciting a response, as though the sensitivity was shifted from the trigger to the cue containing information about the upcoming direction of movement. Figure 5A (right) shows an example of late-increase-type neuron with a phasic increase in activity after the trigger stimulus on both uncued and cued trials, with the activation being well aligned to movement onset in the two conditions.
Fig. 5.
Effect of movement preparation on FSI activity. A: comparison of the activity of two individual neurons during uncued and cued trials. Conventions are the same as in Fig. 3A, except that the superimposed average firing rate for the two types of trial is plotted as colored curves smoothed by a three-point median filter. B: time courses of the population activity preceding the trigger onset under the uncued and cued conditions for early-increase-type neurons (n = 11) and late-increase-type neurons (n = 5) recorded in both conditions.
To give an overview of changes in activity of the whole sample of neurons tested in the two task conditions, we averaged separately the activity from early-increase-type (n = 11) and late-increase-type neurons (n = 5). As can be observed in Fig. 5B (left), when early-increase-type neurons were grouped together, a brief activation in response to the cue was visible at the population level. Also, the population level analysis for this category revealed that the magnitude of activation in response to the trigger stimulus was affected by the task condition, being higher in uncued trials than in cued trials (MANOVA during the 500-ms period after the trigger onset, P < 0.05). On the other hand, the activity across the sample of late-increase-type neurons was not significantly modulated during the cue period, and the magnitude of activation after the trigger onset was not significantly different between the two conditions (MANOVA during the 500-ms period after the trigger onset, P > 0.05) (Fig. 5B, right).
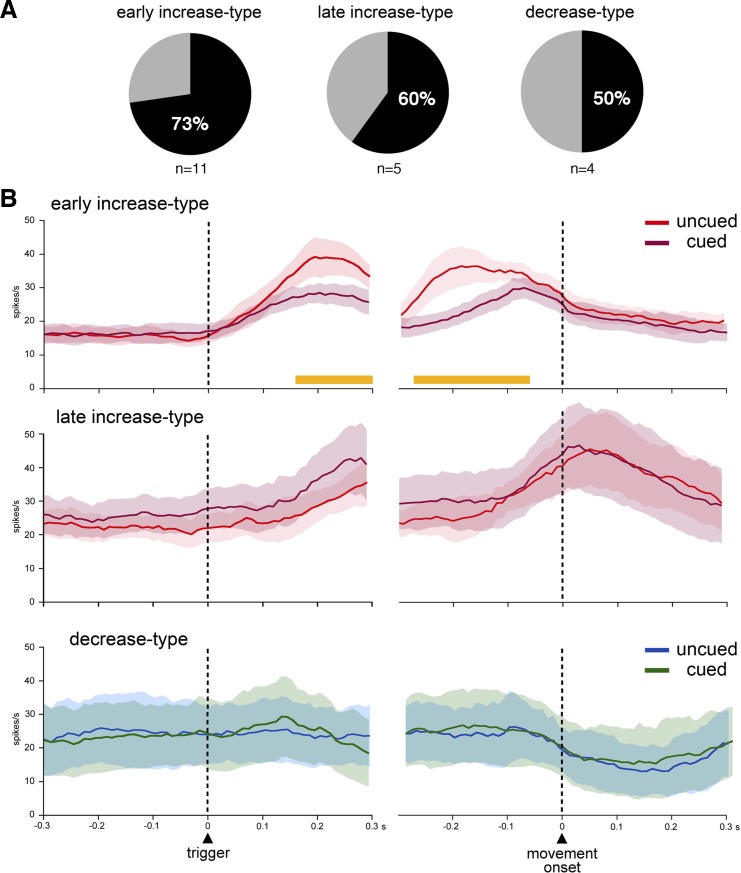
To address the influence of motor preparation more quantitatively, we compared, for each neuron, the magnitude of activity in the uncued and cued conditions. We performed this analysis on averaged spike counts during the same 300-ms intervals defined before. If the activity change was significantly larger (MANOVA during the 300-ms period after the trigger onset, P < 0.05) in one condition than the other, the neuron was judged to be modulated by the task condition. A total of 13 of the 20 movement-related neurons (65%) showed statistically significant differences in their modulations between the two conditions. As shown in Fig. 6A, it was found that neurons displaying a preferential sensitivity for either uncued or cued condition occurred more frequently in the early-increase-type category than other categories [early increase type: 73% (8 of 11), 3 and 5 in monkeys F and T, respectively; late increase type: 60% (3 of 5), all in monkey T; decrease type 50% (2 of 4), both in monkey F]. Figure 6B shows the superimposed averaged activity for the FSIs tested in the two conditions, separately for the three categories of neurons. For the early-increase-type category, the two curves were virtually identical during the period immediately preceding the onset of the trigger stimulus with a rise in activity starting just after the trigger onset and reaching a peak before the movement onset, the level of activation during the cued trials being significantly less than during uncued trials, as mentioned before (MANOVA during the 300-ms period after the trigger onset, P < 0.05). To estimate the timing of when FSI activity in uncued trials differed from that in the cued trials, we performed time series analysis for every 50-ms window moved in 10-ms steps over the period from 300 ms before to 300 ms after the trigger and movement onsets. Statistical analyses revealed that differences between uncued and cued trials became significant (MANOVA, P < 0.05) 160 ms after the trigger presentation and were maintained until 60 ms before movement onset (Fig. 6B). For the late-increase-type category, the population average showed a gradual increment for both cued and uncued trials, which reached a peak very close to movement onset. There was an extensive overlap between the two curves, without significant differences in the level of activation between the two conditions (MANOVA, P > 0.05). Although the small number of decrease-type neurons tested in both conditions makes analysis of the data less reliable, the average activity was very similar between uncued and cued trials for this category of neurons.
Fig. 6.
Comparison of FSI activity in the presence or absence of movement preparation. A: relative proportions of neurons with statistically significant differences in movement-related activity between the uncued and cued conditions. The percentages are calculated from the total number of recorded neurons of each category. n, No. of neurons. B: population average activity included all FSIs recorded in the two task conditions. Each panel shows the averaged activity for uncued and cued trials for a category of FSIs aligned on trigger presentation and movement onset. The orange horizontal lines below the curves indicate the periods when neuronal activity during uncued trials differed significantly from that during cued trials.
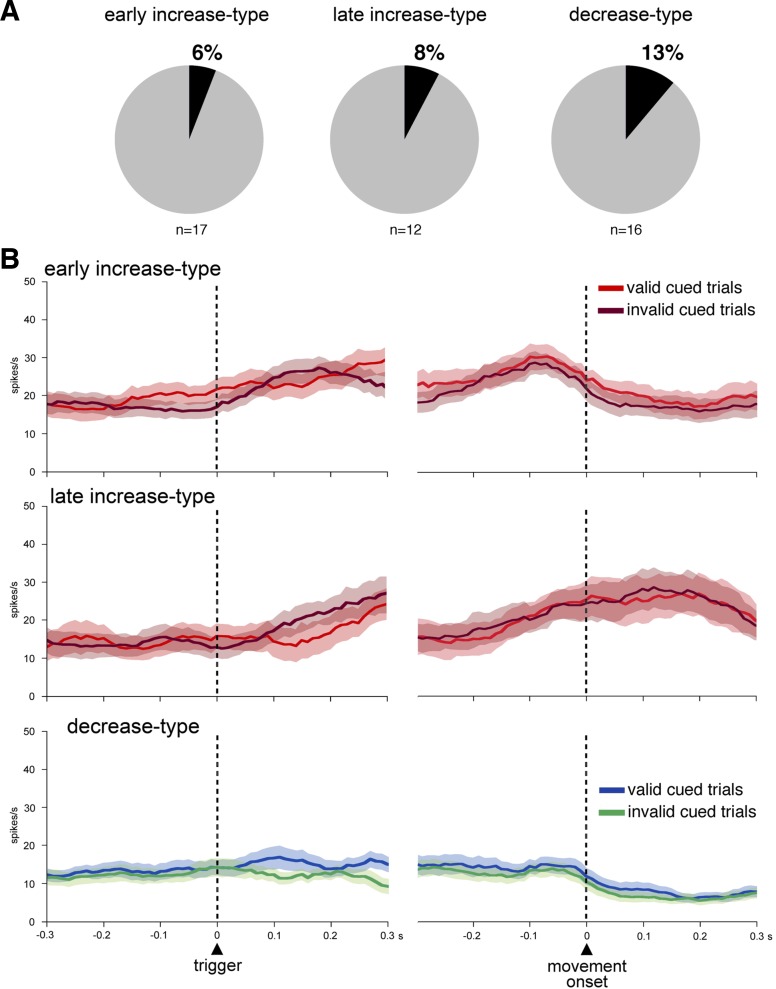
Finally, we further investigated the impact of motor preparation on FSI activity by testing the monkeys on the unreliable cued condition, in which a prepared action was unexpectedly replaced by the other movement option when the trigger occurred. As mentioned in the behavioral results, both monkeys took longer to respond to the trigger stimulus in invalid cued trials than in valid cued trials. This condition thus appeared well suited for examining whether an increase in demands for response initiation would elicit differences in FSI activity. A statistical analysis using the same two 300-ms windows as previously defined was performed to examine, for each neuron in our sample, whether the activity differed as a function of trial types. This comparison indicated that the activity of only 4 neurons of the 45 movement-modulated neurons (9%) varied significantly, depending on the trial type, with these few neurons being assigned to all three categories. Among 58 neurons that were tested in the unreliable cued condition, 45 (78%) showed significant changes in activity surrounding the initiation of movement. Of these, 17, 12, and 16 were categorized as early increase type, late increase type, and decrease type, respectively, based on the temporal profiles of their activity changes in the valid cued trials. The activity modulation occurred in relation to any of the three classes of FSIs we have categorized (Fig. 7A) [6% (1/17) monkey T; 8% (1/12) monkey F; 13% (2/16) monkey T]. We further examined FSI activity across the population. Figure 7B shows that FSI activity averaged over all neurons of each category overlapped almost completely in the two types of trials.
Fig. 7.
Comparison of FSI activity during invalid and valid trials. A: relative proportions of neurons with statistically significant differences in movement-related activity between the invalid and valid trials. The percentages are calculated from the total number of recorded neurons of each category. n, No. of neurons. B: same conventions as in Fig. 3B.
In sum, FSI activity did not vary markedly during the preparatory period preceding the initiation of the movement. Specifically, the level of activity of early-increase-type neurons appeared to be influenced by motor preparation, thus indicating that cognitive demands, and not just movement initiation, might be encoded by changes in FSI activity. The similarity of the level of changes in FSI activity in the valid and invalid cued trials seen at the population level indicates that increased latency of movement initiation was not associated with altered FSI activity when an action should be re-specified at the time the trigger became available for selection.
Recording positions.
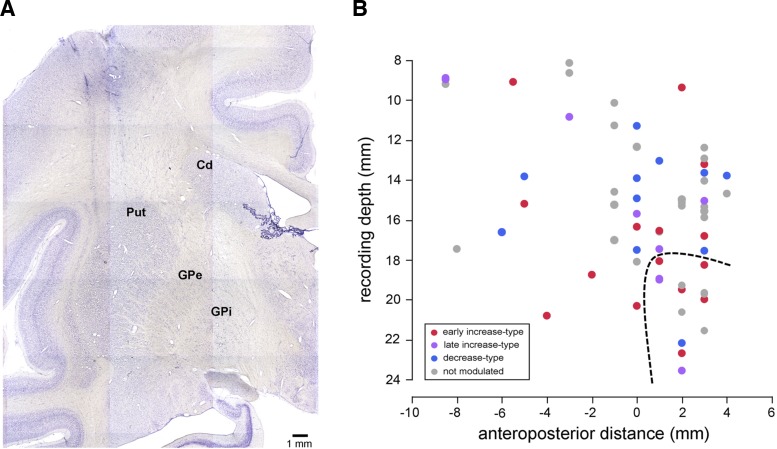
Finally, we took into account the functional specialization within the striatum and examined whether the responses of FSIs could be related to their location in the ventral and dorsal striatal regions. Recording sites were verified histologically in monkey T (Fig. 8A), while we confirmed that our recordings were in the striatum based on characteristic patterns of neuronal activity in monkey F. The majority of the neurons were recorded in the putamen, some of them being in the rostroventral putamen, which was identified as part of the ventral striatum (see materials and methods). Figure 8B illustrates the distribution of the neurons we recorded. Among 64 neurons, 50 were considered to be located within the dorsal part (28 and 22 in monkeys F and T, respectively) and 14 within the ventral part (4 and 10 in monkeys F and T, respectively). The three categories of FSIs were present in equal proportions and showed a considerable overlap in their distributions throughout the anteroposterior extent of the striatum explored. In particular, we found that FSIs with movement-related activity were not only restricted to the posterior putamen, which subserves motor functions, but were also found in the anterior putamen, including its ventral part. Early-increase-type and late-increase-type neurons were found in the dorsal (14 of 64 neurons; 22%) and ventral striatum (8 of 64 neurons; 13%). The frequency of neurons with a sensitivity to movement failed to vary significantly between the two regions (χ2 = 0.411, degrees of freedom = 1, P > 0.05).
Fig. 8.
Recording positions of FSIs. A: coronal section stained with cresyl violet at the level of the striatum, showing traces of electrode paths above the putamen. B: sagittal view of positions of recorded neurons plotted along the reconstructed electrode penetrations. Each dot corresponds to the position of a single FSI. For each neuron, the anteroposterior location (zero indicates the approximate level of the anterior commissure) is plotted against the depth of the electrode at which it was recorded. The recording depth was measured from a zero reference point as determined by using microdrive depth readings. Colored and gray dots indicate neurons that were or were not modulated during movement, respectively. The dashed line indicates the approximate boundary between dorsal and ventral striatum. Cd, caudate nucleus; Put, putamen; GPe, globus pallidus external segment; GPi, globus pallidus internal segment.
DISCUSSION
This study provides the first detailed analysis of the potential contribution of fast-spiking interneurons (FSIs) in the monkey striatum to information processing during movement preparation, initiation, and execution. Our analysis of changes in FSI activity highlighted the following points. First, the variety of changes in FSI activity, in terms of timing relative to movement initiation and the direction of these changes, suggests that these presumed interneurons do not provide a homogeneous signal at a particular moment during reaching task performance. Second, modulations of FSI activity occurring relatively early in relation to movement initiation all consisted of increases in firing rate, whereas those that followed the onset of movement consisted of either increases or decreases in firing rate. Third, increases in FSI activity reaching a peak before movement onset appear to be more influenced by the state of motor preparation, compared with those which peaked after movement onset. Fourth, movement-related changes in FSI firing were not influenced by an unexpected change in target location when monkeys were preparing a movement toward a specific location and instead elicited a movement in the opposite direction. We interpret the variety of these modulation types as reflecting a contribution of presumed GABAergic interneurons to different aspects of motor performance through an inhibitory influence on striatal output circuits.
Characterization of FSIs in the monkey striatum.
In line with previous single-neuron recording studies in the striatum of behaving monkeys (Adler et al. 2013; Yamada et al. 2016), we found that a particular class of neurons can be identified as FSIs on the basis of spike waveform and firing rate criteria. In particular, they are clearly distinguishable from the two other electrophysiologically determined types of neurons in the primate striatum, i.e., PANs and TANs (Aosaki et al. 1994; Apicella 2002; Kimura et al. 1984). It is noteworthy that studies of the functional properties of striatal FSIs, particularly in behaving monkeys, have been limited due to the difficulty in targeting these rarely encountered neurons for recordings. In the present study, the proportion of FSIs that we detected (12%) is higher than reported in previous studies (∼3–5%) (Adler et al. 2013; Yamada et al. 2016), possibly because we paid special attention to the detection of these neurons. Studies in freely moving rats have reported that the proportions of striatal FSIs may vary from 11% (Gage et al. 2010) to 33% (Stalnaker et al. 2012), highlighting the fact that extracellular recordings can be subjected to considerable sampling biases. Putative FSIs in the monkey striatum have firing properties resembling those of FSIs described in the rodent striatum, which are thought to correspond to the parvalbumin-containing group of GABAergic interneurons and are the source of strong inhibitory influences on striatal output pathways (Gittis et al. 2010; Koós and Tepper 1999). However, given the variety of striatal GABAergic interneurons that have not been characterized in vivo, we cannot rule out that neurons classified electrophysiologically into FSIs include, at least in part, one or more other classes of interneurons.
The role of FSIs in the initiation and execution of movement.
Although there is considerable interest in the role of FSIs in regulating the excitability of striatal output pathways, the exact nature of their contribution to movement generation remains unclear. Accordingly, the present study was designed to determine how FSIs modulate their firing in monkeys performing a reaching task that allows to distinguish between initiation and execution of movements. We found that the modulation of FSI firing rate was heterogeneous, with some neurons modulated at the presentation of the trigger stimulus and others being modulated later during the execution of movement. Most modulations of FSI firing around the time of movement initiation consisted of increases, whereas other FSIs changed their firing in the opposite direction. The variety of these modulation types may indicate the presence of distinct FSI subgroups involved in the processing of different movement-related information.
There are only a few studies in behaving monkeys that have reported changes in FSI activity consisting in phasic increases in activity after cue presentation and outcome delivery in a Pavlovian conditioning protocol (Adler et al. 2013) or more complex changes related to actions and outcomes in instrumental conditioning paradigms (Yamada et al. 2016). A comparatively large number of studies in rats trained in a variety of behavioral procedures have shown that FSIs can display changes in activity at certain phases of task performance, such as stimulus presentation, initiation of movement, position in space, and outcome delivery (Berke 2008; Gage et al. 2010; Lansink et al. 2010; Schmitzer-Torbert and Redish 2008; Stalnaker et al. 2012). It therefore appears that FSIs do not modify their activity in a homogeneous manner during task performance in behaving animals, suggesting that they are involved in different processes, depending on the task demands. As discussed further below, variations in the location of the recording sites could also account for differences in task-related activities of FSIs in the striatum.
Although the sample of FSIs recorded in the present study was heterogeneous in terms of the timing of activity changes and the direction of these changes (i.e., increased or decreased in firing rates), the limited diversity of FSI modulations around the time of movement contrasted with the wide variety of task-related activations observed in output neurons of the striatum (i.e., PANs). Numerous studies in behaving monkeys have reported that PANs display transient or sustained increases in firing rate occurring in several distinctive forms during the initiation and execution of actions (Crutcher and Alexander 1990; Kimura 1990). PAN activations may also reflect representational processes, such as preparation of movement, expectation of a trigger stimulus for movement (Alexander and Crutcher 1990; Apicella et al. 1992), or the motivational value of reward obtained at trial end (Cromwell and Schultz 2003; Hassani et al. 2001; Hollerman et al. 1998).
In rats performing a nose-poke task with a choice between two movement options, a relatively uniform increase in FSI activity has been reported at the time of the initiation of movement, with most activations reaching their peak just after choice execution (Gage et al. 2010). These findings have led to the hypothesis that changes in FSI activity constitute signals for selecting between competing actions, by suppressing unwanted action commands to facilitate the selection of the appropriate response. Our task design did not allow us to examine whether FSI activity can be preferentially modulated in the presence of a choice requirement, an issue which remains to be investigated with a task designed specifically to add demands for decision making.
We found that the target location and/or the direction of movement associated with reaching influenced the firing rate changes of about one-third of the FSIs (24%), with no systematic preference for one against the other movement direction. This modest selectivity for target location is somewhat different from the study of Gage et al. (2010), showing that a large majority of FSIs (78%) displayed directional selectivity with either ipsilateral or contralateral movements. It is possible that we recorded from fewer direction-selective FSIs because our task design did not require sufficient demands on response selection. In addition, we found only few FSIs whose magnitude of changes in activity was correlated with latency or duration of the reaching movement, indicating that the firing level of FSIs may not be particularly predictive of variability in the monkey's performance speed.
The role of FSIs in the preparation for movement.
Until now, studies of the role of striatal FSIs have not examined in detail their possible involvement in the preparation of movements and the expectation of task events. In the present study, we tested if FSI activity was sensitive to the preparation component of the task when the monkeys could improve the speed of their movements by using advance information about target location. We found that 65% of the recorded FSIs exhibited transient changes in firing during the cue-trigger interval. Furthermore, these changes were mostly observed in the subset of FSIs that we classified as early-increase-type, whereas late-increase-type neurons did not change the magnitude of their movement-related activity, with or without motor preparation. Although the functional implications of these distinct groups of FSIs remain to be determined, our findings suggest that perhaps the FSIs are functionally more heterogeneous than currently appreciated, with the activity of early-increase-type neurons time locked to the trigger onset being more influenced by the state of preparation for movement, compared with late-increase-type neurons. Specifically, modulation by predictive cues indicates that cognitive processes, and not just the process of movement initiation, might influence FSI activity.
Some FSIs showed a transient activation (less than 500 ms in duration) toward the end of the delay period of the task, just before trigger presentation, but none of them displayed activity changes that were maintained for a longer duration through the cue-trigger interval. This suggests that, even if the preparatory period may introduce additional demands for behavioral inhibition (i.e., active withholding of movement until trigger onset), sustained changes in FSI activity did not appear to be necessary for the behavioral expression of that preparation. On the other hand, short-lasting activations immediately preceding the trigger onset might represent a FSI signal in adjusting the movement preparation to the initiation of the subsequent movement. In the monkey putamen, it is relatively common to find striatal PANs displaying sustained activations that are thought to reflect movement preparation and/or stimulus expectation during delayed-response tasks (Alexander and Crutcher 1990; Apicella et al. 1992), while other PANs are phasically activated during movement initiation and execution (Crutcher and Alexander 1990; Kimura 1990). Different populations of PANs are therefore activated in sequence during the delay period and during the motor components of the task. It is possible that the observed changes in FSI activity may be required to facilitate the transition between subsets of PANs representing distinct steps of movement generation.
To further examine the influence of motor preparation on FSI activity, we also tested monkeys on a condition in which they were preparing a movement toward a specific location and instead must move in the opposite direction when the trigger occurred. Both monkeys slowed down in the trials where the cue was invalid, indicating that the recalibration of the movement direction places increased demands on the timing of movement initiation. We then examined whether this movement recalibration was accompanied by a change in FSI activity. We found that very few FSIs were differentially modulated on invalid and valid cued trials, despite the fact that the stimulus-response remapping had a strong impact on task performance. This finding indicates that the firing level of FSIs around movement initiation was not correlated with how quickly the monkey responds to the trigger stimulus, further confirming that FSI activity was not directly correlated with changes in the monkey's performance. However, in our task design, the switch in movement direction occurred when the prepared movement was not yet initiated, and it is possible that FSIs are critical for the behavioral adjustment when the decision on the movement direction has to be replaced by another after the movement onset.
Functional properties of FSIs and regional specializations of the striatum.
Based on evidence of regional specializations of information processing within the striatum, we examined the possibility that the functional properties of FSIs could be related to their location in different parts of the striatum. Neuronal recordings in behaving rats have outlined differences in the way striatal FSIs are modulated during task performance, according to their anatomical location, namely FSIs in the dorsolateral striatum may be important for controlling motor functions (Gage et al. 2010), whereas those in the dorsomedial and ventromedial striatum may contribute to outcome processing (Lansink et al. 2010; Stalnaker et al. 2012). In our study, no difference was found in the distribution of movement-related FSIs over the extent of the putamen sampled, encompassing motor putamen caudal to the anterior commissure and associative putamen rostral to it. This appears to confirm findings from previous recording studies in behaving monkeys (Adler et al. 2013; Yamada et al. 2016) in which FSI modulations related to task events were evenly distributed throughout the explored striatum. Interestingly, we found that a subset of FSIs located in the most ventral part of the anterior putamen, which is to say in limbic striatum, also exhibited movement-related activity. This differs from PANs recorded in the ventral striatum which have been reported to be specialized in processing motivational information (Apicella et al. 1991). However, given the low neuron number obtained in the present study, it is premature to draw any firm conclusions concerning a variation of functional properties of FSIs that may be related to the regional specializations of the striatum. In addition, it remains to be determined, in our study, whether the impact of reward on FSI activity may be particularly strong in the ventral striatum, as has been observed in behaving rats (Lansink et al. 2010).
How FSI signals are used by the striatal output pathways that control action?
It is thought that FSIs form a local inhibitory circuit that targets selectively the output of the striatum to downstream targets (Szydlowski et al. 2013). Increases in FSI activity are supposed to lead to inhibition of striatal output pathways and therefore suppression of movements by decreasing inhibition of basal ganglia output structures. One can assume that the reverse may occur in the case of decreases in FSI activity, resulting in facilitation of movements. This suggests that the two types of FSI modulations described in the present study might be differently involved in movement generation, one suppressing and the other facilitating movements. Our findings have shown that increases in FSI activity can occur sufficiently early to be involved in preparation and initiation of movements. It is possible that this type of FSI modulation may reflect an enhanced inhibitory level necessary for the expression of the correct response by preventing inappropriate motor commands from being selected. On the other hand, we observed a prevalence of decreases in FSI activity after the onset of movement, suggesting that these modulations may play a role in ongoing motor performance. This may be associated with a release of output neurons from inhibition and therefore facilitation of motor commands. This late FSI signal may be related to the inhibition of competing responses when the action has already been initiated. It is still an open question whether increased and decreased FSI firing rates might reflect differential influences on functionally distinct output pathways thought to exert opposing effects on motor behavior (Gittis et al. 2010; Planert et al. 2008).
In conclusion, evidence in favor of prominent involvement of striatal FSIs in motor control has come from inactivation studies in animals (Burguière et al. 2013; Gernert et al. 2000; Gittis et al. 2011; Xu et al. 2016), as well as from clinical studies in humans (Kalanithi et al. 2005; Kataoka et al. 2010; Reiner et al. 2013). Our results are the first demonstration of modulations of activity from individual FSIs during the successive stages of movement generation, reflecting contributions to different aspects of motor performance, rather than an exclusive involvement in movement initiation. Although it is still unclear what features are precisely encoded by these changes, it is conceivable that disturbed FSI signaling might interfere with action selection, resulting in an impaired ability to inhibit inappropriate behaviors.
GRANTS
This work was supported by Association Française du Syndrome de Gilles de la Tourette and by Centre National de la Recherche Scientifique.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.M. and P.A. performed experiments; K.M. and P.A. analyzed data; K.M. and P.A. interpreted results of experiments; K.M. and P.A. prepared figures; K.M. and P.A. drafted manuscript; K.M. and P.A. edited and revised manuscript; K.M. and P.A. approved final version of manuscript; P.A. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. I. Balansard and L. Renaud for assistance with surgery, Dr. M. Esclapez for providing the histological micrograph reported in Fig. 8, and E. Legallet for designing the computer programs.
REFERENCES
- Adler A, Katabi S, Finkes I, Prut Y, Bergman H. Different correlation patterns of cholinergic and GABAergic interneurons with striatal projection neurons. Front Syst Neurosci 7: 47, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol 64: 133–150, 1990. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci 14: 3969–3984, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P. Tonically active neurons in the primate striatum and their role in the processing of information about motivationally relevant events. Eur J Neurosci 16: 2017–2026, 2002. [DOI] [PubMed] [Google Scholar]
- Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res 85: 491–500, 1991. [DOI] [PubMed] [Google Scholar]
- Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol 68: 945–960, 1992. [DOI] [PubMed] [Google Scholar]
- Berke JD. Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance. J Neurosci 28: 10075–10080, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43: 883–896, 2004. [DOI] [PubMed] [Google Scholar]
- Burguière E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science 340: 1243–1246, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol 89: 2823–2838, 2003. [DOI] [PubMed] [Google Scholar]
- Crutcher MD, Alexander GM. Movement-related neuronal activity selectively coding either direction or muscle pattern in three motor areas of the monkey. J Neurophysiol 64: 151–163, 1990. [DOI] [PubMed] [Google Scholar]
- Deffains M, Legallet E, Apicella P. Modulation of neuronal activity in the monkey putamen associated with changes in the habitual order of sequential movements. J Neurophysiol 104: 1355–1369, 2010. [DOI] [PubMed] [Google Scholar]
- Gage GJ, Stoetzner CR, Wiltschko AB, Berke JD. Selective activation of striatal fast-spiking interneurons during choice execution. Neuron 67: 466–479, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernert M, Hamann M, Bennay M, Los̈cher W, Richter A. Deficit of striatal parvalbumin-reactive GABAergic interneurons and decreased basal ganglia output in a genetic rodent model of idiopathic paroxysmal dystonia. J Neurosci 20: 7052–7058, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Leventhal DK, Fensterheim BA, Pettibone JR, Berke JD, Kreitzer AC. Selective inhibition of striatal fast-spiking interneurons causes dyskinesias. J Neurosci 31: 15727–15731, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci 30: 2223–2234, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Ann N Y Acad Sci 877: 33–48, 1999. [DOI] [PubMed] [Google Scholar]
- Hassani OK, Cromwell HC, Schultz W. Influence of expectation of different rewards on behavior-related neuronal activity in the striatum. J Neurophysiol 85: 2477–2489, 2001. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Influence of reward expectation on behavior-related neuronal activity in primate striatum. J Neurophysiol 80: 947–963, 1998. [DOI] [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci U S A 102: 13307–13312, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol 518: 277–291, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci 13: 4908–4923, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. Behaviorally contingent property of movement-related activity of the primate putamen. J Neurophysiol 63: 1277–1296, 1990. [DOI] [PubMed] [Google Scholar]
- Kimura M, Rajkowski J, Evarts EV. Tonically discharging putamen neurons exhibit set dependent responses. Proc Natl Acad Sci U S A 81: 4998–5001, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koós T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 2: 467–472, 1999. [DOI] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, Pennartz CM. Fast-spiking interneurons of the rat ventral striatum: temporal coordination of activity with principal cells and responsiveness to reward. Eur J Neurosci 32: 494–508, 2010. [DOI] [PubMed] [Google Scholar]
- Mallet N, LeMoine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci 25: 3857–3869, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planert H, Szydlowski SN, Hjorth JJ, Grillner S, Silberberg G. Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways. J Neurosci 30: 3499–3507, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Shelby E, Wang H, Demarch Z, Deng Y, Guley NH, Hogg V, Roxburgh R, Tippett LJ, Waldvogel HJ, Faull RL. Striatal parvalbuminergic neurons are lost in Huntington's disease: implications for dystonia. Mov Disord 28: 1691–1699, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitzer-Torbert NC, Redish AD. Task-dependent encoding of space and events by striatal neurons is dependent on neural subtype. Neuroscience 153: 349–360, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharott A, Doig NM, Mallet N, Magill PJ. Relationships between the firing of identified striatal interneurons and spontaneous and driven cortical activities in vivo. J Neurosci 32: 13221–13236, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G, Bolam JP. Local and afferent synaptic pathways in the striatal microcircuitry. Curr Opin Neurobiol 33: 182–187, 2015. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Calhoon GG, Ogawa M, Roesch MR, Schoenbaum G. Reward prediction error signaling in posterior dorsomedial striatum is action specific. J Neurosci 32: 10296–10305, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowski SN, Dorocic IP, Planert H, Carlen M, Meletis K, Silberberg G. Target selectivity of feedforward inhibition by striatal fast-spiking interneurons. J Neurosci 33: 1678–1683, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Tecuapetla F, Koós T, Ibáñez-Sandoval O. Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat 4: 150, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Li L, Pittenger C. Ablation of fast-spiking interneurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neuroscience 324: 321–329, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Inokawa H, Hori Y, Pan X, Matsuzaki R, Nakamura K, Samejima K, Shidara M, Kimura M, Sakagami M, Minamimoto T. Characteristics of fast-spiking neurons in the striatum of behaving monkeys. Neurosci Res 105: 2–18, 2016. [DOI] [PubMed] [Google Scholar]