Abstract
Central nervous system lesions are common in HIV-infected patients. In the combination anti-retroviral therapy (ART) era, Toxoplasma reactivation has been observed only in patients with unrecognized HIV infection or refusing therapy. We present the case of 10-year-old girl with AIDS who initially presented with pneumonia. She was treated for pneumonia and thereafter started on ART as her CD4 count was low. However, 5 days after starting ART she presented with left ptosis and right-sided monoparesis. She was diagnosed with neurotoxoplasmosis and responded successfully to pyrimethamine–sulfadoxine therapy. Though she had no vision difficulties, her fundus examination revealed chorioretinitis during the hospital stay. We emphasize the importance of routine fundus examination prior to starting ART to rule out chorioretinitis even in an older child with no visual complaints.
INTRODUCTION
Central nervous system (CNS) lesions are common in HIV-infected patients [1]. The most common being neurotoxoplasmosis, progressive multifocal leukoencephalopathy, primary CNS lymphoma, the severe form of HIV-associated neurocognitive disorder, cryptococcal encephalitis and lesions of undetermined origin. The incidence of neurotoxoplasmosis in HIV-infected patients has decreased following the introduction of combined anti-retroviral therapy (cART). In the cART era, Toxoplasma reactivation was observed only in patients with unrecognized HIV infection or those refusing therapy [2]. Toxoplasmosis in HIV-infected children is rare with very few case reports [3] of the same and is commonly seen in adults [4]. We present the case of 10-year-old girl with AIDS who presented with neurotoxoplasmosis after 5 days of starting ART. Her fundus examination revealed chorioretinitis during the hospital stay though she had no visual complaints. She responded to pyrimethamine–sulfadoxine. We emphasize the need for regular fundus examination even in older children and in patients with normal vision as part of the routine screening in HIV-infected children.
CASE REPORT
A 10-year-old girl presented to us in August 2015 with cough for 20 days and fever for 1 day. She was diagnosed to have HIV infection one week prior to being referred to us. Her mother was also HIV infected and had died in July 2015. She had no visual complaints.
On examination, her weight was 15 kg (less than third centile) and height was 111 cm (less than third centile). She had bilateral otorrhoea and bilateral chest crepitations. The examination of other systems was normal.
Investigations revealed haemoglobin of 10.2 g/dl, total leucocyte count: 7500 cells/cumm (polymorphs: 84%; lymphocytes: 14%), ESR: 60 mm at the end of 1 h and Mantoux test was negative. Absolute CD4 count was 14 cells/cumm (below 4%). Chest X-ray showed right parahilar basal bronchitis and bilateral upper zone haziness. Echocardiogram and urine examination were normal.
She was diagnosed with pneumonia and treated with oral amoxicillin–clavulanic acid for 1 week. After resolution of the pneumonia, she was started on ART in September 2015 with zidovudine, lamivudine and efavirenz along with trimethoprim–sulfamethoxazole prophylaxis.
Five days later, she presented with bilateral ear discharge and lethargy for 2 days. On examination, she was afebrile, heart rate: 90 beats/min, respiratory rate: 38 breaths/min, BP: 88/64 mmHg, maintaining oxygen saturation on non-rebreather mask with oxygen at 10 l/min. Respiratory system examination revealed bilateral crepitations. On neurological examination, she was drowsy, Glasgow coma scale score—E2M4V4, deep tendon reflexes were brisk and plantars extensor. She had left ptosis and right upper limb monoparesis. Fundus examination revealed bilateral chorioretinitis.
Investigations revealed haemoglobin: 8.0 g/dl, total leucocyte count: 15 200/cumm (polymorphs: 87%, lymphocytes: 13%), ESR: 105 mm at the end of 1 h. Chest X-ray showed diffuse haziness in both lung fields. Urine examination was normal. Ear swab showed Pseudomonas aeruginosa on culture and gastric lavage showed no acid fast bacilli. Serum IgG for toxoplasma was positive but IgM was negative.
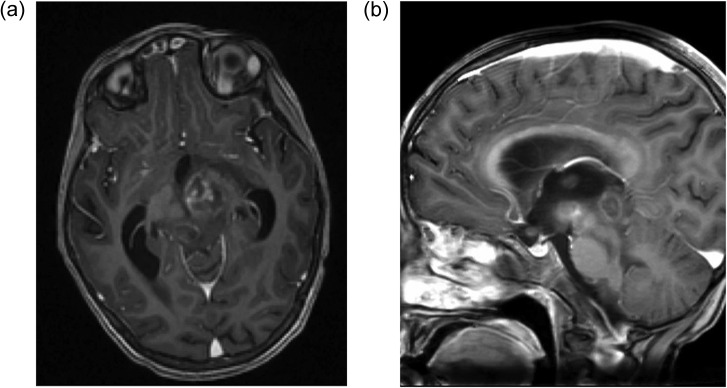
Magnetic resonance imaging (MRI) brain showed a 22 × 19 mm sized, heterogeneous nodular lesion with mixed signal intensity and predominance of hyperintense signal seen involving the left cerebral peduncle as seen in Fig. 1. Similar nodular lesions were seen in the right dorsal midbrain and globus pallidus. The lesion revealed a mild thick peripheral capsular enhancement with ill-defined eccentric enhancement within. There was significant perifocal oedema seen in the left half of midbrain and upper pons and inferior aspect of left thalamus. Mass effect was noted on the inferior third ventricle resulting in obstructive dilatation of both lateral ventricles and superior third ventricle with mild periventricular cerebrospinal fluid ooze. These features were suggestive of toxoplasmosis with obstructive hydrocephalus.
Figure 1:
MRI brain with contrast (a) axial view and (b) sagittal view showing large heterogeneous nodular lesion in the left subthalamic region involving the left cerebral peduncle with perifocal oedema and mass effect.
ART was continued. Measures to decrease the intracranial pressure were started, including oral acetazolamide and intravenous dexamethasone, and these were gradually tapered off after 10 days. One packed cell transfusion was given. Fluid boluses were given followed by inotropic support with dopamine for the initial 48 h. IV meropenem and IV metronidazole were started in view of the otitis media and continued for 10 days. Pyrimethamine (2 mg/kg) and sulfadoxine (100 mg/kg/day) were given orally for 6 weeks for CNS toxoplasmosis. Folinic acid was added.
The child gradually improved. She was weaned off oxygen and inotropic support after 48 h. Her sensorium improved. Nasogastric feeds were started after 48 h and later changed to oral feeds. However, residual left ptosis and right upper arm monoparesis were seen. Repeat MRI after 3 weeks of therapy showed moderate regression of the CNS lesions with no mass effect and no hydrocephalus. Absolute CD4 count improved to 86 cells/cumm and HIV viral load showed 2199 copies/ml. She is on regular follow-up.
DISCUSSION
Toxoplasma gondii is an obligate intracellular protozoan parasite [5]. Neurotoxoplasmosis in HIV-infected children is associated with a low CD4 count (<50 cells/cumm) and seen in patients presenting late in the illness in whom ART has not been started [6]. Our patient presented to us late in her illness with an absolute CD4 count of 14 cells/cumm and had been started on ART 5 days prior to her neurological illness.
Provisional diagnosis of CNS toxoplasmosis is considered in patients with (a) CD4 count <100 cell/cumm, (b) compatible neurological symptoms, (c) positive T. gondii IgG antibody and (d) brain imaging. The diagnosis of neurotoxoplasmosis is usually suspected based on MRI findings of multiple lesions. The characteristic sign of CNS toxoplasmosis is the asymmetrical target sign, which is detectable on CT and MRI scans, although MRI is more sensitive. On T1-weighted precontrast MRIs, the lesions are hypointense relative to brain tissue [7]. For AIDS patient, the differential diagnosis of multiple ring enhancing lesions includes both CNS lymphoma and toxoplasmosis. The presence of a solitary lesion should lead us towards CNS lymphoma rather than toxoplasmosis [3]. Our patient had typical MRI features suggestive of toxoplasmosis and positive toxoplasma IgG along with chorioretinitis. She responded to sulfadoxine–pyrimethamine.
Isolated ocular toxoplasmosis is rare and usually is seen in association with CNS manifestation [8]. A neurologic examination or brain imaging is indicated for children who have been diagnosed with toxoplasma chorioretinitis. Ocular toxoplasmosis appears as white retinal lesions with little associated haemorrhage; visual loss might be seen [9]. In our patient, a fundus examination was not done at the first clinical examination because vision was normal. However, fundus examination done later revealed chorioretinitis. This shows the importance of routine fundus examination in all HIV-positive children even with no visual complaints to look for chorioretinitis as an early manifestation of neurotoxoplasmosis.
Fundus examination should be routinely done in all HIV-infected children even with no visual complaints for early diagnosis of ocular toxoplasmosis so that they can be screened for neurotoxoplasmosis.
ACKNOWLEDGEMENTS
The authors would like to thank Dr Y.K. Amdekar, Medical Director, Bai Jerbai Wadia Hospital for Children, Parel, Mumbai 400012, India.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICAL APPROVAL
Taken from Hospital Ethics Committee.
INFORMED CONSENT
Patient's written consent was taken for publication.
GUARANTOR
Dr Noella Pereira, Assistant Professor, Bai Jerbai Wadia Hospital for Children, Parel, Mumbai, India.
REFERENCES
- 1.Matinella A, Lanzafame M, Bonometti MA, Gajofatto A, Concia E, Vento S, et al. . Neurological complications of HIV infection in pre-HAART and HAART era: a retrospective study. J Neurol 2015;262:1317–27. [DOI] [PubMed] [Google Scholar]
- 2.Kodym P, Malý M, Beran O, Jilich D, Rozsypal H, Machala L, et al. . Incidence, immunological and clinical characteristics of reactivation of latent Toxoplasma gondii infection in HIV-infected patients. Epidemiol Infect 2015;143:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doraiswamy V, Vaswani RK, Lahiri KR, Kondekar SS. Neurotoxoplasmosis mimicking intracranial tuberculoma. J Postgrad Med 2010;56:31–4. [DOI] [PubMed] [Google Scholar]
- 4.Berhe T, Melkamu Y, Amare A.. The pattern and predictors of mortality of HIV/AIDS patients with neurologic manifestation in Ethiopia: a retrospective study. AIDS Res Ther 2012;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAuley JB, Boyer KH, Remington JS, McLeod RL. Toxoplasmosis In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ. Feigin and Cherry's Textbook of Pediatric Infectious Diseases, 7th edn Philadelphia: Saunders, 2014,2986–3005. [Google Scholar]
- 6.Liu YC, Li XH, Li XW, Lun WH, Yan HW, Ge ML, et al. . Study on the association of clinical characteristic, CD4+ and level of HIV viral load among 690 initial HIV-infection. Zhonghua Liu Xing Bing Xue Za Zhi 2007;28:1026–9. Article in Chinese. [PubMed] [Google Scholar]
- 7.Sokolska V, Knysz B, Czapiga E, Gasiorowski J, Sasiadek M, Gładysz A. The role of brain magnetic resonance studies in the diagnostics of central nervous system lesions in HIV-1 positive patients. Wiad Lek. 2006;59:805–13. [PubMed] [Google Scholar]
- 8.Panel on Opportunistic Infections in HIV-Exposed and HIV-Infected Children Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Exposed and HIV-Infected Children Department of Health and Human Services; http://aidsinfo.nih.gov/contentfiles/lvguidelines/oi_guidelines_pediatrics.pdf, Z1–12 (16 October 2016, date last accessed). [Google Scholar]
- 9.Shivananda Sanjeeva GN. Management of Opportunistic infections In: Shah I, Shah N, Manglani M.. IAP Speciality Series on Pediatric HIV (Under IAP Action Plan 2006), 1st edn Mumbai: Indian Academy of Pediatrics, 2006,46–62. [Google Scholar]