Abstract
Purpose
We aimed to evaluate the effect of testosterone replacement therapy (TRT) on cognitive function and depression in men with testosterone deficiency syndrome.
Materials and Methods
We carried out a prospective, placebo-controlled trial involving 106 men with total testosterone levels <3.3 ng/mL and symptoms of hypogonadism. Based on whether the patients received TRT (injection with 1,000 mg testosterone undecanoate) or a placebo (advice to modify lifestyle), the study population was divided into a TRT group (n=54) and a control group (n=52).
Results
The age among patients in the TRT and control groups was 56.7±12.6 years and 57.8±11.4 years, respectively (p> 0.05). At baseline, no significant differences between the TRT and control groups were noted regarding serum testosterone or prostate-specific antigen levels, or regarding the scores for aging symptoms (Aging Males' Symptoms scale), erectile function (5-item International Index of Erectile Function questionnaire), cognitive function (Korean Mini-Mental State Examination), and depression (Beck Depression Inventory). At 8 months after intervention total serum testosterone levels and erectile function scores had significantly increased (p<0.05), whereas the scores for aging symptoms and depression had significantly decreased (p<0.05) in the TRT group; no significant improvement in any parameters was noted for the control group. Notably, significant improvement in cognitive function was noted among patients with cognitive impairment at baseline (cognitive function score <25) who received TRT.
Conclusions
TRT may be considered in men with testosterone deficiency syndrome if low testosterone levels are associated with depression or cognitive impairment.
Keywords: Cognition, Depression, Testosteron
INTRODUCTION
The prevalence of low levels of total serum testosterone is approximately 20% in individuals younger than 50 years, and 50% in those younger than 80 years [1]. Testosterone deficiency syndrome (TDS) represents a clinical and biochemical syndrome associated with advancing age, and is characterized by a total level of serum testosterone below the reference range calculated based on the levels noted among healthy adult men. TDS may have adverse effects on the function of multiple organ systems. Common symptoms of TDS include low libido, decreased vitality, fatigue, mood changes, insomnia, anemia, flushes, erectile dysfunction, decreased muscle mass, increased visceral body fat, ejaculatory dysfunction, and loss of concentration [2]. Low testosterone levels have also been associated with impaired quality of life due to symptoms such as depression and cognitive impairment [3], as well as poorer sense of psychological well-being [4] and subsyndromal levels of depression and anxiety in otherwise healthy older men [5]. Testosterone replacement therapy (TRT) is a widely-used treatment for men with TDS, with well documented beneficial effects on libido, energy levels, bone density, strength, and muscle mass [6]. While a meta-analysis has suggested that TRT may improve depression scores [7], reports regarding the beneficial effects of TRT on quality of life and depressive mood have not been consistent across trials [8]. Lower levels of testosterone appear to affect spatial abilities, verbal abilities and cognitive function [9]. Reports regarding the effects of TRT on cognitive function and memory scores have described mixed results [10,11]. In the present study, we investigated the efficacy and safety of TRT in patients with TDS, as well as its effect on cognitive performance and depression.
MATERIALS AND METHODS
The present study was conducted as a prospective, placebo-controlled study. The study protocol was approved by the Institutional Review Board of Daegu Catholic University Medicine Center and all participants provided informed consent. TDS patients aged >40 years were recruited between April and December 2011. TDS was defined as a total serum testosterone level of <3.3 ng/mL, with symptoms of hypogonadism. The exclusion criteria were: prostate cancer; suspicion of prostate cancer based on elevated levels of serum prostate-specific antigen (PSA, >4.0 ng/mL), and a digital rectal examination; high value (>20) of the self-reported International Prostate Symptom Score; and comorbid pathologies including decreased liver function, chronic renal insufficiency requiring hemodialysis, and severe cardiovascular co-morbidities such as acute myocardial infarction. Use of concomitant medication involving alpha adrenergic blockers, 5-alpha reductase inhibitors, and phosphodiesterase type 5 inhibitors, which can influence urination and the function of sexual hormones, was not permitted during the study.
A total of 106 patients (mean age, 55.8 years; age range, 42~67 years) with TDS were enrolled in the study. The blood sampling for the measurement of serum total testosterone was performed between 9:00 AM and 11:00 AM. The study population was divided into two groups according to whether the patients received TRT (TRT group, n=54) or a placebo (control group, n=52). TRT was performed in the form of several injections of 1,000 mg testosterone undecanoate. The patients from the TRT group were injected upon enrollment, at 8 weeks, and then every 3 months; these patients also received recommendations for lifestyle modification. The patients from the control group received recommendations to change their lifestyle, but did not undergo any treatment with injections.
The study outcomes were evaluated in terms of efficacy and safety of the TRT treatment. The parameters describing efficacy were the total serum testosterone levels (measured between 09:00 AM and 11:00 AM) and the change in the scores for Aging Males' Symptoms (AMS) and 5-item International Index of Erectile Function questionnaire (IIEF-5), which were monitored at baseline and at the 8-month follow-up to calculate the body mass index (BMI). To assess the condition of the prostate, the following evaluations were performed at baseline and at the 8-month follow-up: measurement of PSA levels, uroflowmetry, digital rectal examination, and transrectal ultrasound to assess prostate volume.
Cognitive function was assessed at baseline and at the 8-month follow-up using the Korean Mini-Mental State Examination (K-MMSE), which represents an established instrument to screen for cognitive impairment or dementia in a community setting [12].
Depression was assessed at baseline and at the 8-month follow-up using the 21-item Beck Depression Inventory (BDI), which is one of the most popular measures of depressive symptoms worldwide [13].
Sample size calculations were conducted using G*Power version 3.1 (Düsseldorf University, Düsseldorf, Germany). For a significance level of 0.05 and statistical power of 80%, the minimal sample size necessary to observe an effect was calculated to be 92. Statistical analysis was performed using the Korean version of PASW Statistics ver. 18.0 (IBM Co., Armonk, NY, USA). Data regarding the TRT and control groups was compared using Student's t-test and the Mann-Whitney U-test. Baseline and follow-up values of continuous variables were compared using the paired t-test. Statistical significance was set at a p-value of <0.05.
RESULTS
Among the 106 patients enrolled, 54 received TRT and were included in the TRT group (age, 56.7±12.6 years), while 52 received the placebo treatment and were included in the control group (age, 57.8±11.4 years). At baseline, there were no statistically significant differences between the TRT and control groups regarding BMI, PSA levels, prostate volume, maximal urinary flow rate, postvoid residual volume, total serum testosterone levels, or scores of IIEF-5, AMS, BDI, and K-MMSE (Table 1). The baseline testosterone levels in the TRT and control groups were 2.5±0.6 ng/mL and 2.6±0.7 ng/mL, respectively, whereas the baseline BDI scores were 26.6±4.2 and 27.2±5.3, respectively. The baseline K-MMSE scores in the TRT and control groups were 24.8±4.4 and 23.9±4.6, respectively.
Table 1. Baseline characteristics of patients with testosterone deficiency syndrome.
| Parameter | TRT group (n=54) | Control group (n=52) | p-value |
|---|---|---|---|
| Age (yr) | 56.7±12.6 | 57.8±11.4 | 0.180 |
| BMI (kg/m2) | 26.2±2.0 | 26.3±3.1 | 0.291 |
| PSA (ng/mL) | 0.8±1.3 | 0.9±1.2 | 0.967 |
| Prostate volume (mL) | 23.8±8.8 | 22.9±7.2 | 0.447 |
| Hemoglobin (g/dL) | 12.7±1.1 | 12.4±1.3 | 0.498 |
| Hematocrit (%) | 36.8±3.4 | 36.6±4.1 | 0.346 |
| Qmax (mL/s) | 14.2±5.6 | 14.1±6.1 | 0.659 |
| PVR (mL) | 14.9±10.6 | 14.6±9.5 | 0.766 |
| Total testosterone (ng/mL) | 2.5±0.6 | 2.6±0.7 | 0.841 |
| IIEF-5 score | 20.7±4.8 | 20.6±4.3 | 0.646 |
| AMS scale | 41.4±9.7 | 39.6±7.4 | 0.361 |
| BDI score | 26.6±4.2 | 27.2±5.3 | 0.272 |
| K-MMSE score | 24.8±4.4 | 23.9±4.6 | 0.395 |
Values are presented as mean±standard deviation.
TRT: testosterone replacement therapy, BMI: body mass index, PSA: prostate-specific antigen, Qmax: maximal urinary flow rate, PVR: postvoid residual volume, IIEF-5: 5-item version of the International Index of Erectile Function questionnaire, AMS: Aging Males' Symptoms, BDI: Beck Depression Inventory, K-MMSE: Korean Mini-Mental State Examination.
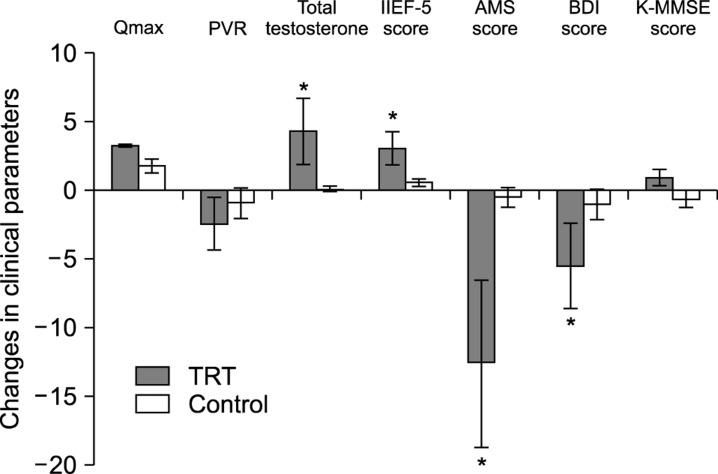
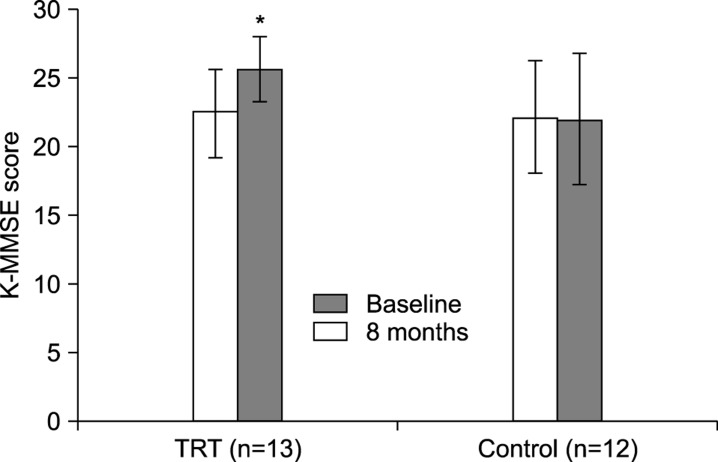
At the 8-month follow-up, patients in the TRT group showed significantly higher levels of total serum testosterone and IIEF-5 scores compared to the baseline values. The patients in the TRT group also showed significantly lower AMS and BDI scores compared to the baseline values (Table 2). No statistically significant differences between baseline and follow-up values were observed regarding BMI, PSA levels, prostate volume, maximal urinary flow rate, and postvoid residual volume in the TRT group. No significant differences between baseline and follow-up values were observed regarding serum testosterone levels or scores for AMS, IIEF-5, BDI, and K-MMSE in the control group (Table 3). At 8 months after intervention, the improvement in total testosterone levels, IIEF-5 score, AMS score, and BDI score was significantly higher for the TRT group than for the control group (Fig. 1). While the overall K-MMSE scores did not significantly increase over the baseline values in either group, significant improvement was noted for patients with cognitive impairment (K-MMSE scores <25) who received TRT (Fig. 2).
Table 2. Clinical parameters of patients with testosterone deficiency syndrome who received TRT (n=54).
| Parameter | Baseline | After TRT | p-value |
|---|---|---|---|
| BMI (kg/m2) | 26.2±2.0 | 26.3±2.1 | 0.320 |
| PSA (ng/mL) | 0.8±1.3 | 1.1±1.2 | 0.113 |
| Prostate volume (mL) | 23.8±8.8 | 25.5±7.4 | 0.546 |
| Hemoglobin (g/dL) | 12.7±1.1 | 13.5±1.4 | 0.071 |
| Hematocrit (%) | 36.8±3.4 | 39.6±5.1 | 0.065 |
| Qmax (mL/s) | 14.2±5.6 | 17.5±4.6 | 0.076 |
| PVR (mL) | 14.9±10.6 | 12.5±8.7 | 0.093 |
| Total testosterone (ng/mL) | 2.5±0.6 | 6.8±3.0 | 0.010 |
| IIEF-5 score | 20.7±4.8 | 23.8±3.6 | 0.031 |
| AMS score | 41.4±9.7 | 28.8±3.6 | 0.047 |
| BDI score | 26.6±4.2 | 20.8±7.3 | 0.033 |
| K-MMSE score | 24.8±4.4 | 25.8±3.8 | 0.094 |
Values are presented as mean±standard deviation.
TRT: testosterone replacement therapy, BMI: body mass index, PSA: prostate-specific antigen, Qmax: maximal urinary flow rate, PVR: postvoid residual volume, IIEF-5: 5-item version of the International Index of Erectile Function questionnaire, AMS: Aging Males' Symptoms, BDI: Beck Depression Inventory, K-MMSE: Korean Mini-Mental State Examination.
Table 3. Clinical parameters of patients with testosterone deficiency syndrome who did not undergo testosterone replacement therapy (control group, n=52).
| Parameter | Baseline | After 8 months | p-value |
|---|---|---|---|
| BMI (kg/m2) | 26.3±3.1 | 26.2±2.6 | 0.219 |
| PSA (ng/mL) | 0.9±1.2 | 0.9±1.1 | 0.477 |
| Prostate volume (mL) | 22.9±7.2 | 22.8±9.1 | 0.549 |
| Hemoglobin (g/dL) | 12.4±1.3 | 12.3±1.2 | 0.491 |
| Hematocrit (%) | 36.6±4.1 | 36.5±3.7 | 0.573 |
| Qmax (mL/s) | 14.1±6.1 | 15.9±5.6 | 0.117 |
| PVR (mL) | 14.6±9.5 | 13.7±8.4 | 0.170 |
| Total testosterone (ng/mL) | 2.6±0.7 | 2.7±0.5 | 0.146 |
| IIEF-5 score | 20.6±4.3 | 21.2±4.6 | 0.172 |
| AMS score | 39.6±7.4 | 39.1±8.1 | 0.324 |
| BDI score | 27.2±5.3 | 26.2±6.4 | 0.139 |
| K-MMSE score | 23.9±4.6 | 23.3±5.2 | 0.248 |
BMI: body mass index, PSA: prostate-specific antigen, Qmax: maximal urinary flow rate, PVR: postvoid residual volume, IIEF-5: 5-item version of the International Index of Erectile Function questionnaire, AMS: Aging Males' Symptoms, BDI: Beck Depression Inventory, K-MMSE: Korean Mini-Mental State Examination.
Fig. 1. Changes in clinical parameters at 8 months after intervention with either testosterone replacement therapy (TRT group, n=54) or placebo (control group, n=52) in patients with testosterone deficiency syndrome. At 8 months after intervention, the improvement in total testosterone levels, IIEF-5 score, AMS score, and BDI score was significantly higher (*) in the TRT group than in the control group. *p<0.05 compared with control group. Qmax: maximal urinary flow rate, PVR: postvoid residual volume, IIEF-5: 5-item version of the International Index of Erectile Function questionnaire, AMS: Aging Males' Symptoms, BDI: Beck Depression Inventory, K-MMSE: Korean Mini-Mental State Examination.
Fig. 2. Overview of K-MMSE scores for patients with testosterone deficiency syndrome, stratified according to whether they received testosterone replacement therapy (TRT group, n=13) or a placebo (control group, n=12). Only data for patients with cognitive impairment at baseline (K-MMSE score <25) are given. At 8 months after intervention, the K-MMSE scores improved significantly (*) in the TRT group but not in the control group. *p<0.05 compared with baseline values. K-MMSE: Korean Mini-Mental State Examination.
There were few serious adverse events, and the incidence of such events did not differ significantly between the TRT and control groups. Prostate biopsy was performed in 3 patients who showed PSA levels >4 ng/mL after TRT, but no carcinoma was noted. No participant experienced an increase in hematocrit levels to >54%. No cardiovascular events, including stroke, myocardial infarction, or unstable angina were reported in either group.
DISCUSSION
The effect of testosterone administration on cognitive function and depression in hypogonadal men is believed to be related to the enhancement of brain perfusion, which is supported by the fact that there are androgen receptors in the brain, suggesting that steroid hormones play a role in neuronal function. Zitzmann et al [14] used 18F-deoxyglucose positron emission tomography to study cerebral glucose metabolism during a standardized mental rotation task in six hypogonadal men. All patients performed the test before and during TRT. While receiving TRT, four of six patients exhibited improved visuospatial performance, which corresponded with enhanced cerebral glucose metabolism during the test. Azad et al [15] used single photon emission computed tomography and showed that, after 3~5 weeks of TRT, cerebral perfusion was increased in the midbrain and the superior frontal gyrus in seven men with hypogonadism. After 12~14 weeks, increased perfusion was still observed in the midbrain as well as in the midcingulate gyrus.
In the present study, TDS patients who received TRT showed significant improvement in cognitive function only if mild cognitive impairment was present at baseline (K-MMSE score <25).
Mild cognitive impairment is believed to be associated with increased risk of developing Alzheimer's disease, as an estimated 6% to 25% individuals with mild cognitive impairment will develop Alzheimer's disease compared with a conversion rate of 0.2% to 3.9% in the general population [16].
The effects of TRT in men with mild cognitive impairment or Alzheimer's disease have been evaluated in small placebo-controlled trials. For example, Cherrier et al [17] evaluated a sample of 32 subjects, which included 17 men with mild cognitive impairment and 15 with Alzheimer's disease. At the 6-week follow-up, patients who received TRT showed significantly better scores regarding spatial memory, constructional abilities, and verbal memory compared to those noted in the placebo group. Taken together, these results suggest that TRT has a beneficial effect on cognitive function.
The baseline BDI scores in the TRT and control groups were 26.6±4.2 and 27.2±5.3, respectively indicating higher depression scores among TDS patients compared to those noted in the general population. In the present study, patients who received TRT showed significantly improved BDI scores at 8 months after the intervention, which supports previous reports that TRT improved mood and well-being, and reduced fatigue and irritability in hypogonadal men [18,19]. The study by Pope et al [20] involved men with depression refractory to standard anti-depressants, and found that TRT lowered the Hamilton Depression score, which is a standard indicator of depression. Since it has been well-documented that depression tends to increase as testosterone levels decrease [21], it is highly likely that TRT improved symptoms of depression by increasing testosterone levels.
The present study has several limitations related to the fact that we did not evaluate the levels of free or bioavailable testosterone, the assessment of cognitive function did not consider specific types of skills (spatial, verbal, visual, etc.), and the follow-up was relatively short. Large-scale studies with long-term follow-up are warranted to fully characterize the effect of TRT on cognitive function and depression.
CONCLUSIONS
TRT effectively improved total serum testosterone levels as well as IIEF-5, AMS, and BDI scores in men with TDS, suggesting that TRT may indeed improve some aspects of depression and cognitive ability in such patients. Therefore, TRT may be considered for men presenting with depression or cognitive impairment in addition to low testosterone levels.
ACKNOWLEDGEMENTS
This study was supported by young scientist research grants from the Korean Society for Sexual Medicine and Andrology (2011).
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166:1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder ET, Zheng L, Ong MD, Martinez C, Flores C, Stewart Y, et al. Effects of androgen therapy on adipose tissue and metabolism in older men. J Clin Endocrinol Metab. 2004;89:4863–4872. doi: 10.1210/jc.2004-0784. [DOI] [PubMed] [Google Scholar]
- 3.Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- 4.Finas D, Bals-Pratsch M, Sandmann J, Eichenauer R, Jocham D, Diedrich K, et al. Quality of life in elderly men with androgen deficiency. Andrologia. 2006;38:48–53. doi: 10.1111/j.1439-0272.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 5.Berglund LH, Prytz HS, Perski A, Svartberg J. Testosterone levels and psychological health status in men from a general population: the Tromsø study. Aging Male. 2011;14:37–41. doi: 10.3109/13685538.2010.522276. [DOI] [PubMed] [Google Scholar]
- 6.Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003;51:101–115. doi: 10.1034/j.1601-5215.2002.51018.x. discussion 115. [DOI] [PubMed] [Google Scholar]
- 7.Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract. 2009;15:289–305. doi: 10.1097/01.pra.0000358315.88931.fc. [DOI] [PubMed] [Google Scholar]
- 8.Seidman SN, Spatz E, Rizzo C, Roose SP. Testosterone replacement therapy for hypogonadal men with major depressive disorder: a randomized, placebo-controlled clinical trial. J Clin Psychiatry. 2001;62:406–412. doi: 10.4088/jcp.v62n0602. [DOI] [PubMed] [Google Scholar]
- 9.Muller M, Aleman A, Grobbee DE, de Haan EH, van der Schouw YT. Endogenous sex hormone levels and cognitive function in aging men: is there an optimal level? Neurology. 2005;64:866–871. doi: 10.1212/01.WNL.0000153072.54068.E3. [DOI] [PubMed] [Google Scholar]
- 10.Cherrier MM, Craft S, Matsumoto AH. Cognitive changes associated with supplementation of testosterone or dihydrotestosterone in mildly hypogonadal men: a preliminary report. J Androl. 2003;24:568–576. doi: 10.1002/j.1939-4640.2003.tb02708.x. [DOI] [PubMed] [Google Scholar]
- 11.Kenny AM, Bellantonio S, Gruman CA, Acosta RD, Prestwood KM. Effects of transdermal testosterone on cognitive function and health perception in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2002;57:M321–M325. doi: 10.1093/gerona/57.5.m321. [DOI] [PubMed] [Google Scholar]
- 12.Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300–308. [Google Scholar]
- 13.McDowell I. Measuring health: a guide to rating scales and questionnaires. 3rd ed. New York: Oxford University Press, Inc.; 2006. [Google Scholar]
- 14.Zitzmann M, Weckesser M, Schober O, Nieschlag E. Changes in cerebral glucose metabolism and visuospatial capability in hypogonadal males under testosterone substitution therapy. Exp Clin Endocrinol Diabetes. 2001;109:302–304. doi: 10.1055/s-2001-16351. [DOI] [PubMed] [Google Scholar]
- 15.Azad N, Pitale S, Barnes WE, Friedman N. Testosterone treatment enhances regional brain perfusion in hypogonadal men. J Clin Endocrinol Metab. 2003;88:3064–3068. doi: 10.1210/jc.2002-020632. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 17.Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, et al. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- 18.Lunenfeld B, Nieschlag E. Testosterone therapy in the aging male. Aging Male. 2007;10:139–153. doi: 10.1080/13685530701485998. [DOI] [PubMed] [Google Scholar]
- 19.Morley JE. Testosterone replacement in older men and women. J Gend Specif Med. 2001;4:49–53. [PubMed] [Google Scholar]
- 20.Pope HG, Jr, Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry. 2003;160:105–111. doi: 10.1176/appi.ajp.160.1.105. [DOI] [PubMed] [Google Scholar]
- 21.Barrett-Connor E, Von Mühlen DG, Kritz-Silverstein D. Bio-available testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1999;84:573–577. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]