Abstract
Yeast Dre2 (anamorsin or CIAPIN1) is an essential component for cytosolic Fe/S cluster biosynthesis. The C-terminal domain contains eight evolutionarily conserved cysteine residues, and we previously demonstrated that the yeast Dre2 overexpressed in Escherichia coli contains one binuclear ([2Fe–2S]) cluster and one tetranuclear ([4Fe–4S]) cluster. In this study, we replaced each conserved cysteine with alanine and analyzed the effects by Electron Paramagnetic Resonance. Although the C311A mutant lacked both signals, our data clearly suggest that the [2Fe–2S] cluster is ligated to Cys252, Cys263, Cys266 and Cys268, whereas the [4Fe–4S] cluster is ligated to Cys311, Cys314, Cys322 and Cys325. By simulation analysis of the C263A and C322A data, we obtained the g-values for the [4Fe–4S] cluster (gx,y,z = 1.830, 1.947 and 2.018) and for the [2Fe–2S] cluster (gx,y,z =1.919, 1.962 and 2.001). We also observed spin–spin interaction between the two clusters, suggesting their close proximity. Chemically reconstituted Dre2 showed air sensitivity of the [4Fe–4S] cluster converting to a [2Fe–2S] cluster. Furthermore, using a yeast shuffle strain, we demonstrated for the first time that each of the Cys Fe–S cluster ligands with the exception of C252 is essential, indicating that both Dre2 clusters are needed for cell viability.
Keywords: anamorsin, CIAPIN1, cytosolic iron–sulfur cluster biosynthesis, Dre2, EPR, iron–sulfur clusters
Iron–sulfur (Fe/S) clusters are evolutionarily ancient prosthetic groups required by many proteins for their essential activities. Some of these proteins were critical for the emergence of fundamental processes during the evolution of our planet, such as nitrogen fixation and photosynthesis (1). The discovery of Azotobacter vinelandii cysteine desulfurase in the 1990s, an enzyme providing the sulfur for Fe/S cluster biosynthesis in nitrogenase, led to the subsequent identification of the complex multi-component machinery responsible for producing Fe/S clusters in all three kingdoms of life (2). The eukaryotic counterpart consists of up to 17 proteins located in mitochondria, namely the ISC (Iron Sulfur Cluster) machinery (3). The first essential extra-mitochondrial Fe/S cluster protein to be discovered was Rli1 (4), a [4Fe–4S] cluster protein required for ribosome biogenesis and translation initiation. Since then, other essential proteins required for DNA replication and genome stability have been found to contain Fe/S clusters, including DNA polymerases, primases and helicases (5–7). The biosynthesis of these extra-mitochondrial Fe/S clusters is dependent not only on the mitochondrial ISC components but also on proteins of the CIA (Cytosolic Iron–sulfur cluster Assembly) pathway. These components function in processing or sensing of an unknown sulfur compound exported by the mitochondrial ATP-binding cassette (ABC) transporter (ATM1 in yeast or ABCB7 in humans) (Fig. 1) (8–10). Our group was the first to identify and characterize Dre2, one of these CIA members (11). A hypomorphic dre2 allele was associated with impaired activities of Fe/S cluster proteins in the cytosol and nucleus but fully functional mitochondrial Fe/S cluster proteins (11). Such phenotypes are typical of CIA defects. We have also shown that Dre2 protein contains a [2Fe–2S] and a [4Fe–4S] cluster (11). Our finding has been reproduced by several other studies (12–14). Lill’s group further demonstrated that a Dre2 interacting partner, diflavo-protein Tah18, can reduce the [2Fe–2S] cluster in Dre2 using electrons from NADPH. The recipient of the Tah18/Dre2 donated electrons during physiologic Fe/S cluster assembly, however, remains to be characterized, and the role of the redox active [4Fe–4S] remains mysterious (12).
Fig. 1.
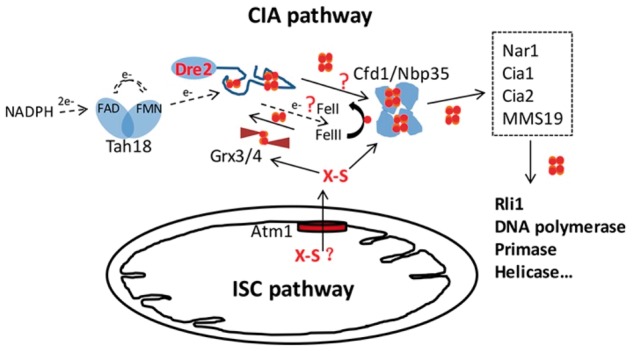
Schematic diagram shows Fe/S cluster biosynthesis in yeast cells and the possible roles of Dre2. The mitochondrial ISC machinery, and the CIA pathway and the ATM1 transport system connecting the two are shown.
The Stubbe and Huang group found that Tah18/Dre2 activity is also required for the de novo synthesis of the diferric-tyrosyl radical cofactor in ribonucleotide reductase (RNR) (15, 16). The human Dre2 (hDre2) homolog, anamorsin, can complement loss of Dre2 function in yeast (11, 12). Anamorsin knockout mice are embryonic lethal and exhibit erythroid cell maturation defects (10). Taken together, these findings suggest that the Tah18/Dre2 complex may play a more general role in iron metabolism, for example, acting as a ferric reductase. However, the nature of the prosthetic groups in Dre2 has been controversial. Several recent studies of recombinant anamorsin reported the detection of only [2Fe–2S] cluster(s) (17–21).
In this study, we performed site-directed mutagenesis to replace individual cysteine residues in Dre2 including eight evolutionarily conserved cysteines, changing each one to alanine. The recombinant proteins were expressed and purified from Escherichia coli. The proteins were then subjected to Electron Paramagnetic Resonance (EPR) analyses. Our results clearly demonstrate that the eight conserved cysteine residues can be divided into two groups, with Cys252, Cys263, Cys266 and Cys268 (A site) serving as the ligands of the [2Fe–2S] cluster and Cys311, Cys314, Cys322 and Cys325 (B site) serving as the ligands of the [4Fe–4S] cluster. By simulation analyses of EPR data from mutants that lack one or the other cluster, we were able to obtain the g-values for each cluster. In order to evaluate the biological roles of these two Fe/S clusters, the Dre2 substitution mutants were expressed in a yeast shuffle strain and the Dre2 covering plasmid was removed. Substitutions of [2Fe–2S] ligands with the exception of C252A or substitutions of [4Fe–4S] cluster ligands did not sustain growth, suggesting that both clusters are essential.
Experimental Procedures
Strains and plasmids
Yeast strains used in this study are listed in Table I, and plasmids are listed in Table II. Escherichia coli expression plasmid pET21b-Dre2-His6 was constructed as previously described (11). Site-directed mutagenesis was performed using Qiagen QuikChange mutagenesis kit, with primers designed to replace each of the nine cysteine residues with alanine. For E. coli expression, the hDre2 open reading frame was moved to pET21b, by introducing NdeI and XhoI ends and a C-terminal His6 tag (pET21b-hDre2-His6). For the study of mutant viability in yeast, a yeast shuffle strain was constructed in which the Δdre2 deletion was covered by a URA3 plasmid pRS416-Dre2. Exposure of this strain to FOA (5-fluoroorotic acid) led to lethality indicating the essential nature of Dre2. The plasmid pRS415-Dre2prom-Dre2 containing the wild-type (WT) Dre2 was modified to introduce the various Cys to Ala substitutions. The plasmid YCplac22-hDre2 containing the hDre2 was also modified. These plasmids were tested in the shuffle strain by selecting for leucine or tryptophan prototrophy, respectively, followed by counterselection with FOA.
Table I.
Yeast strains used in this study
| Strain no. | Strain name | Relevant genotype |
|---|---|---|
| 103-19 | DRE2 shuffle | MATa ura3-52 lys2-801(amber) ade2-101(ochre) trp1-Δ63 his3-Δ200 leu2-Δ1 cyh2 dre2::kanMX [pRS416-DRE2] |
| 98-67 | Gal-Dre2 | MATa His3MX6-PGAL1-Dre2::DRE2 ura3-52 lys2-801(amber) ade2-101(ochre) trp1-Δ63 leu2-Δ1 |
| 66-17 | BY4742 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 |
| 98-68 | Dre2-TAP | MATa His3MX6-Dre2-TAP::DRE2 his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 |
Table II.
Plasmids used in this study
| Plasmid no. | Plasmid name | Expression system |
|---|---|---|
| 17-64 | pET21b-Dre2-His6 | Escherichia coli |
| 18-22 | pET21b-Dre2(C116A)-His6 | Escherichia coli |
| 18-23 | pET21b-Dre2(C252A)-His6 | Escherichia coli |
| 18-24 | pET21b-Dre2(C263A)-His6 | Escherichia coli |
| 18-25 | pET21b-Dre2(C266A)-His6 | Escherichia coli |
| 18-27 | pET21b-Dre2(C311A)-His6 | Escherichia coli |
| 18-28 | pET21b-Dre2(C314A)-His6 | Escherichia coli |
| 18-29 | pET21b-Dre2(C322A)-His6 | Escherichia coli |
| 19-24 | pET21b-Dre2(C325A)-His6 | Escherichia coli |
| 30-10 | pET21b-hDre2-His6 | Escherichia coli |
| 18-73 | pRS416-Dre2 | Saccharomyces cerevisiae |
| 18-30 | pRS415-Dre2prom-Dre2 | Saccharomyces cerevisiae |
| 18-13 | pRS415-Dre2prom-Dre2(C116A) | Saccharomyces cerevisiae |
| 18-14 | pRS415-Dre2prom-Dre2(C252A) | Saccharomyces cerevisiae |
| 18-15 | pRS415-Dre2prom-Dre2(C263A) | Saccharomyces cerevisiae |
| 18-16 | pRS415-Dre2prom-Dre2(C266A) | Saccharomyces cerevisiae |
| 18-18 | pRS415-Dre2prom-Dre2(C311A) | Saccharomyces cerevisiae |
| 18-19 | pRS415-Dre2prom-Dre2(C314A) | Saccharomyces cerevisiae |
| 18-20 | pRS415-Dre2prom-Dre2(C322A) | Saccharomyces cerevisiae |
| 18-21 | pRS415-Dre2prom-Dre2(C325A) | Saccharomyces cerevisiae |
| 27-7 | pRS415-Dre2prom-Dre2(C116A, C252A) | Saccharomyces cerevisiae |
| 27-8 | pRS415-Dre2prom-Dre2(C252A, E272A, E275A) | Saccharomyces cerevisiae |
| 27-19 | YCplac22-hDre2 | Saccharomyces cerevisiae |
| 27-19a | YCplac22-hDre2 (C237A) | Saccharomyces cerevisiae |
| 27-19b | YCplac22-hDre2 (C246A) | Saccharomyces cerevisiae |
| 27-19c | YCplac22-hDre2 (C288A) | Saccharomyces cerevisiae |
Media composition
Rich media, yeast extract, peptone, dextrose (YPD) and yeast extract, peptone, raffinose (YPR) affinose + 0.5% galactose were prepared as described before. Defined complete synthetic media (CSM) and drop out media (CSM-Ura, CSM-Leu) were made as previously described (22).
Protein purification
Escherichia coli BL21 (DE3) (codon plus) harboring pET21b-Dre2-His6, various Dre2 mutants or pET21b-hDre2-His6 was cultured with antibiotics to maintain selection, and subjected to gene induction and protein purification using the protocols previously described (11). Briefly, 125 μM ferric-nitrilotriacetate was added to the medium and cells were grown at 25 °C. Isopropyl-β-d-thiogalactoside (0.2 mM) was added to induce protein expression for 3 h. Cells were lysed by sonication in degassed buffer containing 50 mM Tris–HCl, pH 8.0, 10% glycerol and lysozyme 133 μg/ml. Nickel-nitrilotriacetic acid (Ni-NTA) (Qiagen) affinity columns were used for protein purification. Eluted protein was concentrated using Amicon filters after imidazole was removed.
Electron paramagnetic spectroscopy
EPR samples were prepared as previously described (11). Dithionite (10 mM) was used to reduce the clusters. EPR spectra were recorded by a Bruker Elexsys E500 or ESP 300E spectrometers at X-band (9.4 GHz) using an Oxford Instrument ESR900 helium flow cryostat, a Hewlett-Packard 5350B microwave frequency counter and an ITC4 temperature controller. EPR spectra of the Fe/S clusters were simulated by using Easyspin (http://www.easyspin.org).
Chemical reconstitution
Purified WT Dre2 or C263A proteins were brought into a glove box for chemical reconstitution of Fe/S clusters. The protein was incubated with 10 mM DTT for 1.5 h, followed by stepwise addition of iron ammonium sulfate and Na2S both at 6-fold molar excess relative to protein. The mixture was incubated for 1 h. The protein solution (800 μl) was then passed through a G25 column to remove unbound iron and sulfate or, alternatively, concentrated twice to 50 μl using an YM30 centricon to exchange the reconstitution buffer with 25 mM Tris–HCl, pH 8.0, 150 mM NaCl. Immediately, afterwards, the protein was diluted to 400 μl with the same buffer and transferred to an anaerobic cuvette before removing it from the glove box for spectroscopic analyses.
Western blot
Yeast WT BY4742 or Dre2-TAP cells was transformed with pRS415 or pRS415 carrying different Dre2 alleles, and plasmid selection was maintained by growing in CSM-Leu medium. Gal-Dre2 cells were grown in YPD, CSM-Leu or YPRaffinose + galactose. Cells from individual cultures were lysed in 6× Laemmli buffer by heating at 100 °C for 10 min (23). Samples were normalized such that lysates from equivalent numbers of cells were separated by 12% SDS–PAGE. The gel was run at 200 V for 45 min, and the proteins were transferred to nitrocellulose membranes at 90 V and 4 °C for 2 h in 50 mM Tris, 40 mM glycine, 20% (v/v) methanol and 0.03% (w/v) SDS. Polyclonal anti-Dre2 antibody was raised by immunizing rabbits with the full-length protein expressed and purified from E. coli. This serum was used at 1:1,000 dilution. Stabilized peroxidase-conjugated goat anti-rabbit (H + L) secondary Ab (CWBIO cat# CW0103) was used at a 1:5,000 dilution. The nitrocellulose membranes were developed using enhanced chemiluminescence (ECL) and exposed to film.
Results
Dre2 homologs contain two highly conserved cysteine-rich motifs
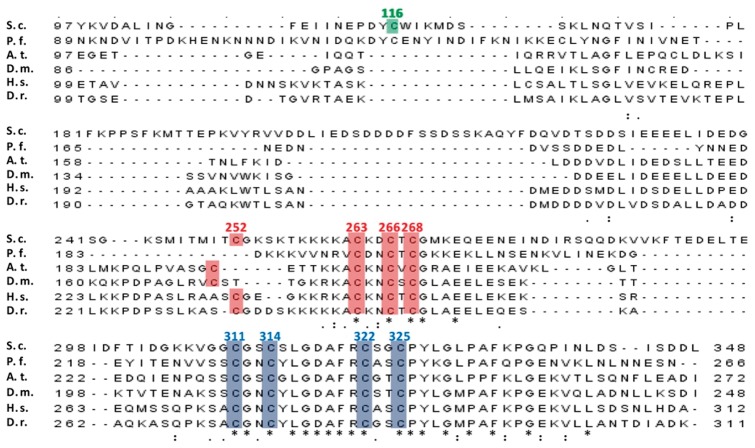
Homologs of yeast Dre2 are found in most eukaryotes including protozoa, plants, insects, vertebrates and mammals. In the C-terminal domain, there are two cysteine-rich motifs, CXnCX2CXC and twin CX2C as shown in red and blue in Fig. 2. These motifs are highly conserved from yeast to humans. We previously reported that the WT Dre2 protein contains one [2Fe–2S] cluster and one [4Fe–4S] cluster by EPR analysis (11). Although this finding has been supported by more recent studies (12–14), some other studies on anamorsin, the hDre2 homolog, claimed that these two cysteine motifs independently bind a [2Fe–2S] cluster in a mutually exclusive manner (19). To resolve the discrepancy, we decided to perform site-directed mutagenesis by mutating each of the cysteine residues to alanine, specifically aiming to disrupt one cluster while not affecting the other. Although the cysteine residue, Cys116, in the N-terminal methyltransferase domain of yeast Dre2 is not present in most of the listed Dre2 homologs shown in green in Fig. 2, we also included the mutant of this cysteine residue in our study.
Fig. 2.
Multiple sequence alignment of essential eukaryotic Dre2 proteins from different organisms. The highlighted amino acids with their numbers (according to the yeast Dre2 sequence) were mutated in this study. S.c., Saccharomyces cerevisiae S288c (the GenBank Accession Number is P36152.1); P.f., Plasmodium falciparum (NC_004329.2); A.t., Arabidopsis thaliana (NP_001078602.1); D.m., Drosophila melanogaster (Q9V3Y2.1); H.s., Homo sapiens (NP_001078602.1); D.r., Danio rerio (NP_001003738.1). Clustal Omega was used to perform multiple sequence alignment. Underneath the alignment, asterisks represent identical residues, and dots or colons represent lesser degrees of conservative changes. Conserved cysteines colored in red or blue are postulated to coordinate a [2Fe–2S] cluster or a [4Fe–4S] cluster, respectively. The non-conserved cysteine in yeast Dre2 is shown in green.
EPR analyses of various Dre2 cysteine mutants
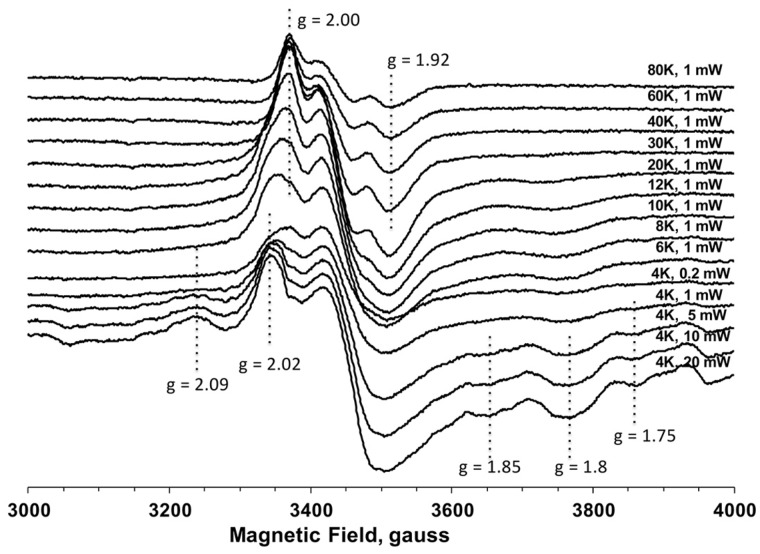
We constructed nine Cys to Ala Dre2 mutants including the non-conserved C116A mutant. Dre2 recombinant proteins were individually overexpressed in E. coli, semi-anaerobically isolated, reduced with sodium dithionite and frozen in EPR tubes. The EPR spectrum of the C116A mutant is very similar to that of the WT protein as previously reported. Using this sample, we measured EPR signals from the Fe/S clusters in Dre2 over a wide range of temperatures from 80 to 6 K at 1 mW microwave power (Fig. 3). At higher temperature (>30 K), only the EPR signal from [2Fe–2S] clusters can be observed, because the spin relaxation time for [2Fe–2S] clusters is slower compared with [4Fe–4S] clusters. However, as sample temperatures are decreased (<20 K), EPR signals from [2Fe–2S] clusters start decreasing due to the broadening effect, whereas EPR signals from [4Fe–4S] clusters become detectable. The shapes of EPR spectra (g-values) from the same cluster usually remain constant at various temperature and microwave powers. Based on these principles of EPR analysis, the EPR data of the non-conserved C116A mutant clearly suggest that there are two distinct Fe/S clusters, a [2Fe–2S] cluster observed at only higher temperatures and a [4Fe–4S] cluster observed at only <20 K, with different g-values. In addition, when the temperature was further lowered to 4 K and the microwave power was increased to >5 mW, other EPR signals (g = 1.75 and 2.09) appeared in the gx and gz regions (Fig. 3). It is highly likely that these very fast relaxing signals are hyperfine signals derived from some spin–spin interaction between the two Fe/S clusters possibly due to their short distance.
Fig. 3.
EPR spectra of the non-conserved C116A Dre2 mutant at different temperatures and microwave powers. The recombinant yeast C116A Dre2 protein was expressed in Escherichia coli and purified by Ni-NTA column, and reduced with 10 mM dithionite. The positions of EPR signals from Fe/S clusters are indicated with their g-values. EPR spectra were recorded under the following conditions: microwave frequency, 9.45 GHz; modulation amplitude, 10G; modulation frequency, 100 kHz; time constant, 82 ms.
The A site [2Fe–2S] cluster is coordinated by Cys252, Cys263, Cys266 and Cys268
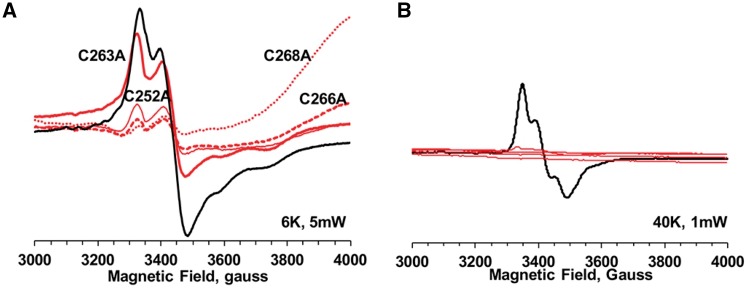
However, EPR spectra of the conserved cysteine mutants are very different from those of the C116A or WT. They can be divided into two groups. As shown in Fig. 4, mutants for the first cysteine-rich motif, C252A, C263A, C266A and C268A (A site), each displayed no EPR signals at 40 K, while retaining small but clear EPR signals similar to those of the WT at 6 K. This strongly suggests that all these cysteine residues are ligands for the [2Fe–2S] cluster in Dre2. The shapes of the EPR spectra in these mutants at 6 K, which represent signals from a [4Fe–4S] cluster, were slightly different from those of the WT, with the exception of C263A (Fig. 4A). However, as the principle g-values were mostly retained, it is very likely that these small changes were caused by some disturbance in the local environment of the [4Fe–4S] cluster.
Fig. 4.
EPR spectra of the Dre2 variants mutated in the CXnCX2CXC domain (A site mutated, B site unmutated) at 6 K, 5 mW (A) and at 40 K, 1 mW (B). C252A, C263A, C266A and C268A Dre2 mutants were overexpressed and purified from Escherichia coli. The samples were reduced with 10 mM sodium dithionite. EPR spectra from these Dre2 mutants are shown in red, whereas EPR spectra from WT are shown in black for comparison. EPR conditions are the same as described in Fig. 3.
The B site [4Fe–4S] cluster is coordinated by Cys311, Cys314, Cys322 and Cys325
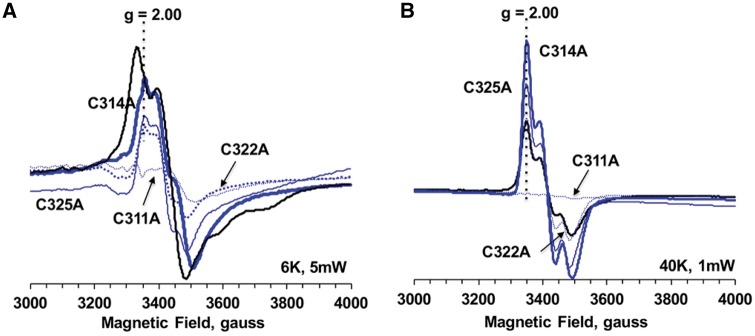
The spectra of the mutants in the second cysteine-rich motif, C311A, C314A, C322A and C325A (B site), displayed almost perfect retention of EPR signals from a [2Fe–2S] cluster at 40 K. These spectra were like those of the WT. The C311A mutant appeared to lose those signals (Fig. 5B). On the other hand, the EPR spectra in these C314A, C322A and C325A mutants at 6 K were clearly different from those in WT, but they were very similar to their [2Fe–2S] signals at 40 K. Indeed, the g-values for their spectra at 6 and 40 K are the same (Fig. 5). In addition, in contrast to the WT and cysteine mutant samples for the first cysteine-rich motif, no [3Fe–4S] cluster EPR signals were detected in the oxidized (non-reduced) mutant samples for the second cysteine-rich motif including C311A (data not shown). [3Fe–4S] cluster EPR signals are often observed as an oxidation product of labile [4Fe–4S] clusters in EPR samples containing [4Fe–4S] clusters after aerobic isolation. Thus, these data strongly suggest that the EPR spectra observed at 6 K for the Dre2 mutants in the second cysteine motif are not EPR signals from the [4Fe–4S] cluster but rather attenuated signals from the remaining [2Fe–2S] cluster. Therefore, Cys311, Cys314, Cys322 and Cys325 are all involved in the coordination of the [4Fe–4S] cluster in Dre2. Probably, the Cys311 residue resides close to the [2Fe–2S] cluster and the Cys to Ala mutation destabilized the [2Fe–2S] cluster.
Fig. 5.
EPR spectra of the Dre2 variants mutated in the twin CX2C motifs (A site unmutated, B site mutated) at 6 K, 5 mW (A) and at 40 K, 1 mW (B). C311A, C314A, C322A and C325A Dre2 mutants were overexpressed and purified from Escherichia coli. The samples were reduced with 10 mM sodium dithionite. EPR conditions are the same as described in Fig. 3.
Next, to obtain accurate g-values for each Fe/S cluster, we used the EPR data of the C263A and C322A mutants, which contain only one Fe/S cluster for simulation analysis, because the EPR data obtained at 6 and 40 K from WT still contained some overlapping signals from the other Fe/S cluster. As shown in Fig. 6, we successfully obtained the g-values for the [4Fe–4S] cluster (gx,y,z = 1.830, 1.947 and 2.0181) and the [2Fe–2S] cluster (gx,y,z =1.919, 1.962 and 2.001). Here, we confirmed that Cys252, Cys263, Cys266 and Cys268 in the first cysteine-rich motif (A site) ligate the [2Fe–-2S] cluster, whereas Cys311, Cys314, Cys322 and Cys325 (B site) are ligands for the [4Fe–4S] cluster.
Fig. 6.
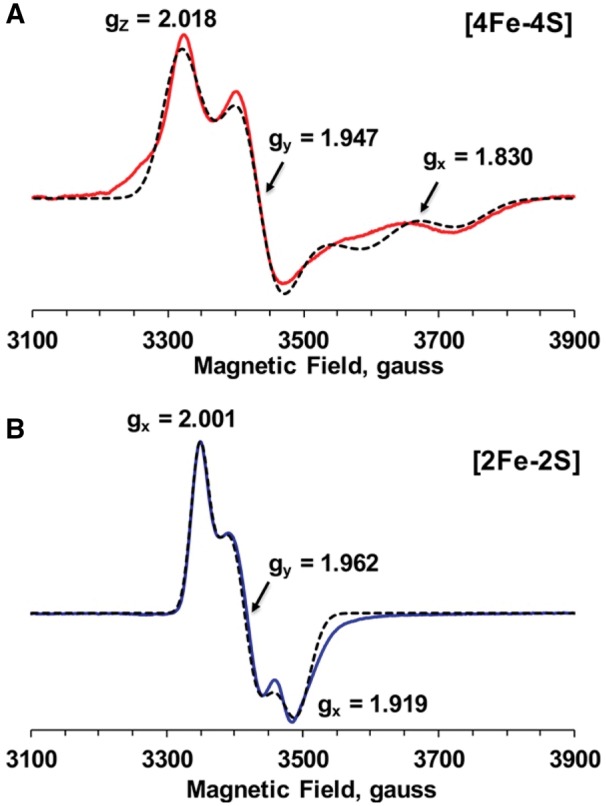
Isolated EPR spectra of the [4Fe–4S] (A) and [2Fe–2S] (B) clusters in Dre2. Simulated [4Fe–4S] and [2Fe–2S] spectra (dotted lines) were obtained from the EPR data of C263A at 10 K and 5 mW (red) and C322A at 40 K and 1 mW (blue), respectively. The g-tensor principal values are shown in the Fig. 6. The EPR conditions were the same as described in Fig. 3.
Human anamorsin (hDre2) contains both [2Fe–2S] and [4Fe–4S] clusters
As recent studies by Banci’s group showed that the hDre2 homolog anamorsin binds only [2Fe–2S] clusters, we cloned, expressed and purified human anamorsin WT protein from E. coli, and we investigated Fe/S clusters in human anamorsin in our hands. As we did for yeast Dre2 proteins, we measured EPR spectra of anamorsin in a wide range of temperatures from 120 K down to 4.3 K (Fig. 7). At higher temperature (>45 K), where only the EPR signal from [2Fe–2S] clusters can be observed, we observed clear rhombic EPR signals with gx,y,z = 1.92, ∼1.96 and 2.00, which were derived from only one paramagnetic species. The intensity of these EPR signals decreased as the temperatures went higher, but the shape and the g-values stayed the same. Thus, this strongly suggests that these signals belong to a [2Fe–2S] cluster. However, as the sample temperature was lowered to around 10 K or below, the EPR signals from the [2Fe–2S] cluster decreased due to the broadening effect. In contrast, meanwhile EPR signals from a [4Fe–4S] cluster became detectable and dominant, which can easily be recognized by the shift of the g = 2.0 signal toward g = 2.02 and the appearance of the g = 1.89 signal (Fig. 7). The EPR spectrum at 4 K could be regarded as a pure [4Fe–4S] cluster free from overlapping with the [2Fe–2S] cluster. These data clearly suggest that human anamorsin also contains two distinct Fe/S clusters, a [2Fe–2S] cluster and a [4Fe–4S] cluster, as seen in yeast Dre2. The g-values for both clusters in human anamorsin were very similar to those in yeast Dre2.
Fig. 7.
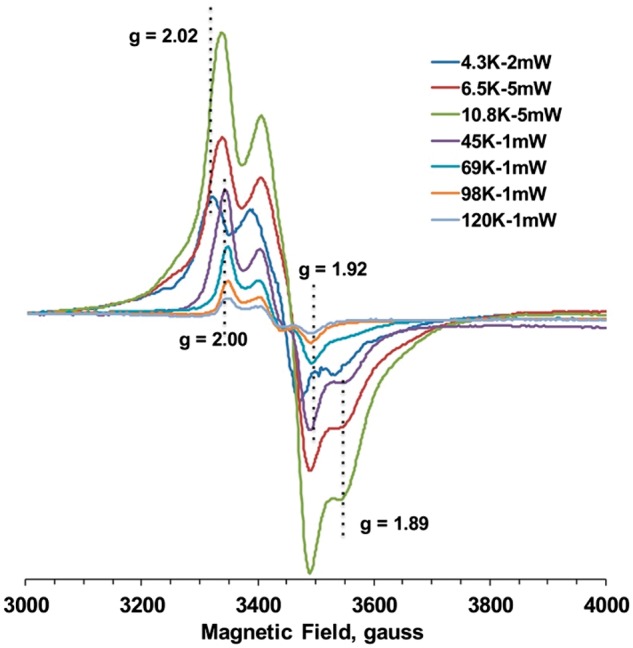
EPR spectra of human Dre2 (anamorsin) at different temperatures and microwave powers. Human anamorsin was expressed and purified from Escherichia coli using the same methods described for the yeast Dre2 protein. The purified human anamorsin was then reduced with 10 mM sodium dithionite and subjected to EPR analysis. EPR conditions are the same as described in Fig. 3.
Effects of chemical reconstitution and air exposure on the Dre2 bound Fe–S clusters
The iron and sulfur content of the as-isolated recombinant Dre2 was substoichiometric, equaling 1:1 iron to sulfur but only about 15% of the predicted cofactor binding (11). We considered that this might be due to air sensitivity of the bound Fe–S clusters. Therefore, an effort was made purify Dre2 anaerobically. The procedure used all degassed buffers and centrifugation and Ni purification steps in the anaerobic chamber. Even working rapidly, the procedure took 3.5 h and brief air exposure may have occurred. The cluster occupancy was slightly improved but still low. After chemical reconstitution with iron and sulfide in the anaerobic chamber, the cluster signals were much improved (Fig. 8A).
Fig. 8.
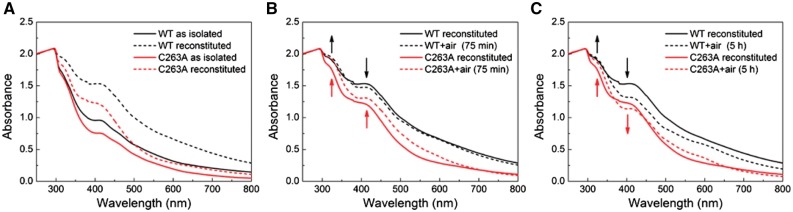
UV/Vis spectra of purified WT (black) and C263A (red) Dre2 proteins, indicating an air sensitive, labile [4Fe–4S] cluster. (A) UV/Vis spectra of WT and C263A as isolated semi-anaerobically from Escherichia coli using degassed buffer (solid lines) and after Fe-S cluster reconstitution (broken lines). (B) UV/Vis spectra of WT and C263A upon reconstitution (solid lines) and after 75-min air exposure (broken lines). (C) UV/Vis spectra of WT and C263A upon reconstitution (solid lines) and after 5-h air exposure (broken lines).
An experiment was then performed in which the reconstituted protein was shifted to air and serial spectra measurements were made, paying particular attention to the shoulder at 410 and 420 nm, and the peak at 320 nm. The 410 nm plateau represents the [4Fe–4S] feature, and the 420 nm peak includes [2Fe–2S] and [4Fe–4S] absorbances, whereas the 320 nm feature represents a [2Fe–2S] cluster signal. The spectra of the reconstituted Dre2 are consistent with the EPR findings showing the presence of both [2Fe–2S] and [4Fe–4S] clusters features.
Over time in air, the 410–420 nm shoulder declined whereas the 320 nm signal was stable and even increased slightly (Fig. 8B and C). A similar experiment was performed with the reconstituted C263A Dre2 protein which is lacking a ligand for the A site cluster. In this case, we expected that only the [4Fe–4S] cluster should be present. The changes in air were very clearly biphasic, showing initial increases at 320 and 420 nm (up to 75 min) suggesting an increase in [2Fe–2S] clusters followed by subsequent decreases up to 5 h (Fig. 8B and C). All in all, the data support the EPR findings regarding the presence of [2Fe–2S] clusters and [4Fe–4S] clusters in WT Dre2 and the presence of [4Fe–4S] clusters on the C263A mutant. They also suggest that the cluster occupancy is air sensitive, especially the [4Fe–4S] cluster on site B which may be converted to a [2Fe–2S] cluster and then subsequently further decay in air. A similar finding has been reported in the recently published manuscript on Dre2 (24).
Both clusters are essential for Dre2 or hDre2 function
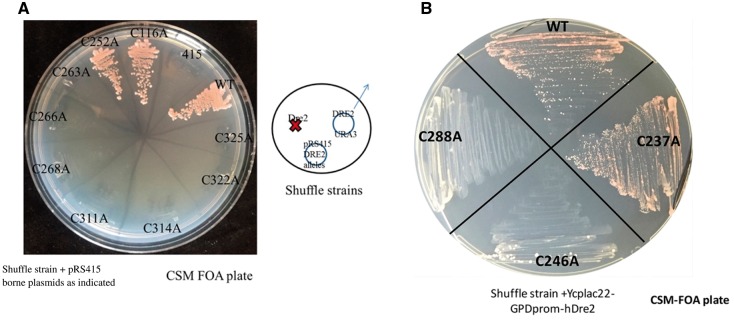
Successfully identifying the cluster ligands and making mutant forms of Dre2 with only one or the other of the two clusters encouraged us to test the in vivo requirement for each of the clusters. Toward this end, we introduced the Cys to Ala substitutions into a yeast expression plasmid pRS415-Dre2. The WT and mutated plasmids were transformed into a Dre2 shuffle strain, and the transformants were selected on FOA containing plates (Fig. 9A). Consistent with SGD (Saccharomyces Genome Database) and our previous finding that Dre2 is an essential gene, we can see in Fig. 9A that the shuffle strain harboring an empty plasmid pRS415 is non-viable on the FOA plate and that the WT Dre2 gene allows growth following FOA counterselection. The non-conserved C116A mutant, similar to the WT allele, sustained growth of the shuffle strain. Mutants of each of the [4Fe–4S] cluster ligands, C311A, C314A, C322A and C325A were non-viable, indicating that the unknown function of this cluster is essential. Three of the four mutants of the [2Fe–2S] cluster ligands, C263A, C266A and C268A, were also non-viable. Surprisingly, the C252A mutant grew robustly similar to WT cells, even though the [2Fe–2S] cluster appeared to be completely abolished in the E. coli-expressed C252A mutant (Fig. 4B). We constructed the C116A/C252A double mutant to test whether these two cysteine residues are redundant in coordinating the essential [2Fe–2S] cluster. However, the double mutant, expressed in the shuffle strain, was still viable and grew like the WT Dre2 following counterselection (data not shown). hDre2 expressed in yeast is able to completely replace the yeast gene in complementation experiments ((11) and Fig. 9B). Several alanine substitution mutants of hDRE2 were evaluated for complementing activity. C246A or C288A was unable to complement lack of Dre2 in yeast (Fig. 9B). C246 in hDre2 aligns with C263, and C288 aligns with C325, and thus these residues are predicted to provide ligands for the site A or site B Fe–S clusters, respectively. The implication is that both clusters are necessary for functioning of hDre2 just as for yeast Dre2. The C237A (aligned with C252 in the yeast protein) was able to complement, however, consistent with a less essential role for this ligand in both the yeast and human proteins.
Fig. 9.
Yeast shuffle strains show that both clusters are essential for viability. (A) Yeast Dre2. Plasmids carrying various Dre2 alleles including WT and Cys to Ala mutants along with the pRS415 plasmid were transformed into the Dre2 shuffle strain and selected on CSM-Leu-Ura medium. The transformants were then passaged on FOA medium to counterselect against the URA3-containing pRS416-Dre2 plasmid. (B) hDre2. YCPlac22 plasmids carrying various human Dre2 (anamorsin) alleles including WT and Cys to Ala mutants were transformed into the Dre2 shuffle strain and selected on CSM-Trp-Ura medium. The transformants were then passaged on FOA medium to counterselect against the URA3-containing pRS416-Dre2 shuffle plasmid.
Dre2 mutant expression levels
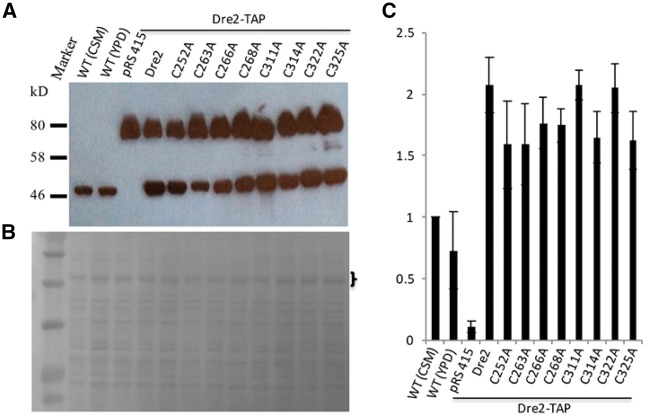
The inability of several Cys to Ala Dre2 mutants to support viability could be due to loss of function of the protein or alternatively due to lack of protein expression. In order to distinguish the two, we decided to test the expression levels of these mutants in a Dre2-TAP strain. The Dre2-TAP cells are as healthy as WT cells, indicating that the TAP tag does not compromise the function of the Dre2 protein. We transformed the pRS415-Dre2prom-Dre2 plasmids with the various Cys mutations or the empty pRS415 plasmid control into the Dre2-TAP strain, growing the cells in CSM-Leu defined medium to maintain plasmid selection. Cell lysate was then subjected to separation by SDS–PAGE, followed by transfer to nitrocellulose membrane for immunoblotting using anti-Dre2 polyclonal antibody. Both WT BY4742 and the Dre2-TAP strain transformed with the pRS415 plasmid contain a single Dre2 protein band, with the Dre2-TAP band migrating slower due to the addition of the 30 kDa TAP tag. In contrast, the Dre2-TAP cells transformed with various Dre2 alleles including WT Dre2 and different mutants contain two Dre2 bands. In addition to the chromosomally encoded Dre2-TAP, a plasmid-borne Dre2 protein was also observed for the various mutant forms of Dre2, and the expression levels were comparable (Fig. 10A). Prior to blotting with anti-Dre2 antibody, the nitrocellulose membrane was stained with amido black and photographed (Fig. 10B). We quantitated the Dre2 protein on the blot (Fig. 10A) by measuring the intensities of the lower Dre2 bands and normalizing them with the intensities of three visible amido black-stained bands in the bracketed area (Fig. 10B) using Image J software. Three blots were performed with three sets of samples from independent cultures. Dre2 levels were compared with those of WT control cells grown in CSM media. Compared with the endogenous Dre2 level in WT control cells, the plasmid-borne Dre2 levels were higher (Fig. 10A and C), perhaps because the plasmid was maintained at more than one copy per cell. Taken together, the results show that substitutions of the Dre2 Fe/S cluster ligands do not affect protein expression or stability. The lethal phenotypes resulting from the substitutions are therefore most likely caused by loss of function of the Fe/S clusters.
Fig. 10.
WT and mutant Dre2 expression levels are comparable. WT (BY4742) cells were grown in either CSM or YPD medium. Dre2-TAP cells carrying various Dre2 alleles on pRS415 plasmid and empty pRS415 plasmid as control were grown in CSM-Leu medium. Equivalent cell numbers (4 × 106) were loaded in each lane of a 12% SDS–PAGE gel and proteins were electrophoretically separated. Proteins were then transferred to a nitrocellulose membrane for immunoblotting using polyclonal anti-Dre2 antibody. (A) Amido black staining of the membrane was photographed to show equal protein loading. (B) Statistical comparison of Dre2 levels in different cells. (C) Triplicate Western blots were performed. In each blot, Dre2 levels were normalized to the protein levels in the bracketed area using Image J software. Dre2 level in WT cells grown in CSM medium was set to 1.
Discussion
The hDre2 homolog anamorsin was initially identified as an anti-apoptotic molecule (10). Knockout in mice caused embryonic lethality and defective hematopoiesis. Impaired red blood cell maturation was suggestive of a problem with iron metabolism (10). The link to cytosolic Fe/S cluster biosynthesis was later established in a yeast genetic screen and biochemical studies (11). However, the detailed molecular function of Dre2 has remained elusive. The methyltransferase domain in its N-terminus seems to be dispensable and not to have methyltransferase activity (15). It may play a non-essential role in protein recognition and interaction. The essential role of Dre2 is mostly dependent on its C-terminus that contains redox active Fe/S cluster(s) (18, 20, 21). However, the types of the two Fe/S cluster(s) accommodated in Dre2/anamorsin and their roles have been controversial. Thus, in this study, we further characterized these clusters using EPR and site-directed mutants and identified the Fe/S cluster ligands. This study confirms the presence of one [2Fe–2S] and one [4Fe–4S] cluster in human anamorsin as well as in yeast Dre2. The ligands for both clusters have been identified. A [2Fe–2S] cluster is bound to the first cysteine-rich motif consisting of Cys252, Cys263, Cys266 and Cys268, whereas a [4Fe–4S] cluster is bound to the second cysteine-rich motif consisting of Cys311, Cys314, Cys322 and Cys325. In addition, we demonstrated that both the [2Fe–2S] cluster and [4Fe–4S] cluster in Dre2 are essential for cell viability.
Although our EPR results clearly show that Dre2/anamorsin contains one [2Fe–2S] cluster and one [4Fe–4S] cluster, the [4Fe–4S] cluster was not detected by some other groups. What is the explanation for this discrepancy? (i) Compared with the [2Fe–2S] cluster, the [4Fe–4S] cluster in Dre2 is sensitive to oxygen, thus, it is likely that the full-length Dre2/anamorsin samples prepared by these groups already lost the [4Fe–4S] cluster during purification. We have noticed from the earliest stage of our study that although we bubbled all the buffers with pure nitrogen and added 1–2 mM DTT before use, the content of the [4Fe–4S] cluster in purified WT Dre2 was about 10% that of the [2Fe–2S] cluster. The [2Fe–2S] cluster was always predominant in our Dre2 samples. We think that sonication is most likely the critical step during which the [4Fe–4S] cluster can be lost. In fact, very recently, anaerobically purified Dre2 including the cell disruption step by sonication was shown to contain much higher content of [4Fe–4S] cluster (24). In addition, in our experiments with anaerobically reconstituted WT and C263A Dre2, air-dependent spectral changes indicate loss of the [4Fe–4S] clusters and maintenance and increase of [2Fe–2S] cluster signals suggesting that there may be conversion of the B site cluster from one form to another (Fig. 8). Subsequent decrease in absorbances in air suggests loss of [2Fe–2S] clusters as well. However, we disagree with the result shown in Fig. 6A of the reference (20). The authors measured the difference spectrum of a Dre2 sample (10 μM) purified anaerobically, comparing the sample reduced by 10 mM sodium dithionite with the non-reduced sample. However, the EPR spectrum appears to be from an unknown source in the sample rather than from the [2Fe–2S] cluster in Dre2, because it is theoretically established that EPR spectra for [2Fe–2S] clusters are anisotropic based on the chemical structures of [2Fe–2S] clusters. This indicates that the sample contained very low amounts of Fe/S clusters, perhaps because they were lost during purification. Thus, the microwave power half-saturation (P1/2) value calculated from these data is misleading. (ii) The second possibility is that the redox potential of the [4Fe–4S] cluster is too low to be efficiently reduced with sodium dithionite. If this is the case, the majority of the [4Fe–4S] cluster in Dre2 remains as the oxidized [4Fe–4S]2+ state, which is EPR silent. In fact, Lill’s group has demonstrated that this [4Fe–4S] cluster has a lower redox potential and is incapable of receiving electron from NADPH through Tah18 (12). Adding some redox mediators like methyl viologen might be helpful to more efficiently reduce the [4Fe–4S] cluster with sodium dithionite, as shown for biotin synthase (25). (iii) The third possibility is that the [4Fe–4S] was reduced, but it has the S = 3/2 spin ground state. EPR signals of [4Fe–4S] clusters at the S = 3/2 state can be detected only in the g =5 region, and a much higher enzyme concentration is required than for detecting EPR signals of [4Fe–4S] clusters in the S =1/2 state in the g = 2.0 region (26, 27). But since we observed the [4Fe–4S] signal in the g = 2.0 regions, it is less likely that Dre2/anamorsin as purified in other labs has exclusively the g = ∼5 signals.
The results that the C252A mutant expressed in yeast maintained cell viability and grew robustly like the WT were completely unexpected because mutation of each of the other conserved cysteines in Dre2 was associated with non-viability of the yeast shuffle strain. Our EPR data clearly showed that there was no EPR signal from the [2Fe–2S] cluster in the C252A mutant as observed in other three C263A, C266A and C268A mutants. There might be two possibilities to explain this. (i) One possible explanation is that Cys252 is not a ligand for the [2Fe–2S] cluster. However, the reason why we could not see the EPR [2Fe–2S] signals in this mutant is that as Cys252 is located very close to the [2Fe–2S] cluster, the C252A mutation indirectly destabilizes the [2Fe–2S] cluster, resulting in the loss of the EPR signal after the aerobic isolation process. On the other hand, under in vivo condition, the [2Fe–2S] cluster in the C252A mutant may still be stable enough to maintain its function and support survival and growth of yeast cells. It is interesting to note that the Dre2 homolog from Plasmodium falciparum lacks the 252-corresponding cysteine but contains all other seven cysteines (Fig. 2). This fact also seems to favor the hypothesis that Cys252 is not a ligand. If Cys252 is not a ligand for the [2Fe–2S] cluster, possible other candidates for a ligand of Fe/S clusters are histidine, arginine, aspartic acid and glutamic acid. For example, a conserved glutamate residue was found to be essential for coordinating the [2Fe–2S] cluster in the iron–sulfur regulator, IscR (28). Then, we found that Glu272 and Glu275 residues are evolutionarily conserved in Dre2. We mutated and generated the C252A/E272A/E275A triple mutant to test this hypothesis. However, our very recent data showed that this mutant was still viable in shuffle strain experiments, implying that those conserved glutamate residues are not ligands for the [2Fe–2S] cluster either (data not shown). As there is no other conserved ligand candidate nearby, so far, it is less likely that another residue could be the fourth ligand of the [2Fe–2S] cluster. (ii) Another possibility is that Cys252 is a ligand for the [2Fe–2S] cluster but a less critical ligand than the others. When Cys252 is not available, Dre2 can accommodate nearby residues as an alternative ligand to form the [2Fe–2S] cluster and sustain its physiological function in yeast in vivo. This phenomenon is called cysteine ligand swapping, and it has been reported for [2Fe–2S] proteins (29). An example of this situation is reported for the ABC protein ABCE1, which contains a unique N-terminal region with eight conserved cysteines, predicted to coordinate Fe/S clusters. Based on biophysical, biochemical and yeast genetic analyses, it was found that ABCE1 harbors two essential [4Fe–4S] clusters (30). Later, the 3D crystal structure of ABCE1 showed that these eight conserved cysteines were their ligands (31). However, mutational analysis revealed that only seven of the eight conserved cysteines were essential and mutagenesis of the cysteine at position 6 yielded a functional ABCE1. Furthermore, the lethal mutation at position 4 can be rescued by ligand swapping with an adjacent, extra cysteine conserved among all eukaryotes (30). Another example similar to our results was reported for biotin synthase, which contains a [2Fe–2S] cluster coordinated by three cysteines and an atypical arginine residue (25). In that study, the evolutionary conserved Arg was mutated to Ala, Cys, His and Met. Although perturbations in a number of characteristics of the [2Fe–2S] cluster were noted, in vivo expression of each Arg mutant enzyme was sufficient to sustain growth of a bioB− mutant (25), perhaps because very small threshold amounts of biotin are needed for viability.
Currently, we favor the hypothesis that C252A is a crucial but not an essential ligand of the Dre2 [2Fe–2S] cluster. The discrepancy that the EPR data from the mutant showed lack of the [2Fe–2S] cluster and yet the C252A mutant in yeast is viable and grows normally could be due to a threshold effect such that the mutant binds a small amount of [2Fe–2S] clusters in vivo but enough to sustain normal growth. Further studies will be needed to fully resolve this mystery. Interestingly in a paper published while our manuscript was under review, Lill et al. showed that the C252A Dre2 mutant expressed in yeast bound about 30% of the WT level of iron (24), indicating an in vivo effect of this mutant as a cluster ligand but a less marked effect than for the other ligands. The spectroscopy and complementation studies with the hDre2 protein show essentiality of the C246 (predicted A site ligand) and C288 (predicted B site ligand) and not of C237 (predicted A site ligand), thus behaving exactly as the yeast Dre2. The likelihood is that the C237 is a genuine ligand for the hDre2 [2Fe–2S] cluster but less essential than the other ligands, just as is the case for the C252 in the yeast protein.
Dre2 acts in a complex with diflavoprotein Tah18 providing electrons for cytosolic Fe/S cluster biosynthesis (12). However, two major unresolved questions with this model need further investigation: first, what is the electron recipient? Second, what is the role of the [4Fe–4S] cluster in Dre2? Electrons for Fe/S cluster assembly in the cytoplasm, donated by Dre2/Tah18 may be needed to reduce the key constituents of the Fe/S cluster, iron and sulfur. Alternatively, recipients of the electrons may be proteins involved in the CIA pathway, such as the Nbp35/Cfd1 scaffold. The role of a reductase in cytoplasmic Fe/S cluster assembly in some ways parallels the role played by Arh1/Yah1in mitochondrial Fe/S cluster assembly. In that case, NADPH donates electrons to Arh1 and the latter interacts and reduces Yah1, which may provide reducing power to release sulfur from the cysteine desulfurase. Yah1 is a ferredoxin with only a [2Fe–2S] cluster, and thus no [4Fe–4S] cluster is present in this mitochondrial electron transfer pathway. The role of the [4Fe–4S] cluster of Dre2 may be special to the cytoplasmic setting. Lill’s group reported that although the [4Fe–4S] cluster in Dre2 is not involved in receiving electrons from NADPH through Tah18 (12), the absence of Fe/S in aerobically purified Dre2 prevents the binding with Tah18 (21), and thus the [4Fe–4S] cluster might have a structural role in Tah18–Dre2 interaction. Or, it could be speculated that the [4Fe–4S] cluster in Dre2 might be transferable to a scaffold protein as an intermediate in Fe/S biogenesis (Fig. 1).
In addition to the defect in synthesis of cytosolic Fe/S clusters, RNR is also impaired in the dre2-depleted cells. RNR needs electrons and iron to assemble its essential diferric tyrosyl radical, and physical interaction between RNR β subunit and Dre2 has been reported (15, 16). Based on these observations, a role of Dre2 as a ferric reductase has been proposed (15). Ferric reductase activity is likely needed for cytosolic Fe/S cluster biosynthesis as well, although the source of iron has not been well understood. In fact, little is known about intracellular iron trafficking. The bioavailable form of iron being trafficked and delivered to different iron utilization pathways is still mysterious. A recent study found that the labile Fe/S clusters on the dimer interface of glutaredoxins Grx3 and Grx4 contribute to the long sought cytosolic iron pool (32). Disruption of this iron pool causes a global defect of all iron utilization pathways including Fe/S cluster, heme and diiron enzymes (32), but how iron is mobilized from this labile Fe/S cluster is not known and an intriguing question associated with this model is about the fate of the sulfide in the cluster. Dre2 has been found physically interacting with Grx3/4 and may play a role in releasing iron from the reductively labile cluster on Grx3/4 (16, 33). A recent NMR study interestingly showed that the human apo-Grx3 binds to apo-anamorsin through their N-terminal domains, and the holo-Grx3 can transfer its [2Fe–2S] to anamorsin (18). These models may not be mutually exclusive. A key redox reaction required for iron mobilization and/or the biogenesis of the [2Fe–2S] cluster in Dre2 is dependent on the interaction of Dre2 and Grx3/4. More recently, however, it was published that two of the human members of the NEET family of [2Fe–2S] cluster proteins, nutrient-deprivation autophagy factor-1 and mitoNEET, can also transfer their [2Fe–2S] clusters to apo-anamorsin (34). It would be of great importance to define the true physiological donor(s) and biogenesis pathways of the Dre2/anamorsin [2Fe–2S] cluster and [4Fe–4S] cluster.
In summary, we unambiguously demonstrated that Dre2 contains a [2Fe–2S] cluster coordinated by Cys252, Cys263, Cys266 and Cys268 and a [4Fe–4S] cluster coordinated by the Cys311, Cys314, Cys322 and Cys325 residues. Both clusters are essential for cell viability. As human anamorsin can complement a yeast dre2 strain (11), it is highly likely that the human anamorsin also likely contains the same sets of prosthetic groups, as strongly supported by our EPR data and yeast complementation data (Fig. 7).
Acknowledgements
We thank Ms Diana Orlandi and Mr Yuta Inaba for their technical assistance and Dr Yifeng Wei for discussion on bioinformatics.
Glossary
Abbreviations
- CIA
Cytosolic Iron cluster Assembly
- CIAPIN1
cytokine-induced apoptosis inhibitor 1
- EPR
Electron Paramagnetic Resonance
- Fe/S
Iron–sulfur
- FOA
5-fluoroorotic acid
- hDre2
human Dre2
- IMS
intermembrane space
- ISC
Iron Sulfur Cluster
- SGD
Saccharomyces Genome Database
- WT
wild type
Funding
This work was supported by NSFC grant 31570060 to Y.Z., NIH grant R01GM097409 and AHA grant 11SDG5560001 to E.N.-O and NIH grant R37DK053953 to A.D. and NIH grant R01GM107542 to Debkumar Pain, Timothy Stemmler and A.D.
Conflict of Interest
None declared.
References
- 1.Rouault T.A. (2015) Iron-Sulfur clusters in Chemistry and Biology. Walter de Gruyter, Berlin/Boston.
- 2.Zheng L., White R.H., Cash V.L., Dean D.R. (1994) Mechanism for the desulfurization of L-cysteine catalyzed by the nifS gene product. Biochemistry 33, 4714–4720 [DOI] [PubMed] [Google Scholar]
- 3.Stehling O., Wilbrecht C., Lill R. (2014) Mitochondrial iron-sulfur protein biogenesis and human disease. Biochimie 100, 61–77 [DOI] [PubMed] [Google Scholar]
- 4.Kispal G., Sipos K., Lange H., Fekete Z., Bedekovics T., Janaky T., Bassler J., Aguilar Netz D.J., Balk J., Rotte C., Lill R. (2005) Biogenesis of cytosolic ribosomes requires the essential iron-sulphur protein Rli1p and mitochondria. EMBO J. 24, 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klinge S., Hirst J., Maman J.D., Krude T., Pellegrini L. (2007) An iron-sulfur domain of the eukaryotic primase is essential for RNA primer synthesis. Nat. Struct. Mol. Biol. 14, 875–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Netz D.J., Stith C.M., Stumpfig M., Kopf G., Vogel D., Genau H.M., Stodola J.L., Lill R., Burgers P.M., Pierik A.J. (2012) Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat. Chem. Biol. 8, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudolf J., Makrantoni V., Ingledew W.J., Stark M.J., White M.F. (2006) The DNA repair helicases XPD and FancJ have essential iron-sulfur domains. Mol. Cell 23, 801–808 [DOI] [PubMed] [Google Scholar]
- 8.Lee J.Y., Yang J.G., Zhitnitsky D., Lewinson O., Rees D.C. (2014) Structural basis for heavy metal detoxification by an Atm1-type ABC exporter. Science 343, 1133–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lill R., Dutkiewicz R., Freibert S.A., Heidenreich T., Mascarenhas J., Netz D.J., Paul V.D., Pierik A.J., Richter N., Stumpfig M., Srinivasan V., Stehling O., Muhlenhoff U. (2015) The role of mitochondria and the CIA machinery in the maturation of cytosolic and nuclear iron-sulfur proteins. Eur. J. Cell Biol. 94, 280–291 [DOI] [PubMed] [Google Scholar]
- 10.Shibayama H., Takai E., Matsumura I., Kouno M., Morii E., Kitamura Y., Takeda J., Kanakura Y. (2004) Identification of a cytokine-induced antiapoptotic molecule anamorsin essential for definitive hematopoiesis. J. Exp. Med. 199, 581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Lyver E.R., Nakamaru-Ogiso E., Yoon H., Amutha B., Lee D.W., Bi E., Ohnishi T., Daldal F., Pain D., Dancis A. (2008) Dre2, a conserved eukaryotic Fe/S cluster protein, functions in cytosolic Fe/S protein biogenesis. Mol. Cell Biol. 28, 5569–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netz D.J., Stumpfig M., Dore C., Muhlenhoff U., Pierik A.J., Lill R. (2010) Tah18 transfers electrons to Dre2 in cytosolic iron-sulfur protein biogenesis. Nat. Chem. Biol. 6, 758–765 [DOI] [PubMed] [Google Scholar]
- 13.Bernard D.G., Netz D.J., Lagny T.J., Pierik A.J., Balk J. (2013) Requirements of the cytosolic iron-sulfur cluster assembly pathway in Arabidopsis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basu S., Netz D.J., Haindrich A.C., Herlerth N., Lagny T.J., Pierik A.J., Lill R., Lukes J. (2014) Cytosolic iron-sulphur protein assembly is functionally conserved and essential in procyclic and bloodstream Trypanosoma brucei. Mol. Microbiol. 93, 897–910 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Li H., Zhang C., An X., Liu L., Stubbe J., Huang M. (2014) Conserved electron donor complex Dre2-Tah18 is required for ribonucleotide reductase metallocofactor assembly and DNA synthesis. Proc. Natl Acad. Sci. U. S. A. 111, E1695–E1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Liu L., Wu X., An X., Stubbe J., Huang M. (2011) Investigation of in vivo diferric tyrosyl radical formation in Saccharomyces cerevisiae Rnr2 protein: requirement of Rnr4 and contribution of Grx3/4 AND Dre2 proteins. J. Biol. Chem. 286, 41499–41509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banci L., Bertini I., Calderone V., Ciofi-Baffoni S., Giachetti A., Jaiswal D., Mikolajczyk M., Piccioli M., Winkelmann J. (2013) Molecular view of an electron transfer process essential for iron-sulfur protein biogenesis. Proc. Natl Acad. Sci. U. S. A. 110, 7136–7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banci L., Ciofi-Baffoni S., Gajda K., Muzzioli R., Peruzzini R., Winkelmann J. (2015) N-terminal domains mediate [2Fe-2S] cluster transfer from glutaredoxin-3 to anamorsin. Nat. Chem. Biol. 11, 772–778 [DOI] [PubMed] [Google Scholar]
- 19.Banci L., Ciofi-Baffoni S., Mikolajczyk M., Winkelmann J., Bill E., Pandelia M.E. (2013) Human anamorsin binds [2Fe-2S] clusters with unique electronic properties. J. Biol. Inorg. Chem. 18, 883–893 [DOI] [PubMed] [Google Scholar]
- 20.Soler N., Craescu C.T., Gallay J., Frapart Y.M., Mansuy D., Raynal B., Baldacci G., Pastore A., Huang M.E., Vernis L. (2012) A S-adenosylmethionine methyltransferase-like domain within the essential, Fe-S-containing yeast protein Dre2. FEBS J. 279, 2108–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soler N., Delagoutte E., Miron S., Facca C., Baille D., d'Autreaux B., Craescu G., Frapart Y.M., Mansuy D., Baldacci G., Huang M.E., Vernis L. (2011) Interaction between the reductase Tah18 and highly conserved Fe-S containing Dre2 C-terminus is essential for yeast viability. Mol. Microbiol. 82, 54–67 [DOI] [PubMed] [Google Scholar]
- 22.Sherman F. (2002). Getting started with yeast. Methods Enzymol. 350, 3–41 [DOI] [PubMed] [Google Scholar]
- 23.Laemmli U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 24.Netz D.J., Genau H.M., Weiler B.D., Bill E., Pierik A.J., Lill R. (2016) The conserved protein Dre2 uses essential [2Fe-2S] and [4Fe-4S] clusters for its function in cytosolic iron-sulfur protein assembly. Biochem. J. 473, 2073–2085 [DOI] [PubMed] [Google Scholar]
- 25.Broach R.B., Jarrett J.T. (2006) Role of the [2Fe-2S]2+ cluster in biotin synthase: mutagenesis of the atypical metal ligand arginine 260. Biochemistry 45, 14166–14174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindahl P.A., Day E.P., Kent T.A., Orme-Johnson W.H., Munck E. (1985) Mossbauer, EPR, and magnetization studies of the Azotobacter vinelandii Fe protein. Evidence for a [4Fe-4S]1+ cluster with spin S = 3/2. J. Biol. Chem. 260, 11160–11173 [PubMed] [Google Scholar]
- 27.Ohnishi T., Nakamaru-Ogiso E. (2008) Were there any “misassignments” among iron-sulfur clusters N4, N5 and N6b in NADH-quinone oxidoreductase (complex I)? Biochim. Biophys. Acta 1777, 703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng J., Zhang X., Wang Y., Ai C., Liu Q., Qiu G. (2008) Glu43 is an essential residue for coordinating the [Fe2S2] cluster of IscR from Acidithiobacillus ferrooxidans. FEBS Lett. 582, 3889–3892 [DOI] [PubMed] [Google Scholar]
- 29.Golinelli M.P., Akin L.A., Crouse B.R., Johnson M.K., Meyer J. (1996) Cysteine ligand swapping on a deletable loop of the [2Fe-2S] ferredoxin from Clostridium pasteurianum. Biochemistry 35, 8995–9002 [DOI] [PubMed] [Google Scholar]
- 30.Barthelme D., Scheele U., Dinkelaker S., Janoschka A., Macmillan F., Albers S.V., Driessen A.J., Stagni M.S., Bill E., Meyer-Klaucke W., Schunemann V., Tampe R. (2007) Structural organization of essential iron-sulfur clusters in the evolutionarily highly conserved ATP-binding cassette protein ABCE1. J. Biol. Chem. 282, 14598–14607 [DOI] [PubMed] [Google Scholar]
- 31.Karcher A., Schele A., Hopfner K.P. (2008) X-ray structure of the complete ABC enzyme ABCE1 from Pyrococcus abyssi. J. Biol. Chem. 283, 7962–7971 [DOI] [PubMed] [Google Scholar]
- 32.Muhlenhoff U., Molik S., Godoy J.R., Uzarska M.A., Richter N., Seubert A., Zhang Y., Stubbe J., Pierrel F., Herrero E., Lillig C.H., Lill R. (2010) Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab. 12, 373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Netz D.J., Pierik A.J., Stumpfig M., Muhlenhoff U., Lill R. (2007) The Cfd1-Nbp35 complex acts as a scaffold for iron-sulfur protein assembly in the yeast cytosol. Nat. Chem. Biol. 3, 278–286 [DOI] [PubMed] [Google Scholar]
- 34.Lipper C.H., Paddock M.L., Onuchic J.N., Mittler R., Nechushtai R., Jennings P.A. (2015) Cancer-related NEET proteins transfer 2Fe-2S clusters to anamorsin, a protein required for cytosolic iron-sulfur cluster biogenesis. PLoS One 10, e0139699. [DOI] [PMC free article] [PubMed] [Google Scholar]