Abstract
Ambient air pollution and temperature have been linked with cardiovascular morbidity and mortality. Metabolic syndrome and its components—abdominal obesity, elevated fasting blood glucose concentration, low high-density lipoprotein cholesterol concentration, hypertension, and hypertriglyceridemia—predict cardiovascular disease, but the environmental causes are understudied. In this study, we prospectively examined the long-term associations of air pollution, defined as particulate matter with an aerodynamic diameter less than or equal to 2.5 µm (PM2.5), and temperature with the development of metabolic syndrome and its components. Using covariate-adjustment Cox proportional hazards models, we estimated associations of mean annual PM2.5 concentration and temperature with risk of incident metabolic dysfunctions between 1993 and 2011 in 587 elderly (mean = 70 (standard deviation, 7) years of age) male participants in the Normative Aging Study. A 1-μg/m3 increase in mean annual PM2.5 concentration was associated with a higher risk of developing metabolic syndrome (hazard ratio (HR) = 1.27, 95% confidence interval (CI): 1.06, 1.52), an elevated fasting blood glucose level (HR = 1.20, 95% CI: 1.03, 1.39), and hypertriglyceridemia (HR = 1.14, 95% CI: 1.00, 1.30). Our findings for metabolic syndrome and high fasting blood glucose remained significant for PM2.5 levels below the Environmental Protection Agency's health-safety limit (12 μg/m3). A 1°C increase in mean annual temperature was associated with a higher risk of developing elevated fasting blood glucose (HR = 1.33, 95% CI: 1.14, 1.56). Men living in neighborhoods with worse air quality—with higher PM2.5 levels and/or temperatures than average—showed increased risk of developing metabolic dysfunctions.
Keywords: air pollution, blood glucose, high-density lipoprotein cholesterol, hypertension, metabolic syndrome, obesity, temperature, triglycerides
Metabolic syndrome is an urgent public health concern that affects 10%–25% of the global population and is associated with increased risk of cardiovascular disease, asthma, sleep apnea, and selected malignancies and with higher total and cause-specific mortality (1). Metabolic syndrome is defined as having at least 3 of the following conditions: abdominal obesity (waist circumference ≥102 cm for men or ≥88 cm for women), high fasting blood glucose (≥100 mg/dL or medication to treat elevated blood glucose), low high-density lipoprotein (HDL) cholesterol (<40 mg/dL for men, <50 mg/dL for women, or medication to treat low HDL cholesterol), hypertension (systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥85 mm Hg, or medication to treat elevated blood pressure), and hypertriglyceridemia (triglyceride level ≥150 mg/dL or medication to treat elevated triglycerides) (2) (see Web Table 1, available at http://aje.oxfordjournals.org/). In the United States, metabolic syndrome affects an estimated 47–68 million people, with males aged 60 years or older being among those at greatest risk (3–5).
Rates of metabolic syndrome are increasing worldwide and show geographic variations, largely due to lifestyle, socioeconomic, and ethnic differences (6–8). However, local environmental conditions might also contribute to variations in metabolic syndrome rates. Air pollution (including atmospheric particulate matter with an aerodynamic diameter less than or equal to 2.5 µm (PM2.5)) and outdoor temperature are 2 essential environmental factors that have been shown in experimental and in vitro studies to affect cellular and systemic metabolic processes (9, 10).
Nonetheless, the possible relationships of local levels of PM2.5 and temperature with metabolic syndrome are still underinvestigated in human populations. In particular, previous epidemiologic studies have investigated the relationship of single exposures with individual metabolic components rather than with the entire complex of metabolic alterations that constitute metabolic syndrome (11–15). Furthermore, previous studies have focused mainly on the associations between short-term temperature exposure and metabolic outcomes, which may reflect transient modifications not necessarily related to long-term risks (15, 16). Additionally, to our knowledge, no investigators have published an incidence analysis on the topic.
In the current study, we prospectively examined the association of the average levels of ambient PM2.5 and temperature at the participants’ addresses with the development of metabolic syndrome and of the individual conditions that constitute metabolic syndrome. We used the prospective follow-up of participants in the Normative Aging Study (NAS), a Department of Veterans Affairs longitudinal study of a cohort of older men living across eastern Massachusetts, southern New Hampshire, and southern Maine.
METHODS
Study population
The present analysis included 587 men who were active participants between 1993 and 2011 in the NAS, a longitudinal study (Web Table 2) (17). Each participant included in the analysis was free of at least 1 component of metabolic syndrome at his first visit between 1993 and 2011 and received comprehensive outpatient medical evaluations every 3–7 years. During the visits, participants provided detailed information about their lifestyles, dietary habits, activity levels, and demographic factors. In each diagnosis-specific analysis, we excluded participants who met pathological criteria for that disorder at the baseline visit (Table 1, Web Table 2). All participants gave written informed consent in accordance with the VA Boston Healthcare System Institutional Review Board and the institutional review boards of all participating institutions.
Table 1.
Sociodemographic Characteristics of Active Participants at the Baseline Visit and Prevalence of Metabolic Syndrome and Its Components, Normative Aging Study, 1993–2011
| Characteristic | Entire Population | Subset Used in Metabolic Component Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdominal Obesity | High Fasting Blood Glucose | Low HDL Cholesterol | Hypertension | Hypertriglyceridemia | Metabolic Syndrome | |||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. of participants | 587 | 396 | 293 | 326 | 116 | 316 | 271 | |||||||
| No. of excluded participants | 191 | 294 | 261 | 471 | 271 | 316 | ||||||||
| No. of visitsa | 2.2 (6.0) | 2.3 (6.0) | 2.1 (6.0) | 2.1 (6.0) | 2.1 (6.0) | 2.1 (6.0) | 2.1 (6.0) | |||||||
| Age, yearsb | 70.4 (6.9) | 70.8 (6.8) | 70.5 (6.9) | 70.5 (7.1) | 68.4 (7.6) | 70.8 (7.1) | 70.4 (7.3) | |||||||
| Nonwhite race/ethnicity (% of census tract)b | 11.9 (13.0) | 12 (12.6) | 11.2 (11.9) | 12.5 (13.6) | 10.8 (11.6) | 12.2 (13.3) | 12.1 (12.9) | |||||||
| Education, years | ||||||||||||||
| ≤12 | 171 | 29.1 | 116 | 29.3 | 86 | 29.4 | 89 | 27.3 | 33 | 28.5 | 86 | 27.2 | 72 | 26.6 |
| 12.1–16 | 294 | 50.1 | 196 | 49.5 | 138 | 47.1 | 166 | 50.9 | 54 | 46.6 | 161 | 51.0 | 134 | 49.5 |
| >16 | 122 | 20.8 | 84 | 21.2 | 69 | 23.6 | 71 | 21.8 | 29 | 25.0 | 69 | 21.8 | 65 | 24.0 |
| Physical activity, MET-hours/week | ||||||||||||||
| <12 | 342 | 58.3 | 206 | 52.0 | 152 | 51.9 | 192 | 58.9 | 59 | 50.9 | 191 | 60.4 | 140 | 51.7 |
| 12–29.9 | 149 | 25.4 | 111 | 28.0 | 87 | 29.7 | 86 | 26.4 | 42 | 36.2 | 79 | 25.0 | 85 | 31.4 |
| ≥30 | 96 | 16.4 | 79 | 20.0 | 54 | 18.4 | 48 | 14.7 | 15 | 12.9 | 46 | 14.6 | 46 | 17.0 |
| Smoking status | ||||||||||||||
| Never smoker | 169 | 28.8 | 121 | 30.6 | 95 | 32.4 | 94 | 28.8 | 36 | 31.0 | 94 | 29.8 | 87 | 32.1 |
| Former smoker | 384 | 65.4 | 253 | 63.9 | 184 | 62.8 | 211 | 64.7 | 69 | 59.5 | 202 | 63.9 | 165 | 60.9 |
| Current smoker | 34 | 5.8 | 22 | 5.6 | 14 | 4.8 | 21 | 6.4 | 11 | 9.5 | 20 | 6.3 | 19 | 7.0 |
| Alcohol consumption, drinks/day | ||||||||||||||
| <2 | 453 | 77.2 | 311 | 78.5 | 230 | 78.5 | 235 | 72.1 | 92 | 79.3 | 243 | 76.9 | 206 | 76.0 |
| ≥2 | 134 | 22.8 | 85 | 21.5 | 63 | 21.5 | 91 | 27.9 | 24 | 20.7 | 73 | 23.1 | 65 | 24.0 |
| Consumption of dark fish, times/week | ||||||||||||||
| <1 | 507 | 86.4 | 335 | 84.6 | 245 | 83.6 | 285 | 87.4 | 99 | 85.3 | 270 | 85.4 | 231 | 85.2 |
| ≥1 | 80 | 13.6 | 61 | 15.4 | 48 | 16.4 | 41 | 12.6 | 17 | 14.7 | 46 | 14.6 | 40 | 14.8 |
| Permanent residency, Greater Boston, Massachusetts | ||||||||||||||
| Yes | 514 | 87.6 | 346 | 87.4 | 254 | 86.7 | 286 | 87.7 | 100 | 86.2 | 280 | 88.6 | 237 | 87.5 |
| No | 73 | 12.4 | 50 | 12.6 | 39 | 13.3 | 40 | 12.3 | 16 | 13.8 | 36 | 11.4 | 34 | 12.6 |
| Use of statins | ||||||||||||||
| No | 449 | 76.5 | 288 | 72.7 | 217 | 74.1 | 326 | 100.0 | 91 | 78.5 | 316 | 100.0 | 262 | 96.7 |
| Yes | 138 | 23.5 | 108 | 27.3 | 76 | 25.9 | 0 | 0.0 | 25 | 21.6 | 0 | 0.0 | 9 | 3.3 |
| Use of diabetes medication | ||||||||||||||
| No | 560 | 95.4 | 379 | 95.7 | 293 | 100.0 | 316 | 96.9 | 114 | 98.3 | 307 | 97.2 | 268 | 98.9 |
| Yes | 27 | 4.6 | 17 | 4.3 | 0 | 0.0 | 10 | 3.1 | 2 | 1.7 | 9 | 2.9 | 3 | 1.1 |
| Use of antihypertensive medication | ||||||||||||||
| No | 300 | 51.1 | 209 | 52.8 | 166 | 56.7 | 185 | 56.8 | 116 | 100 | 177 | 56.0 | 179 | 66.1 |
| Yes | 287 | 48.9 | 187 | 47.2 | 127 | 43.3 | 141 | 43.3 | 0 | 0.0 | 139 | 44.0 | 92 | 34.0 |
Abbreviations: HDL, high-density lipoprotein; MET, metabolic equivalent.
a Values expressed as the mean (maximum).
b Values expressed as the mean (standard deviation).
Spatiotemporally resolved modeling of particulate matter level
To assign exposure we used a recently published, validated spatiotemporal hybrid model, developed by our group, to estimate daily PM2.5 level (in μg/m3) across the study area (18). We used the average PM2.5 level during the year prior to each visit at each participant's residential address to assess the long-term association of air pollution with metabolic dysfunction. The model incorporates fine-scale local land-use regression analysis and satellite-measured aerosol optical depth (18). This hybrid model provides address-level resolution for long-term PM2.5 measurements collected between May 2000 and December 2011. Due to limitations in data availability, we used a similar hybrid model based on the moderate resolution imaging spectroradiometer model, albeit at a lower spatial resolution (a 10-km × 10-km grid) for May 2000–October 2003 and on a 1-km × 1-km grid for October 2003–December 2011 (18, 19). Missing observations on the 1-km × 1-km grid for the period of October 2003–December 2011 were also replaced with measurements from the 10-km × 10-km grid. Out-of-sample cross-validation showed a good fit of the 10-km × 10-km model (R2 = 0.81) and an excellent fit of the 1-km × 1-km model (R2 = 0.87) (18, 19).
Spatiotemporally resolved modeling of temperature level
We used the average daily temperature during the year before each visit at a 1-km × 1-km resolution as a proxy for the temperatures at the participants’ addresses in order to assess the long-term association of outdoor temperature with metabolic dysfunction. Daily temperature with 1-km × 1-km resolution was predicted using a spatiotemporally resolved, satellite-based model similar to that used for PM2.5 (20, 21). Daily estimates were averaged to produce average annual temperatures. Ten-fold out-of-sample cross-validation was used to validate the accuracy of the predictions, and it showed good fit (mean out-of-sample R2 = 0.94) (20, 21).
Assignment of PM2.5 and temperature levels
In this analysis, we defined as baseline the first NAS visit conducted prior to May 2000, that is, the first month with available PM2.5 and temperature estimates. We used, as a proxy measure for between-visit exposure, the average of PM2.5 or temperature levels at each participant's address in the year before each subsequent follow-up visit. The 1-year average was selected because it correlates well with averages of PM2.5 over longer time windows (e.g., 2–5 years) in this study population (Web Table 3), and it was available for a higher number of visits than were the 2- to 5-year averages.
Clinical measures
At each visit, anthropometric measurements were performed with participants in undershorts and socks. Waist circumference was measured in centimeters at the umbilical level, perpendicular to the axis of the upper body (22). Participants were considered to have abdominal obesity if their waist circumference was ≥102 cm. At each visit, fasting blood glucose and lipid levels were measured in blood samples drawn on the morning of the medical evaluation. In keeping with the diagnostic criteria for metabolic syndrome, high blood sugar was defined as a fasting blood glucose ≥100 mg/dL or medication to treat elevated blood glucose, low HDL cholesterol was defined as any value <40 mg/dL or medication to treat low HDL cholesterol, and hypertriglyceridemia was any triglyceride measure ≥150 mg/dL or medication to treat elevated triglycerides. Individuals were diagnosed with hypertension if their systolic blood pressure was ≥130 mm Hg or diastolic blood pressure was ≥85 mm Hg, or they were taking medication to treat elevated blood pressure. Participants who met 3 or more of the above diagnostic criteria for abdominal obesity, high fasting blood glucose, low HDL, hypertension, or hypertriglyceridemia were considered to have metabolic syndrome (2, 23). A structured, in-person questionnaire was used to collect information about lifestyle and dietary habits, including physical activity, smoking status, alcohol consumption, and average dark fish consumption.
Statistical analyses
The risk of future metabolic dysfunction associated with 1-year average ambient PM2.5 level or temperature estimated at the participants’ addresses was evaluated using Cox proportional hazards modeling. This method's ability to handle censored data and time-dependent covariates makes it well suited for this analysis (24). Metabolic syndrome and 5 individual measures of metabolic function—waist circumference, fasting blood glucose, HDL cholesterol, blood pressure, and triglycerides—were modeled separately.
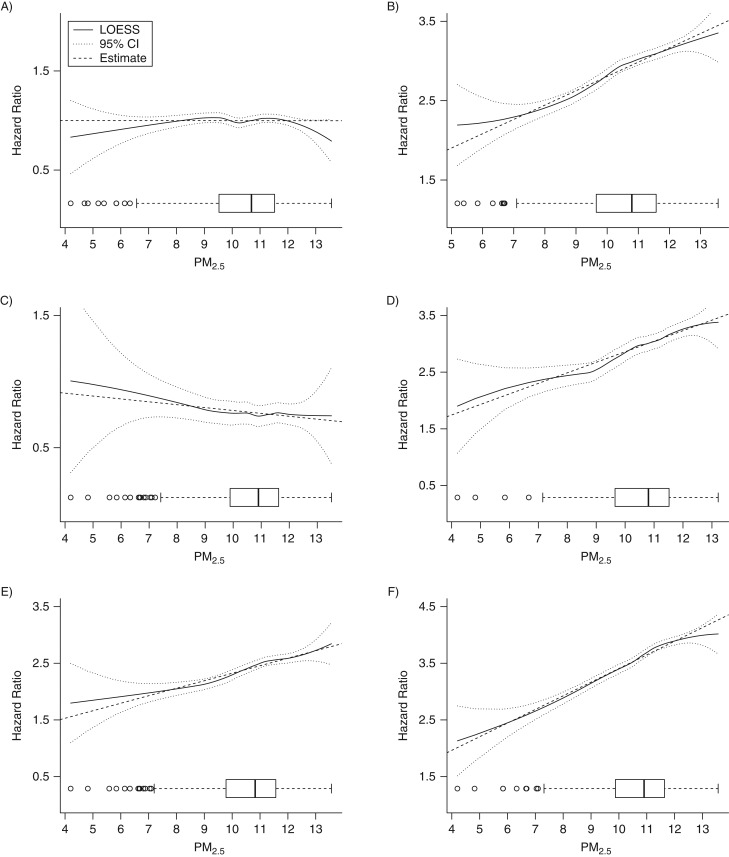
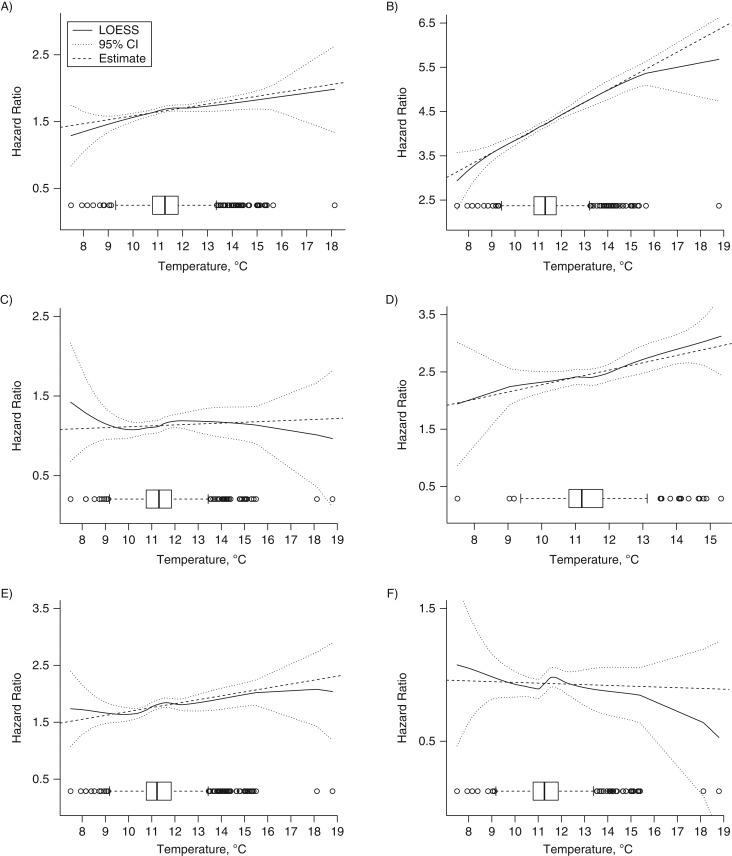
Each model included time-dependent variables (the participant's age at the time of the visit (years; continuous), dark fish consumption (less than once a week, at least once a week), alcohol consumption (<2 drinks/day, ≥2 drinks/day), smoking status (never, former, current), and physical activity (metabolic equivalent hours per week: <12, 12–29.9, ≥30)) and variables measured at the baseline visit (education as a proxy for socioeconomic status (years: ≤12, 12.1–16, >16), an indicator for whether the participant was a permanent resident of the Greater Boston area (yes/no), and percentage of the participant's census tract that was nonwhite (continuous)). Lipid-lowering (statins), antihypertensive, and diabetes medications were included in the model for metabolic syndrome as a whole and were considered to be time-dependent variables. PM2.5 models adjusted for 1-year average temperature levels and vice versa. Each model included strata for variables that did not meet the Cox proportional hazards assumptions. We also represented the significant associations and verified graphically that PM2.5 and temperature levels did not show major departures from the linear association with the outcomes (Figures 1 and 2).
Figure 1.
Level of exposure to fine particulate matter and the hazard ratio for the composite diagnosis of metabolic syndrome and each individual component according to level of exposure to fine particulate matter (box-and-whisker plots) among Normative Aging Study participants, 2000–2011. A) Abdominal obesity; B) high fasting blood glucose; C) low high-density lipoprotein cholesterol; D) hypertension; E) hypertriglyceridemia; F) metabolic syndrome. CI, confidence interval; LOESS, locally weighted scatterplot smoothing; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm.
Figure 2.
Level of exposure to temperature and the hazard ratio for the composite diagnosis of metabolic syndrome and each individual component according to level of exposure to fine particulate matter (box-and-whisker plots) among Normative Aging Study participants, 2000–2011. A) Abdominal obesity; B) high fasting blood glucose; C) low high-density lipoprotein cholesterol; D) hypertension; E) hypertriglyceridemia; F) metabolic syndrome. CI, confidence interval; LOESS, locally weighted scatterplot smoothing.
We also reanalyzed the results from each PM2.5 model after restricting the analysis to observations with PM2.5 levels lower than 12 µg/m3, which is the primary annual fine-particle standard set by the Environmental Protection Agency for public health protection (25).
As a sensitivity analysis, we excluded outliers for PM2.5 and temperature levels for all models, and we excluded physical activity from the list of covariates because including it might have resulted in overadjustment. We further investigated the interaction between air pollution and ambient temperature in relation to metabolic syndrome and its individual components.
We additionally evaluated changes in each cardiometabolic measure over time relative to air pollution and temperature using a linear model with repeated observations for each participant. The analysis was subsequently stratified according to the presence or absence of metabolic syndrome at baseline.
We used SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina), and R (R Foundation for Statistical Computing, Vienna, Austria) for all models (26–28).
RESULTS
Descriptive statistics
Table 1 shows demographic and clinical data for all participants. At baseline, of the 587 NAS participants, 191 (33%) had abdominal obesity, 294 (50%) had high blood glucose, 261 (44%) had low HDL cholesterol, 471 (80%) had hypertension, 271 (46%) had hypertriglyceridemia, and 316 (53%) met criteria for metabolic syndrome. Participants with specific metabolic dysfunctions were excluded from the corresponding analysis groups. The most common conditions in both preexisting and incident cases of metabolic syndrome were hypertension, hypertriglyceridemia, and low HDL cholesterol levels (Web Table 4). Participants were 70 years old, on average, at baseline and made up to 6 visits (mean = 2.2). Most participants were white (88%), had at least a college degree (71%), and were permanent residents in the Greater Boston area (86%). Most men reported low physical activity (58%), light or lower alcohol consumption (77%), and being former smokers (65%). Twenty-four percent of the participants used statins, 5% used diabetes medications, and 49% used antihypertensive medications. The percentage of users of prescribed medications and numbers of participants varied across the data subsets used for analysis of the individual components of metabolic syndrome.
Ambient air pollution and temperature associations
Estimated PM2.5 levels ranged between 4.2 μg/m3 and 13.6 μg/m3 (mean = 10.5 (standard deviation, 1.4) μg/m3). A 1-μg/m3 increase in annual PM2.5 level was significantly associated with a 27% higher risk of developing metabolic syndrome (hazard ratio (HR) = 1.27, 95% confidence interval (CI): 1.06, 1.52; P = 0.01), a 20% higher risk of developing elevated fasting blood glucose (HR = 1.20, 95% CI: 1.03, 1.39; P = 0.02), and a 14% increased risk of hypertriglyceridemia (HR = 1.14, 95% CI: 1.00; 1.30; P = 0.05) (Table 2, Figure 1). Estimated annual temperature levels ranged between 7.5°C and 18.8°C (mean = 11.4 (standard deviation, 1.1) °C). A 1°C increase in annual temperature was associated with a 33% higher risk of elevated fasting blood glucose (HR = 1.33, CI: 1.14, 1.56; P < 0.001) (Table 2, Figure 2). However, temperature was not significantly associated with the risk of metabolic syndrome as a whole (HR = 0.99, 95% CI: 0.82, 1.21; P = 0.95).
Table 2.
Associations of 1-Year PM2.5 a and Temperatureb Exposure With Risk of Metabolic Syndrome and Its Components, Normative Aging Study, 2000–2011
| Metabolic Syndrome or Component | No. of Participants | No. of Observations | No. of Events | PM2.5 Level | Temperature | ||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P Valuec | Hazard Ratio | 95% CI | P Valuec | ||||
| Abdominal obesityd | 396 | 857 | 107 | 1.00 | 0.86, 1.16 | 1.00 | 1.06 | 0.86, 1.31 | 0.58 |
| High fasting blood glucosed | 293 | 562 | 118 | 1.20 | 1.03, 1.39 | 0.02 | 1.33 | 1.14, 1.56 | <0.001 |
| Low HDL cholesterold | 326 | 625 | 165 | 0.98 | 0.85, 1.13 | 0.76 | 1.01 | 0.85, 1.20 | 0.90 |
| Hypertensiond | 116 | 207 | 82 | 1.20 | 0.97, 1.49 | 0.09 | 1.14 | 0.86, 1.50 | 0.37 |
| Hypertriglyceridemiad | 316 | 598 | 154 | 1.14 | 1.00, 1.30 | 0.05 | 1.07 | 0.92, 1.24 | 0.36 |
| Metabolic syndromed , e | 271 | 517 | 140 | 1.27 | 1.06, 1.52 | 0.01 | 0.99 | 0.82, 1.21 | 0.95 |
Abbreviations: HDL, high-density lipoprotein; CI, confidence interval; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm.
a PM2.5 concentration (for each 1 µg/m3) was a 1-year average from daily estimates including aerosol-optical-depth data from the 1-km × 1-km model and 10-km × 10-km model.
b Temperature (for each 1°C) was a 1-year average from daily estimates including aerosol-optical-depth data from the 1-km × 1-km model.
c Two-sided P value.
d The model included both time-dependent variables and variables measured at baseline. Time-dependent variables: age at the visit (years; continuous), dark fish consumption (less than once a week, at least once a week), alcohol consumption (<2 drinks/day, ≥2 drinks/day), smoking status (current, former, never), and physical activity level (metabolic equivalent hours per week, <12, 12–29.9, ≥30). Variables measured at baseline: education (years, ≤12, 12.1–16, >16), whether the participant was a permanent resident of the Greater Boston area (yes/no), and percentage of the participant's census tract that was nonwhite (continuous).
e Category included medications varying over time: diabetes medication (no/yes), statins (no/yes), and antihypertensive medication (no/yes).
After restricting the analysis only to observations with 1-year average PM2.5 levels less than 12 µg/m3 (Table 3), PM2.5 results for the analyses of metabolic syndrome and high fasting blood glucose remained significant and showed stronger associations: 40% higher risk of developing metabolic syndrome (HR = 1.40, CI: 1.11, 1.77; P = 0.005) and 31% higher risk of elevated fasting blood glucose (HR = 1.31, CI: 1.05, 1.63; P = 0.02).
Table 3.
Association of 1-Year PM2.5 a Levels With Risk of Metabolic Syndrome and Its Components, Restricted to Observations With PM2.5 Levels Lower Than 12 µg/m3, Normative Aging Study, 2000–2011
| Metabolic Syndrome or Component | No. of Participants | No. of Observations | No. of Events | PM2.5 Level | ||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P Valueb | ||||
| Abdominal obesityc | 368 | 753 | 102 | 1.12 | 0.94, 1.34 | 0.21 |
| High fasting blood glucosec | 261 | 484 | 101 | 1.31 | 1.05, 1.63 | 0.02 |
| Low HDL cholesterolc | 287 | 536 | 141 | 0.98 | 0.83, 1.16 | 0.85 |
| Hypertensionc | 101 | 179 | 67 | 1.36 | 0.81, 2.29 | 0.24 |
| Hypertriglyceridemiac | 282 | 515 | 135 | 1.14 | 0.96, 1.35 | 0.12 |
| Metabolic syndromec , d | 243 | 441 | 125 | 1.40 | 1.11, 1.77 | 0.005 |
Abbreviations: HDL, high-density lipoprotein; 95% CI, 95% confidence interval; PM2.5, particulate matter with diameter under 2.5 μm.
a PM2.5 (for each 1 µg/m3) was 1-year average from daily estimates including AOD data from the 1 × 1 km model and 10 × 10 km model.
b Two sided P.
c The model included both time-dependent variables and variables measured at baseline. Time-dependent variables: age at the visit (years; continuous), dark fish consumption (less than once a week, at least once a week), alcohol consumption (<2 drinks/day, ≥2 drinks/day), smoking status (current, former, never), physical activity level (<12, 12–29.9, ≥30 metabolic equivalent hours per week), and 1-year average temperature levels. Variables measured at baseline: education (≤12, 12.1–16, >16 years), whether the participant was a permanent resident of the greater Boston area (yes/no), and percentage of the participant's census tract that was nonwhite (continuous).
d Included medications varying over time: diabetes medication (no/yes), statins (no/yes), and antihypertensive medication(no/yes).
The 1-year average PM2.5 levels and temperatures included outliers, defined as having an absolute deviation of 3 standard deviations from the mean. Analyses excluding the 33 outliers confirmed results for PM2.5 and temperature on metabolic syndrome and fasting blood glucose (Web Table 5). Results from analyses excluding physical activity from the list of covariates were also consistent with previous findings (Web Table 6). Additionally, we found significant interaction between particulate matter and temperature in none of the models (Web Table 7).
The association of temperature with waist circumference increased significantly over time (Web Table 8). Our findings suggested that air pollution and temperature have homogeneous associations with the progression of each component for persons with and without defined metabolic syndrome (Web Table 8).
DISCUSSION
The present study showed associations of long-term exposure to higher ambient PM2.5 levels and outdoor temperatures with increased risk of developing several components of metabolic dysfunction. PM2.5 was associated with an increased risk of metabolic syndrome and some of its individual metabolic components: elevated fasting blood glucose and triglycerides. Temperature was associated with an increased risk of developing elevated fasting blood glucose. To our knowledge, this was the first study to prospectively examine both PM2.5 and temperature exposure in relation to the risk of metabolic syndrome and of its individual components. Our results indicated that individuals who lived in neighborhoods with worse air quality, as reflected in higher PM2.5 levels and/or higher temperatures than average, had a greater risk of developing components of metabolic syndrome. The associations of PM2.5 with metabolic syndrome and fasting blood glucose remained significant when restricting the analysis to observations below the Environmental Protection Agency's health-safety threshold for primary annual fine-particle exposure (25). This finding may support the need for more stringent measures to further reduce PM2.5 levels, as well as a need to begin considering ambient temperature—an environmental variable that has become key for climate-change predictions—as a novel risk factor for long-term metabolic risk.
Our results were consistent with recent research into the biological mechanisms through which high levels of PM2.5 and temperature exposure might affect metabolic regulation. PM2.5 exposure may predispose individuals to metabolic dysfunction through the generation of oxygen-centered free radicals, which can disrupt insulin signaling and impair vasorelaxation, thus contributing to insulin resistance and vascular disease (29, 30). PM2.5 exposure has recently been shown to contribute to the generation and increase of reactive oxygen species, which have been associated with metabolic dysfunction (31–33). Additionally, PM2.5 exposure may contribute to the risk of metabolic syndrome by activating cell-signaling pathways implicated in metabolic dysfunction. Sun et al. (34) demonstrated that long-term PM2.5 exposure leads to systemic inflammation, increased visceral adiposity, and insulin resistance in male mice on a high-fat diet. Exposure to air pollution caused several signaling abnormalities associated with insulin resistance in the mice, including decreased Akt/protein kinase B and endothelial nitric oxide synthase phosphorylation in endothelial cells and elevated protein kinase C expression. In a study by Mendez et al. (35), the same research group showed that long-term PM2.5 exposure causes upregulation of genes involved in lipogenesis, lipolysis, adipocyte differentiation, and lipid-droplet formation in mouse adipose tissue. These studies are highly supportive of our findings and suggest possible mechanisms for PM2.5-induced metabolic dysfunction.
The relationship between temperature and high blood sugar that we identified might be due to the role of adipose tissue in adaptation to temperature differences—a hypothesis consistent with prior obesity research (36). Indeed, in the presence of colder temperatures, fat stored in adipocytes is mobilized and used to produce heat to keep body temperature constant. Brown adipose tissue, in particular, burns energy and glucose to generate heat (10, 37, 38), and individuals with higher proportions of brown adipose tissue are protected from diabetes and obesity (39). People exposed to comparatively higher temperatures burn fewer calories to maintain body temperature, have less brown adipose tissue, and therefore, as shown in our analysis, may be more prone to developing insulin resistance.
Interestingly, neither PM2.5 nor temperature was significantly associated with abdominal obesity, low HDL cholesterol, or hypertension, all of which are key components of metabolic syndrome that are often presented as underlying and/or preceding the other components (40) and cardiovascular morbidity (41, 42). This finding may indicate that PM2.5 and temperature activate metabolic mechanisms such as inflammation, which might increase the risk of developing elevated fasting blood glucose and hypertriglyceridemia without substantially increasing the risk of abdominal obesity, low HDL cholesterol, or hypertension. On the other hand, the lack of association with abdominal obesity indicated that our findings were not confounded by possible correlations with obesity-associated lifestyles, such as those resulting in a high-calorie diet or low physical activity, which would be expected to also significantly increase obesity risk.
In the present study, the estimated magnitude of the association between PM2.5 and metabolic syndrome was relatively larger than that found in other studies of cardiovascular events or death (9). Older individuals are especially susceptible to pollution-triggered morbidity and mortality (43). The risk of developing metabolic syndrome increases with age; metabolic syndrome affects approximately 24% of the US adult population, and the prevalence is dramatically higher in older adults (≥50 years of age), at 44% (3, 23). Therefore, NAS participants represent a particularly vulnerable population, composed of older male participants (mean age 70 years). Previous studies suggested that metabolic syndrome is a precursor for coronary heart disease (44), and it contributes to the risk of cardiovascular mortality (45). Increased risk for developing metabolic syndrome in response to air pollution might serve as one of the primary intermediate outcomes contributing to air pollution–related cardiovascular disease events.
We used validated spatiotemporally resolved models to estimate PM2.5 levels and temperature at each participant's home address at a resolution of 1 km × 1 km. All of the NAS participants lived in the same metropolitan area, which was relatively cool and had a low penetrance of air conditioning (46). However, 1-year averages of both PM2.5 and temperature levels showed wide variations across participants, mostly due to differences in residential address characteristics such as proximity to roadways or water and amounts of green space and concrete. In our study, we were also able to control for variables related to personal and residence-based characteristics, which should have limited residual confounding by those factors. Finally, our results included a substantial number of observations; each of our 587 participants completed 2.2 study visits, on average.
Some limitations could have affected our study. The study sample was over 88% white and all male, which limits the generalizability of our results to other racial/ethnic groups and to women. Other possible sources of bias should be considered in the interpretation of our results, including the use of exposure estimates based on residential address or 1-km × 1-km resolution, which may misclassify personal exposure levels. Measurement error for air pollution or temperature is a common limitation of epidemiologic studies, which usually leverage PM2.5 and temperature measurements of ambient particulate concentrations and temperature, respectively, from stationary monitors to obtain exposure estimates for study participants. The difference between the ambient estimate and a participant's average personal exposure is a potentially important source of bias, for measures of both air pollution and temperature. This bias would be unlikely to alter our conclusions on statistically significant findings, because misclassification from using the ambient measurements is most likely to attenuate statistical associations (47). It is possible, however, that measurement error is responsible for the lack of association observed with some metabolic components. Further, because NAS participants are largely retired, estimates of levels at the participants’ addresses or at a 1-km × 1-km resolution are expected to correlate well with personal exposures due to the lack of commuting to work (48).
In conclusion, our results add evidence that long-term exposure to higher PM2.5 levels and warmer temperatures increases the risk of metabolic dysfunction. People living in neighborhoods with worse air quality—in terms of higher PM2.5 levels and/or higher temperatures than average—showed an increased risk of developing metabolic dysfunctions. These metabolic associations may represent intermediate factors that could help to explain the link of increased exposure to PM2.5 and higher temperature with cardiovascular morbidity and long-term mortality risks. Our findings may indicate the need for more stringent control of ambient PM2.5 levels and for revising estimates of the health impact of climate changes to account for the long-term risk of metabolic dysfunction and its sequelae.
Supplementary Material
ACKNOWLEDGMENTS
Authors affiliations: Department of Environmental Health, Harvard Medical School, Harvard University, Boston, Massachusetts (Rachel S. Wallwork); Department of Environmental Health, Harvard T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts (Elena Colicino, Jia Zhong, Brent A. Coull, Joel D. Schwartz, Andrea A. Baccarelli); Department of Geography and Environmental Development, Faculty of Humanities and Social Sciences, Ben-Gurion University of the Negev, Beer Sheva, Israel (Itai Kloog); Department of Epidemiology, VA Boston Healthcare System, Boston, Massachusetts (Pantel Vokonas); and Department of Epidemiology, School of Public Health and Department of Medicine, School of Medicine, Boston University, Boston, Massachusetts (Pantel Vokonas). E.C. is currently at the Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, New York.
R.S.W. and E.C. contributed equally to this work.
This work was supported by the National Institute of Environmental Health Sciences (grants R01ES021733 and R01ES015172). The Normative Aging Study (NAS) is supported by the Cooperative Studies Program, Massachusetts Veterans Epidemiology Research and Information Center. Additional support for the NAS was provided by the US Department of Agriculture, Agricultural Research Service (contract 53-K06-510).
The views expressed in this paper are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs.
Conflict of interest: none declared.
REFERENCES
- 1.Byrne CD, Wild SH, eds. The Metabolic Syndrome. 2nd ed Chichester, West Sussex, UK: Wiley-Blackwell, Inc.; 2011. [Google Scholar]
- 2.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. [DOI] [PubMed] [Google Scholar]
- 3.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report. 2009;(13):1–7. [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care. 2004;27(10):2444–2449. [DOI] [PubMed] [Google Scholar]
- 5.Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes Care. 2011;34(1):216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misra R, Patel T, Kotha P, et al. . Prevalence of diabetes, metabolic syndrome, and cardiovascular risk factors in US Asian Indians: results from a national study. J Diabetes Complications. 2010;24(3):145–153. [DOI] [PubMed] [Google Scholar]
- 7.Rochlani Y, Pothineni NV, Mehta JL. Metabolic syndrome: does it differ between women and men. Cardiovasc Drugs Ther. 2015;29(4):329–338. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28(4):629–636. [DOI] [PubMed] [Google Scholar]
- 9.Pope CA 3rd, Turner MC, Burnett R, et al. . Relationships between fine particulate air pollution, cardiometabolic disorders and cardiovascular mortality. Circ Res. 2015;116(1):108–115. [DOI] [PubMed] [Google Scholar]
- 10.Ouellet V, Routhier-Labadie A, Bellemare W, et al. . Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96(1):192–199. [DOI] [PubMed] [Google Scholar]
- 11.Brook RD, Kousha T. Air pollution and emergency department visits for hypertension in Edmonton and Calgary, Canada: a case-crossover study. Am J Hypertens. 2015;28(9):1121–1126. [DOI] [PubMed] [Google Scholar]
- 12.Eze IC, Schaffner E, Fischer E, et al. . Long-term air pollution exposure and diabetes in a population-based Swiss cohort. Environ Int. 2014;70:95–105. [DOI] [PubMed] [Google Scholar]
- 13.Christensen JS, Raaschou-Nielsen O, Tjonneland A, et al. . Road traffic and railway noise exposures and adiposity in adults: a cross-sectional analysis of the Danish Diet, Cancer, and Health cohort. Environ Health Perspect. 2016;124(3):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valdés S, Maldonado-Araque C, García-Torres F, et al. . Ambient temperature and prevalence of obesity in the Spanish population: the Di@bet.es study. Obesity (Silver Spring). 2014;22(11):2328–2332. [DOI] [PubMed] [Google Scholar]
- 15.Halonen JI, Zanobetti A, Sparrow D, et al. . Outdoor temperature is associated with serum HDL and LDL. Environ Res. 2011;111(2):281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keatinge WR, Coleshaw SR, Easton JC, et al. . Increased platelet and red cell counts, blood viscosity, and plasma cholesterol levels during heat stress, and mortality from coronary and cerebral thrombosis. Am J Med. 1986;81(5):795–800. [DOI] [PubMed] [Google Scholar]
- 17.Bell B, Rose CL, Damon A. The Veterans Administration longitudinal study of healthy aging. Gerontologist. 1966;6(4):179–184. [DOI] [PubMed] [Google Scholar]
- 18.Kloog IC, Chudnovsky AA, Just AC, et al. . A new hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmos Environ. 2014;95:518–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloog I, Nordio F, Coull BA, et al. . Incorporating local land use regression and satellite aerosol optical depth in a hybrid model of spatiotemporal PM2.5 exposures in the Mid-Atlantic states. Environ Sci Technol. 2012;46(21):11913–11921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kloog I, Chudnovsky AA, Koutrakis P, et al. . Temporal and spatial assessments of minimum air temperature using satellite surface temperature measurements in Massachusetts, USA. Sci Total Environ. 2012;432:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kloog I, Nordio F, Coull BA et al. . Predicting spatiotemporal mean air temperature using MODIS satellite surface temperature measurements across the Northeastern USA. Remote Sens Environ. 2014;150:132–139. [Google Scholar]
- 22.Troisi RJ, Heinold JW, Vokonas PS, et al. . Cigarette smoking, dietary intake, and physical activity: effects on body fat distribution—the Normative Aging Study. Am J Clin Nutr. 1991;53(5):1104–1111. [DOI] [PubMed] [Google Scholar]
- 23.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 24.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–157. [DOI] [PubMed] [Google Scholar]
- 25.US Environmental Protection Agency National ambient air quality standards for particulate matter. Final rule. Fed Reg. 2013;78(10):3086–3287. [Google Scholar]
- 26.R Development Core Team R: A Language and Environment for Statistical Computing. Version 3.2.3. https://cran.r-project.org/doc/manuals/r-release/fullrefman.pdf. Published December 10, 2015. Accessed March 8, 2016.
- 27. Therneau T. A Package for Survival Analysis in S. Version 2.38. http://CRAN.R-project.org/package=survival. Published June 12, 2015. Accessed August 24, 2015.
- 28. Therneau TM, Grambsch PM, eds. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer Science+Business Media; 2000. [Google Scholar]
- 29.Perticone F, Ceravolo R, Candigliota M, et al. . Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50(1):159–165. [DOI] [PubMed] [Google Scholar]
- 30.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. [DOI] [PubMed] [Google Scholar]
- 31.Araujo JA, Nel AE. Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Part Fibre Toxicol. 2009;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutcheson R, Rocic P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: the great exploration. Exp Diabetes Res. 2012;2012:271028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marques de Mattos A, Marino LV, Ovidio PP, et al. . Protein oxidative stress and dyslipidemia in dialysis patients. Ther Apher Dial. 2012;16(1):68–74. [DOI] [PubMed] [Google Scholar]
- 34.Sun Q, Yue P, Deiuliis JA, et al. . Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119(4):538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendez R, Zheng Z, Fan Z, et al. . Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am J Transl Res. 2013;5(2):224–234. [PMC free article] [PubMed] [Google Scholar]
- 36.Richards JB, Valdes AM, Gardner JP, et al. . Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. Am J Clin Nutr. 2007:86(5):1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. . High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cypess AM, Lehman S, Williams G, et al. . Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. . Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–1508. [DOI] [PubMed] [Google Scholar]
- 40.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. [DOI] [PubMed] [Google Scholar]
- 41.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81(4A):7B–12B. [DOI] [PubMed] [Google Scholar]
- 42.Conti CR. Diabetes, hypertension, and cardiovascular disease [editorial]. Clin Cardiol. 2001;24(1):1. [PubMed] [Google Scholar]
- 43.Simoni M, Baldacci S, Maio S, et al. . Adverse effects of outdoor pollution in the elderly. J Thorac Dis. 2015;7(1):34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexander CM, Landsman PB, Teutsch SM, et al. . NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52(5):1210–1214. [DOI] [PubMed] [Google Scholar]
- 45.Katzmarzyk PT, Church TS, Janssen I, et al. . Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care. 2005;28(2):391–397. [DOI] [PubMed] [Google Scholar]
- 46.Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2007;17(3):279–287. [DOI] [PubMed] [Google Scholar]
- 47.Hart JE, Spiegelman D, Beelen R, et al. . Long-term ambient residential traffic–related exposures and measurement error-adjusted risk of incident lung cancer in the Netherlands Cohort Study on Diet and Cancer. Environ Health Perspect. 2015;123(9):860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Power MC, Weisskopf MG, Alexeeff SE, et al. . Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect. 2011;119(5):682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.