Abstract
Extracellular ATP is a known receptor agonist in animals and was previously shown to alter plant growth, and so we investigated whether ATP derivatives could function outside plant cells as signaling agents. Signaling responses induced by exogenous nucleotides in animal cells typically include increases in free cytoplasmic calcium concentration ([Ca2+]cyt). We have evaluated the ability of exogenously applied adenosine 5′-[γ-thio]triphosphate (ATPγS), adenosine 5′-[β-thio]diphosphate (ADPβS), and adenosine 5′-O-thiomonophosphate to alter [Ca2+]cyt in intact apoaequorin transgenic Arabidopsis thaliana seedlings. ATPγS and ADPβS increase [Ca2+]cyt, and this increase is enhanced further when the nucleotides are added with the elicitor oligogalacturonic acid. Exogenous treatment with ATP also increases the level of transcripts encoding mitogen-activated protein kinases and proteins involved in ethylene biosynthesis and signal transduction. The increase in [Ca2+]cyt induced by nucleotide derivatives can be ablated by Ca2+-channel blocking agents and by the calcium chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), and the changes in gene expression can be partially blocked by these agents. These observations suggest that extracellular ATP can activate calcium-mediated cell-signaling pathways in plants, potentially playing a physiological role in transducing stress and wound responses.
INTRODUCTION
Extracellular ATP (xATP) and ADP and other nucleoside triphosphates and diphosphates function as both autocrine and paracrine signaling molecules in the animal extracellular matrix (ECM) where they affect a broad range of physiological processes by activating specialized P2 receptors and subsequent downstream signal transduction cascades. Unique physiological responses to nucleotide-based extracellular signaling agents in animals include neurotransmission, the regulation of blood pressure, and the immune response (Ralevic and Burnstock, 1998). Fundamental cellular processes mediating these physiological effects include those common to both animals and plants. Among these may include the regulation of cell growth, the induction of programmed cell death, secretory processes, and the modulation of ion channel activity (Ralevic and Burnstock, 1998).
ATP is typically abundant inside living cells. This ubiquitous intracellular metabolite may exit the cell and enter the ECM by both passive means, resulting from the loss of plasma membrane integrity and by various active transport mechanisms. These include the exocytosis of ATP packaged in synaptic vesicles, as well as ATP release occurring via unknown anion channels (Sauer et al., 2000; Bowler et al., 2001), and in association with multidrug resistance transporter activity (Boyum and Guidotti, 1997). ATP release sites have been observed to colocalize with ecto-ATPase enzymatic activity (Joseph et al., 2003). Among these enzymes are ectoapyrases, also known as ecto-nucleoside triphosphate diphosphohydrolase, catalyzing the hydrolysis of extracellular nucleoside triphosphates (NTPs) and nucleoside diphosphates to nucleoside monophosphates and Pi, thus playing a key regulatory role in modulating P2 receptor activity (Clifford et al., 1997; Ostrom et al., 2000; Zimmermann, 2001).
The physiological effects of xATP on plant growth and development are less well documented. Early studies demonstrated that exogenous application of ATP could modulate stomatal aperture in Commelina communis and the stimulation of pollen tube generative nuclear divisions in Lilium longiflorum (Kamizyo and Tanaka, 1982; Nejidat et al., 1983). Recent results show that micromolar levels of exogenous ADP increase the growth rate of Arabidopsis thaliana root hairs (Lew and Dearnaley, 2000), and millimolar concentrations of xATP suppress the gravitropic growth of Arabidopsis roots (Tang et al., 2003) as well as inhibit Arabidopsis pollen germination (Steinebrunner et al., 2003).
Previous research has also shown that Arabidopsis leaf tissue can release measurable ATP, with increasing concentrations released from transgenics overexpressing a plant multidrug resistance gene homolog, MDR1 (Thomas et al., 2000). Additionally, plants express ectoapyrases that can control xATP concentrations (Thomas et al., 1999, 2000), and the activity of these enzymes can alter plant metabolic functions. For example, Arabidopsis plants overexpressing a pea (Pisum sativum) apyrase have increased toxin resistance (Thomas et al., 2000) and export radiolabeled herbicide more effectively than the wild-type controls (Windsor et al., 2003). Apyrase knockout studies in Arabidopsis indicate that apyrase is essential for pollen germination, and low concentrations of apyrase inhibitors or high concentrations of ATP inhibit pollen tube emergence (Steinebrunner et al., 2003). The mechanisms underlying these effects are unknown, but an intriguing possibility is that plant apyrases may perform a regulatory function similar to that of their animal counterparts, and correspondingly, that ATP functions as a signal in the plant cell wall.
There is increasing evidence that plants have cellular mechanisms capable of sensing and responding to extracellular nucleotides. Arabidopsis root hairs have been reported to undergo plasma membrane depolarization upon perfusion with various nucleoside triphosphates and diphosphates (Lew and Dearnaley, 2000). For NTPs to function as a signal outside the cell, there is a requirement for a signal source. The [ATP] in the cytoplasm of plant cells is estimated at 1.3 mM (Blatt, 1987), so an obvious potential source of NTP/NDP release into the extracellular space would be wound-induced cell damage. Furthermore, wound responses, and indeed many stimulus-response pathways in plants, are mediated by the intracellular messenger calcium (Knight et al., 1993; reviewed in Trewavas and Malho, 1997; Sanders et al., 1999), and all known classes of P2 receptor in animal systems induce an increase in the free calcium ion concentration in the cytoplasm ([Ca2+]cyt) as a critical downstream step in the signaling pathway (Ralevic and Burnstock, 1998).
In light of the physiological and cellular effects of xATP and the conservation in plants of a key regulatory enzyme, apyrase, we have investigated the possibility of a signaling function for extracellular nucleoside triphosphates and diphosphates in the plant apoplasm. Here, we report that applying ATP and ADP analogs to intact Arabidopsis seedlings induces significant increases in [Ca2+]cyt and that this effect is enhanced when these analogs are added together with oligogalacturonic acid (OGA), a cell wall derivative that is released during wounding and induces Ca2+ transients in tobacco (Nicotiana tabacum) suspension culture (Chandra and Low, 1997; Cessna and Low, 2001a). We also show that externally applied nucleotide derivatives increase the mRNA abundance of genes responsive to touch and osmotic stress in both Arabidopsis suspension culture cells and whole seedlings. Taken together with observations that stress and wounding are stimuli that can induce the release of ATP, these data suggest that plants, like animals, may have cell-signaling pathways that couple the stimulus of extracellular nucleoside triphosphates and diphosphates to adaptive physiological responses.
While this article was in preparation, Demidchik et al. (2003) reported that ATP derivatives can induce changes in [Ca2+]cyt in cells of excised Arabidopsis roots. These results are in accord with our findings and underscore the possibility that nucleotide derivatives are acting as local signaling agents in the plant apoplastic space. Our results provide additional evidence that Ca2+ signaling is an important secondary messenger mediating responses to xATP, document effects downstream of the Δ[Ca2+]cyt, and reveal a physiological significance for extracellular nucleotide-mediated cell signaling in plants.
RESULTS
ATPγS and ADPβS Increase [Ca2+]cyt in Arabidopsis Seedlings
To evaluate the potential of ATP derivatives to induce a change in the [Ca2+]cyt in plants, we selected the well-established and highly sensitive aequorin Ca2+ reporter system (Knight and Knight, 1995). The aequorin apoprotein and the coelenterazine luminophore cofactor form a complex that emits photonic energy upon Ca2+ binding, thereby providing a quantitative measure of increased [Ca2+]cyt, as reported by relative luminescent units (RLUs). We also developed a unique 96-well plate format allowing for increased seedling numbers and statistical significance. Using this approach, we observed a consistent increase in [Ca2+]cyt in response to exogenous treatment with various nucleoside triphosphates and diphosphates.
The rate of phosphate liberation because of ATP/ADP hydrolysis by apyrase or phosphatase activity is unknown, so the stable analogs adenosine 5′-[γ-thio]triphosphate (ATPγS) and adenosine 5′-[β-thio]diphosphate (ADPβS) were used in the majority of the experiments described here. Known P2 agonists in animal systems, ATPγS and ADPβS induce a dose-dependent increase in aequorin luminescence relative to the buffer only control (Mes), whereas treatment with even high concentrations of adenosine 5′-O-thiomonophosphate (AMPS) has minimal effects (Figure 1A). The increase in percentage of luminescence relative to the Mes control (normalized with the discharge control; see Methods) has a linear relationship with increasing concentrations of ATPγS and ADPβS from 250 μM to 1 mM. ATPγS and ADPβS are very similar in their ability to increase the luminescence signal, with 500 μM of either P2 agonist causing an increased luminescence that averaged ∼400% of the Mes control (Figure 1A). Similar results have been achieved with a wide variety of hydrolysable ATP/ADP salts regardless of the molecular identity of the counter ion, whereas AMP and adenosine have no significant effect (data not shown). Free phosphate occasionally causes a slight (on average ≤200%) increase in aequorin luminescence (data not shown). Because calcium signaling can be decoded by both the magnitude and the duration of the response, we performed estimates of the maximum [Ca2+]cyt peak heights in response to given stimuli. The formula converting aequorin luminescence to [Ca2+]cyt makes use of the known relationship between the amount of light released and the maximum amount of light possible (Lmax) (see Methods). Analysis of the kinetics of the response to ATPγS revealed both an increase in the magnitude of a rapid response peak (a brief transient within the first 10 s) and a second peak of longer duration occurring between 30 s and 1 min (peak-2). The rapid response peak reaches 1.2 ± 0.25 μM, more than fourfold higher than that induced by the Mes control (285 ± 39 nM) (P < 0.001). The change induced by the Mes control is presumably a rapid touch response because of cotyledon movement during injection of the solution. Peak-2, which is never observed after Mes control injections, reaches 327 ± 63 nM (Figure 1B). Resting [Ca2+]cyt was estimated at 80 ± 15 nM. As a positive control, 0.666 M mannitol, previously reported to induce a peak height of 1.7 μM [Ca2+]cyt in the aequorin whole seedling system (Knight et al., 1997), was assayed, revealing a similar peak height of 2.1 ± 0.7 μM (Figure 1B).
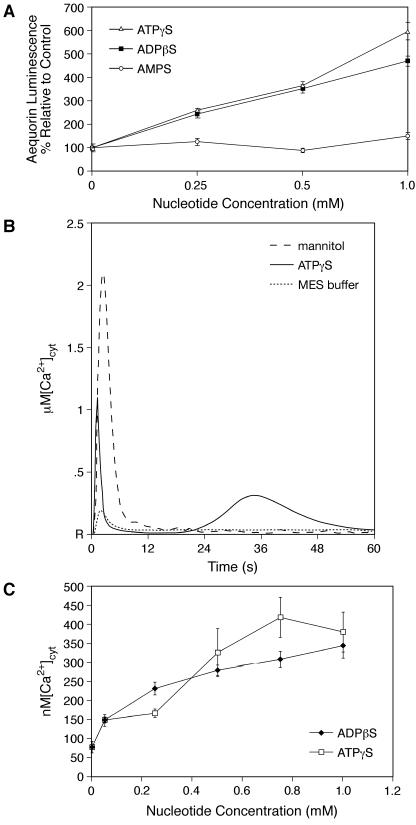
Figure 1.
Apoaequorin Transgenic Arabidopsis Respond to Nucleoside Triphosphates and Diphosphates by Increasing the [Ca2+]cyt.
(A) ATPγS and ADPβS induce a dose-dependent increase in aequorin luminescence relative to the control (P < 0.001). Total seedling numbers exceed 130 with a minimum of 8 seedlings/treatment group. Error bars indicate ±se.
(B) Average [Ca2+]cyt peak heights (6 to 10 seedlings/treatment group) plotted over a 1-min time course. Representative traces induced by 500 μM ATP, 50 mM Mes (buffer only), and 0.666 M mannitol. R indicates resting calcium levels.
(C) ATPγS and ADPβS induce a dose-dependent and saturable increase in [Ca2+]cyt peak-2 heights (P < 0.001). Seedling numbers range from 6 to 12 per treatment group.
Even though the height of the rapid response peak increased significantly in the presence of both ATPγS and ADPβS, the effect was only weakly dose dependent (data not shown). The peak-2 height (Figure 1C) for ATPγS and ADPβS effects on [Ca2+]cyt was dose dependent over a concentration range of 50 to 750 μM and saturated above 750 μM, raising the [Ca2+]cyt to ∼300 to 400 nM. AMPS was not observed to affect significantly either the rapid response peak height or the resting [Ca2+]cyt (data not shown). As observed with the total integrals (Figure 1A), ADPβS and ATPγS were similar in their ability to induce a Δ[Ca2+]cyt with a midrange of activity between 250 and 500 μM (all P < 0.001, as compared with Mes). Although at the low end of the concentration range, ADPβS appeared to be more effective and at the high end of the titration curve, ATPγS appeared to be so, the differences in [Ca2+]cyt at the same concentration of ADPβS and ATPγS were not statistically significant.
The report of nucleotide-induced increases in [Ca2+]cyt in Arabidopsis roots (Demidchik et al., 2003) made it of interest to determine how much of the luminescent signal we observed from the whole seedling was derived from the root. We performed simple shoot dissections, leaving the root embedded in the agar growth medium. Upon treatment with 500 μM ADPβS, the shoot and the root together released 11.7% ± 1% of Lmax, as compared with the roots only releasing 1.3% ± 0.1% of Lmax (data not shown), suggesting that ∼90% of the luminescent signal is derived from the shoot. Intact seedlings in which signals from the root were masked by a foil screen responded to 500 μM ATPγS with a normalized aequorin luminescence that was 78.8% ± 16.4% of the whole seedling luminescence signal (n = 6; data not shown), further supporting the conclusion that the shoot portion is contributing a significant fraction of the aequorin signal resulting from the increase in [Ca2+]cyt.
xATP/ADP Enhance OGA-Induced [Ca2+]cyt Response
Both OGA and ATP/ADP are presumably released into the plant apoplastic space as a result of wound-induced cell damage. To address the possibility that these molecules function together to signal wound responses, we compared the ability of OGA to induce Δ[Ca2+]cyt alone and together with various ATP derivatives.
Previous studies in soybean (Glycine max) suspension culture using similar OGA (degree of polymerization 6 to 18) have shown that 5 to 10 μg/mL OGA can induce an increased [Ca2+]cyt sufficient to activate downstream oxidative bursts (Navazio et al., 2002). In early experiments using our system, we observed similar responsiveness to 10 and 30 μg/mL of OGA, so we selected a concentration range of 2.5 to 10 μg/mL OGA to evaluate the potential of xATP/ADP to further increase [Ca2+]cyt.
In agreement with previous reports using apoaequorin tobacco suspension culture (Chandra and Low, 1997), OGA alone does induce a measurable dose-dependent increase in aequorin luminescence in intact Arabidopsis seedlings (Figure 2A). Both ATPγS and ADPβS at 250 μM were able to increase the luminescence signal induced by 2.5 μg/mL OGA as compared with the same concentration of AMPS (Figure 2A).
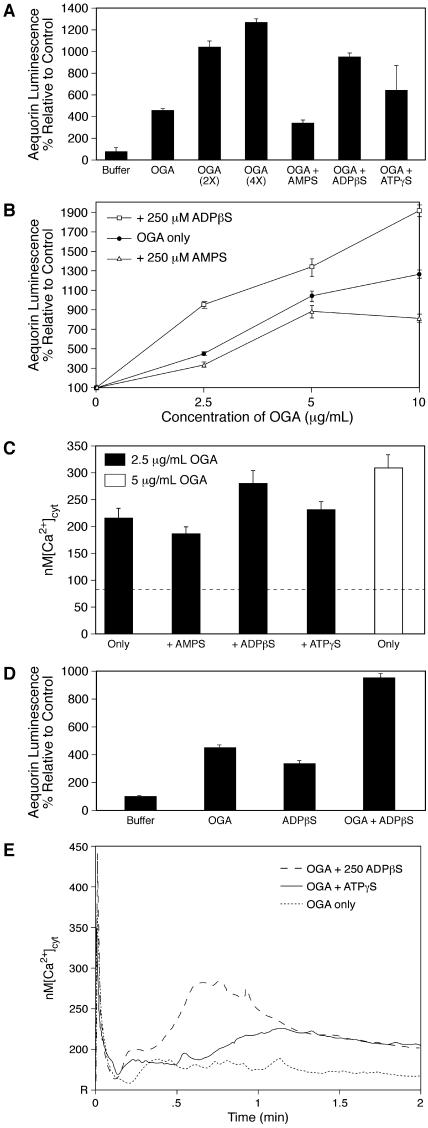
Figure 2.
xATP/ADP Enhance OGA-Induced [Ca2+]cyt Response.
(A) Aequorin luminescence as an indicator of dose responsiveness to OGA alone (2.5 μg/mL, 5 μg/mL, and 10 μg/mL are represented by [OGA] at 1×, 2×, and 4× respectively) and together with 250 μM ATPγS and ADPβS (P < 0.001) versus AMPS. Total seedling numbers exceed 180 with a minimum of 13 seedlings/treatment group. Error bars indicate ±se.
(B) Curve shift of relative aequorin luminescence to the left in the presence of 250 μM ADPβS and to the right in the presence of 250 μM AMPS (P < 0.01 at all concentrations of OGA for both ADPβS and AMPS). Total seedling numbers exceed 200 with a minimum of eight seedlings/treatment group. Error bars indicate ±se.
(C) ADPβS (250 μM) induces an increase in OGA-induced [Ca2+]cyt peak-2 heights (P < 0.05). Seedling numbers range from 6 to 12 per treatment group. The dashed line represents resting [Ca2+]cyt.
(D) ADPβS (250 μM) and 2.5 μg/mL OGA increase relative aequorin luminescence additively. Total seedling numbers exceed 110 with a minimum of 20 seedlings/treatment group. Error bars indicate ±se.
(E) Kinetic analysis of the calcium response to 2.5 μg/mL of OGA ± 250 μM ADPβS or ATPγS. Average [Ca2+]cyt peak heights (6 to 12 seedlings/treatment group) plotted over a 2-min time course. R indicates resting calcium levels.
Although both ATPγS and ADPβS displayed similar efficacy in this assay, the ADPβS enhancement of OGA-induced Δ[Ca2+]cyt was consistently the stronger of the two. We therefore selected 250 μM ADPβS to evaluate the effects over the full OGA titration range. It induced a leftward shift in the OGA response curve, halving the concentration of OGA required to cause the same increase in RLUs, such that 2.5 μg/mL OGA and 250 μM ADPβS together induced the same apparent increase in [Ca2+]cyt as 5 μg/mL OGA alone (Figure 2B). Unexpectedly, AMPS had a significant inhibitory effect on all concentrations of OGA, decreasing the luminescence signal relative to the control and thereby shifting the OGA response curve to the right. Concentrations as low as 100 μM ATPγS, ADPβS, and AMPS affected the luminescence signal generated by 2.5 μg/mL of OGA. Relative to 2.5 μg/mL of OGA, 100 μM ATPγS increased the signal by 62% (P < 0.01), 100 μM ADPβS increased the signal by 30% (P < 0.05), and 100 μM AMPS decreased the signal by 34% (P < 0.001; data not shown).
Evaluations of the magnitude of the OGA-induced Δ[Ca2+]cyt, both alone and together with either ATPγS or ADPβS, were also performed. When assessed by the measure of [Ca2+]cyt peak-2 height, the level of response induced by 2.5 μg/mL of OGA plus 250 μM ADPβS was near the level induced by 5 μg/mL of OGA only, whereas the effects of ATPγS and AMPS were not statistically significant (Figure 2C). The overall change induced by OGA plus ADPβS was less than the additive levels expected if two distinct signaling pathways were responding to both of these signaling agents. This contrasts with the additive and possibly synergistic effect observed for 2.5 μg/mL of OGA + 250 μM ADPβS as measured by the relative luminescence assay, compared with either OGA or ADPβS alone (Figure 2D). This disparity could be accounted for by an increase in the duration of the calcium flux, which would increase the change measured by the relative luminescence assay but not the measure of [Ca2+]cyt peak-2 height. Evaluations of the kinetics of the response provide evidence that this is the case because OGA in the presence of either ADPβS or ATPγS display elevated calcium levels for a longer time course than OGA alone (Figure 2E). Increasing the duration of the flux would alter the calcium signature, an important means by which calcium signals are decoded (Sanders et al., 2002).
An increase in [Ca2+]cyt upon exogenous addition of ADPβS at saturating concentrations of OGA would be indicative of distinct receptor activity. Alternatively, observations of discrete sources of the calcium ions contributing to the Ca2+ flux induced by these signaling molecules would also provide evidence of separate receptors responding to OGA and nucleotide derivatives. OGA-induced Ca2+ transients and the subsequent downstream oxidative burst have been previously shown to be mediated by Ca2+ release from intracellular stores in tobacco cells (Cessna and Low, 2001a). Is the nucleotide-induced increase in [Ca2+]cyt observed in the Arabidopsis whole seedling system similarly mediated by internal Ca2+ release?
Inhibitor and Chelator Assays Indicate That the Calcium Source Is the ECM
To address the question of the source of the calcium ions contributing to the increase in [Ca2+]cyt, various pharmacological agents were assayed as Ca2+ flux inhibitors. Pretreatment with several putative inhibitors of Ca2+ influx across the plasma membrane were effective in ablating the luminescent signal induced by 500 μM ADPβS (Figure 3). The Ca2+ channel inhibitors LaCl3 and GdCl3 were completely effective at 1 and 10 mM, respectively, and 1 mM GdCl3 reduced the apparent ADPβS-stimulated increase in [Ca2+]cyt by 50%, providing evidence of Ca2+ channel activity. Pretreatment with some of these pharmacological agents also altered the aequorin luminescent signal induced by Mes buffer (Figure 3), presumably by affecting the touch-induced calcium response. Because both Gd3+ (Ding and Pickard, 1993) and La3+ (Sato et al., 2001) inhibit mechanosensitive responses in plant cells, all of the pharmacological data are presented relative to the Mes buffer pretreated with purified water. Although these two agents have been frequently used as calcium influx inhibitors, other experimental evidence suggests that La3+ and Gd3+ may have intracellular effects (Polisensky and Braam, 1996; Cessna and Low, 2001a). As such, these agents are not sufficiently specific to exclude a release of Ca2+ from intracellular stores via endomembrane-localized Ca2+ channels.
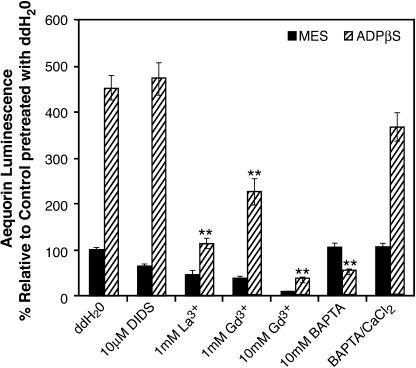
Figure 3.
Ca2+ Flux Inhibitors Affect Aequorin Luminescence Induced by 500 μM ADPβS.
Seedlings were pretreated for 30 min as indicated. Total seedling numbers exceed 80 with a minimum of six seedlings/group. Double asterisks indicate P < 0.01. Error bars indicate ±se. ddH2O, purified water.
The anion transport inhibitor 4,4′-diisothiocyanato-stilbene-2-2′-disulfonic acid (DIDS) blocks cytokinin-induced changes in membrane polarity and root hair growth in alfalfa (Medicago sativa) (Silverman et al., 1998). Cytokinin is an adenine derivative reported to activate some animal P2 receptors (Froldi et al., 1999), and DIDS is also an antagonist of some classes of animal P2 receptors (Ralevic and Burnstock, 1998), so DIDS was selected as a possible Ca2+ flux modulator. When the seedlings were pretreated with 10 μM DIDS, we observed no change in the ADPβS-induced increase in luminescence (Figure 3), and concentrations of DIDS as high as 300 μM did not significantly reduce either the ATPγS or ADPβS peak-2 height (data not shown). The phospholipase C inhibitor U73122 was assayed at 50 μM and also did not block the ADPβS-induced luminescence relative to the control (data not shown).
These results are consistent with the possibility that the source of the Ca2+ ions contributing to the ADPβS-induced increase in relative luminescence is the extracellular environment. Studies using the calcium chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), thought to remain extracellular (Polisensky and Braam, 1996), provide further evidence to support this hypothesis. As low as 1 mM BAPTA was sufficient to completely block the ADPβS-induced Ca2+ flux (Figure 3). The specificity of this effect was demonstrated by the inclusion of excess Ca2+ in the BAPTA pretreatment incubation, which restores responsiveness to ADPβS.
Demidchik et al. (2003) have reported that pyridoxal phosphate-6-azo-(benzene-2,4-disulfonic acid) (PPADS), a P2 receptor antagonist in animal systems, effectively blocks the Ca2+ transients induced by an ATP analog in excised Arabidopsis roots. We assayed the effects of PPADS on nucleotide-induced Δ[Ca2+]cyt in intact aequorin-expressing seedlings and found that PPADS pretreatment reduced the peak-2 height in response to 500 μM ATPγS to 187 ± 15 nM (n = 12) and ADPβS to 153 ± 13 nM (n = 12), a statistically significant inhibition of the calcium response (P < 0.01; data not shown). However, because this pretreatment typically also reduced the plants' responses to Mes and mannitol controls, a clear interpretation of its effects will require additional tests.
ATP Is Released from Arabidopsis Seedlings in Response to Touch and Osmotic Stresses
The results above are consistent with the postulate that exogenous ATP or ADP can function as cell–cell signaling agents in plants. However, for ATP (and ADP) to function as extracellular signals, these molecules must be released into the plant apoplasm in response to specific stimuli. Presumably, ATP and ADP along with OGA are released during wounding as a result of the loss of cellular integrity. However, in the absence of cell damage events, are there any other conditions in which ATP release might be expected?
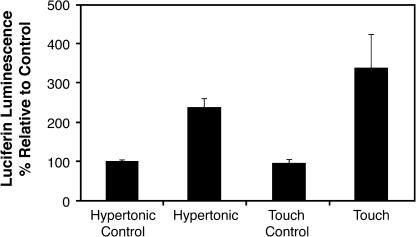
To address this question, we used luciferin-luciferase as a bioluminescent xATP reporter to assay a variety of conditions known to induce ATP release from animal cells (Ostrom et al., 2000; Sauer et al., 2000). Touch and osmotic stresses were able to induce measurable increases in ATP release as measured by an increase in luciferin luminescence relative to the control (P = 0.01 and P < 0.001, respectively) (Figure 4). The touch stimulus induced by shaking the seedlings had the highest signal strength at 350% relative luminescence. Hypertonic stress was also able to induce measurable ATP release, and this cannot be attributable to touch responses accounted for in the osmotic control. Similar results were observed using both NaCl and mannitol as the osmoticum for stress treatments (data not shown). Seedlings were assayed for their ability to recover from the various treatments, and both touch and hypertonic seedlings appeared phenotypically normal within 24 h of the experiment, suggesting that minimal cell damage is associated with the ATP release observed. Hypotonic stress also induced an increase in the luminescent signal as compared with the osmotic control stimuli (P < 0.001; data not shown). However, these seedlings displayed chlorosis after the treatment, so it was not clear whether the ATP release we measured from these seedlings was because of hypotonic stress or because of loss of cell integrity, and these results are not shown.
Figure 4.
Stress Stimuli Induce ATP Release as Measured by Luciferin-Luciferase.
Seedlings were stimulated by mechanical stimulation and hypertonic stress (300 mM NaCl). P < 0.05. Error bars indicate ±se.
xATP Treatment Alters Stress-Responsive Gene Expression Profiles
The above results indicate that various stress conditions can induce ATP release into the ECM, and they raise the question of whether ATP is a mediator of stress-related signaling. Various stresses, including those that we have shown to be capable of inducing ATP release, can activate downstream kinase cascades in tomato (Lycopersicon esculentum) cells (Felix et al., 2000). More specifically, the level of transcripts for members of the mitogen-activated protein kinase (MAPK) family in Arabidopsis has been shown to be upregulated in response to touch and osmotic stresses (Mizoguchi et al., 1996) as well as wounding (Reymond et al., 2000), so we decided to evaluate the ability of exogenously applied ATP to alter protein kinase transcript levels.
In initial studies, we found that the touch stimulation of plants that accompanied treatment with the buffer control produced a significant background increase in the level of mRNA for MAPK genes. Theoretically, it seemed likely that this background noise could be attributed to the ATP released by the mechanical stimulation associated with the buffer treatment. Even though in these initial studies the MAPK mRNA level induced by nucleotides was higher than that induced by the buffer control, we sought a plant system that was adapted (desensitized) to touch stimulation and submergence for the gene expression studies. We found that tissue culture cells and liquid-grown whole seedlings, which are constantly shaken during their growth, met this criterion, and we used these for most of the assays of nucleotide effects on gene expression changes.
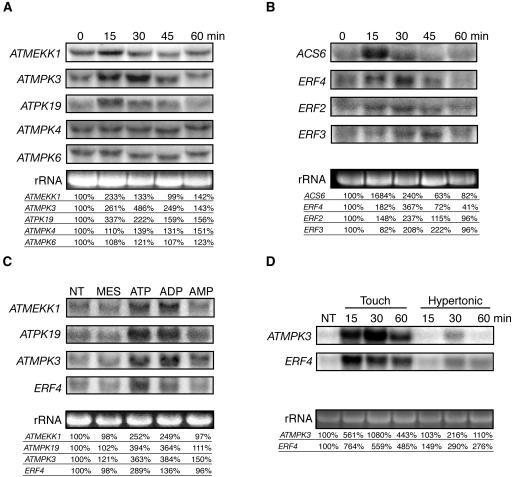
Treatment of Arabidopsis suspension culture cells with 500 μM ATP (equal to midrange for the Ca2+ response) increased the steady state level of mRNAs for mitogen-activated protein kinase kinase kinase1 (ATMEKK1), mitogen-activated protein kinase3 (ATMPK3), and ribosomal protein S6 kinase (ATPK19), but not ATMPK4 and ATMPK6, in a time-dependent manner (Figure 5A). Using liquid-grown whole Arabidopsis seedlings, 500 μM of either ATPγS or ADPβS were shown to be equally effective in increasing the mRNA transcript levels of ATMEKK1, ATMPK3, and ATPK19, in contrast with the minimal effects of AMPS (Figure 5C), and similar results were achieved using seedlings grown on agar (data not shown). Although cold and salt stresses are able to increase the mRNA levels of ATMEKK1, ATMPK3, and ATPK19, the kinetics of the response to xATP is more similar to that induced by touch and wounding (Mizoguchi et al., 1996; Reymond et al., 2000). Similarity in the characteristically rapid and transient regulation of the level of each mRNA suggests the possibility that these various stimuli induce activation of a common cell-signaling pathway.
Figure 5.
xATP Upregulates the mRNA Levels Encoding Various MAP Kinases and Proteins Involved in Ethylene Biosynthesis and Signaling.
RNA gel blots were probed with partial cDNAs of ATMEKK1, ATMPK3, ATMPK4, ATMPK6, ATPK19, ACS6, ERF2, ERF3, and ERF4 as indicated. Blots were stripped and rehybridized with different probes as indicated. Quantifications of mRNA levels were performed by densitometry using a PhosphorImager and normalized against the ribosomal 25S rRNA. Transcript level values are shown relative to the control.
(A) Time course of MAPK mRNA levels in Arabidopsis suspension culture cells treated with 500 μM ATP.
(B) Time course of changes in the level of ethylene-signaling mRNAs in Arabidopsis suspension culture cells treated with 500 μM ATP.
(C) Effect of 500 μM ATPγS, ADPβS, and AMPS treatment on genes encoding three MAPK family members and an ethylene response transcription factor in liquid-grown whole seedlings at the 30-min time point, as compared with the no treat (NT) control.
(D) Hypertonic stress and touch stimuli induce a similar increase in ATMPK3 and ERF4 mRNA levels. Seedlings were treated by a 1-min mechanical stimulation (touch) or 300 mM NaCl (hypertonic), and RNA was extracted from tissue collected at the indicated time point.
Ethylene levels also increase as a consequence of wounding or stress (Morgan and Drew, 1997). Preliminary microarray results suggested that the level of mRNA transcripts for many genes involved in ethylene biosynthesis and response were upregulated by external application of ATPγS to whole seedlings. RNA gel blot analysis of suspension culture cells showed that the steady state level of mRNA for the gene encoding 1-aminocyclopropane-1-carboxylic acid synthase 6 (ACS6), a critical enzyme catalyzing the rate-limiting step in ethylene biosynthesis, was also increased by exogenous ATP in suspension culture cells (Figure 5B). Downstream of ethylene biosynthesis and perception, ethylene responsive factors (ERFs), a multigene family of transcription factors, are regulated by ethylene and stress stimulation (Fujimoto et al., 2000), and ATP treatments were sufficient to increase the mRNA levels of various ERFs (ERF2, ERF3, and ERF4) as well (Figure 5B). The time lag between the peak increase in mRNA levels for ACS6 (at 15 min) and that for ERF mRNA (at ∼30 to 45 min) correlates with the expectation that ethylene biosynthesis would precede a requirement for ethylene responses. In comparison with the equal ability of ATPγS and ADPβS to increase MAPK mRNA levels, the expression of the ethylene-related ERF4 is more strongly induced by ATPγS treatment under the conditions tested (Figure 5C).
Although touch and osmotic stresses have been previously shown to alter MAPK family transcript levels (Mizoguchi et al., 1996; Reymond et al., 2000) and the levels of mRNA encoding proteins involved in ethylene biosynthesis and response (Arteca and Arteca, 1999; Reymond et al., 2000), a direct correlation had not previously been demonstrated in our experimental system. Consequently, we tested whether the same conditions we used to show touch- and hypertonic stress–induced ATP release could also induce comparable gene expression changes and selected ATMPK3 and ERF4 as the genes to assay for this purpose. The message abundance of these genes was upregulated by touch and osmotic stress in a pattern similar to that induced by ATP analog treatments (Figure 5D), suggesting that nucleotide-based signaling may be contributing to the observed changes in gene expression.
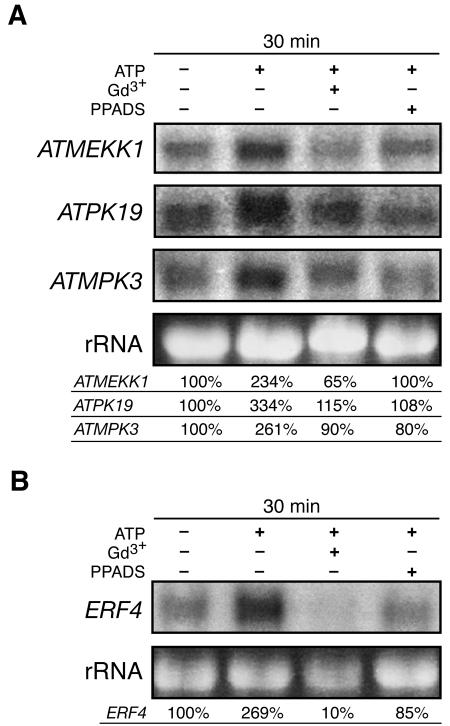
To assay the involvement of calcium signaling in mediating these responses, we measured the ability of the Ca2+ channel blocker Gd3+ and the Ca2+ chelator BAPTA to inhibit the ATPγS-induced accumulation of ATMEKK1, ATPK19, ATMPK3, and ERF4 mRNAs. Pretreatment of Arabidopsis suspension culture with 10 mM Gd3+ partially inhibited these changes in gene expression (Figure 6A), and similar results have been observed using 10 mM BAPTA in the whole seedling system (data not shown). Pretreatment with 300 μM PPADS similarly blocked these responses to ATP, suggesting the existence of a P2-like receptor in plants (Figure 6B). To confirm that touch-desensitized systems can still respond to ATP analogs by increasing [Ca2+]cyt, a final calcium flux assay was performed on liquid-grown whole seedlings. In these seedlings, 500 μM ATP induced a 16-fold increase in aequorin luminescence relative to the Mes control (n = 6).
Figure 6.
Gadolinium and PPADS Suppress ATP-Induced Changes in Gene Expression.
Blots were stripped and rehybridized with different probes as indicated. Quantitations of mRNA levels were performed by densitometry using a PhosphorImager and normalized against the ribosomal 25S rRNA. Transcript level values are shown relative to the control.
(A) Gadolinium (Gd3+; 10 mM) and 300 μM PPADS pretreatment reduce the ATP-mediated increase in the level of mRNAs encoding various MAPKs in Arabidopsis suspension culture cells treated with 500 μM ATP for 30 min.
(B) Gadolinium (Gd3+; 10 mM) and 300 μM PPADS pretreatment reduces the ATP-mediated increase of ERF4 mRNA levels in Arabidopsis suspension culture cells treated with 500 μM ATP for 15 min.
DISCUSSION
xATP Sensing in the Plant Apoplasm
We have presented significant evidence to support the hypothesis that extracellular nucleotide derivatives can function in plant cell signaling processes. Rapid induction of the secondary messenger Ca2+ suggests that extracellular ATP, ADP, and their thiol derivatives are directly perceived by sensing mechanisms capable of transmitting the signal across the plasma membrane. Although the nature of the nucleotide sensor is unknown, the apparent dose dependency of the calcium response (Figures 1A and 1C) and the saturation of the Δ[Ca2+]cyt peak height (Figure 1C) suggests receptor mediation.
Early studies showed that exogenous application of ATP could affect some plant physiological processes, but the possibility that xATP could have an intracellular effect by crossing the plasma membrane and altering the metabolic status of the cell confounded the interpretations of these results. However, simple diffusion of ATP into cells is unlikely because of the high negative charge of ATP and the unfavorable electrochemical gradient for entry into the cell. Instead, a variety of nucleotidases (including apyrase) participate in the step-wise hydrolysis of the nucleotide to the corresponding base, allowing for the subsequent uptake of the phosphates and the base by distinct transporters (Komoszynski and Wojtczak, 1996; Zimmermann, 1996). However, the addition of a thiol group to the terminal phosphate of ATP (ATPγS) and ADP (ADPβS) results in the resistance of these stable analogs to nucleotidase degradation (Zimmermann, 1996). Thus, it is extremely unlikely that the results we are reporting are because of intracellular effects.
A wide variety of stimuli induce a biologically significant increase in [Ca2+]cyt with the appropriate response being decoded by the localization (both cellular and subcellular), magnitude, duration, and oscillation frequency of the Ca2+ flux (Trewavas and Malho, 1997). The [Ca2+]cyt reported to be physiologically relevant in many plant stimulus response-coupling mechanisms ranges from a few hundred nanomolar up to 10 μM. We report that ATPγS can increase the magnitude of the rapid response peak up to micromolar levels and can induce a second peak reaching a maximum of >300 nM [Ca2+]cyt, an increase of fourfold to fivefold over the resting levels measured in our system (Figures 1B and 1C).
Although the Δ[Ca2+]cyt profiles induced by ATPγS and ADPβS are similar (Figures 1A and 1C), it remains to be determined whether these results represent a single sensor for both ATP and ADP or (assuming receptor mediation) multiple receptors with distinct substrate specificities. Also, the heterogeneous nature of the cell type responding limits the usefulness of a pharmacological ranking of Δ[Ca2+]cyt in whole seedlings or root tissue to exogenously applied nucleotide derivatives. As little as a threefold increase in [Ca2+]cyt is sufficient to fully activate calmodulin (Trewavas and Malho, 1997). Presumably, the xATP/ADP-induced transient increase in [Ca2+]cyt we report is at least sufficient to activate a sensitive signal transducer like calmodulin. Alternatively, it is also possible that only some cells are responding to xATP/ADP and other cells are not responding at all, which would mean that the global signal we measure greatly underestimates the increases in [Ca2+]cyt happening in the responding cells. Regardless, ATP derivatives induce an increase in [Ca2+]cyt that is in the physiologically relevant range and thus has the potential to induce downstream responses in Arabidopsis.
Our data are consistent with the data and conclusions of Demidchik et al. (2003). The nucleotide concentrations needed to induce a significant aequorin signal in the intact seedlings we used are higher than the levels required to induce similar or higher aequorin signals in the excised roots used by Demidchik et al. This difference may be attributable, in part, to the greater accessibility of cuticleless epidermal cells of excised roots to the treatment solution or to methodological differences, for example, the inclusion of 10 mM CaCl2 in the root system recording solution, a notable difference considering the extracellular source of the calcium ions contributing to the calcium flux. Alternatively, the response differences may reflect a greater sensitivity of root cells to exogenous ATP, just as they are much more sensitive to exogenous auxin applications.
Calcium Channel Involvement: The Search for a Plant xATP Receptor
Identification of a plant receptor responsive to nucleotide derivatives would further validate the xATP-signaling hypothesis and allow for functional studies to examine the biological roles of this signal transduction pathway. Animal P2 receptors include both ligand-gated ion channels (the P2X family) and seven-pass transmembrane heterotrimeric G-protein–coupled receptors (the P2Y family) (Ralevic and Burnstock, 1998).
If the nucleotide-induced changes in [Ca2+]cyt we observe are mediated by receptors similar to known mammalian P2-receptors, the data argue more for a ligand-gated ion channel than for a classical heterotrimeric G-protein–coupled receptor. We observed no apparent effect on the ADPβS-induced increase in [Ca2+]cyt from pretreatment with the phospholipase C inhibitor U73122 (data not shown), whereas the Ca2+ chelator BAPTA at low concentrations, presumed to remain extracellular, was able to ablate the response (Figure 3). Additionally, the ability of the Ca2+ channel inhibitors Gd3+ and La3+ to block the response (Figure 3) suggests the involvement of a Ca2+ channel, although because these agents have been observed to have both extracellular and intracellular effects (Polisensky and Braam, 1996; Cessna and Low, 2001a), the channel could be localized to either the plasma membrane or an endomembrane. A simple model to explain our results would be a nucleotide-induced opening of voltage-gated Ca2+ channels in response to membrane depolarization. Alternatively, the data can also be explained by membrane depolarization concurrent with Ca2+ influx into the cytoplasm as a result of ligand-gated ion channel activity. However, because the opening of Ca2+ channels in tomato has been reported to be mediated by a nonclassical heterotrimeric G-protein (Aharon et al., 1998; Hooley, 1999), we cannot eliminate the possibility of indirect coupling through heterotrimeric G-proteins.
The ability to specifically block stimulus response coupling with a P2 antagonist would be informative in determining whether xATP is a necessary component in wound and stress response signaling. As such, our finding that PPADS pretreatment of suspension culture cells can reduce the ATP-induced increases in stress responsive mRNA levels (Figure 6B) with similar efficacy to Gd3+ pretreatment suggests receptor mediation of these responses. Additional PPADS studies are required to further evaluate the role of nucleotide-based signaling in mediating plant responses to stress stimuli.
Extracellular nucleotides are capable of lowering the extracellular [Ca2+] by binding to positively charged ions, including Ca2+ and Mg2+, and the Ca2+ chelation studies of Cessna and Low (2001b) indicate that plants have a sensor for extracellular [Ca2+]. However, the possibility that ATP- and ADP-induced Ca2+ flux is simply because of an ion chelation effect is unlikely. Because ATP has a higher degree of negative charge than ADP and can chelate calcium better, the expectation would be that ATP would have a stronger effect on the extracellular Ca2+ sensor than ADP. This is apparently not the case because ATPγS and ADPβS at 250 to 500 μM are extremely similar in their ability to induce an increase in [Ca2+]cyt (Figure 1A). Moreover, previous studies have shown that the active form of OGA is associated with Ca2+ as a cofactor (Cessna and Low, 2001a), so OGA would be expected to be less effective in inducing an increase in the [Ca2+]cyt in the presence of any agent that significantly chelates external Ca2+. On the contrary, we observe that ADPβS enhances OGA activity (Figure 2), suggesting that a mechanism distinct from chelation is responsible for the ADPβS effects observed.
ATP Release by Wounding and Stress
The cytoplasmic concentration of ATP in plant cells is near millimolar or higher (Blatt, 1987), so one could reasonably expect that when the cell membrane is broken by a wound event, the extracellular [ATP] would rise to near this level, at least transiently. In addition, there is increasing evidence that stresses such as mechanical stimulations (touch, shear stress, and stretch), osmotic shock, and hypoxia can induce ATP release from various animal cells (Ostrom et al., 2000; Gerasimovskaya et al., 2002), possibly by a nonlytic active transport mechanism (Dubyak and El-Moatassim, 1993; Bowler et al., 2001). The released ATP can induce propagation of Ca2+ waves that are mediated by P2 receptor activation (Sauer et al., 2000). We have shown that plant responses to touch and osmotic stresses, previously shown to be associated with elevated [Ca2+]cyt (Knight et al., 1991, 1997), are also able to induce measurable ATP release (Figure 4).
Only a relative quantification of the released ATP can be measured by the method used for Figure 4 because the released ATP is highly diluted into the surrounding media. However, depending on the ATP release mechanism (Joseph et al., 2003), the [xATP] could transiently approach a concentration near cytoplasmic levels (millimolar) in ECM microenvironments adjacent to the release site. Furthermore, even concentrations in the low micromolar range can induce a measurable Δ[Ca2+]cyt in Arabidopsis root cells (Demidchik et al., 2003).
The fact that various stress stimuli induce the release of ATP into the ECM may be related to the fact that many of these same stimuli, including touch, wounding, and the osmotic changes brought on by drought and salt stresses, also induce an increase in [Ca2+]cyt (Sanders et al., 1999). This increase in [Ca2+]cyt may be a direct result of the perception of the stress stimulus or a result of the production of signaling agents, such as released ATP. In this regard, we note that the root has been reported to be the primary sensor of drought and salt stresses (Davies and Zhang, 1991; Kiegle et al., 2000). It would be interesting to evaluate whether these stresses induce the release of ATP from roots and, if so, the degree to which xATP (or extracellular ADP) is contributing to the Δ[Ca2+]cyt and downstream adaptive responses.
xATP, Wounding, Touch, and Osmotic Stresses Induce Common Downstream Responses: Altered mRNA Levels Encoding MAPK and Ethylene Signaling Proteins
Ethylene is a major plant hormone known to be a critical mediator of stress responses (reviewed in Wang et al., 2002), and it is of special interest that we report that treatment with ATP (both hydrolyzable and stable analogs) can upregulate the mRNA levels of ACS6 as well as the mRNA levels of various ERFs (ERF2, ERF3, and ERF4; Figure 5B). Furthermore, 1-aminocyclopropane-1-carboxylic acid synthase is a key rate-limiting enzyme catalyzing ethylene biosynthesis, and ACS6 has been shown to be upregulated by touch stimuli (Figure 5D; Arteca and Arteca, 1999). The expression pattern of various ERFs in Arabidopsis has been shown to be differentially upregulated by ethylene as well as stress conditions (Figure 5D; Fujimoto et al., 2000).
Protein phosphorylation cascades are also important members of the signal transduction machinery activated during stress responses. Correspondingly, in response to exogenous treatment with ATP, we have repeatedly observed the upregulation of the level of transcripts for the protein kinases ATMEKK1, ATPK19, and ATMPK3 (Figure 5A) that is strikingly similar in kinetics to those previously reported in response to touch and osmotic stresses (Figure 5D; Mizoguchi et al., 1996) as well as to wounding (Reymond et al., 2000). By comparison, the mRNA level of ATMPK4 and ATMPK6, which is not upregulated by wounding, is not upregulated by ATP treatment, a result that underscores the specificity of ATP-induced MAPK expression. MAPK family members have recently been implicated in the regulation of both ethylene biosynthesis (Kim et al., 2003) and transmittance of the ethylene signal after perception at constitutive triple response, the ethylene receptor (Ouaked et al., 2003). OGA, generated by cell wall fragmentation as a result of wounding or herbivory, is a known elicitor of Ca2+ flux and downstream defense responses, including the oxidative burst (Chandra and Low, 1997). It is reasonable to assume that both OGA and ATP (along with other intracellular components, including ADP) would be present together in the plant apoplasm near a wound site. Because ATPγS and ADPβS enhance the ability of OGA to induce an increase in aequorin luminescence (Figure 2), it is reasonable to postulate that OGA and ATP (or ADP) in the plant apoplasm could function together as coelicitors to activate defense response signaling pathways. ADPβS was observed to be more effective than ATPγS when applied in concert with OGA, possibly suggesting distinct receptors for ADP and ATP in vivo. Also, the inhibitory effect of AMPS on OGA responses (Figures 2A and 2B) is intriguing, and it is possible that AMP, as a breakdown product of ATP, can feed back and inhibit the signaling pathway. Further evaluations of downstream responses, such as effects on the oxidative burst, are required to address a role for ATP derivatives as elicitors.
Ethylene has been proposed to function as a local signaling agent downstream of OGA, interacting with the complex signaling networks that mediate wound responses (Rojo et al., 1999). We propose that ATP and OGA signaling, although acting via distinct calcium signaling pathways, converge at ethylene signaling. Ethylene is also a major hormone transducing stress responses (Wang et al., 2002), and we have shown that ATP signaling alone could potentially be sufficient to induce ethylene production. Further studies are in progress to examine the physiological changes of seedlings grown in the presence of ATP, and preliminary data support the hypothesis that ethylene production is increased (data not shown).
Stress responses are multifactorial, and neither xATP nor any other single signaling agent is likely to mediate all physiological responses to stress stimuli. Drought, salt, and touch-induced calcium transients have been shown to be at least partially sensitive to the calcium chelator EGTA in a cell- and tissue-type dependent manner (Haley et al., 1995; Knight et al., 1997). Stress-induced increases in xATP could play an important role in mediating some of these signaling pathways, and this is proposed in the model described below.
A Model for xATP Involvement in Wound and Stress Responses
Taken together, the data suggest that plant physiological responses to touch, wounding, and various forms of stress may be mediated at least in part by xATP signaling and that [Ca2+]cyt transients and MAPK cascades may play a role in transducing responses further downstream. We propose that increased xATP is one of the primary chemical signals indicating stress conditions and that ethylene may represent a secondary stress signal, induced by the action of the xATP released by stress (and wound) stimuli. The fact that gadolinium and BAPTA reduce the ability of ATP to increase mRNA transcript levels further substantiates a role for Ca2+ in mediating downstream responses to xATP.
Lower vertebrates, insects, paramecium, and even prokaryotes have been observed to respond to exogenous application of ATP (Burnstock, 1996), so xATP signaling may predate the divergence of plant and mammalian kingdoms. xATP has been shown to have wide-ranging effects on plant physiology, including the modulation of toxin efflux (Thomas et al., 2000), the inhibition of auxin transport root gravitropism (Tang et al., 2003), and the inhibition of pollen germination (Steinebrunner et al., 2003). We have provided evidence to support the existence of a novel plant cell-signaling pathway involving extracellular nucleotide derivates. Ultimately, for any molecule to function as a cell-to-cell signal, there is a requirement for a signal source, a change in intracellular signal transduction machinery, and a measurable downstream biological response. We have shown that various stimuli known to alter [Ca2+]cyt can also induce ATP release into the ECM, suggesting the possibility that xATP can mediate downstream effects of these stimuli, including changes in gene expression.
Clearly, the ATP response system described here will be extremely useful for evaluating potential P2 receptor antagonists, signal transduction modulators, and candidate P2 receptor mutants. Functional studies using these tools will facilitate our further understanding of this plant cell-signaling pathway, with clear implications for stress and defense responses and possibly many other aspects of plant physiological processes.
METHODS
Plant Material
Plants from the transgenic Arabidopsis thaliana line pMAQ2 (a kind gift from J. Braam, Rice University, Houston, TX, with permission from patent holder Chemicon International), which express cytosolic apoaequorin, were used to measure [Ca2+]cyt. Single seeds were plated in the central wells of opaque Microlite 1+ 96-well luminometer plates (Dynex Technologies, Chantilly, VA) on 20 μL of full-strength MS medium (Life Technologies, Paisley, UK), 0.85% agar, and 1% sucrose, pH 5.8. The plates were wrapped with parafilm and incubated at 4°C in the dark for 2 to 4 d. To form a humidity chamber, ddH20 was added to all the peripheral wells, and the plates were resealed with a porous medical tape. Seedlings were cultivated at 22 to 25°C with both 16-h and 24-h photoperiods to day 7. Liquid-grown apoaequorin transgenic Arabidopsis were cultivated directly in the luminometer plates on a thin layer of agar plus 100 μL of half-strength MS media with shaking on a rotator at 135 rpm. The wild-type Columbia seedlings were used for the remainder of the experiments and were similarly grown on agar-containing MS media in 96-well plates or 35-mm Petri dishes or in half-strength liquid MS media in 24-well plates to day 7.
Reconstitution of Aequorin
Reconstitution of aequorin in vivo was performed essentially as previously described (Knight et al., 1991, 1996) with some modifications. Coelenterazine-cp (2.5 μM; Molecular Probes, Eugene, OR; Sigma, St. Louis) was added to each well in sufficient volume to submerge the seedlings in the solution, and incubation was performed overnight in the dark at 25°C. The remaining solution not taken up by the seedlings was aspirated from each well, and after a 30-min recovery period, the plates were assayed in a luminometer.
Luminescence Measurements: Data Acquisition and Processing
Luminescence measurements were performed by placing the 96-well plate in the light-tight housing of the luminometer plate reader (Dynatech, Burlington, MA). Experimental solutions were injected with an automatic injector in sufficient volume to submerge the seedling (120 μL per well, one seedling per well). Luminescence measurements were initiated coincident with the injection of the solution, and luminescence counts were integrated over 1 min. To estimate the amount of aequorin remaining, a discharge control solution (previously established; Knight et al., 1996) of 3 M CaCl2 and 30% ethanol was applied through a second injector in sufficient volume (60 μL per well) to achieve a final concentration of 1 M CaCl2 and 10% ethanol, and luminescence was integrated for an additional 30 s.
The data were then processed as a ratio of the RLUs acquired during the experimental treatment to the discharge control, thus normalizing for the mass of individual seedlings and the degree of coelenterazine reconstitution. A computer software program designed to present the experimental data as a percentage of luminescence relative to the control performed further data processing. Individual experiments involved treatment groups of varying size, with 6 to 10 seedlings per group (minus high and low outliers as required). This approach also allowed repeat experimental groups on distinct days to be compiled into larger data sets for increased statistical significance. Statistical significance was calculated using the Student's t test. For the kinetic analyses, RLUs generated by the experimental treatment were plotted over a 1-min time course, and luminescence values were converted to peak Δ[Ca2+]cyt as described below.
In separate experiments designed to allow for quantification of the peak Δ[Ca2+]cyt, the luminescence data were converted from RLU using a modified procedure and calibration calculation previously established (Brini et al., 1995; Baum et al., 1999). In brief, the calculation makes use of an experimentally proven relationship between the amount of luminescence released by aequorin (reconstituted with the coelenterazine-cp derivative) and [Ca2+]cyt and is as follows: [Ca2+]cyt (nM) = {[ X1/3 + (X1/3 × 57) − 1]/(1 − X1/3)}/0.026, where X is RLU/s divided by the maximum amount of luminescence possible (Lmax). Data were collected every one-tenth of a second for a duration of one-hundredth of a second (and multiplied by 100) to obtain the maximum RLU/s during the first 10 s (the rapid response peak), and a second maximum RLU/s during the remainder of the 2-min reading (peak-2). Discharge control-treated seedlings were used to estimate Lmax for each treatment group. For all treatments, six to seven replicates were performed per group (minus high and low outliers), and the individual [Ca2+]cyt from repeat experiments on different days was averaged together.
To dissect the shoot away from the root, the shoot was gently grasped with forceps, and a scalpel was used to cleanly cut through the stem, leaving the root embedded in agar. Following a brief rest period, the root tissue and shoot tissue were assayed separately in parallel with whole seedlings. Foil masking of the root was performed by placing a small strip of aluminum foil in the bottom of each well to cover the exposed area surrounding the shoot before analysis in parallel with whole seedlings.
Experimental Treatments
Experimental solutions were prepared from the following stocks and diluted as required: 50 mM stocks of ATPγS, ADPβS, and AMPS; 250 mM ATP stock (adenosine 5′triphosphate), 0.5 mM CaCl2 stock, and 0.666 M mannitol (all Sigma). OGA with a 6 to 20 degree of polymerization was prepared according to Hahn et al. (1992) (a kind gift from Mona Mehdy, University of Texas, Austin) and diluted from 0.39 μg/μL as required. All experimental working solutions (except mannitol) were prepared fresh on the day of the experiment and buffered to pH 5.7 with 50 mM Mes [2-(N-morpholino)-ethanesulfonic acid]. Note that 50 mM Mes is sufficient to prevent the acidic effect of ATP.
Pretreatment with Inhibitors
The following pharmacological agents were solubilized as recommended by the manufacturer and diluted to the final concentration in ddH20: lanthanum chloride (La3+), gadolinium chloride (Gd3+), BAPTA, 1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione (U73122), DIDS, and PPADS (all Sigma). Pretreatment was performed immediately after the coelenterazine reconstitution by a 30-min incubation with the appropriate inhibitor. The solutions were aspirated from the wells, and the seedlings were allowed to acclimate for 30 min before proceeding as indicated above.
ATP Release Assays
Luciferin-luciferase assay to determine ATP release was performed using an ATP bioluminescent assay kit (Sigma). The wild-type Columbia seedlings grown in 96-well plates to day 7 were stimulated by touch and osmotic stresses either in the well or removed from the well and transferred to microcentrifuge tubes as described below. When removed from the well, the seedlings were transferred to microcentrifuge tubes (10 seedlings per tube) and submerged in 500 μL of purified water. For comparison, seedlings were also evaluated directly in the well (one seedling per well) and simply submerged in 100 μL of purified water at the onset of the treatment. Touch stimulus was applied by shaking the seedlings for 2 min on a rotator at 150 rpm. Fifty microliters of the purified water surrounding the seedlings of the various treatment groups were immediately removed and frozen in liquid nitrogen for assay by luciferin-luciferase. The control groups were identically handled except for the absence of a stimulus during the treatment time course. Subsequent luciferin-luciferase treatment and luminescence measurements were performed at the luminometer using a 6-s integration time.
For hypotonic stress, seedlings were grown in 96-well plates (one seedling per well) for 1 week under continuous light. Seedlings were pretreated with 100 μL of 600 mM NaCl followed by a 30-min incubation for ion equilibration. An equal volume of water was then added into each well to achieve a final concentration of 300 mM of NaCl. One minute later, 100 μL of the NaCl solution containing released ATP was transferred into a new centrifuge tube and frozen in liquid nitrogen. For hypertonic stress and the touch controls, 200 μL of 300 mM NaCl or water were added directly into the well with seedlings. Again, the NaCl solution or water was aliquoted and frozen in liquid nitrogen after 1 min. To measure the released ATP in the solution, 10 μL of an aliquoted solution was added into a 150-μL bioluminescent assay solution (Sigma-Aldrich). The luminescence signal was integrated for 1 min.
RNA Gel Blot Analysis
Stress-induced treatments to evaluate changes in gene expression were performed as described above for the ATP release assay. Arabidopsis suspension culture cells were maintained in Gamborg's B5 media, and the cells were split into equivalent flasks 3.5 d before the assay. Pretreatments with gadolinium, PPADS (2 h before the treatment), were performed in phosphate drop-out Gamborg's B5 media to prevent precipitate formation, and the ATP treatments (hydrolysable disodium salt) were performed in media buffered with 50 mM Mes to prevent pH drift. The cells were collected at the appropriate time points by filtration through Miracloth (Calbiochem, La Jolla, CA), and the cells were frozen in liquid nitrogen. The liquid-grown wild-type Columbia seedlings were grown in a 24-well plate (12 seedlings per well) to day 7 in half-strength MS media. The media was aspirated, and treatments with 500 μM of ATPγS, ADPβS, and AMPS were performed for 30 min. Samples were collected and frozen in liquid nitrogen. RNA was extracted with an RNeasy plant mini kit (Qiagen, Valencia, CA). cDNA fragments of ATMEKK1 (1 to 308, AT4G08500), ATMPK3 (1 to 259, AT3G45640), ATPK19 (1 to 343, AT3G08720), ATMPK4 (90 to 430, AT4G01370), ATMPK6 (189 to 522, AT2G43790), ACS6 (1 to 300, AT4G11280), ERF2 (291 to 623, AT5G47220), ERF4 (242 to 620, AT3G15210), and ERF3 (244 to 609, AT5G61590) were amplified by PCR and used for probe labeling. Probes were random labeled with 32P with a DECAprime II kit (Ambion, Austin, TX). Gel separation, blot, and hybridization were performed according to the instruction manual for the NorthernMax-Gly kit (Ambion). RNA gel blots were repeated at least twice, and representative blots are presented.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under the following accession numbers: ATMEKK1 (D50468), ATMPK3 (D21839), ATPK19 (D42061), ATMPK4 (D21840), ATMPK6 (D21842), ACS6 (AF361097), ERF2 (AB008104), ERF4 (AB008106), and ERF3 (NM_103946).
Acknowledgments
We thank Janet Braam for the PMAQ2 seeds, Diana Polisensky for technical advice with aequorin, Mona Mehdy for the OGA and critical reading of the manuscript, and Stephen Stout for critical reading of the manuscript. This work was supported in part by grants to S.J.R. from the National Science Foundation (IBN-0080363 and IBN 0344221) and the National Aeronautics and Space Administration (NAG2-1586).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Stanley J. Roux (sroux@uts.cc.utexas.edu).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.023945.
References
- Aharon, G.S., Gelli, A., Sneddon, W.A., and Blumwald, E. (1998). Activation of a plant plasma membrane Ca2+ channel by TGα1, a heterotrimeric G protein α-subunit homologue. FEBS Lett. 424, 17–21. [DOI] [PubMed] [Google Scholar]
- Arteca, J., and Arteca, N. (1999). A multi-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase (ACS6) in mature Arabidopsis leaves. Plant Mol. Biol. 39, 209–219. [DOI] [PubMed] [Google Scholar]
- Baum, G., Long, J.C., Jenkins, G.I., and Trewavas, A.J. (1999). Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc. Natl. Acad. Sci. USA 96, 13554–13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler, W.B., Buckley, K.A., Gartland, A., Hipskind, R.A., Bilbe, G., and Gallagher, J.A. (2001). Extracellular nucleotide signaling: A mechanism for integrating local and systemic responses in the activation of bone remodeling. Bone 28, 507–512. [DOI] [PubMed] [Google Scholar]
- Boyum, R., and Guidotti, G. (1997). Glucose-dependent, cAMP-mediated ATP efflux from Saccharomyces cerevisiae. Microbiology 143, 1901–1908. [DOI] [PubMed] [Google Scholar]
- Brini, M., Marsault, R., Bastianutto, C., Alvarez, J., Pozzan, T., and Rizzuto, R. (1995). Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]c). J. Biol. Chem. 270, 9896–9903. [DOI] [PubMed] [Google Scholar]
- Burnstock, G. (1996). Purinoceptors: Ontogeny and phylogeny. Drug Dev. Res. 39, 204–242. [Google Scholar]
- Cessna, S.G., and Low, P.S. (2001. a). Activation of the oxidative burst in aequorin-transformed Nicotinia tabacum cells is mediated by protein kinase- and anion channel-dependent release of Ca2+ from internal stores. Planta 214, 126–134. [DOI] [PubMed] [Google Scholar]
- Cessna, S.G., and Low, P.S. (2001. b). An apoplastic Ca2+ sensor regulates internal Ca2+ release in aequorin-transformed tobacco cells. J. Biol. Chem. 276, 10655–10662. [DOI] [PubMed] [Google Scholar]
- Chandra, S., and Low, P.S. (1997). Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J. Biol. Chem. 272, 28274–28280. [DOI] [PubMed] [Google Scholar]
- Clifford, E.E., Martin, K.A., Dalal, P., Thomas, R., and Dubyak, G.R. (1997). Stage-specific expression of P2Y receptors, ecto-apyrase, and ecto-5′nucleotidase in myeloid leukocytes. Am. J. Physiol. 273, C973–C987. [DOI] [PubMed] [Google Scholar]
- Davies, W.J., and Zhang, J.H. (1991). Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 55–76. [Google Scholar]
- Demidchik, V., Nichols, C., Oliynyk, M., Dark, A., Glover, B.J., and Davies, J.M. (2003). Is ATP a signaling agent in plants? Plant Physiol. 133, 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, J.P., and Pickard, B.G. (1993). Mechanosensory calcium-selective cation channels in epidermal cells. Plant J. 3, 83–110. [DOI] [PubMed] [Google Scholar]
- Dubyak, G.R., and El-Moatassim, C. (1993). Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 265, C577–C606. [DOI] [PubMed] [Google Scholar]
- Felix, G., Regenass, M., and Boller, T. (2000). Sensing of osmotic pressure changes in tomato cells. Plant Physiol. 124, 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froldi, G., Gallo, U., Ragazzi, E., and Caparrotta, L. (1999). 6-Benzylaminopurine: A plant derived cytokinin inducing positive inotropism by P2-receptors. Planta Med. 65, 245–249. [DOI] [PubMed] [Google Scholar]
- Fujimoto, S.Y., Ohta, M., Usui, A., Shinshi, H., and Ohme-Takagi, M. (2000). Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 12, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimovskaya, E.V., Ahmad, S., White, C., Jones, P., Carpenter, T.C., and Stenmark, K.R. (2002). Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. J. Biol. Chem. 277, 44638–44650. [DOI] [PubMed] [Google Scholar]
- Hahn, M., Darvill, A., Albersheim, P., Bergmann, C., Cheong, J., Koller, A., and Lo, V. (1992). Preparation and characterization of oligosaccharide elicitors of phytoalexin accumulation. In Molecular Plant Pathology: A Practical Approach 2, S.J. Gurr, M.J. McPherson, and D.J. Bowles, eds (Oxford: IRL Press), pp. 132–139.
- Haley, A., Russell, A.J., Wood, N., Allan, A., Knight, M., Campbell, A.K., and Trewavas, A. (1995). Effects of mechanical signaling on plant cell cytosolic calcium. Proc. Natl. Acad. Sci. USA 92, 4124–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley, R. (1999). A role for G proteins in plant hormone signalling? Plant Physiol. Biochem. 37, 393–402. [Google Scholar]
- Joseph, S.M., Buchakjian, M.R., and Dubyak, G.R. (2003). Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J. Biol. Chem. 278, 23331–23342. [DOI] [PubMed] [Google Scholar]
- Kamizyo, A., and Tanaka, N. (1982). Studies on the generative nuclear divisions. III. Effects of exogenous ATP on the generative nuclear divisions in Lilium longiflorum. Cytologia 47, 195–205. [Google Scholar]
- Kiegle, E., Moore, C., Haseloff, J., Tester, M.A., and Knight, M.R. (2000). Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J. 23, 267–278. [DOI] [PubMed] [Google Scholar]
- Kim, C.Y., Liu, Y., Thorne, E.T., Yang, H., Fukushige, H., Gassmann, W., Hildebrand, D., Sharp, R., and Zhang, S. (2003). Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell 15, 2707–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, H., and Knight, M.R. (1995). Recombinant aequorin methods for intracellular calcium measurement in plants. Methods Cell Biol. 49, 201–216. [DOI] [PubMed] [Google Scholar]
- Knight, H., Trewavas, A.J., and Knight, M.R. (1996). Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8, 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, H., Trewavas, A.J., and Knight, M.R. (1997). Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 12, 1067–1078. [DOI] [PubMed] [Google Scholar]
- Knight, M., Read, N.D., Campbell, A., and Trewavas, A.J. (1993). Imaging calcium dynamics in living plants using semi-synthetic recombinant aequorins. J. Cell Biol. 121, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, M.R., Campbell, A.K., Smith, S.M., and Trewavas, A.J. (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352, 524–526. [DOI] [PubMed] [Google Scholar]
- Komoszynski, M., and Wojtczak, A. (1996). Apyrases (ATP diphosphohydrolases, EC 3.6.1.5): Function and relationship to ATPases. Biochim. Biophys. Acta 1310, 233–241. [DOI] [PubMed] [Google Scholar]
- Lew, R.R., and Dearnaley, J.D.W. (2000). Extracellular nucleotide effects on the electrical properties of growing Arabidopsis thaliana root hairs. Plant Sci. 153, 1–6. [Google Scholar]
- Mizoguchi, T., Irie, K., Hirayama, T., Hayashida, N., Yamaguchi-Shinozaki, K., Matsumoto, K., and Shinozaki, K. (1996). A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold and water stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 93, 765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, P.W., and Drew, M.C. (1997). Ethylene and plant responses to stress. Physiol. Plant. 100, 620–630. [Google Scholar]
- Navazio, L., Moscatiello, R., Bellincampi, D., Baldan, B., Meggio, F., Brini, M., Bowler, C., and Mariani, P. (2002). The role of calcium in oligogalacturonide-activated signaling in soybean cells. Planta 215, 596–605. [DOI] [PubMed] [Google Scholar]
- Nejidat, A., Itai, C., and Roth-Bejerano, N. (1983). Stomatal response to ATP mediated by phytochrome. Physiol. Plant. 57, 367–370. [Google Scholar]
- Ostrom, R.S., Gregorian, C., and Insel, P.A. (2000). Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J. Biol. Chem. 275, 11735–11739. [DOI] [PubMed] [Google Scholar]
- Ouaked, F., Rozhon, W., Lecourieux, D., and Hirt, H. (2003). A MAPK pathway mediates ethylene signaling in plants. EMBO J. 22, 1282–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisensky, D.H., and Braam, J. (1996). Cold-shock regulation of the Arabidopsis TCH genes and the effects of modulating the intracellular calcium levels. Plant Physiol. 111, 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic, V., and Burnstock, G. (1998). Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413–492. [PubMed] [Google Scholar]
- Reymond, P., Weber, H., Damond, M., and Farmer, E.E. (2000). Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12, 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo, E., Leon, J., and Sanchez-Serrano, J.J. (1999). Cross-talk between wound signaling pathways determines local versus systemic gene expression in Arabidopsis thaliana. Plant J. 20, 135–142. [DOI] [PubMed] [Google Scholar]
- Sanders, D., Brownlee, C., and Harper, J. (1999). Communicating with calcium. Plant Cell 11, 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, D., Pelloux, J., Brownlee, C., and Harper, J. F .(2002). Calcium at the crossroads of signaling. Plant Cell 14 (suppl.), S401–S417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, Y., Wada, M., and Kadota, A. (2001). External Ca2+ is essential for chloroplast movement induced by mechanical stimulation but not by light stimulation. Plant Physiol. 127, 497–504. [PMC free article] [PubMed] [Google Scholar]
- Sauer, H., Hescheler, J., and Wartenberg, M. (2000). Mechanical strain-induced Ca2+ waves are propagated via ATP release and purinergic receptor activation. Am. J. Physiol. Cell Physiol. 279, C295–C307. [DOI] [PubMed] [Google Scholar]
- Silverman, F.P., Assiamah, A.A., and Bush, D.S. (1998). Membrane transport and cytokinin action in root hairs of Medicago sativa. Planta 205, 23–31. [Google Scholar]
- Steinebrunner, I., Wu, J., Sun, Y., Corbett, A., and Roux, S.J. (2003). Disruption of apyrases inhibits pollen germination in Arabidopsis. Plant Physiol. 131, 1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W.Q., Brady, S.R., Sun, Y., Muday, G.K., and Roux, S.J. (2003). Extracellular ATP inhibits root gravitropism at concentrations that inhibit polar auxin transport. Plant Physiol. 131, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, C., Rajagopal, A., Windsor, B., Dudler, R., Lloyd, A., and Roux, S.J. (2000). A role for ectophosphatase in xenobiotic resistance. Plant Cell 12, 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, C., Sun, Y., Naus, K., Lloyd, A., and Roux, S.J. (1999). Apyrase functions in plant phosphate nutrition and mobilizes phosphate from extracellular ATP. Plant Physiol. 119, 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas, A.J., and Malho, R. (1997). Signal perception and transduction: The origin of the phenotype. Plant Cell 9, 1181–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K.L., Li, H., and Ecker, J.R. (2002). Ethylene biosynthesis and signaling networks. Plant Cell 14 (suppl.), S131–S151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor, B., Roux, S.J., and Lloyd, A. (2003). Multiherbicide tolerance conferred by AtPgp1 and apyrase in Arabidopsis: A novel mechanism of herbicide resistance. Nat. Biotechnol. 21, 428–433. [DOI] [PubMed] [Google Scholar]
- Zimmermann, H. (1996). Extracellular purine metabolism. Drug Dev. Res. 39, 337–352. [Google Scholar]
- Zimmermann, H. (2001). Ectonucleotidases: Some recent developments and a note on nomenclature. Drug Dev. Res. 52, 44–56. [Google Scholar]