Abstract
IgE is an immunoglobulin that plays a central role in acute allergic reactions and chronic inflammatory allergic diseases. The development of a drug able to neutralize this antibody represents a breakthrough in the treatment of inflammatory pathologies with a probable allergic basis. This review focuses on IgE-related chronic diseases, such as allergic asthma and chronic urticaria (CU), and on the role of the anti-IgE monoclonal antibody, omalizumab, in their treatment. We also assess the off-label use of omalizumab for other pathologies associated with IgE and report the latest findings concerning this drug and other new related drugs. To date, omalizumab has only been approved for severe allergic asthma and unresponsive chronic urticaria treatments. In allergic asthma, omalizumab has demonstrated its efficacy in reducing the dose of inhaled corticosteroids required by patients, decreasing the number of asthma exacerbations, and limiting the effect on airway remodeling. In CU, omalizumab treatment rapidly improves symptoms and in some cases achieves complete disease remission. In systemic mastocytosis, omalizumab also improves symptoms and its prophylactic use to prevent anaphylactic reactions has also been discussed. In other pathologies such as atopic dermatitis, food allergy, allergic rhinitis, nasal polyposis, and keratoconjunctivitis, omalizumab significantly improves clinical manifestations. Omalizumab acts in two ways: by sequestering free IgE and by accelerating the dissociation of the IgE-Fcε receptor I complex.
1. Introduction
IgE has unique properties among immunoglobulin isotypes and plays a central role in the pathophysiology of acute allergic reactions and chronic inflammatory allergic diseases. In genetically susceptible individuals, exposure to specific allergens results in an increase of specific IgE, which can bind onto effector cells through a high affinity receptor known as FcεRI expressed in mast cells and basophils [1].
IgE is very short-lived in plasma (about 1 day), but receptor-bound IgE can remain fixed to cells in tissues for weeks or months. Moreover, IgE binding to FcεRI increases cell survival and receptor upregulation [2, 3] and upon contact with a specific allergen induces the release of pharmacologically active mediators stored in the granules of mast cells (MC) and blood basophils (BS), resulting in clinical manifestations of type 1 hypersensitivity. In type 1 hypersensitivity, in the initial phase, an antigen (the allergen) is presented to antigen-specific CD4+ Th2 cells, which stimulate B-cell production of IgE antibodies that are also antigen-specific. During sensitization, the IgE antibodies bind to FcεRI on the surface of tissue MC and blood BS. Later exposure to the same allergen cross-links the bound IgE on sensitized cells, resulting in degranulation and secretion of preformed pharmacologically active mediators such as histamine. All of this occurs as an immediate reaction, starting within seconds. A late reaction caused by the induced synthesis and release of leukotrienes, chemokines, and cytokines by the activated mast cells allows the recruitment of other leukocytes, eosinophils, basophils, and Th2 lymphocytes to the site of inflammation. The allergic reaction includes symptoms like cough, bronchospasm, wheezing, diarrhea, and urticaria due to this process [1, 4].
IgE-mediated chronic diseases have classically been treated with antihistamines, corticoids, and other anti-inflammatory medications, but a number of patients do not respond to these treatments. The discovery and characterization of the pathways that drive different asthma phenotypes and our growing understanding of the pathophysiology of chronic urticaria (CU) have opened up new avenues for their treatment. To target the IgE with biological drugs has been pursued in the treatment of more severe cases of these pathologies.
The use of omalizumab (OmAb), an anti-IgE drug, is approved in severe allergic asthma not controlled by conventional treatment and in CU [5, 6]. IgE is known to be involved in other pathologies, and for this reason omalizumab is currently being assessed in conditions such as allergic rhinitis, atopic dermatitis, food allergies, mastocytosis, and eosinophilic gastrointestinal disease [7].
At present, the most interesting feature of omalizumab is its efficacy in conditions in which no successful treatment has previously been reported [5]. Perhaps unexpectedly, some reports have noted the drug's beneficial role in conditions, which apparently are not IgE-mediated [8]. If this efficacy is demonstrated, the uses of this biological drug may be extended to the treatment of other diseases.
In recent years, a number of other anti-IgE drugs have been developed. They will be discussed in this review, but it is by no means clear whether they will eventually be used in humans.
2. IgE-Related Chronic Diseases
2.1. Asthma
2.1.1. Clinical Manifestations and Epidemiology
Asthma is a chronic inflammatory disease of the airways characterized by intermittent chest symptoms, variable airway obstruction, and bronchial hyperresponsiveness. In recent years, there has been a shift in the conception of asthma, which is no longer seen as a single disease but as a chronic condition with marked clinical heterogeneity over time [9]. Today, asthma is considered a complex syndrome with different phenotypes that share similar clinical manifestations but probably have different etiologies. The circumstances in which the symptoms appear are important because they can explain whether the condition is related to exposure to cold air, to pollen, or to other stimuli. Asthma symptoms occur paroxistically; that is, the patient is healthy for long periods although in severe cases the clinical manifestations persist. Various cells and inflammatory mediators are involved in this pathogenic process, which is conditioned partially by genetic factors [10]. Approximately 300 million people worldwide currently have asthma. In childhood, this disease is more frequent in males, but, in puberty, both sexes are affected equally and, in adulthood, it is more common in women. Mortality is around 180,000 deaths every year [11].
Classically, asthma has been divided into extrinsic and intrinsic phenotypes. Extrinsic asthma is characterized by hypersensitivity to a foreign molecule (substances, proteins) and is always associated with allergy. Intrinsic asthma covers all cases of asthma not attributable to allergies, such as asthma caused by sinus infections, chronic sinusitis, nasal polyps, acute bronchitis, colds, stress, or exercise. The attempts to understand the complexity of asthma presentation and the emergence of biological agents have led to a renewed interest in identifying clinically significant phenotypes. Currently, the study of asthma phenotypes is evolving, with a growing focus on their genetic base and corresponding biomarkers; but rather than creating an ever-expanding list of specific phenotypes, future research is likely to center on dissecting out the clinically relevant ones. The elucidation of asthma phenotypes has been further refined by the study of endotypes, which has provided information on the pathophysiological mechanisms present in different phenotypes [9, 12].
2.1.2. Pathophysiology
Asthma has largely been viewed as a Th2-mediated process strongly linked to atopy and eosinophilic inflammation. However, a significant proportion of asthma cases do not present an increase in Th2 cytokines [13]. Non-Th2-mediated asthma is not as well understood as Th2-mediated asthma. In this review, we focus on Th2-mediated asthma, in which the role of IgE is well known.
Phenotyping studies have identified an early-onset allergic Th2-asthma phenotype (usually during preadolescence) and several late-onset Th2-related phenotypes (often at the age of 20 or later). The clinical phenotype of exercise-induced asthma (EIA) is also likely to have a Th2 component, given its eosinophil- and mast cell-related profile [14].
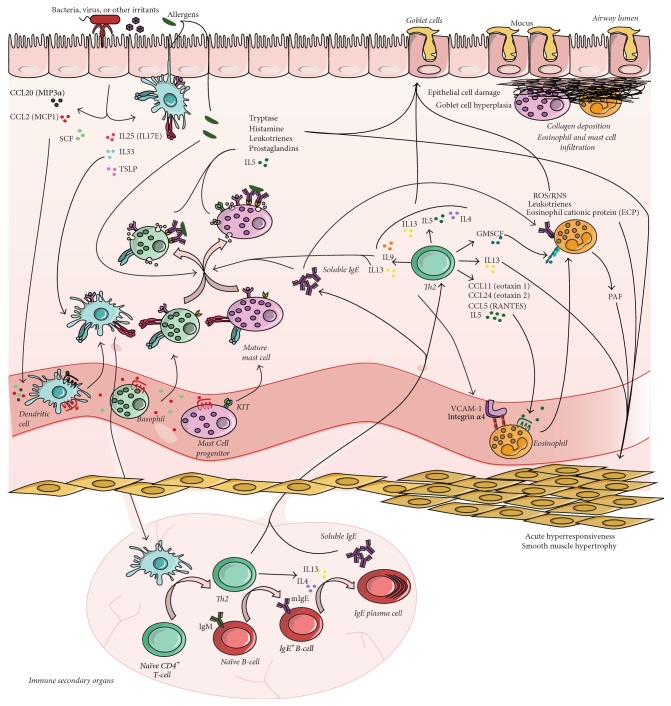
Early-onset allergic Th2 asthma is the most studied phenotype, accounting for 50% of subjects with asthma, and it is linked with other allergic diseases such as allergic rhinitis and atopic dermatitis. The impairment it causes ranges from mild to severe [15]. This phenotype is associated with an increase in total and specific IgE [16]. There appears to be a genetic component to early-onset asthma, as evidenced by the family history of asthma in this group [17]. In allergic asthma, the allergen can directly activate sentinel dendritic cells (DC) present in the airway epithelium [18, 19] (Figure 1). However, bacterial epitopes or other injuries caused by virus or pollutants can act as initiators, as they can activate airway epithelial cells. These cells secrete several cytokines such as thymic stromal lymphopoietin (TSLP), IL25, and IL33, which can directly activate DC [20–22], and chemokines such as monocyte chemoattractant protein-1 (MCP1/also called CCL2) and macrophage inflammatory protein-3 (MIP3a/also called CCL20), which recruit basophils (BS). This also causes an increase in mast cells (MC) in the area [23] but it is not clear whether this is due to recruitment of MC progenitors, mainly by stem cell factor (SCF), or to proliferation of resident MC. DC then migrate to secondary immune organs and, via major histocompatibility complex class II (MHCII) and OXO40L, activate GATA3 transcription by naïve T-cells. The resulting Th2 cells will promote IgG to IgE switching of B-cells. In germinal centers, IL4 and IL13 cytokines, secreted by Th2 cells, cause IgE+ B-cells to become IgE plasma cells and to secrete soluble IgE against allergens. Soluble IgE, together with Th2 cells, return to the pulmonary tissue [24]. These IgE bind to FcεRI on the MC and BS cell surface. IgE-allergen complex promotes MC and BS degranulation of preformed mediators (histamine, tryptase, etc.) and secretion of de novo soluble components, including leukotrienes, prostaglandins, and other Th2 cytokines, which contribute to the prolonged inflammation and to the recruitment of more immune cells [25, 26]. Th2 cell-secreted IL9 cytokine contributes to this MC and BS activation [27].
Figure 1.
Pathophysiology of allergic asthma. Volatile allergens and/or other irritants can activate sentinel dendritic cells and/or epithelial cells in the airway epithelium that will also recruit and activate dendritic cells. The activation of dendritic cells will trigger Th2 responses, leading to the accumulation of soluble IgE as well as several cytokines. These cytokines will recruit mast cell progenitors, as well as basophils and eosinophils, which will be activated and secrete proinflammatory cytokines and other soluble factors such as histamine, tryptase, prostaglandins, and leukotrienes. As a consequence, there will be an increase in mucus production and bronchoconstriction (for mechanistic details, see text). If this activation is maintained, the airway will suffer persistent structural changes that will cause chronic bronchoconstriction due to fibrosis in the subepithelium and smooth muscle hypertrophy. mIgE: membrane IgE; PAF: platelet-activating factor.
Secretion of IL4 by Th2 cells promotes ICAM-1 and VCAM-1 expression on the surface of blood vessels [28], which allows eosinophils to attach and to be recruited, attracted by the action of eosinophil-attractant chemokines secreted by MC, BS, and Th2 cells such as IL5, eotaxin 1 and eotaxin 2, or RANTES, which also increase BS and MC recruitment and proliferation [29, 30]. All these cytokines and soluble molecules secreted by T-cells, MC, BS, and eosinophils cause inflammation, increase in goblet cell-produced mucus, and bronchoconstriction characteristic of an acute exacerbation. However, if this situation persists, these substances cause permanent epithelial damage and lead to structural changes, known as airway remodeling. This airway remodeling is marked by subepithelial fibrosis, due to an increase in the epithelial-mesenchymal trophic unit and collagen deposition characterized by eosinophil and mast cell infiltration, as well as smooth muscle hypertrophy, leading to chronic bronchoconstriction and reduced airway responses to bronchodilators [31] (Figure 1).
Late-onset Th2 asthma is characterized by marked eosinophilia, less atopy, and recurrent exacerbations. This form of asthma is believed to be unrelated to allergic triggers. A family history of this asthma is also less commonly observed and the genetics of this phenotype have not been specifically studied. The lack of clinical allergy in this phenotype suggests that the Th2 process differs from and is probably more complex than the early-onset allergic phenotype [13]. Aspirin-exacerbated respiratory disease (AERD) is a subphenotype of persistent eosinophilic asthma and is widely believed to be an endotype. It comprises a type of adult-onset, highly eosinophilic asthma with inflammation of nasal and bronchial tissues and non-IgE-mediated response to aspirin or other cyclooxygenase-1 inhibitors. AERD pathophysiology is characterized by increased cysteinyl leukotriene production [32].
Furthermore, numerous environmental factors such as smoking, hormonal changes, infections, and obesity are comorbidities and confounders that can alter asthma phenotypes and influence the underlying immunoinflammatory process.
2.2. Chronic Urticaria
2.2.1. Clinical Manifestations, Classification, and Epidemiology
Urticaria is characterized by pruritic wheals that develop quickly with a central edema and a surrounding area of erythema. The size of the wheals is variable and the lesions last from one to 24 hours. The disease may be accompanied by angioedema, defined as cutaneous or mucosal swelling that is generally nonpruritic but is painful and lasts from one to three days [6]. Urticaria can be divided into two groups on the basis of its clinical manifestations: the acute form, which lasts less than six weeks and is often allergic, and chronic spontaneous urticaria (CSU), also known as chronic spontaneous/idiopathic urticaria, which presents daily or almost daily wheals for more than six weeks. This condition affects 0.1%–0.8% of the population [33, 34]. It includes a subpopulation of patients with positive autoimmune serology (up to 30%) to the IgE receptor, IgE, and antithyroid antibodies [35]. The persistence and severity of the symptoms correlate with positive autoimmune serology, more intense inflammation in skin biopsy, and resistance to antihistamines [36]. A third general form of the condition is known as inducible urticaria (physical, cold, cholinergic urticaria, or dermatographism) but will not be addressed here.
2.2.2. Pathophysiology
Chronic spontaneous urticaria may occur as a result of mast cell and basophil release of bioactive mediators. However, the mechanism of mast cell degranulation in urticaria patients remains unclear. Currently, we know that immunological and nonimmunological factors are involved. The key role in the pathogenesis of CSU is played by the vasoactive mediators released from dermal mast cells. Histamine is the most prominent of these mediators although there are others such as eicosanoids, cytokines, and proteases. Histamine acts on H1 receptors (85%) and on H2 (15%) in the skin. Histamine binding to H1 receptors provokes pruritus, vasodilatation, and edema [37]. Mechanisms other than histamine release which have been implicated in CSU include autoimmunity and abnormalities in basophil signal transduction and basopenia [37].
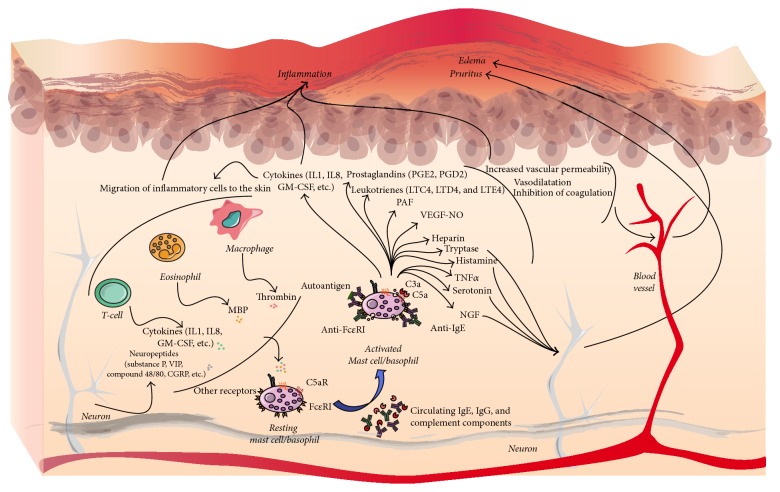
In terms of pathophysiology, three categories of CSU have been defined (Figure 2).
Figure 2.
Pathophysiology of urticaria. In CSU patients, stimulation of mast cells (MC) and basophils (BS) can be triggered by IgE against autoantigens, by IgG against FcεRI or IgE against IgE itself, or by complement. Moreover, MC and BS can be stimulated by molecules secreted by other immune cells or neurons. Once activated, MC and BS secrete several preformed mediators, such as histamine or tryptase, and other de novo mediators, such as prostaglandins and leukotrienes, will promote inflammation, vascular permeability, and vasodilatation, as well as neuron stimulation. These effects are transduced into edema and pruritus. Secretion of cytokines by MC and BS triggers migration of other immune cells to the skin, which will contribute to skin inflammation. MBP: major basic protein; NFG: nerve growth factor; PAF: platelet-activating factor; NO: nitric oxide.
(1) Allergic. In this case, an allergen acts by stimulating the production of IgE, which binds to the FcεRI, leading to mast cell and basophil degranulation.
(2) Autoimmune. An autoimmune etiology is suggested by several findings. Autologous intradermal injection of sera from patients with CSU causes wheal and flare reactions [38]. Moreover, the analysis of urticaria patients' sera reveals IgG autoantibodies to the alpha subunit of FcεRI or to IgE itself [39]. IgG autoantibodies against IgE or the IgE high affinity receptor are produced in almost half of the patients with CSU. The autoantibody cross-linking against the alpha subunit of FcεRI induces degranulation of the mast cells and blood basophils, which is followed by the release of histamine [40]. IgG1 and IgG3 are the main anti-FcεRI autoantibody subclasses found in CSU [41]. The role of complement has been demonstrated, since the presence of C5a increases the histamine released by anti-FcεRI autoantibodies in normal human mast cells and basophils in vitro [42].
Furthermore, a small percentage of blood basophils, histamine-releasing autoantibodies, and HLA-DR alleles that are generally associated with autoimmune diseases are frequently increased in CSU [43].
(3) Nonimmunological. The mechanisms are independent of IgE and FcεRI. This group includes inducible urticaria and urticaria secondary to drugs. Moreover, other CSU patients without autoimmunity or increased serum IgE could also be included: in these cases, the trigger is not known, but it may involve alterations in other unknown molecular mechanisms.
3. Anti-IgE-Based Treatments
Since the identification of IgE as major stimuli in the inflammatory cascade, the development of agents to target IgE has thrived. Among them, the anti-IgE biological omalizumab has been one of the most successful.
3.1. Anti-IgE Drug Omalizumab: Mechanism of Action
Omalizumab (OmAb) is a recombinant humanized monoclonal antibody that was designed to bind to IgE on the Fc (constant fragment) portion, C epsilon 3 locus, in the same domain where IgE is bound to FcεRI [44, 45]. This drug was synthetized with the aim of sequestering free IgE and reducing allergic inflammation [5]. The drug is administered subcutaneously and is absorbed slowly. The peak of serum concentration is reached after 7-8 days [5] and it is eliminated via the reticuloendothelial system, having a half-life of around 26 days.
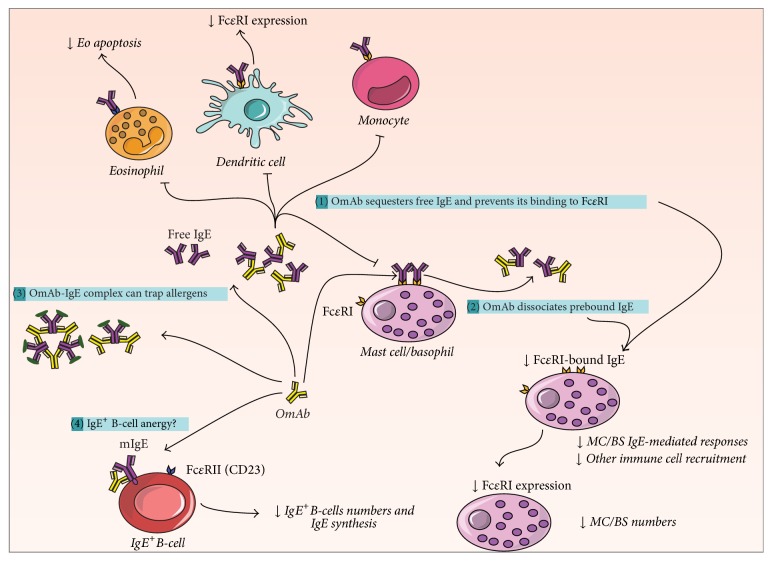
It has been accepted for a long time that OmAb acts on the free IgE (mechanism (1) in Figure 3) and may abolish the binding of IgE to FcεRI+ or FcεRII+ (CD23) cells, B-cells, dendritic cells (DC), eosinophils (Eo), and monocytes. Interestingly, in recent years, the drug's action has been shown to go further, dissociating bound IgE from the IgE-FcεRI complex (mechanism (2) in Figure 3) [46, 47]. Thus, at a physiological concentration range, OmAb may accelerate the dissociation of the preformed IgE-FcεRI complex on the surfaces of mast cells and basophils in addition to its ability to neutralize the free IgE, leading to an impairment of the IgE-inflammatory signaling cascade [47]. Moreover, the density of FcεRI expression on basophils, mast cells, and dendritic cells falls notably in patients receiving anti-IgE treatment within the first week of OmAb application [8]. This may be because the IgE stabilizes the receptor on the cell surface and prevents its internalization; thus, a reduction of the immunoglobulin leads to a decrease in receptor expression [48–50]. All these events make these cells unresponsive to IgE triggering and reduce symptoms such as inflammation, edema, and pruritus. Finally, this leads to a reduction in MC/BS numbers. Interestingly, the reduction in FcεRI expression has also been demonstrated in dendritic cells [51].
Figure 3.
Mechanism of action of OmAb. The two main described mechanisms of action of OmAb are (1) its ability to sequester free IgE and block its binding to IgE receptors (FcεRI) and (2) its ability to accelerate the dissociation of IgE bound to FcεRI in mast cells (MC) and basophils (BS). As a consequence, there is a reduction of IgE-triggered responses, as well as a reduction of the number of eosinophils (Eo), mast cells (MC), and basophils (BS). As a complementary mechanism, IgE complexed with OmAb may trap allergens (3). Another less understood mechanism would lead to a reduction of IgE+ B-cell numbers and a decrease of IgE synthesis (4). mIgE: membrane IgE.
Likewise, as a complementary mechanism, it has been proposed that OmAb-IgE complexes can bind to antigens and act as competitive inhibitors (mechanism (3) described in Figure 3) [52].
It has also been published that OmAb may target membrane-IgE (mIgE) in IgE+ B-cells, reducing IL4R expression and IgE synthesis and decreasing the number of these cells, possibly by causing B-cell anergy [53] (mechanism (4) described in Figure 3). OmAb has also been reported to cause eosinophil apoptosis [54], a finding that is in agreement with the fall in blood eosinophilia found in asthma patients after OmAb administration [55]. However, it is not clear whether this is due to a direct effect of OmAb or is caused by the reduction of IgE or the reduced secretion of cytokines by T-cells.
To sum up, OmAb may act via several mechanisms, which appear to affect not only IgE-triggered events, but also the viability of the different cells, involved in these pathologies. This leads to a rapid and prolonged reduction of the symptomatology.
3.2. Omalizumab in Asthma
While most asthma is controlled with anti-inflammatory and bronchodilator medications irrespective of phenotype, a minority of patients, around 10%, respond poorly. Thus, the definition of “severe” asthma is applied to patients whose symptoms or exacerbations require the use of a high-dose inhaled corticosteroid plus a second controller, or whose disease persists in spite of treatment [56, 57]. The understanding of asthma physiopathology has allowed the design of treatments for these persistent cases based on anti-IgE monoclonal antibodies such as OmAb.
Many clinical studies have reported the effectiveness of OmAb and the use of the drug has been extensively reviewed in the literature. Among the largest studies, a systematic study in 2006 analyzed its efficacy in allergic asthma, based on data obtained from 14 studies including a total of 3,143 patients. The results showed that inhaled corticosteroid therapy, which is the mainstay of asthma therapy, was reduced by more than 50% in a significant number of patients after OmAb treatment, and some patients were able to discontinue inhaled corticosteroid therapy entirely [58]. Other clinical trials carried out in recent years have confirmed that OmAb treatment improves symptoms and reduces the frequency of asthma exacerbations and the need for high doses of inhaled corticosteroids [8].
In several real-life studies, the use of omalizumab has been associated with an absence of exacerbations and an improvement in quality of life, which is reflected in reduced hospital admissions and emergency visits [8].
In an attempt to elucidate the drug's mechanism of action, another placebo-controlled trial was conducted in 41 adult patients with severe, nonatopic refractory asthma. Interestingly, OmAb regulated FcεRI expression negatively on basophils and plasmacytoid dendritic cells and increased forced expiratory volume in the first minute (FEV1) compared with baseline after 16 weeks in patients with severe nonatopic asthma, as it does in severe atopic asthma [59]. This finding points to a possible role of IgE in nonatopic asthma.
The standard duration of treatment with OmAb has not been established to date. A follow-up study showed that, after six years of OmAb treatment, most patients had mild and stable asthma in the ensuing three years after treatment discontinuation [60]. It has been suggested that the persistence of the effects of OmAb may be due to its ability to curtail airway remodeling in patients with asthma. In fact, it has been found that OmAb significantly decreased the airway wall area, the percentage of wall area, and the luminal area of the right apical bronchial segments, whereas no change was achieved with conventional therapy [61]. After one year of OmAb treatment, a significant mean reduction in eosinophilic infiltration was recorded as well as a reduction in reticular base membrane in bronchial biopsies from patients with severe persistent allergic asthma. These findings indicate that OmAb may modify the course of the disease due to their possible influence curtailing airway remodeling.
3.3. Omalizumab in Chronic Urticaria
Nowadays, several options are available for treating chronic urticaria. Practical measures include the avoidance of aggravating factors such as drugs (nonsteroidal anti-inflammatory drugs, NSAIDs), alcohol, stress, and local heat and friction. Currently, the guidelines recommend a stepwise approach, which includes nonsedating antihistamines as a first line of treatment or combinations with oral corticosteroids. Control is often insufficient and additional therapies have included leukotriene antagonists or cyclosporine, and more recently OmAb has been added if symptoms persist [6].
The use of OmAb in urticaria has focused mainly on CSU with autoimmune form [62]. The effect of OmAb on CSU with or without angioedema has been demonstrated in several double-blinded randomized placebo-controlled studies including almost 1200 patients, with relatively few side effects [63–66]. Although the OmAb dose for CSU is set at 300 mg every 4 weeks, a dose of 150 mg every 4 weeks also achieves an effect in some patients, and in other cases the dose needs to be increased to 300 mg every two weeks. In some cases, signs and symptoms of urticaria cease after a few days of treatment, a faster effect than in asthma, which begins to present improvement after a week at the earliest [62]. These findings suggested another mechanism of action for OmAb, apart from its ability to sequester free IgE. Lowering free IgE levels may downregulate the levels of IgE receptor expression density on the surface of mast cells in the long term, but this would not appear to be responsible for the rapid improvement reported in the clinical symptoms; a more likely reason is OmAb's ability to dissociate prebound IgE from FcƐRI [46, 47]. Nevertheless, it is still unclear how OmAb works in CSU: in addition, the fact that OmAb is not effective in all patients suggests the involvement of mechanisms/pathways in CSU other than the IgE cascade.
Interestingly, OmAb has also been reported to be effective in treating other forms of urticaria: cold urticaria, solar urticaria, cholinergic urticaria, delayed pressure, and symptomatic urticaria factitia, although the role of IgE in these urticarial conditions is unknown [67–71].
3.4. Off-Label Use of Omalizumab in Other Diseases
3.4.1. Systemic Mastocytosis, Hyperimmunoglobulin E Syndrome, and Eosinophilic Gastroenteritis
Mastocytosis is a heterogeneous disorder that results from abnormal proliferation and accumulation of mast cells in one or more organs. When this infiltration extends to extracutaneous organs such as bone marrow, liver, spleen, and gastrointestinal tract in spite of the cutaneous infiltration, systemic mastocytosis is diagnosed. Increased local concentration of soluble mast cell growth factors in lesions is believed to stimulate mast cell proliferation. Impaired mast cell apoptosis and interleukin-6 have also been implicated, as evidenced by BCL-2 upregulation and high IL6 levels in tissue. Most patients with the systemic form present an activating point mutation in the c-kit gene in codon 816 (D816V), which is thought to contribute to the abnormal proliferation of mast cells and enhanced mast cell survival [72]. OmAb has been reported to be safe and effective in preventing recurrent anaphylaxis [73]. Since OmAb reduces the expression of FcεRI on circulating basophils and mast cells, it seems to lower their activity and thus reduces their potential reactivity [74, 75]. Curiously, there is no evidence of the capacity of OmAb to decrease mast cell numbers, because the serum tryptase levels in several patients with mastocytosis do not vary during the period of response [76]. In another study, serum tryptase was reported to decrease during OmAb therapy in two mastocytosis patients, but it remained unchanged in two others [77]. The mechanisms underlying the symptomatic improvement in patients with systemic mastocytosis treated with OmAb are still not fully understood.
Hyperimmunoglobulin E syndrome (HIES) is a heterogeneous group of immune disorders characterized by very high levels of serum IgE, dermatitis, and recurrent skin and lung infections. There are two forms of HIES: a dominant form caused by mutations in STAT3 and a recessive form for which a genetic cause is unclear. These syndromes have distinct presentations, courses, and outcomes but both present clear increases in IgE levels. Studies report clinical improvements in patients with high serum IgE levels and presenting severe atopic eczema and in patients presenting several other symptoms after OmAb treatment [78, 79].
Eosinophilic gastroenteritis is characterized by patchy or diffuse eosinophilic infiltration of any part of the gastrointestinal tract. Anti-IgE treatment with OmAb is associated with a 35–45% drop in peripheral blood eosinophil count and decreases in duodenal and antral eosinophils. It effectively blocks CD23-mediated allergen binding to B-cells [80].
3.4.2. Allergic Rhinitis, Nasal Polyposis, and IgE-Related Respiratory Diseases
There is a close relationship between asthma and allergic rhinitis. For this reason, OmAb was expected to be effective in the treatment of concomitant rhinitis in patients with asthma. Indeed, in one trial, the odds ratio for a positive effect on rhinitis was 3.56, indicating that the probability of improvement was three and a half times higher in subjects treated with OmAb [81]. In a 2002 double-blinded, randomized trial, combination therapy of OmAb with specific immunotherapy (SIT) for birch and pollen reduced symptom load over two pollen seasons by 48% compared with SIT alone [82].
In nasal polyposis, the results of using OmAb are less obvious since this condition appears in nonallergic patients. Significantly, high levels of IgE in polyps have been related to Staphylococcus aureus enterotoxin acting as a superantigen rather than atopy. Interestingly, IgE antibodies against S. aureus enterotoxin were found in a significant higher concentration in severe asthma patients compared to controls suggesting a relationship between these antibodies and asthma severity [83]. In this context, several studies with OmAb have also demonstrated clinical efficacy in the treatment of nasal polyps with comorbid asthma [84, 85].
Omalizumab has also demonstrated its clinical relevance in patients with allergic bronchopulmonary aspergillosis (ABPA), an allergic reaction to Aspergillus characterized by high IgE levels, which usually occurs in combination with cystic fibrosis (CF) [86, 87]. Several case series have also reported success with OmAb in ABPA patients without CF [88, 89].
3.4.3. Atopic Dermatitis and Bullous Pemphigoid
Atopic dermatitis (AD) is one of the most frequent chronic inflammatory skin disorders associated with elevated serum IgE levels. Acute AD skin lesions are characterized by intensely pruritic, erythematous papules associated with epidermal intercellular edema as well as increased Langerhans cells, inflammatory dendritic epidermal cells, macrophages, eosinophils, and activated CD4-positive Th2 cells. The results with the use of OmAb for atopic dermatitis are controversial: several case reports investigating anti-IgE therapy in patients with AD have found symptomatic improvement [90, 91], but others report negative responses in patients with severe AD treated with a four-month course of OmAb [92], or a favorable response in only 6 of 11 patients [78]. More randomized controlled trials including a placebo control group are needed to validate the effectiveness of OmAb for AD.
Bullous pemphigoid (BP) is an acquired autoimmune disease presenting subepidermal blistering, eosinophilia, and severe itching. It is characterized by the presence of autoantibodies against the 230 kDa bullous pemphigoid antigen within basal keratinocytes and the 180 kDa type XVII collagen in the basement membrane zone lying between the epidermis and dermis. IgE-specific antibodies against type XVII collagen were detected in sera and biopsy samples from the majority of BP patients and these IgE autoantibodies have been shown to be pathogenic. Clinical trials with OmAb showed effectiveness in several cases [93, 94].
3.4.4. Food Allergy and Food-Related Anaphylaxis
OmAb induced a significant increase in the threshold dose for an oral food challenge with peanuts causing allergic symptoms [95]. OmAb has also been useful in introducing oral immunotherapy (OIT) in food-allergic patients. In a pilot study with children with clinical reactions to cow's milk, OmAb treatment combined with oral milk desensitization permitted rapid milk dose escalation in the majority of subjects. [96]. OmAb was also reported to be effective in tolerability of various food allergies during an OIT protocol in 25 patients [97]. Thus, OmAb in combination with oral desensitization has a potential value for the treatment of food allergy.
3.4.5. Atopic Keratoconjunctivitis
Atopic keratoconjunctivitis is a severe ocular disorder of the cornea with immediate and delayed hypersensitivity reactions, which can produce loss of visual acuity and blindness. In an open-label trial, six patients treated with OmAb showed an improvement in their ocular symptoms [98].
3.5. Other Anti-IgE-Based Therapies
3.5.1. CεmX Monoclonal Antibodies
Membrane-bound IgE (mIgE) is part of the IgE-BCR and is essential for generating isotype-specific IgE responses. On mIgE+ B-cells, the membrane-bound ε-chain exists predominantly in the long isoform, thus providing an attractive site for immunologic targeting of mIgE+ cells. CεmX-specific antibodies have proved potentially useful for targeting mIgE+ cells to control IgE production [99]. These were the bases for the production of quilizumab, a humanized IgG1 monoclonal antibody that binds to the M1-prime segment present only on mIgE, but not on soluble IgE in serum. In phase I and II studies, quilizumab reduced serum total IgE by approximately 25%. These decreases were sustained for at least six months after the last dose, in contrast to OmAb, which must be administered every 2–4 weeks to maintain reduced IgE levels [100]. Unfortunately, in adults with uncontrolled allergic asthma, a 36-week treatment with quilizumab did not have a clinically significant impact on exacerbation rate, lung function, or quality of life [101]. Nor did its use in patients with refractory CSU achieve a clinically significant improvement, although it reduced median serum IgE by approximately 30% [102].
3.5.2. Ligelizumab
Ligelizumab (QGE031) is a humanized IgG1 monoclonal antibody that binds with higher affinity to the C epsilon 3 domain of IgE. Designed to achieve greater IgE suppression than OmAb, it may overcome some of the limitations associated with OmAb dosing and achieve better clinical outcomes. Data from preclinical experiments and two phase I randomized, double-blind, placebo-controlled clinical trials showed QGE031 to be superior to OmAb in suppressing free IgE and basophil surface expression of FcεRI and IgE. These effects allowed almost complete suppression of the skin prick response to the allergen, which was superior in extent and duration compared to the case with OmAb [103].
3.5.3. Bispecific Antibodies and Designed Ankyrin Repeat Proteins (DARPins)
Some studies have shown that the use of bispecific antibodies that cross-link FcεRI and the low-affinity IgG receptor (FcγRIIb) on mast cells and basophils inhibits allergen-induced cell degranulation [104, 105].
DARPins are genetically engineered proteins that typically exhibit highly specific and high affinity target protein binding. One study reported a specific anti-IgE DARPin (DE53-Fc) fused to the Fc part of a human IgG1, which inhibited allergen-induced basophil activation in samples of different donors via FcγRIIb [106]. For its part, DARPin E2_79 is able to block IgE:FcεRI interactions and actively stimulates the dissociation of preformed ligand-receptor complexes [107].
Bispecific antibodies and DARPins represent promising drug candidates for the treatment of IgE-related diseases but their potential for use in humans is still to be confirmed.
3.5.4. IgE-R419N-Fc3-4
Structural studies of OmAb have facilitated the design of an IgE-Fc3-4 mutant (IgE-R419N-Fc3-4) that is resistant to OmAb neutralization but is able to bind FcεRI and FcεRII. The IgE-R419N-Fc3-4 mutant, in combination with OmAb, can effectively exchange cell-bound IgE for IgE-R419N-Fc3-4 and this dual inhibitor treatment blocks basophil activation more powerfully than either inhibitor alone. This approach, involving simultaneous depletion of antigen-specific IgE while engaging FcεRI and FcεRII receptors with an IgE variant, can be used to further test the role of IgE-dependent regulatory pathways during anti-IgE treatment and may provide a promising way forward for enhancing current anti-IgE therapies [108].
4. Conclusion
A better understanding of asthma and chronic urticaria phenotypes and endotypes will allow us to select treatments based on the likelihood of response, thereby improving the control and quality of life of these patients. The number of biological treatments for these diseases continues to grow.
Targeting IgE has proved to be a successful approach to IgE-related diseases with poor response to traditional treatments. Although the indications for OmAb are currently limited to allergic asthma and CSU, its potential in the treatment of other allergic comorbidities is becoming increasingly clear. In fact, the off-label uses of OmAb have shown promising results in a variety of diseases in which IgE has a limited or unconfirmed role. The knowledge of OmAb's mechanism of action may help to elucidate the relationships between all the factors interacting in IgE-mediated pathologies and may shed some light on some others that are not IgE-mediated.
Thus, more studies are needed to uncover the molecular insights of these pathologies and the mechanism of action of anti-IgE biological drugs such as OmAb and to test the efficacy of new IgE-targeted drugs in humans.
Acknowledgments
This work was supported by Fondo de Investigaciones Sanitarias, Ministerio de Economía y Competitividad (Grant PI120032).
Competing Interests
The authors declare that there are no competing interests regarding the publication of this paper.
References
- 1.Oettgen H. C. Fifty years later: emerging functions of IgE antibodies in host defense, immune regulation, and allergic diseases. Journal of Allergy and Clinical Immunology. 2016;137(6):1631–1645. doi: 10.1016/j.jaci.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai K., Kitaura J., Kawakami Y., et al. Regulation of mast cell survival by IgE. Immunity. 2001;14(6):791–800. doi: 10.1016/S1074-7613(01)00157-1. [DOI] [PubMed] [Google Scholar]
- 3.Kalesnikoff J., Huber M., Lam V., et al. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14(6):801–811. doi: 10.1016/S1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 4.Ishizaka K. Basic mechanisms of IgE-mediated hypersensitivity. Current Opinion in Immunology. 1989;1(4):625–629. doi: 10.1016/0952-7915(89)90031-9. [DOI] [PubMed] [Google Scholar]
- 5.Babu K. S., Polosa R., Morjaria J. B. Anti-IgE—emerging opportunities for Omalizumab. Expert Opinion on Biological Therapy. 2013;13(5):765–777. doi: 10.1517/14712598.2013.782391. [DOI] [PubMed] [Google Scholar]
- 6.Zuberbier T., Aberer W., Asero R., et al. The EAACI/GA2LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69(7):868–887. doi: 10.1111/all.12313. [DOI] [PubMed] [Google Scholar]
- 7.Mauro M., Incorvaia C., Formigoni C., Elia R., Russello M., Pellegrino D. The anti-IgE antibody omalizumab as a probe to investigate the role of IgE in pathology. Panminerva Medica. 2012;54(4):305–312. [PubMed] [Google Scholar]
- 8.Incorvaia C., Mauro M., Russello M., Formigoni C., Riario-Sforza G. G., Ridolo E. Omalizumab, an anti-immunoglobulin E antibody: state of the art. Drug Design, Development and Therapy. 2014;8:197–207. doi: 10.2147/dddt.s49409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai M., Oppenheimer J. Elucidating asthma phenotypes and endotypes: progress towards personalized medicine. Annals of Allergy, Asthma & Immunology. 2016;116(5):394–401. doi: 10.1016/j.anai.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J., Paré P. D., Sandford A. J. Recent advances in asthma genetics. Respiratory Research. 2008;9, article 4 doi: 10.1186/1465-9921-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braman S. S. The global burden of asthma. Chest. 2006;130, supplement 1:4S–12S. doi: 10.1378/chest.130.1_suppl.4s. [DOI] [PubMed] [Google Scholar]
- 12.Corren J. Asthma phenotypes and endotypes: an evolving paradigm for classification. Discovery Medicine. 2013;15(83):243–249. [PubMed] [Google Scholar]
- 13.Wenzel S. E. Asthma phenotypes: the evolution from clinical to molecular approaches. Nature Medicine. 2012;18(5):716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 14.Hallstrand T. S., Moody M. W., Aitken M. L., Henderson W. R., Jr. Airway immunopathology of asthma with exercise-induced bronchoconstriction. The Journal of Allergy and Clinical Immunology. 2005;116(3):586–593. doi: 10.1016/j.jaci.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miranda C., Busacker A., Balzar S., Trudeau J., Wenzel S. E. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. Journal of Allergy and Clinical Immunology. 2004;113(1):101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Woodruff P. G., Modrek B., Choy D. F., et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. American Journal of Respiratory and Critical Care Medicine. 2009;180(5):388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bisgaard H., Bønnelykke K., Sleiman P. M. A., et al. Chromosome 17q21 gene variants are associated with asthma and exacerbations but not atopy in early childhood. American Journal of Respiratory and Critical Care Medicine. 2009;179(3):179–185. doi: 10.1164/rccm.200809-1436oc. [DOI] [PubMed] [Google Scholar]
- 18.Lambrecht B. N., Salomon B., Klatzmann D., Pauwels R. A. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. The Journal of Immunology. 1998;160(8):4090–4097. [PubMed] [Google Scholar]
- 19.van Rijt L. S., Jung S., KleinJan A., et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. Journal of Experimental Medicine. 2005;201(6):981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allakhverdi Z., Comeau M. R., Jessup H. K., et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. The Journal of Experimental Medicine. 2007;204(2):253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eiwegger T., Akdis C. A. IL-33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. European Journal of Immunology. 2011;41(6):1535–1538. doi: 10.1002/eji.201141668. [DOI] [PubMed] [Google Scholar]
- 22.Reibman J., Hsu Y., Chen L. C., Bleck B., Gordon T. Airway epithelial cells release MIP-3α/CCL20 in response to cytokines and ambient particulate matter. American Journal of Respiratory Cell and Molecular Biology. 2003;28(6):648–654. doi: 10.1165/rcmb.2002-0095OC. [DOI] [PubMed] [Google Scholar]
- 23.Brightling C. E., Bradding P., Symon F. A., Holgate S. T., Wardlaw A. J., Pavord I. D. Mast-cell infiltration of airway smooth muscle in asthma. The New England Journal of Medicine. 2002;346(22):1699–1705. doi: 10.1056/nejmoa012705. [DOI] [PubMed] [Google Scholar]
- 24.Fahy J. V. Type 2 inflammation in asthma—present in most, absent in many. Nature Reviews Immunology. 2015;15(1):57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng L. E., Sullivan B. M., Retana L. E., Allen C. D. C. C. D. C., Liang H.-E., Locksley R. M. IgE-activated basophils regulate eosinophil tissue entry by modulating endothelial function. Journal of Experimental Medicine. 2015;212(4):513–524. doi: 10.1084/jem.20141671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voehringer D. Protective and pathological roles of mast cells and basophils. Nature Reviews Immunology. 2013;13(5):362–375. doi: 10.1038/nri3427. [DOI] [PubMed] [Google Scholar]
- 27.Matsuzawa S., Sakashita K., Kinoshita T., Ito S., Yamashita T., Koike K. IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. The Journal of Immunology. 2003;170(7):3461–3467. doi: 10.4049/jimmunol.170.7.3461. [DOI] [PubMed] [Google Scholar]
- 28.Schleimer R. P., Sterbinsky S. A., Kaiser J., et al. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM-1. The Journal of Immunology. 1992;148(4):1086–1092. [PubMed] [Google Scholar]
- 29.Collins P. D., Marleau S., Griffiths-Johnson D. A., Jose P. J., Williams T. J. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. Journal of Experimental Medicine. 1995;182(4):1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ying S., Meng Q., Zeibecoglou K., et al. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (intrinsic) asthmatics. Journal of Immunology. 1999;163(11):6321–6329. [PubMed] [Google Scholar]
- 31.Benayoun L., Druilhe A., Dombret M.-C., Aubier M., Pretolani M. Airway structural alterations selectively associated with severe asthma. American Journal of Respiratory and Critical Care Medicine. 2003;167(10):1360–1368. doi: 10.1164/rccm.200209-1030oc. [DOI] [PubMed] [Google Scholar]
- 32.Cowburn A. S., Sladek K., Soja J., et al. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. The Journal of Clinical Investigation. 1998;101(4):834–846. doi: 10.1172/jci620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellgren L. The prevalence of urticaria in the total population. Acta allergologica. 1972;27(3):236–240. doi: 10.1111/j.1398-9995.1972.tb01420.x. [DOI] [PubMed] [Google Scholar]
- 34.Zuberbier T., Balke M., Worm M., Edenharter G., Maurer M. Epidemiology of urticaria: a representative cross-sectional population survey. Clinical and Experimental Dermatology. 2010;35(8):869–873. doi: 10.1111/j.1365-2230.2010.03840.x. [DOI] [PubMed] [Google Scholar]
- 35.Schocket A. L. Chronic urticaria: pathophysiology and etiology, or the what and why. Allergy and Asthma Proceedings. 2006;27(2):90–95. [PubMed] [Google Scholar]
- 36.Sabroe R. A., Fiebiger E., Francis D. M., et al. Classification of anti-FcϵRI and anti-IgE autoantibodies in chronic idiopathic urticaria and correlation with disease severity. Journal of Allergy and Clinical Immunology. 2002;110(3):492–499. doi: 10.1067/mai.2002.126782. [DOI] [PubMed] [Google Scholar]
- 37.Viegas L. P., Ferreira M. B., Kaplan A. P. The maddening itch: an approach to chronic urticaria. Journal of Investigational Allergology and Clinical Immunology. 2014;24(1):1–5. [PubMed] [Google Scholar]
- 38.Grattan C. E. H., Wallington T. B., Warin R. P., Kennedy C. T., Bradfield J. W. A serological mediator in chronic idiopathic urticaria—a clinical, immunological and histological evaluation. British Journal of Dermatology. 1986;114(5):583–590. doi: 10.1111/j.1365-2133.1986.tb04065.x. [DOI] [PubMed] [Google Scholar]
- 39.Khalaf A. T., Li W., Jinquan T. Current advances in the management of urticaria. Archivum Immunologiae et Therapiae Experimentalis. 2008;56(2):103–114. doi: 10.1007/s00005-008-0013-6. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan A. P., Greaves M. Pathogenesis of chronic urticaria. Clinical and Experimental Allergy. 2009;39(6):777–787. doi: 10.1111/j.1365-2222.2009.03256.x. [DOI] [PubMed] [Google Scholar]
- 41.Fiebiger E., Hammerschmid F., Stingl G., Maurer D. Anti-FcepsilonRIalpha autoantibodies in autoimmune-mediated disorders. Identification of a structure-function relationship. The Journal of Clinical Investigation. 1998;101(1):243–251. doi: 10.1172/jci511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrer M., Nakazawa K., Kaplan A. R. Complement dependence of histamine release in chronic urticaria. Journal of Allergy and Clinical Immunology. 1999;104(1):169–172. doi: 10.1016/S0091-6749(99)70129-6. [DOI] [PubMed] [Google Scholar]
- 43.Konstantinou G. N., Asero R., Maurer M., Sabroe R. A., Schmid-Grendelmeier P., Grattan C. E. H. EAACI/GA2LEN task force consensus report: the autologous serum skin test in urticaria. Allergy: European Journal of Allergy and Clinical Immunology. 2009;64(9):1256–1268. doi: 10.1111/j.1398-9995.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 44.Presta L. C., Lahr S. J., Shields R. L., et al. Humanization of an antibody directed against IgE. The Journal of Immunology. 1993;151(5):2623–2632. [PubMed] [Google Scholar]
- 45.Zheng L., Li B., Qian W., et al. Fine epitope mapping of humanized anti-IgE monoclonal antibody omalizumab. Biochemical and Biophysical Research Communications. 2008;375(4):619–622. doi: 10.1016/j.bbrc.2008.08.055. [DOI] [PubMed] [Google Scholar]
- 46.Eggel A., Baravalle G., Hobi G., et al. Accelerated dissociation of IgE-FcepsilonRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. The Journal of Allergy and Clinical Immunology. 2014;133(6):1709–1719.e8. doi: 10.1016/j.jaci.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrano-Candelas E., Martinez-Aranguren R., Valero A., et al. Comparable actions of omalizumab on mast cells and basophils. Clinical and Experimental Allergy. 2016;46(1):92–102. doi: 10.1111/cea.12668. [DOI] [PubMed] [Google Scholar]
- 48.Beck L. A., Marcotte G. V., MacGlashan D., Jr., Togias A., Saini S. Omalizumab-induced reductions in mast cell FcεRI expression and function. The Journal of Allergy and Clinical Immunology. 2004;114(3):527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 49.MacGlashan D., Jr., Lichtenstein L. M., McKenzie-White J., et al. Upregulation of FcεRI on human basophils by IgE antibody is mediated by interaction of IgE with FcεRI. Journal of Allergy and Clinical Immunology. 1999;104(2, part 1):492–498. doi: 10.1016/s0091-6749(99)70399-4. [DOI] [PubMed] [Google Scholar]
- 50.Kubo S., Matsuoka K., Taya C., et al. Drastic up-regulation of FcεRI on mast cells is induced by IgE binding through stabilization and accumulation of FcεRI on the cell surface. The Journal of Immunology. 2001;167(6):3427–3434. doi: 10.4049/jimmunol.167.6.3427. [DOI] [PubMed] [Google Scholar]
- 51.Prussin C., Griffith D. T., Boesel K. M., Lin H., Foster B., Casale T. B. Omalizumab treatment downregulates dendritic cell FcεRI expression. The Journal of Allergy and Clinical Immunology. 2003;112(6):1147–1154. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Chang T. W. The pharmacological basis of anti-IgE therapy. Nature Biotechnology. 2000;18(2):157–162. doi: 10.1038/72601. [DOI] [PubMed] [Google Scholar]
- 53.Chan M. A., Gigliotti N. M., Dotson A. L., Rosenwasser L. J. Omalizumab may decrease IgE synthesis by targeting membrane IgE+ human B cells. Clinical and Translational Allergy. 2013;3, article 29 doi: 10.1186/2045-7022-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noga O., Hanf G., Brachmann I., et al. Effect of omalizumab treatment on peripheral eosinophil and T-lymphocyte function in patients with allergic asthma. Journal of Allergy and Clinical Immunology. 2006;117(6):1493–1499. doi: 10.1016/j.jaci.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 55.Skiepko R., Zietkowski Z., Lukaszyk M., et al. Changes in blood eosinophilia during omalizumab therapy as a predictor of asthma exacerbation. Postepy Dermatologii i Alergologii. 2014;31(5):305–309. doi: 10.5114/pdia.2014.40973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung K. F., Wenzel S. E., Brozek J. L., et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. The European Respiratory Journal. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 57.Lo C.-Y., Michaeloudes C., Bhavsar P. K., et al. Increased phenotypic differentiation and reduced corticosteroid sensitivity of fibrocytes in severe asthma. The Journal of Allergy and Clinical Immunology. 2015;135(5):1186–1195.e6. doi: 10.1016/j.jaci.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 58.Walker S., Monteil M., Phelan K., Lasserson T. J., Walters E. H. Anti-IgE for chronic asthma in adults and children. The Cochrane Database of Systematic Reviews. 2006;(2) doi: 10.1002/14651858.CD003559.pub3.CD003559 [DOI] [PubMed] [Google Scholar]
- 59.Garcia G., Magnan A., Chiron R., et al. A proof-of-concept, randomized, controlled trial of omalizumab in patients with severe, difficult-to-control, nonatopic asthma. Chest. 2013;144(2):411–419. doi: 10.1378/chest.12-1961. [DOI] [PubMed] [Google Scholar]
- 60.Nopp A., Johansson S. G. O., Adédoyin J., Ankerst J., Palmqvist M., Öman H. After 6 years with Xolair; a 3-year withdrawal follow-up. Allergy: European Journal of Allergy and Clinical Immunology. 2010;65(1):56–60. doi: 10.1111/j.1398-9995.2009.02144.x. [DOI] [PubMed] [Google Scholar]
- 61.Hoshino M., Ohtawa J. Effects of adding omalizumab, an anti-immunoglobulin E antibody, on airway wall thickening in asthma. Respiration. 2012;83(6):520–528. doi: 10.1159/000334701. [DOI] [PubMed] [Google Scholar]
- 62.Kaplan A. P. Therapy of chronic urticaria: a simple, modern approach. Annals of Allergy, Asthma and Immunology. 2014;112(5):419–425. doi: 10.1016/j.anai.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 63.Kaplan A., Ledford D., Ashby M., et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. Journal of Allergy and Clinical Immunology. 2013;132(1):101–109. doi: 10.1016/j.jaci.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Maurer M., Altrichter S., Bieber T., et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. The Journal of Allergy and Clinical Immunology. 2011;128(1):202.e5–209.e5. doi: 10.1016/j.jaci.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 65.Maurer M., Rosén K., Hsieh H., et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. The New England Journal of Medicine. 2013;368(10):924–35. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 66.Saini S., Rosen K. E., Hsieh H.-J., et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. Journal of Allergy and Clinical Immunology. 2011;128(3):567–573.e1. doi: 10.1016/j.jaci.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 67.Bindslev-Jensen C., Skov P. S. Efficacy of omalizumab in delayed pressure urticaria: a case report. Allergy. 2010;65(1):138–139. doi: 10.1111/j.1398-9995.2009.02188.x. [DOI] [PubMed] [Google Scholar]
- 68.Boyce J. A. Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. Journal of Allergy and Clinical Immunology. 2006;117(6):1415–1418. doi: 10.1016/j.jaci.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Güzelbey O., Ardelean E., Magerl M., Zuberbier T., Maurer M., Metz M. Successful treatment of solar urticaria with anti-immunoglobulin E therapy. Allergy: European Journal of Allergy and Clinical Immunology. 2008;63(11):1563–1565. doi: 10.1111/j.1398-9995.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- 70.Krause K., Ardelean E., Keãler B., et al. Antihistamine-resistant urticaria factitia successfully treated with anti-immunoglobulin E therapy. Allergy. 2010;65(11):1494–1495. doi: 10.1111/j.1398-9995.2010.02409.x. [DOI] [PubMed] [Google Scholar]
- 71.Metz M., Bergmann P., Zuberbier T., Maurer M. Successful treatment of cholinergic urticaria with anti-immunoglobulin E therapy. Allergy. 2008;63(2):247–249. doi: 10.1111/j.1398-9995.2007.01591.x. [DOI] [PubMed] [Google Scholar]
- 72.Robyn J., Metcalfe D. D. Systemic mastocytosis. Advances in Immunology. 2006;89:169–243. doi: 10.1016/s0065-2776(05)89005-4. [DOI] [PubMed] [Google Scholar]
- 73.Douglass J. A., Carroll K., Voskamp A., Bourke P., Wei A., O'Hehir R. E. Omalizumab is effective in treating systemic mastocytosis in a nonatopic patient. Allergy. 2010;65(7):926–927. doi: 10.1111/j.1398-9995.2009.02259.x. [DOI] [PubMed] [Google Scholar]
- 74.Kibsgaard L., Skjold T., Deleuran M., Vestergaard C. Omalizumab induced remission of idiopathic anaphylaxis in a patient suffering from indolent systemic mastocytosis. Acta Dermato-Venereologica. 2014;94(3):363–364. doi: 10.2340/00015555-1687. [DOI] [PubMed] [Google Scholar]
- 75.Paraskevopoulos G., Sifnaios E., Christodoulopoulos K., Mantopoulou F., Papakonstantis M., Sabaziotis D. Successful treatment of mastocytic anaphylactic episodes with reduction of skin mast cells after anti-IgE therapy. European Annals of Allergy and Clinical Immunology. 2013;45(2):52–55. [PubMed] [Google Scholar]
- 76.Carter M. C., Robyn J. A., Bressler P. B., Walker J. C., Shapiro G. G., Metcalfe D. D. Omalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosis. Journal of Allergy and Clinical Immunology. 2007;119(6):1550–1551. doi: 10.1016/j.jaci.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 77.Molderings G. J., Raithel M., Kratz F., et al. Omalizumab treatment of systemic mast cell activation disease: experiences from four cases. Internal medicine. 2011;50(6):611–615. doi: 10.2169/internalmedicine.50.4640. [DOI] [PubMed] [Google Scholar]
- 78.Belloni B., Ziai M., Lim A., et al. Low-dose anti-IgE therapy in patients with atopic eczema with high serum IgE levels. The Journal of Allergy and Clinical Immunology. 2007;120(5):1223–1225. doi: 10.1016/j.jaci.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 79.Chularojanamontri L., Wimoolchart S., Tuchinda P., Kulthanan K., Kiewjoy N. Role of omalizumab in a patient with hyper-IgE syndrome and review dermatologic manifestations. Asian Pacific Journal of Allergy and Immunology. 2009;27(4):233–236. [PubMed] [Google Scholar]
- 80.Stone K. D., Prussin C. Immunomodulatory therapy of eosinophil-associated gastrointestinal diseases. Clinical and Experimental Allergy. 2008;38(12):1858–1865. doi: 10.1111/j.1365-2222.2008.03122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Humbert M., Boulet L. P., Niven R. M., Panahloo Z., Blogg M., Ayre G. Omalizumab therapy: patients who achieve greatest benefit for their asthma experience greatest benefit for rhinitis. Allergy. 2009;64(1):81–84. doi: 10.1111/j.1398-9995.2008.01846.x. [DOI] [PubMed] [Google Scholar]
- 82.Kuehr J., Brauburger J., Zielen S., et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. Journal of Allergy and Clinical Immunology. 2002;109(2):274–280. doi: 10.1067/mai.2002.121949. [DOI] [PubMed] [Google Scholar]
- 83.Bachert C., Gevaert P., Howarth P., Holtappels G., Van Cauwenberge P., Johansson S. G. O. IgE to Staphylococcus aureus enterotoxins in serum is related to severity of asthma. The Journal of Allergy and Clinical Immunology. 2003;111(5):1131–1132. doi: 10.1016/s0091-6749(03)70044-x. [DOI] [PubMed] [Google Scholar]
- 84.Gevaert P., Calus L., Van Zele T., et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. Journal of Allergy and Clinical Immunology. 2013;131(1):110.e1–116.e1. doi: 10.1016/j.jaci.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 85.Vennera M. D. C., Picado C., Mullol J., Alobid I., Bernal-Sprekelsen M. Efficacy of omalizumab in the treatment of nasal polyps. Thorax. 2011;66(9):824–825. doi: 10.1136/thx.2010.152835. [DOI] [PubMed] [Google Scholar]
- 86.Lehmann S., Pfannenstiel C., Friedrichs F., Kröger K., Wagner N., Tenbrock K. Omalizumab: a new treatment option for allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Therapeutic Advances in Respiratory Disease. 2014;8(5):141–149. doi: 10.1177/1753465814547517. [DOI] [PubMed] [Google Scholar]
- 87.Tanou K., Zintzaras E., Kaditis A. G. Omalizumab therapy for allergic bronchopulmonary aspergillosis in children with cystic fibrosis: a synthesis of published evidence. Pediatric Pulmonology. 2014;49(5):503–507. doi: 10.1002/ppul.22937. [DOI] [PubMed] [Google Scholar]
- 88.Collins J., de Vos G., Hudes G., Rosenstreich D. Allergic bronchopulmonary aspergillosis treated successfully for one year with omalizumab. Journal of Asthma and Allergy. 2012;(5):65–70. doi: 10.2147/JAA.S34579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pérez-de-Llano L. A., Vennera M. C., Parra A., et al. Effects of omalizumab in Aspergillus-associated airway disease. Thorax. 2011;66(6):539–540. doi: 10.1136/thx.2010.153312. [DOI] [PubMed] [Google Scholar]
- 90.Incorvaia C., Pravettoni C., Mauro M., Yacoub M.-R., Tarantini F., Riario-Sforza G. G. Effectiveness of omalizumab in a patient with severe asthma and atopic dermatitis. Monaldi Archives for Chest Disease. 2008;69(2):78–80. doi: 10.4081/monaldi.2008.401. [DOI] [PubMed] [Google Scholar]
- 91.Sheinkopf L. E., Rafi A. W., Do L. T., Katz R. M., Klaustermeyer W. B. Efficacy of omalizumab in the treatment of atopic dermatitis: a pilot study. Allergy and Asthma Proceedings. 2008;29(5):530–537. doi: 10.2500/aap.2008.29.3160. [DOI] [PubMed] [Google Scholar]
- 92.Krathen R. A., Hsu S. Failure of omalizumab for treatment of severe adult atopic dermatitis. Journal of the American Academy of Dermatology. 2005;53(2):338–340. doi: 10.1016/j.jaad.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 93.Dufour C., Souillet A. L., Chaneliere C., et al. Successful management of severe infant bullous pemphigoid with omalizumab. British Journal of Dermatology. 2012;166(5):1140–1142. doi: 10.1111/j.1365-2133.2011.10748.x. [DOI] [PubMed] [Google Scholar]
- 94.London V. A., Kim G. H., Fairley J. A., Woodley D. T. Successful treatment of bullous pemphigoid with omalizumab. Archives of Dermatology. 2012;148(11):1241–1243. doi: 10.1001/archdermatol.2012.1604. [DOI] [PubMed] [Google Scholar]
- 95.Savage J. H., Courneya J.-P., Sterba P. M., Macglashan D. W., Saini S. S., Wood R. A. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. Journal of Allergy and Clinical Immunology. 2012;130(5):1123.e2–1129.e2. doi: 10.1016/j.jaci.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nadeau K. C., Schneider L. C., Hoyte L., Borras I., Umetsu D. T. Rapid oral desensitization in combination with omalizumab therapy in patients with cow's milk allergy. The Journal of Allergy and Clinical Immunology. 2011;127(6):1622–1624. doi: 10.1016/j.jaci.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bégin P., Dominguez T., Wilson S. P., et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using Omalizumab. Allergy, Asthma and Clinical Immunology. 2014;10(1, article 7) doi: 10.1186/1710-1492-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williams P. B., Sheppard J. D., Jr. Omalizumab: a future innovation for treatment of severe ocular allergy? Expert Opinion on Biological Therapy. 2005;5(12):1603–1609. doi: 10.1517/14712598.5.12.1603. [DOI] [PubMed] [Google Scholar]
- 99.Chen J.-B., Wu P. C., Hung A. F.-H., et al. Unique epitopes on CεmX in IgE-B cell receptors are potentially applicable for targeting IgE-committed B cells. The Journal of Immunology. 2010;184(4):1748–1756. doi: 10.4049/jimmunol.0902437. [DOI] [PubMed] [Google Scholar]
- 100.Pelaia G., Gallelli L., Renda T., et al. Update on optimal use of omalizumab in management of asthma. Journal of Asthma and Allergy. 2011;4:49–59. doi: 10.2147/JAA.S14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harris J. M., Maciuca R., Bradley M. S., et al. A randomized trial of the efficacy and safety of quilizumab in adults with inadequately controlled allergic asthma. Respiratory Research. 2016;17(1):p. 29. doi: 10.1186/s12931-016-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harris J. M., Cabanski C. R., Scheerens H., et al. A randomized trial of quilizumab in adults with refractory chronic spontaneous urticaria. The Journal of Allergy and Clinical Immunology. 2016;138(6):1730–1732. doi: 10.1016/j.jaci.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 103.Arm J. P., Bottoli I., Skerjanec A., et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clinical & Experimental Allergy. 2014;44(11):1371–1385. doi: 10.1111/cea.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tam S. W., Demissie S., Thomas D., Daëron M. A bispecific antibody against human IgE and human FcγRII that inhibits antigen-induced histamine release by human mast cells and basophils. Allergy. 2004;59(7):772–780. doi: 10.1111/j.1398-9995.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 105.Zhu D., Kepley C. L., Zhang M., Zhang K., Saxon A. A novel human immunoglobulin Fcγ-Fcε bifunctional fusion protein inhibits Fcε RI-mediated degranulation. Nature Medicine. 2002;8(5):518–521. doi: 10.1038/nm0502-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eggel A., Buschor P., Baumann M. J., Amstutz P., Stadler B. M., Vogel M. Inhibition of ongoing allergic reactions using a novel anti-IgE DARPin-Fc fusion protein. Allergy: European Journal of Allergy and Clinical Immunology. 2011;66(7):961–968. doi: 10.1111/j.1398-9995.2011.02546.x. [DOI] [PubMed] [Google Scholar]
- 107.Kim B., Eggel A., Tarchevskaya S. S., Vogel M., Prinz H., Jardetzky T. S. Accelerated disassembly of IgE-receptor complexes by a disruptive macromolecular inhibitor. Nature. 2012;491(7425):613–617. doi: 10.1038/nature11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pennington L. F., Tarchevskaya S., Brigger D., et al. Structural basis of omalizumab therapy and omalizumab-mediated IgE exchange. Nature Communications. 2016;7, article 11610 doi: 10.1038/ncomms11610. [DOI] [PMC free article] [PubMed] [Google Scholar]