Abstract
Many moths finish their long distance migration after consecutive nights, but little is known about migration duration and distance. This information is key to predicting migration pathways and understanding their evolution. Tethered flight experiments have shown that ovarian development of rice leaf folder (Cnaphalocrocis medinalis [Guenée]) moths was accelerated and synchronized by flight in the first three nights, whereby most females were then matured for mating and reproduction. Thus, it was supposed that this moth might fly three nights to complete its migration. To test this hypothesis, 9 year’s field data for C. medinalis was collected from Nanning, Guangxi Autonomous Region in China. Forward trajectories indicated that most moths arrived at suitable breeding areas after three nights’ flight. Thus, for C. medinalis this migration duration and distance was a reasonable adaptation to the geographic distribution of suitable habitat. The development of female moth ovaries after three consecutive night flights appears to be a well-balanced survival strategy for this species to strike between migration and reproduction benefits. Hence, an optimum solution of migration-reproduction trade-offs in energy allocation evolved in response to the natural selection on migration route and physiological traits.
Many insects undertake long-distance migrations to avoid adverse conditions and exploit temporary or patchy habitats1,2,3. Due to their limited flight capabilities, they perform their annual migration journey with the help of the wind at heights of a hundred or even a thousand meters1,2,4,5. Their flight heights are much too high to be seen by the naked eye. Besides, many of them fly in the dark1,2,6,7. Most insect windborne migrations are therefore invisible to a ground based observer. Although the entomological radar is a very useful tool to observe the movement of insects at high altitude, the farthest distance covered by the radar is only ten kilometers at horizon distance and therefore much less than the migratory insect’s flight distance6. Recent technological advancement of high-resolution Global Positioning Systems and other sensors such as miniaturized tracking tags have dramatically improved our ability to describe animal movements. To date, only a few large day-flying insects have been tracked at low heights at distances of ten kilometers ranges8,9,10,11. Very little is therefore known about the distance covered by insects during their migration. How far an insect can migrate is a fundamental question that needs to be answered to predict insect migration pathways.
Many small insects perform non-stop migration and complete their long-distance journey as one continuous flight1. For example, the flight duration of the brown planthopper was estimated to be over 36 h or more12,13. Based on previous studies, many nocturnal moths always take off at dusk, stop flight at the next dawn, and then take-off again at the next dusk. They refuel themselves by feeding on nectar to build up the energy needed for their next flight. They fly on consecutive nights in order to try to complete their long distance migration in as short a period as possible. This type of migration is known as multi-stop migration, and the Armyworm (Mythimna seperata [Walker]), Silver Y moth (Autographa gamma) and the rice leaf roller (Cnaphalocrocis medinalis [Guenée]) are known to adopt this migration strategy5,14,15. Studies have shown that these nocturnal migrants are capable of travelling up to 300 km per night4,5,7, thus any inaccuracy in predicting the number of flight nights will produce a dramatic error in predicting the migration pathway. Tethered flight experiments have been carried out to test the flight capability of migratory moths. It has been shown that C. medinalis can fly 4–5 and even up to 9 consecutive nights16. Results probably do not reflect the flying behavior of moths in the wild because tethered moths were tested in an unnatural setting17,18.
For a small migratory insect, investments in flight organs are costly and it is widely accepted that this is a costly strategy in terms of reproduction output19. Competition for limited internal resources lead to physiological management of migration-reproduction trade-offs in energy allocation20,21. Johnson (1963, 1969) described this migration-mediated reproductive cost as the ‘oogenesis–flight syndrome’. Previous observations have shown that migration in many insects is restricted to the post-teneral, pre-reproductive period22,23,24. Therefore, approximate flight capacity might be ascertained from studies on the oogenesis-flight relationship. A case in hand is C. medinalis, a major migratory insect pest of rice that has had serious outbreaks in many Asian countries in recent decades, especially in China14,15,25. Their migration/reproduction trade-off and the oogenesis-flight relationship has been well studied25,26. The ovarian development of young female C. medinalis moths was accelerated and oviposition was synchronized by flight in the first three nights25 (F. Yang & G. Hu, unpublished). Ovaries of most females was matured for mating and reproduction after three nights’ flight25 (F. Yang & G. Hu, unpublished). Thus, this may indicate that this moth flies three nights to complete its migration. In addition, no significant differences in mating frequency and lifetime fecundity were observed among the unflown controls and those that had flown for 1–3 nights, but moths which took flight more than 3 consecutive nights had lower mating frequency and lifetime fecundity25. In other words, there were no negative influences of flight on reproduction if female moths flew 3 or fewer nights.
As the ‘oogenesis–flight syndrome is produced from responses to natural selection in energy allocation27, flight duration can be considered the optimum solution for migrants to complete their migration and maximize the benefits for their reproduction. Therefore, flight duration can be used to estimate migration distance. Flight duration is also directly affected by the distribution of suitable habitat in both space and time. Most migratory moths should be able to reach their preferred habitat within their flight capability time. Thus, three consecutive nights’ flight should be a reasonable amount of time for C. medinalis to complete its migration and arrive at suitable habitat. To test this hypothesis, 9-years field data were collected for C. medinalis from Nanning City, Guangxi Autonomous Region (1978, 1979, 1980, 1981, 2004, 2005, 2007, 2010 and 2011). The probabilities for C. medinalis to successfully arrive in its expected destination were calculated by forward trajectories (Fig. 1). By exploring the relationship between migration, reproduction and the geographic distribution of its habitat, in this study we aim to gain a better understanding of insect migration and its consequences.
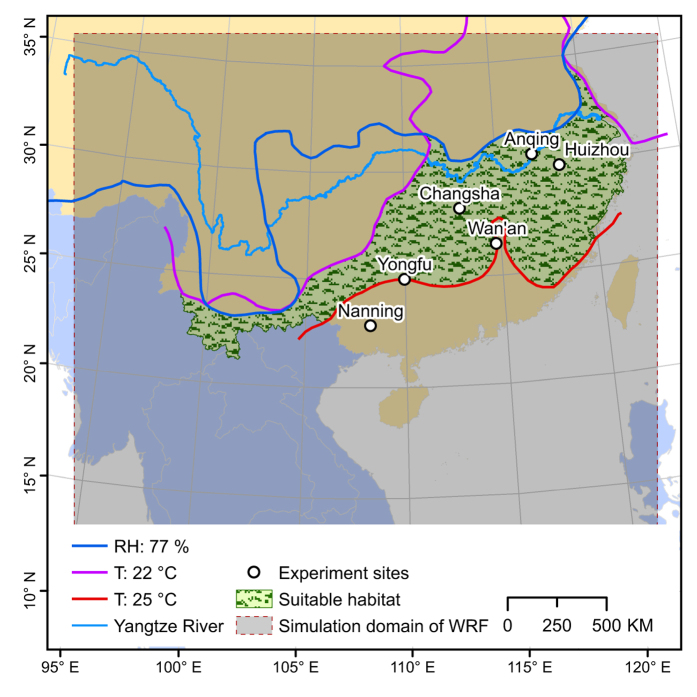
Figure 1. Study area examined using the WRF model to identify suitable habitat for migratory C. medinalis and locations of experimental sites in China.
Suitable habitat was determined by examining temperature, relative humidity and rice plant data considering that (i) the optimal temperature and relative humidity range for C. medinalis eggs is 22–31 °C and 77–100%, respectively48 and (ii) only young rice plants are suitable. Early season rice in southern China is most sensitive to low temperatures during the heading stage52 and rice plants should be too old for C. medinalis if they grew in temperatures greater than 25 °C. Suitable habitat for migratory C. medinalis is to areas where the average temperature is 22–25 °C and the relative humidity is greater than 77%, as indicated on the map. (The map was produced using ArcGIS 10.2 software, http://www.arcgis.com/features/).
Results
Population abundance data from field surveys indicated that there was an emerged peak of C. medinalis adult moths from the end of May to the middle June in Nanning each year (Fig. 2). During this peak, rice plants were at flowering stage and were not suitable for C. medinalis larva to feed on (Table 1). Females with ovarian development Level I - II accounted for more than 65% of the female population in every year (Table 2). This suggested that most adults emerged from the local population and would emigrate before their ovaries matured, according to the criteria described by Zhang et al.26.
Figure 2. Population dynamics of C. medinalis moths in Nanning, China.
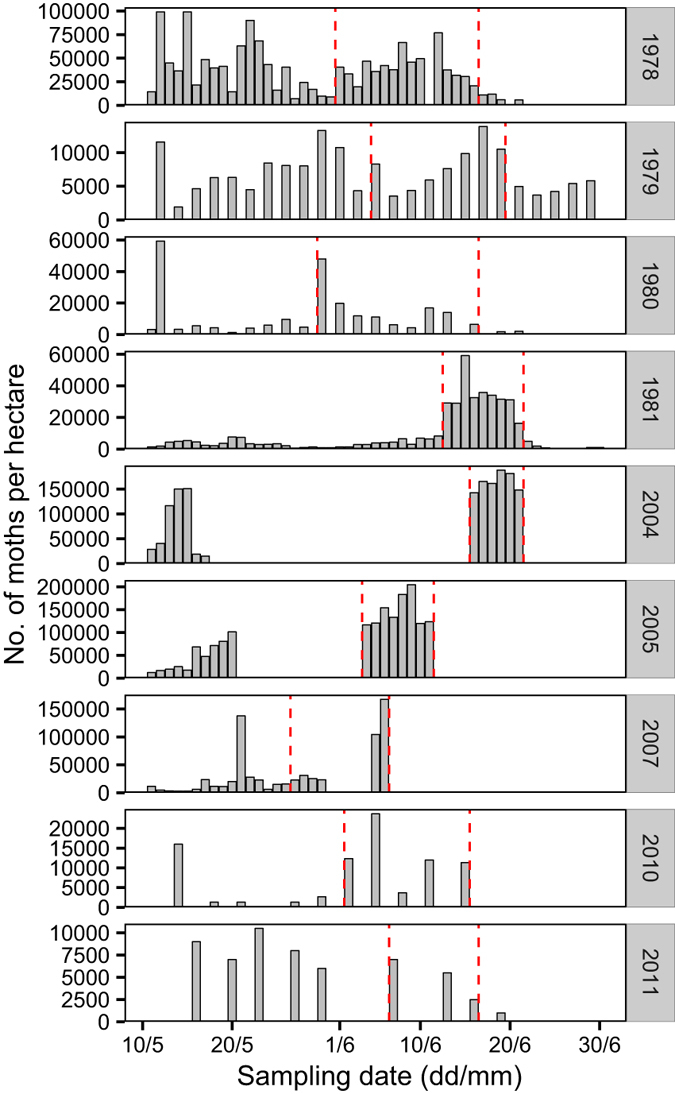
Results of the field survey performed daily in 1978, 1981, 2004, 2005 and 2007, every two days in 1979, 1980, and every 3 days in 2010 and 2011. In 2007, the field survey was performed from 11 May to 6 June but the data from 31 May to 4 June is missing. The period between the 2 dashed red lines indicates the emigration period.
Table 1. Expected growth period of rice in selected sites in China during early and middle June.
| Site | Latitude | Rice varieties | Growth period of rice | Suitable for C. medinalis feeding |
|---|---|---|---|---|
| Nanning | 22.85 | Early season rice | Heading | No |
| Late season rice | Not yet planted | No | ||
| Yongfu | 24.98 | Early season rice | Booting | Yes |
| Late season rice | Not yet planted | No | ||
| Wan’an | 26.46 | Early season rice | Booting to Heading | Yes |
| Late season rice | Sowing | No | ||
| Changsha | 28.20 | Early season rice | Booting to Heading | Yes |
| Middle season rice | Seedling | No | ||
| Late season rice | Sowing | No | ||
| Huizhou | 29.88 | Middle season rice | Tillering | Yes |
| Late season rice | Sowing | No | ||
| Anqing | 30.51 | Early season rice | Booting | Yes |
| Middle season rice | Transplanting | No | ||
| Late season rice | Sowing | No |
Table 2. Abundance of C. medinalis moths and female ovarian development in Nanning, China.
| Year | Period | No. of days | No. of moths observed per ha. | No. of moths dissected | Proportion (No.) of moths in each stage of ovarian development |
||
|---|---|---|---|---|---|---|---|
| Level - I | Level - II | ≥Level - III | |||||
| 1978 | 1–16 Jun | 15 | 616200 | 852 | 74.88 (638) | 7.16 (61) | 17.96 (153) |
| 1979 | 5–19 Jun | 8 | 64020 | 272 | 60.66 (165) | 10.29 (28) | 29.04 (79) |
| 1980 | 30 May–16 Jun | 9 | 138675 | 304 | 74.01 (225) | 8.22 (25) | 17.76 (54) |
| 1981 | 13–21 Jun | 9 | 298650 | 998 | 88.48 (883) | 8.72 (87) | 2.81 (28) |
| 2007 | 27 May–6 Jun | 7 | 374460 | 176 | 36.93 (65) | 28.98 (51) | 34.09 (60) |
Distributions of temperature and relative humidity in early and middle June indicated that the expected destination of migratory C. medinalis was located in the lower and middle reaches of the Yangtze River (Fig. 1). The rice planting schedules surveyed at Yongfu, Changsha, Huizhou and Huaining indicated that farmers practice both single- and double-cropping in this region (see Table 1). The early season rice in this area was at booting stage during this period and was harvested from middle July to early August. The late season rice was transplanted after the harvest of early rice. Single cropping rice planting technique is also known as middle season rice planting. Middle season rice is generally planted later than early rice. The plants of middle season rice were transplanted from late May to early July irregularly and were at different stages from seedling to tillering in early and middle June (Table 1). In summary, the progeny produced from migratory C. medinalis would be able to find suitable host rice plants at the correct growth stage located in the lower and middle reaches of the Yangtze River during early and middle June.
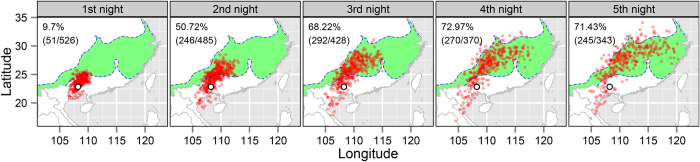
Forward trajectories were calculated for 110 events and the distributions of endpoints are shown in Fig. 3. Most trajectories arrived after three or four consecutive nights to within the area delineated as suitable habitat located in the lower and middle reaches of the Yangtze River (Fig. 3). After three consecutive nights’ flight, only 68.22% (292/406) valid endpoints located to within the suitable habitat, while 72.97% (270/370) did so after four consecutive nights. This may have been because the number of invalid endpoints increased as the number of nights increased (Fig. 3 and Table 3). However, the proportional difference after three and four nights was only 4.75%. Trajectories were calculated with different initial heights (See method, Table 3). Three consecutive nights’ flight was enough for most moths to finish their migration and find out their suitable habitat at all heights except at 1500 m (Table 3).
Figure 3. Distributions of endpoints of forward trajectories starting from Nanning, China.
Red dots indicate the endpoints of the forward trajectories, and the green-filled region delineates the potential suitable habitat of C. medinalis (Fig. 1). The percentage of endpoints that successfully landed in suitable habitat is given with the number of all valid endpoints shown in parenthesis. Trajectory analysis was determined over 5 consecutive nights. (The map is made by R software, https://www.r-project.org/).
Table 3. Proportions of trajectory analysis endpoints that were located in the expected destinations of suitable habitat.
| Height | Percent of valid endpoints (%) |
||||
|---|---|---|---|---|---|
| 1st night | 2nd night | 3rd night | 4th night | 5th night | |
| 500 m | 10.20 (10/98) | 30.99 (22/71) | 41.18 (14/34) | 26.67 (4/15) | 0.00 (0/6) |
| 750 m | 15.89 (17/107) | 46.81 (44/94) | 67.09 (53/79) | 59.62 (31/52) | 56.41 (22/39) |
| 1000 m | 12.15 (13/107) | 56.73 (59/104) | 69.7 (69/99) | 72.73 (64/88) | 65.88 (56/85) |
| 1250 m | 7.48 (8/107) | 57.41 (62/108) | 73.83 (79/107) | 78.1 (82/105) | 75.00 (78/104) |
| 1500 m | 2.80 (3/107) | 54.63 (59/108) | 70.64 (77/109) | 80.91 (89/110) | 81.65 (89/109) |
| Total | 9.70 (51/526) | 50.72 (246/485) | 68.22 (292/428) | 72.97 (270/370) | 71.43 (245/343) |
Analysis was carried out on 5 consecutive nights and for five different altitude heights. This number is also expressed out of a total number of endpoints tested and given in parenthesis.
Discussion
The morphological and physiological traits differences between migrant and non-migrant individuals of the same species have been studied widely27, especially within the context of the oogenesis-flight syndrome22. All these migratory traits were thought to be the consequence of physiology and morphology to adapt to migratory behavior27. Many studies on bird migration have shown that migration distance plays an important role in the expression of migratory traits, including fuel load and aerodynamic properties28,29,30,31. But studies on the driving forces or factors to shape insect migratory traits have been limited. In this study, most C. medinalis moths arrived at suitable breeding areas within three nights of simulated flight, and this migration duration agreed with our supposition as derived from previous studies on its physiological traits of ovarian development25. The distribution of suitable habitat presents the migration distance of flyers should cover, thus our results suggest for the first time, that migration distance represents an important role in determining the migration duration of C. medinalis, which may in turn shape its oogenesis-flight syndrome.
The distributions of rice host, temperature and relative humidity suggested that the expected destination of migratory C. medinalis moths was located in the lower and middle reaches of the Yangtze River during early and middle June. This result was in agreement with previous studies on migration processes in 1977, 1979 and 198014,15. In these three years, C. medinalis moth population peaks occurred simultaneously in a wide area in this region during late May and the middle of June14,15. Based on these observations, its northward migration to eastern Asia was divided into 5 steps. Among these 5 steps, moths migrated from South China to the Yangtze River Valley in the third and fourth steps during late May to middle July14,15. The Yangtze River Valley is one of the most important rice-producing regions in eastern Asia covering the Jiangxi, Anhui, Jiangsu, Zhejiang and Shanghai Provinces32. Thus, it is a very important region available for C. medinalis to build and develop its population. Most individuals from the Yangtze River Valley population migrate to South China in autumn, except for a few individuals that migrate further north33. Compared with the other migration steps, the migration distance of the third step also was the longest. In this paper, only the third migration step during late May and middle June was studied. Our conclusions are very credible and meaningful because this third migration step is the most important for C. medinalis to exploit suitable habitat.
Due to changes in available resources through time and space, exploiting seasonal breeding habitats and escaping deteriorating conditions is the primary driver of the evolution of long-range migration7,27,34. Thus, the spatiotemporal distribution of suitable habitat has a great influence on the determination of migratory pathways, especially for monophagous or oligophagous insects. As expected, major rice crop pests such as C. medinalis and two rice planthopper species Nilaparvata lugens (Stål) and Sogatella furcifera (Horváth) share similar migration pathways in eastern Asia14,15,35,36. Previous studies have shown that population dynamics and migration patterns of C. medinalis and N. lugens have dramatically changed according to the change of rice planting systems32,37,38,39. In the Yangtze River valley, double-cropping rice was mainly planted from 1960 s to 1990 s, but double-cropping technique has now been greatly reduced and single-cropping practice has been rapidly increasing since 199732. Due to the high suitability of single cropping rice, populations of C. medinalis and N. lugens grew so quickly that it caused serious local damage in these regions and mass emigration to the Yangtze River Delta32,37,38,39. It can therefore be inferred that migration distances were determined greatly by the distribution of suitable migratory habitat.
Previous studies also suggested that migration distance was related to body size, behavior strategy and morphological characteristics40,41. Compared to C. medinalis, rice planthoppers perform non-stop migration to finish their long-distance journey in one continuous flight. They use a different migration strategy to cover a similar migration distance. Study between silver Y moths and passerines showed that they achieved extremely similar levels of travel speed and orientation performance during their nocturnal migratory flights in the northern temperate zone by different behavioral strategies3,4. Therefore, it is unclear whether the migration distance was driven by a large variety in characteristics of morphology or animal behavior. Further work needs to be done to answer these complex questions. By contrast, our results suggest that migration distance could represent an important role to determine the migration duration of C. medinalis and to shape its oogenesis-flight syndrome. C. medinalis female moths developed their ovaries after three consecutive flight nights is a well-balanced surviving strategy to strike between migration and reproduction benefits. Hence, three nights’ flight seem to be an optimum solution of migration-reproduction trade-offs in energy allocation evolved in response to the natural selection on migration route and physiological traits.
Method
Survey Data of C. medinalis
Nine years of survey data of C. medinalis was collected from rice paddy fields in Nanning, Guangxi Autonomous Region in China (Fig. 1). Data was collected in May and June during the years 1978, 1979, 1980, 1981, 2004, 2005, 2007, 2010 and 2011, and comprises date recorded, moth abundance and ovary development. The abundance of moths was recorded based on a survey that was carried out in the morning, beginning at 7:00 AM (Beijing Time, BJT). A 2 m stick was swept across the canopy of rice plants, and the number of disturbed moths flying away were counted by eye. The survey covered a sweep area of at least 40 m2 of rice plants each morning. The number of moths per hectare was calculated and recorded. Ovary dissection data are available for the years 1978–1981 and 2007. Female moths of C. medinalis were collected from rice paddies and dissected daily to determine their stage of ovarian development according to the criteria described by Zhang et al.26. Females with ovarian development Level I–II were regarded as ‘sexually immature individuals’ and those with Level III–V were regarded as ‘sexually mature individuals’. The proportion of females with ovarian development categorised as Level–I, Level–II and ≥Level–III was tabulated.
Meteorological data and modelling
The Weather Research and Forecasting (WRF) Model is a next-generation mesoscale numerical weather prediction system42. The model is designed to serve both atmospheric research and operational forecasting needs. The model was used to produce a high resolution atmospheric background for the trajectory analysis carried out in this study. The dimensions of the model domain were 99 × 84 grid points in a resolution of 30 km. Twenty-nine vertical layers were available and the model ceiling was 100 hPa. The scheme selection and parameters used for the WRF are listed in Supplementary Table S1. National Centers for Environmental Prediction (NCEP) Final Analysis (FNL) Data was used as the meteorological data for the model input. FNL is a six-hourly, global, 1-degree grid meteorological dataset. The model forecast time is 72 h with data outputs at 1 h intervals, for horizontal and vertical wind speeds, temperature and precipitation.
Forward Trajectory Analysis
We estimated suitable migratory landing areas for C. medinalis moth by constructing their forward flight trajectories with the following assumptions. Firstly, no orientation pattern of C. medinalis was detected by radar, but moths displaced downwind33. Secondly, results from tethered flight experiments suggested that its self-powered flight speed was about 0.8 m/s and it cannot fly in an atmospheric temperature below 12.9 °C43. Thirdly, C. medinalis take off at dusk, about 20:00 BJT33,43,44. Fourthly, few C. medinalis moths have been observed by radar and aerial net after midnight33,45, and flight activity significantly decreases after 6 hours’ continuous flight (see Supplementary Fig. S1) leading most migrants to descend and land. Finally, the flight altitude of C. medinalis observed by radar was below 1000 m and most moths concentrated at about 500 m in autumn33,45. Forward trajectories on the first migration night began from Nanning with the start time taken at dusk (about 20:00 BJT), using five different initial flight altitude heights: 500, 750, 1000, 1250 and 1500 m above mean sea level. The air speed of migrants was wind speed plus with its self-powered flight (0.8 m/s). Trajectories were terminated when the air temperature fell below 12.9 °C or flight duration was up to six hours. On the second migration night, trajectory analysis continued from the last known locations of the first night’s trajectories with the same take off time and altitude. The complete forward trajectories analysis was calculated continuously for 5 consecutive nights.
This method was first introduced by Zhu and Liao46 with wind field and temperature data from the WRF output46,47. Further details for this method was described by Hu et al. & Lu et al.12,47. The script for calculating this trajectory was written in FORTRAN and run using the operating system Fedora 13 (Fedora Project, http://fedoraproject.org/).
Expected destination of migratory C. medinalis
Previous studies have suggested that the optimal temperature and relative humidity ranges for rice leaf folder eggs were 22–31 °C and 77–100%, respectively48. In addition, C. medinalis moths prefer to lay eggs on rice plants at tillering stage and its larva mostly feed on rice plants between tillering and booting stages49,50. Adult moths emigrate when the rice host is at a deteriorative or heading stage14,51. Therefore, the expected destination of migratory moths should be to areas where rice plants were between tillering and booting stages. In South China, early and late season rice are planted successively in the same year, a planting system known as double-cropping rice32. The early rice must be harvested as early as possible in the summer, so that the fields can be ploughed and levelled for transplanting the late season rice. Rice plants are sensitive to low temperatures during the plant’s reproductive stage and the optimum temperature for indica rice heading is 25–35 °C52. Thus, no suitable rice hosts for C. medinalis larva would be available in areas where the average temperature was greater than 25 °C in South China in the first half of the year. Based on the above analyses, the expected destination of migratory C. medinalis is to areas where the average temperature is 22–25 °C and the relative humidity was greater than 77%. Daily long-term mean temperature and relative humidity were derived from the Japanese 55-year Reanalysis datasets (http://jra.kishou.go.jp/JRA-55). The meteorological information was applied to explore the range of suitable habitat of C. medinalis in South China. In addition, the rice planting schedules were surveyed at Nanning, Yongfu, Changsha, Huizhou and Huaining to check whether suitable rice host were growing (Fig. 1).
Additional Information
How to cite this article: Wang, F.-Y. et al. Determining the migration duration of rice leaf folder (Cnaphalocrocis medinalis (Guenée)) moths using a trajectory analytical approach. Sci. Rep. 7, 39853; doi: 10.1038/srep39853 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was supported by the Fundamental Research Funds for the Central Universities (KJQN201434). GH’s visiting scholarship at Rothamsted was funded by the China Scholarship Council and the Priority Academic Program Development of Jiangsu Higher Education Institutions. Rothamsted Research is a national institute of bioscience strategically funded by the UK Biotechnology and Biological Sciences Research Council (BBSRC).
Footnotes
Author Contributions G.H. and B.P.Z. designed the analysis, F.Y.W. and F.Y. performed the analysis with input from S.Y.L., F.Y.W. and M.H.L. All authors except S.Y.L. contributed to the interpretation of results and manuscript writing.
References
- Dingle H. Migration: the biology of life on the move. New York: Oxford University Press (1999). [Google Scholar]
- Hu G. Lim K. S., Reynolds D. R., Reynolds A. M. & Chapman J. W. Wind-related orientation patterns in diurnal, crepuscular and nocturnal high-altitude insect migrants. Front. Behav. Neurosci. 10, 32 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. W., Reynolds D. R. & Wilson K. Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 18, 287–302 (2015). [DOI] [PubMed] [Google Scholar]
- Alerstam T. et al. Convergent patterns of long-distance nocturnal migration in noctuid moths and passerine birds. Proc. R. Soc. B 278, 3074–3080 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. W. et al. Flight orientation behaviors promote optimal migration trajectories in high-flying insects. Science 327, 682–685 (2010). [DOI] [PubMed] [Google Scholar]
- Chapman J. W., Drake V. A. & Reynolds D. R. Recent insights from radar studies of insect flight. Annu. Rev. Entomol. 56, 337–356 (2011). [DOI] [PubMed] [Google Scholar]
- Chapman J. W. et al. Animal orientation strategies for movement in flows. Curr. Biol. 21, R861–R870 (2011). [DOI] [PubMed] [Google Scholar]
- Cant E. T., Smith A. D., Reynolds D. R. & Osborne J. L. Tracking butterfly flight paths across the landscape with harmonic radar. Proc. R. Soc. B 272, 785–790 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikelski M. et al. Simple rules guide dragonfly migration. Biol. Lett. 2, 325–329 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissling W. D., Pattemore D. E. & Hagen M. Challenges and prospects in the telemetry of insects. Biol. Rev. 89, 511–530 (2014). [DOI] [PubMed] [Google Scholar]
- Kays R., Crofoot M. C., Jetz W. & Wikelski M. Terrestrial animal tracking as an eye on life and planet. Science 348, aaa2478 (2015). [DOI] [PubMed] [Google Scholar]
- Hu G. et al. The influence of typhoon Khanun on the return migration of Nilaparvata lugens (Stål) in Eastern China. PLoS ONE 8, e57277 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otuka A. Migration of rice planthoppers and their vectored re-emerging and novel rice viruses in East Asia. Front Microbiol. 4, 309 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. S. et al. Studies on the migration of rice leaf roller Cnaphalocrocis Medinalis Guenée. Acta Entomologica Sinica 23, 130–140 (1980). [Google Scholar]
- Zhang X., Geng J. & Zhou W. A study on ecological mechanism of the migration of rice leaf roller Cnaphalocrocis Medinalis Guenée. Journal of Nanjing Agricultural College 4, 40–51 (1981). [Google Scholar]
- Wang F. Y., Zhang X. X. & Zhai B. P. Flight and re-migration capacity of the rice leaf folder moth Cnaphalocrocis Medinalis (Guenée) (Lepidoptera Crambidae). Acta Entomologica Sinica, 53, 1265–1272 (2010). [Google Scholar]
- Riley J. R., Downham M. C. A. & Cooter R. J. Comparison of the performance of Cicadulina leafhoppers on flight mills with that to be expected in free flight. Entomol. Exp. Appl. 83, 317–322 (1997). [Google Scholar]
- Jones H. B. C., Lim K. S., Bell J. R., Hill J. K. & Chapman J. W. Quantifying interspecific variation in dispersal ability of noctuid moths using an advanced tethered flight technique. Ecol. Evol. 6, 181–190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff D. A. Dispersal in dipterans: its costs and consequences. J. Anim. Ecol. 46, 443–456 (1977). [Google Scholar]
- Rankin M. A. & Burchsted C. A. The cost of migration in insects. Annu. Rev. Entomol. 37, 533–539 (1992). [Google Scholar]
- Zera A. J. Intermediary metabolism and life history trade-offs: lipid metabolism in lines of the wing-polymorphic cricket, Gryllus firmus, selected for flight capability vs. early age reproduction. Am. Nat. 167, 889–900 (2006). [DOI] [PubMed] [Google Scholar]
- Johnson C. G. Physiological factors in insect migration by flight. Nature 198, 423–427 (1963). [Google Scholar]
- Johnson C. G. Migration and dispersal of insects by flight. Methuen, London (1969). [Google Scholar]
- Kennedy J. S. A turning point in the study of insect migration. Nature 198, 785–791 (1961) [Google Scholar]
- Zhang L. et al. Accelerated and synchronized oviposition induced by flight of young females may intensify larval outbreaks of the rice leaf roller. PLoS ONE 10, e0121821 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. X., Lu Z. Q. & Geng J. G. Application of ovarian dissection of female Cnaphalocrocis medinalis moths in prediction and forecasting system. Entomological Knowledge 16, 97–99 (1979). [Google Scholar]
- Dingle H. & Drake V. A. What is migration? BioScience 57, 113–121 (2007). [Google Scholar]
- Maggini I. & Bairlein F. Endogenous rhythms of seasonal migratory body mass changes and nocturnal restlessness in different populations of northern wheatear Oenanthe oenanthe. J. Biol. Rhythm. 25, 268–276 (2010). [DOI] [PubMed] [Google Scholar]
- Delingat J., Hobson K., Dierschke V., Schmaljohann H. & Bairlein F. Population differentiation of northern wheatears by means of morphometric data and stable isotopes. J. Ornithol. 152, 383–395 (2011). [Google Scholar]
- Förschler M. I. & Bairlein F. Morphological shifts of the external flight apparatus across the range of a passerine (northern wheatear) with diverging migratory behaviour. PLoS ONE 6, e18732 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman A. M., Bairlein F. & Schmaljohann H. The nature of the migration route shapes physiological traits and aerodynamic properties in a migratory songbird. Behav. Ecol. Sociobiol. 68, 391–402 (2014). [Google Scholar]
- Hu G. et al. Rice planting systems, global warming and outbreaks of Nilaparvata lugens (Stål). B. Entomol. Res. 101, 187–199 (2011). [DOI] [PubMed] [Google Scholar]
- Riley J. R. et al. Observations of the autumn migration of the rice leaf roller Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) and other moths in eastern China. B. Entomol. Res. 85, 397–414 (1995). [Google Scholar]
- Alerstam T., Hedenström A. & Åkesson S. Long-distance migration: evolution and determinants. Oikos 103, 247–260 (2003). [Google Scholar]
- Cheng S. N. et al. Studies on the migrations of brown planthopper Nilaparvata lugens Stål. Acta Entomologica Sinica 22, 1–21 (1979). [Google Scholar]
- Lu Z. Q., Shu W. X., Zhu S. D., Hu M. & Yang J. S. Synchronized migrations and population dynamics of rice leaf roller and white backed planthopper. China Plant Protection. 11, 23–32 (1990). [Google Scholar]
- Hu G. et al. Outbreaks of the brown planthopper Nilaparvata lugens (Stål) in the Yangtze River Delta: immigration or local reproduction. PLoS One 9, e88973 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi G. J. et al. Occurrence of the third and fourth generations of Cnaphalocrocis medinalis in the mixed cropping rice region in Anqing, Anhui Province. Chinese J. Rice Sci. 22, 513–518 (2008). [Google Scholar]
- Qi G. J. et al. Effect of the change of rice planting system on the formation of outbreak population of brown planthopper, Nilaparvata lugens (Stål). Acta Phytophylacica Sinica. 37, 193–200 (2010). [Google Scholar]
- Hein A. M., Hou C. & Gillooly J. F. Energetic and biomechanical constraints on animal migration distance. Ecol. Lett. 15, 104–110 (2012). [DOI] [PubMed] [Google Scholar]
- Johansson F., Söderquist M. & Bokma F. Insect wing shape evolution: independent effects of migratory and mate guarding flight on dragonfly wings. Biol. J. Linn. Soc. 97, 362–372 (2009). [Google Scholar]
- Skamarock W. et al. A Description of the Advanced Research WRF Version 3. NCAR Technical Note NCAR/TN-475+STR (2008). [Google Scholar]
- Chen Y. N. & Wang Q. W. The observation of flight characteristics of Cnaphalocrocis medinalis III. The initiative of take-off and passive of operation. Journal of Hunan Agricultural College, 6, 23–33 (1980). [Google Scholar]
- Zhang X. X., Zhou L. Y. & Cheng J. Y. Determination of the parameters for trajectory analysis of in Huaihe and Yangtze rice areas. Journal of Nanjing Agricultural University, 17, 32–38 (1994). [Google Scholar]
- Gao Y. B. et al. Dynamic analysis on the migration of the rice leaf roller Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) by Doppler Insect Monitoring Radar and numerical simulation. Acta Ecologica Sinica 11, 5238–5247 (2008). [Google Scholar]
- Zhu Y. Q. & Liao D. X. An investigation for computing three-dimensional trajectory. Quarterly Journal of Applied Meteorology 3, 328–333 (1992). [Google Scholar]
- Lu F., Zhai B. P. & Hu G. Trajectory analysis methods for insect migration research. Chinese Journal of Applied Entomology 50, 853–862 (2013). [Google Scholar]
- Fang Y. S., Liao H. J., Qian Q. & Liu X. D. Combined effects of temperature and relative humidity on eggs of the rice leaf folder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). Acta Entomologica Sinica 56, 786–791 (2013). [Google Scholar]
- Wu J. C. & Zhang X. X. Effect s of temperature on the growth and the development of experimental populations of the rice leaf roller, Cnaphalocrocis medinalis Guenée. Journal of Nanjing Agricultural College 7, 19–28 (1984). [Google Scholar]
- Liao H. J., Huang J. R., Fang Y. S. & Liu X. D. Biological response of the rice leaf folder Cnaphalocrocis medinalis (Guenée) reared on rice and maize seedling to temperature. Acta Ecologica Sinica 33, 409–415 (2013). [Google Scholar]
- Wu J. C. Effect of changing photoperiod, temperature and food quality on the migration of rice leaf roller Cnaphalocrocis medinalis Guenée. Acta Entomologica Sinica, 28, 398–405 (1985). [Google Scholar]
- Tsunoda S. Adjustment of photosynthetic structures in three steps of rice, evolution. In Biology of Rice (eds Tsunoda S. & Takahashi N.) 89–116 (Tokyo: Jpn. Sci. Sac. Press., 1984). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.