Abstract
In heterogeneous environments, the capacity for colour change can be a valuable adaptation enhancing crypsis against predators. Alternatively, organisms might achieve concealment by evolving preferences for backgrounds that match their visual traits, thus avoiding the costs of plasticity. Here we examined the degree of plasticity in pigmentation of newt larvae (Lissotriton boscai) in relation to predation risk. Furthermore, we tested for associated metabolic costs and pigmentation-dependent background choice behaviour. Newt larvae expressed substantial changes in pigmentation so that light, high-reflecting environment induced depigmentation whereas dark, low-reflecting environment induced pigmentation in just three days of exposure. Induced pigmentation was completely reversible upon switching microhabitats. Predator cues, however, did not enhance cryptic phenotypes, suggesting that environmental albedo induces changes in pigmentation improving concealment regardless of the perceived predation risk. Metabolic rate was higher in heavily pigmented individuals from dark environments, indicating a high energetic requirement of pigmentation that could impose a constraint to larval camouflage in dim habitats. Finally, we found partial evidence for larvae selecting backgrounds matching their induced phenotypes. However, in the presence of predator cues, larvae increased the time spent in light environments, which may reflect a escape response towards shallow waters rather than an attempt at increasing crypsis.
Concealment from predators is of capital importance in an organism’s life, given that predation is one of the strongest selective pressures in nature. As such, camouflage is a widespread behavioural tactic employed by animals in order to reduce the risk of being detected or recognized1,2,3. Camouflage strategies are diverse, including background matching through crypsis4,5,6, disruptive coloration promoting misidentification of the outlines of the body7,8,9,10, or even masquerade (i.e., resemblance of an organism to an inedible object)11,12,13. Although defensive adaptations may target sensory systems other than vision14, most remarkable examples involve visual camouflage and cryptic coloration.
While many animals have evolved constitutive colour patterns matching their habitats as key adaptions, organisms may often face spatial or temporal environmental heterogeneity so that a single pigmentation pattern may not provide crypsis across all environmental conditions15. One way to maximize crypsis in those cases is evolving a coloration constituting a compromise between the requirements of the differing microhabitats16,17,18,19. Alternatively, opposing selection pressures can generate a prey species to evolve two or more distinct morphs, each one adapted to a different part of the environment (i.e., polymorphic crypsis)20. Apart from promoting these constitutive adaptions, selection is expected to favour the capacity to plastically alter coloration depending on the surrounding conditions21. As it is also true for other plastic traits, colour change may be energetically costly, as it requires cell migration and synthesis or recruitment of pigments, and may even compromise the immune system through reallocation of resources to pigment production22,23,24,25. In rapidly changing environments, alterations in the pigmentation pattern must be reversible and promptly elicited to be effective in the short-term26. However, if such alterations are costly, we hypothesize that organisms would more readily trigger colour changes when the perceived risk of predation is higher, so that they would be more prone to pay the cost of shifting pigmentation as the environment changes in the presence of predators than in predator-free environments. Moreover, if changes in pigmentation entail costs and organisms have the chance to move across dissimilar habitat patches, they might have evolved preferences for patches in which their colour patterns grant better crypsis, rather than incurring the costs of altering their pigmentation. Also, prey might have evolved background matching preferences to reduce detectability if their ability to change colour is not quick enough to match the pace of environmental change. Here we tested these ideas about the interplay of pigmentation plasticity, costs of pigment production, and habitat choice using amphibian larvae as study system.
Many amphibians rely on cryptic coloration as their first line of defence27. By achieving colour patterns resembling habitat features, both anurans and salamanders avoid detection from visual predators, thereby improving their fitness28,29,30,31. In addition to constitutive adaptations, amphibians can increase concealment by expressing plasticity in skin coloration. In response to environmental stimuli such as background colour, light intensity, temperature, humidity or stress, melanosomes (i.e., the light absorbing organelles) can be reallocated in the melanophores (i.e., a type of pigment cells within the dermal chromatophore unit), resulting in a lightening (pigment aggregation) or darkening (pigment dispersion) of the skin32,33,34,35,36,37. Although these colour shifts are not instantaneous as those exhibited by chameleons, flatfish or cephalopods38,39,40, both larvae and adults of many amphibian species benefit from relatively rapid (i.e., within minutes) as well as more long-term (i.e., several weeks) colour change, which can be effective in hampering predator detection3,41.
Although the ability of amphibians to adjust skin coloration may result advantageous in achieving crypsis, individuals may incur costs and trade-offs associated to colour change23,24,26. This may represent, for instance, an important constraint for larvae to conceal in dark, low-reflecting environments, since rearranging pigment organelles in the dermis (or even increasing the number of melanosomes and chromatophores) requires energy expenditure, which may result in competing demands with other physiological processes and growth22,23. An accurate assessment of predatory threats seems therefore essential for amphibian larvae to maximize the benefits of pigmentation plasticity. In several anuran species, larvae respond to the presence of predators by increasing tail coloration42,43,44,45. This predator-induced phenotype is interpreted as a defensive strategy to deflect attacks of predators away from critical parts of the body9,46,47. Antipredator responses can be so fine-tuned to the risk of predation that the same amphibian species may markedly increase coloration at the distal portion of the tail in the presence of ambush predators, or reduce pigmentation altogether in the presence of fish, which are much more visually guided predators48.
Because background matching effectively reduces predation17,49,50, a common assumption is that prey have been selected to prefer appropriate habitats matching their visual aspect. Although few studies have found empirical support for such behaviour51,52,53,54,55,56, the idea that prey recognize and show preferences for backgrounds that confer greater crypsis against predators has been often presumed, also in amphibian larvae57,58.
The vast majority of studies on amphibian camouflage have focused on anurans28,33,34,37,59,60 (see ref. 61 for an extensive review), whereas the adaptive value of cryptic coloration and behaviour of salamanders –aside from their aposematic traits62,63,64– has received considerably less investigation. We analysed the expression of phenotypic plasticity in skin pigmentation of larvae of the Iberian newt, Lissotriton boscai, in response to different albedo environments and the presence of chemical cues from a common predator: nymphs of the dragonfly Anax imperator. We tested the following hypotheses: (1) L. boscai larvae have the ability to adjust skin pigmentation to different reflecting microhabitats, (2) such changes in pigmentation are modulated by perceived risk of predation, (3) induced pigmentation changes are reversible, (4) there are metabolic costs associated to increased skin pigmentation, and (5) background choice behaviour is conditional upon individual level of pigmentation. We expected larvae to gradually darken or lighten depending on environmental albedo, in order to match their backgrounds and improve concealment against potential predators. If altering pigmentation entailed substantial metabolic costs, we expected exposure to predator cues to make larval newts more prone to pay such costs in order to achieve fine-tuned background colour matching.
Results
Skin pigmentation
Environmental albedo induced significant changes in skin pigmentation of larval newts throughout the first five weeks of the experiment. Skin pigmentation of larvae in light, high-reflecting microhabitats decreased by 68% on average, whereas it increased by 347% on average in dark, low-reflecting microhabitats (F3,56 = 75.81, p < 0.0001; Fig. 1). At the beginning of the experiment (day 0), skin pigmentation of larvae in light and dark microhabitat conditions was similar (p = 0.76; Fig. 1). Intra-individual changes in skin pigmentation started to be noticeable as early as 3 days after the experiment began, both for light and dark conditions (p < 0.0001 in both cases). From day 3 to week 2, skin pigmentation of larvae in light conditions decreased even further (p = 0.04), but pigmentation of larvae in dark conditions remained invariant (p = 0.99). From week 2 to week 5, skin pigmentation of larvae did not vary within microhabitat (p = 0.21 and p = 0.12 for light and dark conditions, respectively). Differences in skin pigmentation between the two microhabitat treatments were clear at day 3, and continued to be significant during the rest of the experiment (p < 0.0001 in all cases) (Fig. 1). Exposure to predator cues had no significant effect on changes in skin pigmentation (F3,56 = 0.45, p = 0.72), and did not interact with microhabitat conditions (F3,56 = 0.58, p = 0.63).
Figure 1. Mean ± SE of changes in skin pigmentation of larval newts induced by environmental albedo.
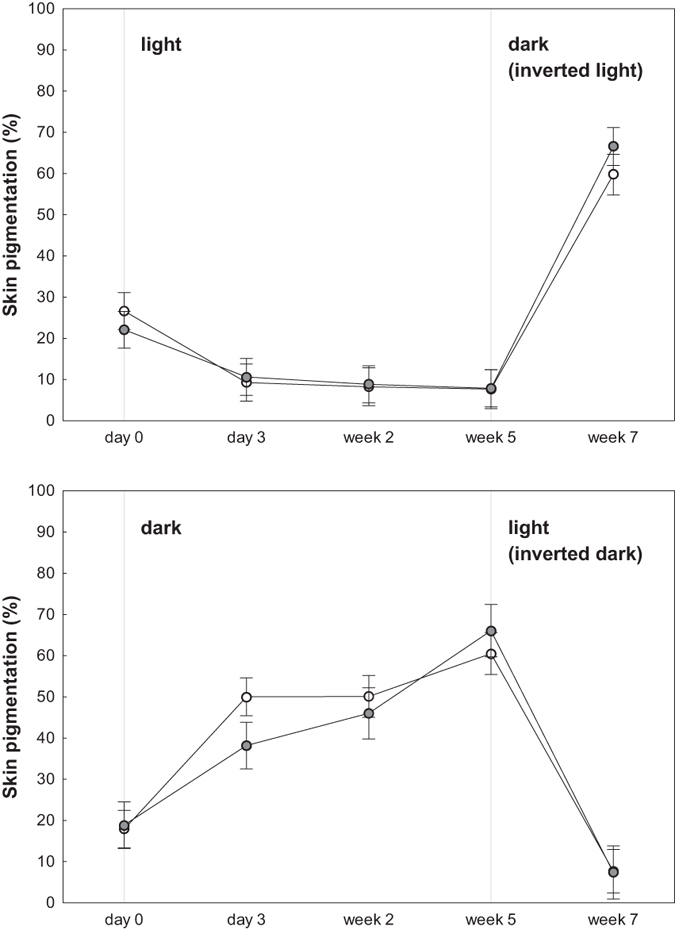
From day 0 to week 5: induced changes under light (upper panel) and dark (bottom panel) microhabitat conditions. From week 5 to week 7: reverted changes after inverting microhabitat conditions. Open circles represent larvae in a predator-free environment; solid circles represent larvae exposed to predator cues.
Reversibility
Changes in skin pigmentation of larval newts induced by microhabitat conditions were reverted from week 5 to week 7 (F1,37 = 915.88, p < 0.0001; Fig. 1). Thus, after inverting microhabitat conditions, larvae in both light and dark treatments adjusted skin pigmentation accordingly: larvae moved from light to dark conditions drastically increased pigmentation from week 5 to week 7 (p = 0.0002). Likewise, larvae moved from dark into light conditions markedly reduced their pigmentation during the same period of time (p = 0.0002) (Fig. 1). Skin pigmentation of larvae between the two microhabitats differed clearly on week 7 (p = 0.0002). Neither the presence of predator cues nor its interaction with microhabitat conditions affected the reversibility of skin pigmentation of larval newts (F1,37 = 0.47, p = 0.5 and F1,37 = 0.06, p = 0.81 respectively).
Metabolic costs
To assess the potential metabolic cost associated to skin pigmentation or depigmentation of larval newts, we measured standard metabolic rate (SMR) of larvae exposed to different microhabitat treatments. SMR of larval newts differed between light and dark conditions (F1,45 = 5.45, p = 0.02). After two weeks of experiment, larvae in dark conditions showed 64% higher rates of oxygen consumption (mean ± SE: 10.73 ± 1.31 μg O2 h−1) than larvae in light conditions (6.55 ± 1.22 μg O2 h−1). Larvae exposed to predator cues showed similar SMR than larvae exposed to clean water, and exposure to predator cues neither affected SMR of larvae within each microhabitat treatment (i.e., predator cue treatment and its interaction with microhabitat had no significant effect and were dropped from the model). When included as a covariate, percentage of skin pigmentation had no significant effect on SMR. However, when the collinear effect of microhabitat was not included in the model, there was a significant positive correlation between percentage of skin pigmentation and SMR, with more pigmented larvae showing higher rates of oxygen consumption (r = 0.32, F1,45 = 5.15, p = 0.03; Fig. 2).
Figure 2. Relationship between skin pigmentation and standard metabolic rate (SMR) of larval newts, after two weeks of experiment.
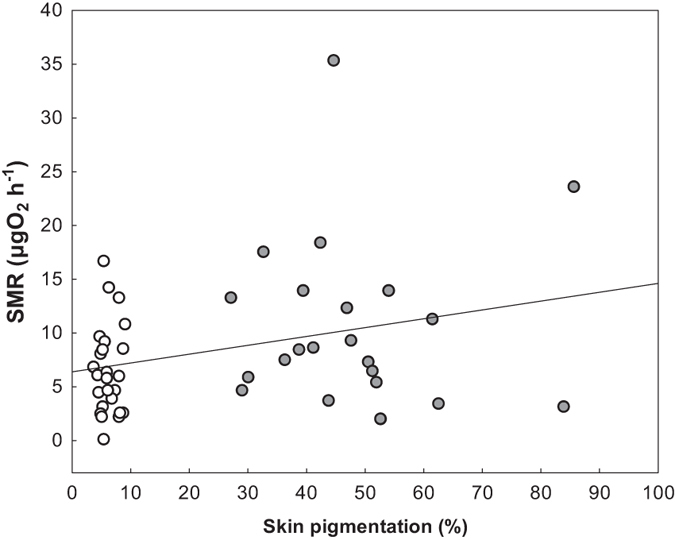
Open circles represent unpigmented larvae subjected to light microhabitat conditions; solid circles represent heavily pigmented larvae subjected to dark microhabitat conditions.
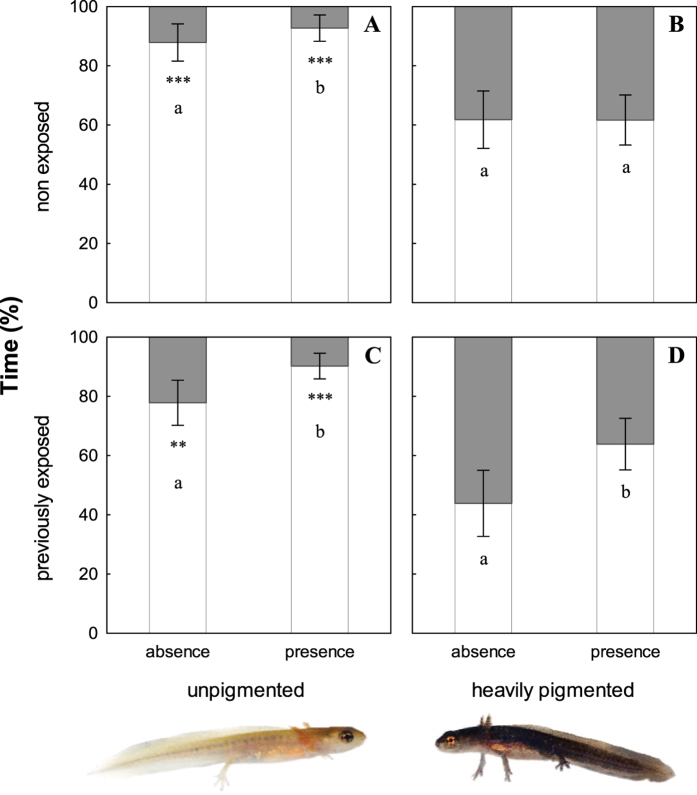
Background choice behaviour
Previous microhabitat conditions significantly affected ulterior background choice by larval newts: unpigmented larvae coming from light conditions spent more time in the light environment than heavily pigmented larvae from dark conditions, whereas the opposite occurred in the dark environment (χ2 = 19.14, n = 48, p < 0.0001; Fig. 3). Unpigmented larvae also spent more time in the light than in the dark environment (two-tailed binomial test, p < 0.0001), whereas heavily pigmented larvae showed no marked preference for either environment (p = 0.18; Fig. 3). Larval preferences were significantly affected by previous experimental exposure to predator cues (χ2 = 4.62, n = 48, p = 0.03), and by the presence or absence of predator cues during the trial (χ2 = 5.29, n = 48, p = 0.02). The effect of the interaction between these two factors was also significant (χ2 = 16.71, n = 48, p < 0.0001). Preferences of non-exposed unpigmented larvae for the light environment were stronger in the presence of predator cues (χ2 = 5.43, n = 11, p = 0.02; Fig. 3A), whereas testing predator cues did not influence choice behaviour in non-exposed heavily pigmented larvae (χ2 = 0.36, n = 11, p = 0.54; Fig. 3B). Both unpigmented and heavily pigmented newts previously exposed to predator cues increased the time spent in the light environment when predator cues were present during the trial (χ2 = 30.10, n = 14, p < 0.0001 and χ2 = 37.22, n = 12, p < 0.0001 respectively; Fig. 3C,D). In the absence of predator cues, non-exposed larvae tended to spend more time in the light environment than previously exposed larvae (χ2 = 2.8, n = 48, p = 0.09), whereas in the presence of predator cues, preferences of exposed and non-exposed larvae did not significantly differ (χ2 = 0.003, n = 47, p = 0.96) (Fig. 3). The mean time spent in the light environment by exposed and non-exposed larvae did not significantly differ within unpigmented or heavily pigmented larvae (χ2 = 2.39, n = 25, p = 0.12 and χ2 = 0.48, n = 23, p = 0.49 respectively; Fig. 3).
Figure 3. Proportion of time (mean percentage ± SE) spent in light (open bars) vs. dark (solid bars) environments by unpigmented and heavily pigmented larval newts subjected to light and dark microhabitat conditions respectively, after five weeks of experiment.
Preferences of the same individual larva are tested in the absence and the presence of predator cues. Upper panels: larvae no previously exposed to predator cues. Bottom panels: larvae previously exposed to predator cues. Different letters indicate significant differences between testing predator cue treatments. Asterisks indicate significant preferences (**P < 0.01; ***P < 0.001).
Discussion
Our study revealed rapid changes in skin pigmentation of L. boscai larvae in response to dissimilar albedo environments. Shifts in coloration were clearly perceptible after only 3 days, and larvae maintained or even enhanced their induced phenotypes during subsequent weeks maintained in their respective light or dark microhabitats (Fig. 4). Moreover, when we inverted the microhabitat treatments, larvae responded to the new backgrounds by quickly reverting pigmentation, evidencing a remarkable ability of larval newts to achieve concealment through plastic changes in coloration in a highly reversible way. These rapid and reversible changes in coloration may constitute a key adaptation enhancing concealment in heterogeneous environments, where animals face a variety of backgrounds as they move across dissimilar habitat patches15.
Figure 4. Phenotypic variation in skin pigmentation of larval newts at week 5 of the experiment.
(A) Depigmentation induced by light microhabitat conditions, (B) over-pigmentation induced by dark microhabitat conditions, (C) microhabitat inversion: unpigmented larvae from light conditions are transferred to new dark conditions, and (D) microhabitat inversion: heavily pigmented larvae from dark conditions are transferred to new light conditions.
Whereas colour change is certainly advantageous in the achievement of crypsis, it may incur in non-trivial physiological costs26,23,24. In amphibians and other vertebrates, pigment organelle translocations may require high energetic expenditure, since it involves complex neuroendocrine control of the chromatophores22,65,66. Additionally to this rapid, physiological colour change, the production of pigment particles and the number of chromatophores can be altered in response to a persistent stimulus22,67,68. For example, dark backgrounds are known to favour the production of melanin and inhibit the production of guanine (i.e., the light-reflecting platelets), whereas light backgrounds cause the reverse effect. This slow (i.e., over weeks to months), morphological colour change may also entail important metabolic costs associated to melanin synthesis or apoptosis32,69,70.
While the capacity of amphibians to change their colour is well known35,67,68,71, most studies have focused on anurans with special attention to post-metamorphic coloration (e.g. refs 72, 73, 74, but see ref. 57). In larval salamanders, changes in pigmentation have been observed mainly in Ambystoma sp. in response to environmental factors such as temperature and ultraviolet (UV) radiation, water turbidity and substrate colour, with degree of plasticity varying over larval ontogeny75,76,77,78. Together with protection against UV-induced damage29,79,80, prevention from visual predators appears to be the most relevant function of such colour plasticity in Ambystoma larvae and the one demonstrated here in the genus Lissotriton3,28,40,41 (but see ref. 81).
In our experiment, SMR in heavily pigmented larvae exposed to dark, low-reflecting microhabitats for two weeks was more than 60% higher than that of unpigmented larvae exposed to light, high-reflecting microhabitats during the same period of time. The fact that more pigmented larvae showed higher rates of oxygen consumption suggests that melanin dispersion in the dermis (physiological colour change) or an increase in the number of melanosomes and/or melanophores (morphological colour change) is physiologically costly. The existence of these production costs for pigmentation plasticity may limit camouflage in dark environments. As a consequence, prey forced to achieve crypsis in dark environments might incur higher fitness costs derived from colour change than prey in light environments.
Due in part to the observed metabolic costs of colour plasticity, the perceived level of risk may play an important role in the achievement of crypsis through colour change. In consequence, we could expect colour change to be a threat-sensitive response, with plastic organisms more readily changing their coloration in environments in which they perceive a high risk of predation. Contrary to our expectations, however, cryptic phenotypes of larval newts were not boosted by the presence of water-borne cues from predatory dragonfly nymphs, suggesting that pigmentation change improving concealment is positively selected with independence of the perceived risk of predation. Garcia and Sih76 also observed rapid colour change in Ambystoma larvae when switching from dark to light backgrounds and vice versa, but, as occurred in our experiment, the presence of chemical cues from fish predators was not found to influence background colour matching. Together, these results suggest that colour change in larval salamanders is not fine-tuned to the level of predation risk, but rather tuned to the surrounding light conditions. Since predation is a great selective force in aquatic ecosystems82 the consequences of suboptimal crypsis due to inaccurate assessment of predation risk might likely exceed the costs of colour plasticity24. Thus, this threat-independent rapid colour change is likely adaptive, since it confers the ability to speedily mimic backgrounds, increasing crypsis and preventing visual detection by predators in heterogeneous environments15,28,75,83.
In addition to colour change, antipredatory responses enhancing crypsis may also involve behavioural adaptations15. For example, prey animals can decrease their probability of being detected by selecting appropriate backgrounds matching their phenotype51,52,56. These preferences of prey might provide concealment against predators without incurring the costs of colour plasticity. Previous research in larval amphibians has pointed out a relationship between background colour and antipredator responses, with individuals selecting backgrounds enhancing their crypsis potential after being disturbed57, or avoiding higher activity rates in non-matching substrates58. Nevertheless, none of these studies have demonstrated larval ability to flexibly choose backgrounds resembling their own appearance. In our study, we found partial evidence for larvae selecting backgrounds matching their induced phenotypes. Although pigmentation induced by environmental albedo subsequently affected habitat choice by L. boscai –unpigmented larvae spent more time in the light environment than heavily pigmented larvae, and the opposite was true in the dark environment–, only unpigmented larvae showed a strong preference for their matching background, whereas heavily pigmented larvae showed no clear preferences at all. Moreover, heavily pigmented newts also increased the time spent in light environments in the presence of predator cues, likely waiving crypsis in favour of reaching the safer and shallower areas of the pond associated with brighter conditions. In sum, larval newts in our experiment preferred the light environment, and these preferences where stronger in the presence of predator cues (Fig. 3). Newts previously exposed to predator cues increased the time spent in light environments in the presence of cues. Predation risk is therefore a contributing factor influencing background choice in L. boscai.
A combination of adaptive preferences for light environments, in which camouflage presumably involves less physiological cost, together with a certain degree of imprinting onto the microhabitat conditions experienced during the previous weeks, might explain the behavioural pattern observed. Animals with colour plasticity may prefer environments where the metabolic costs of colour change are reduced, even if initially they are not well camouflaged. Garcia and Sih76 found evidence for colour plasticity interacting with colour-dependent antipredator behaviour in Ambystoma sp., in a way that species with greater capacity for colour change would undergo weaker pressures to evolve colour-dependent background choice23,76. Though often presumed, the idea that prey recognize and prefer appropriate habitats conferring crypsis based on their own phenotype is not well supported by our data. Visual complexity of the background reducing the risk of detection84,85, and/or behavioural imprinting for particular environments86, might alternatively explain the selection of safe backgrounds by some prey.
Methods
Study animals and experimental setup
We collected L. boscai larvae (n = 60) by dip-netting at several ponds in Doñana National Park, south-west Spain. Natural microhabitats of larval newts at these ponds generally consist of a grey sandy soil and bushes of hygrophytes, submerged macrophytes and floating macrophytes. Light conditions mostly depend on vegetation density and proximity to photic, shallow areas. All larvae were at the same developmental stages (stage stage 45–47, following Shi and Boucaut87) and were similarly sized (mean ± SD = 1.93 ± 0.28). Larvae were transported to Doñana Biological Station in Seville and housed in a walk-in climatic chamber with controlled temperature and photoperiod (20 °C; L:D 12:12). To analyse the capacity of larval newts to change skin coloration in response to different albedo environments, we used 1 L translucent plastic buckets arranged in two opposed microhabitat treatments, i.e., ‘light’ vs. ‘dark’. Buckets in the ‘light’, high-reflecting treatment consisted of a substrate of white gravel at the bottom and a white plastic sleeve covering the walls. Buckets in the ‘dark’, low-reflecting treatment were provided with a black gravel substrate and covered with black plastic sleeves in identical manner. Buckets were filled with carbon-filtered dechlorinated tap water and received one larva each. Water was renewed twice weekly and newts were fed mosquito larvae every other day. We randomly assigned half of the larvae to the ‘light’ treatment and the other half to the ‘dark’ treatment (n = 30 each). To analyse the potential reversibility of inducible changes in coloration, microhabitat treatments were inverted after five weeks of experiment, so that larvae in the ‘light’ treatment were transferred to the ‘dark’ treatment and vice versa. From this moment onwards, we continued the experiment for two additional weeks (until week 7). By the end of the experiment some of the initial larvae had already metamorphosed and a few died, so we tested reversibility on a reduced sample (n = 41).
To test for the effect of predator cues on inducible changes in skin coloration of larval newts, we also dip-netted dragonfly nymphs (A. imperator) at several ponds within the Park, to be used as predator cue donors. Dragonflies were also housed in the climatic chamber and kept individually in 1 L plastic buckets. To prepare predator chemical cues, we filled each donor dragonfly aquarium with 0.5 L of dechlorinated tap water, to be pervaded with predator cues. Since these cues last c. 2–4 days in water88, we extracted and mixed the water from three donor aquaria every 48 h. To prepare control water we followed the same procedure but without placing predators in the aquaria89,90,91. For each microhabitat treatment, we randomly assigned half of the larvae to a ‘predator’ treatment and the other half to a ‘non-predator’ treatment, setting a 2 × 2 factorial design (light vs. dark x predator presence vs. absence; n = 15 in each treatment combination). Every 48 h, 10 mL of water containing predator cues were added to buckets assigned to the ‘predator’ treatment, whereas buckets assigned to the ‘non-predator’ treatment received 10 mL of control water. Similar volumes of predator cues added to water are known to systematically induce antipredator behavior in amphibian larvae91,92,93. Dragonflies were fed anuran tadpoles from a stock tank, once per day. At the end of the experiments, all surviving larval and metamorphic newts were released at their ponds of origin after standard prophylaxis procedures, whereas no dragonflies survived. All experiments were carried out in accordance with all current European directives and Spanish laws, and under permission of the Consejería de Medio Ambiente from Junta de Andalucía. Procedures conformed to the recommended guidelines for use of live amphibians and reptiles in laboratory research94. All experimental protocols were approved by the ‘Comité de Ética de Experimentación Animal CEEA-EBD’.
Image processing
Induced changes in skin pigmentation of larval newts were assessed through quantitative image analysis. A side-view digital image of each larva was taken before the experiment began and then at different moments throughout the experiment (i.e., day 0, day 3, week 2, week 5, and week 7). Images were processed using Adobe Photoshop CS3 Extended, blindly to information about treatments and time of the experiment. We followed the methodological approach of Mayani-Parás et al.95, who estimated avian egg camouflage by quantifying the proportion of eggshell covered with dirt. For each picture, we selected a standardized area (1 × 3 mm) covering the central part of the animal body between the anterior and posterior limbs. Then, we carefully selected every dark pixel within this area, using the “magic wand” tool and refining the selection by picking up pixels discretely. Percentage of skin pigmentation was calculated as the relationship between the number of pixels selected and the total number of pixels in the standardized area. This way, we obtained an accurate measure of pigmentation for each individual larva at different moments in the experiment. We used this measure to compare skin pigmentation of larval newts across microhabitat and predator cue treatments, and within the same individual larva throughout the course of the experiment.
Metabolic cost
To measure SMR on newt larvae, we used a flow-through aquatic respirometer consisting of five cylindrical chambers (44 mm diameter × 163 mm long) that were supplied with oxygen-saturated water from a header tank at a constant flow. Ten optical sensors (optodes; Oxy 10-PreSens, Germany) were mounted at the entrance and exit of the chambers and connected to an oxymeter (Oxy 10-PreSens, Germany) that recorded oxygen concentration every 15 seconds. This way we simultaneously obtained measures of oxygen consumption (mg/L) for five independent individuals. The respirometer was calibrated at least once daily using a saturated sodium sulphite solution and oxygen saturated water to achieve 0 and 100% oxygen concentrations96. Respirometry trials were conducted after two weeks of experiment, when changes in skin pigmentation were clearly observable. All the measurements were taken at 20 °C, the same temperature experienced by larvae in the walk-in climatic chamber during the previous weeks. For the trials, larvae (n = 47) were introduced individually in the chambers. Not all the initial larvae could be tested because some metamorphosed and a few died during the course of the experiment. Thus, the sample distribution across treatment was as follows: light x predator n = 13; light x non-predator n = 12; dark x predator n = 13; dark × non predator n = 9. We allowed larvae to acclimate in the chambers for five minutes and then we recorded oxygen consumption in each chamber for 30 min. SMR was calculated as:
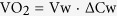 |
where VO2 is the rate of oxygen consumption (μg h−1), Vw is the flow rate of water through the chamber (L h−1), and ΔCw is the instantaneous difference in oxygen concentration between the inflow and outflow96,97. After the trials, each larva was blotted dry and weighed on a high precision balance (CP324S, Sartorius, precision: ±0.1 mg), for inclusion of body mass as a covariate in statistical analyses.
Background choice behaviour
In a subsequent experiment, we compared preferences for light vs. dark environments of larval newts that had been previously subjected to light or dark microhabitat conditions, in the presence and absence of predator cues. These behavioural assays took place after five weeks of experiment, right before inverting microhabitat conditions. Larvae were tested in transparent aquaria (32 × 17 × 18 cm, 10 L) that were divided in two spaces of equal surface and volume, but offering two opposed microhabitat conditions, light vs. dark. The light side of each experimental aquarium was provided with a white gravel substrate and we covered the walls with a white plastic sleeve, whereas the dark side was provided with a black gravel substrate and the walls were covered with a dark plastic sleeve. There was no physical separation between the two sides, so that larvae were free to move from one environment to the other. We tested each individual larva (n = 48) in two predator cue treatments (‘presence’ vs. ‘absence’) in a random sequence. In the ‘presence’ treatment, we added to the aquaria 20 mL of water pervaded with predator cues from three dragonfly nymphs, whereas in the ‘absence’ treatment we added 20 mL of control water. Larvae were given 24 h between trials to rest. The position of the light and the dark environments in experimental aquaria was randomized across trials and treatments.
At the beginning of the trials, we placed a single larva in the middle of each experimental aquarium, so it was given the choice between the two environments. We waited 5 min before the trials began to allow larvae to acclimate, and then we monitored each larva for 30 min, using the instantaneous scan sampling method, and recording every 1 min the side that each larva occupied in the aquarium (30 scans per larva in total). Preferences of larvae for light vs. dark environments were assessed from the proportion of scans they spent in each side of the aquarium. Then, we used this measure to estimate the effects of previous microhabitat conditions (‘light’ vs. ‘dark’) and previous exposure to predator cues (‘predator’ vs. ‘non-predator’) on larval preferences for light vs. dark environments in the presence or absence of predator cues.
Data analyses
To analyse changes in skin coloration of larval newts induced by microhabitat conditions or exposure to predator cues, we used a linear mixed model (LMM) with time of the experiment (four levels: ‘day 0’, ‘day 3’, ‘week 2’ or ‘week 5’), microhabitat (‘ligh’ vs. ‘dark’) and predator cue (‘predator’ vs. ‘non-predator’) as three independent factors, individual larva as random factor, and percentage of skin pigmentation as dependent variable. To analyse the reversibility of inducible changes in pigmentation, we used a LMM with time of the experiment (two levels: ‘week 5’ vs. ‘week 7’), microhabitat (‘light’ vs. ‘dark’) and predator cue (‘predator’ vs. ‘non-predator’) as three independent factors, individual larva as random factor, and percentage of skin pigmentation as dependent variable. Percentage of skin pigmentation was non-normally distributed and was transformed using Box-Cox power transformations. Post-hoc comparisons among treatments were made using Tukey’s honestly significant difference tests98.
To analyse differences in SMR of larval newts associated to skin pigmentation we used a forward stepwise general regression model (GRM) with microhabitat (‘light’ vs. ‘dark’) and predator cue (‘predator’ vs. ‘non-predator’) treatments as two categorical independent predictors, weight and percentage of pigmented skin (week 2) as two continuous independent predictors, and SMR as dependent variable. To analyse background choice behaviour by larval newts we used a generalized linear mixed model (GLMM) with a binomial distribution. Microhabitat (‘light’ vs. ‘dark’), predator cue (‘predator’ vs. ‘non-predator’) and testing predator cue (‘presence’ vs. ‘absence’) were included as three independent factors, individual larva as random factor, and percentage of time spent in light vs. dark environments as dependent variable. All analyses were performed in Statistica 12.0 and R 3.1.3.
Additional Information
How to cite this article: Polo-Cavia, N. and Gomez-Mestre, I. Pigmentation plasticity enhances crypsis in larval newts: associated metabolic cost and background choice behaviour. Sci. Rep. 7, 39739; doi: 10.1038/srep39739 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We thank P. Burraco, R. Arribas, L. Asencio and C. Diaz-Paniagua for their help in the field and with animal husbandry, and two anonymous reviewers for their helpful comments. Financial support was provided by the Spanish Ministry of Science and Innovation (MICINN), Grant CGL2012-40044 to IGM, and by the Universidad Autónoma de Madrid, Short Stay Grant to NPC. Additional financial support was provided by the MICINN, Grant CGL2015-68670-R to NPC.
Footnotes
Author Contributions Conceived and designed the experiments: N.P.C. and I.G.M. Performed the experiments: N.P.C. and I.G.M. Analysed the data: N.P.C. and I.G.M. Contributed reagents/materials/analysis tools: N.P.C. and I.G.M. Wrote the manuscript: N.P.C. and I.G.M.
References
- Hanlon R. Cephalopod dynamic camouflage. Curr. Biol. 17, 1–5 (2007). [DOI] [PubMed] [Google Scholar]
- Manríquez K. C., Pardo L. M., Wells R. J. D. & Palma A. T. Crypsis in Paraxanthus barbiger (Decapoda: Brachyura): mechanisms against visual predators. J. Crust. Biol. 28, 473–479 (2008). [Google Scholar]
- Stevens M. & Merilaita S. Animal camouflage: current issues and new perspectives. Philos. Trans. R. Soc. Lond. B 364, 423–427 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace A. R. Darwinism. An exposition of the theory of natural selection with some of its applications. London, UK: Macmillan & Co (1889). [Google Scholar]
- Cott H. B. Adaptive coloration in animals. London, UK: Methuen (1940). [Google Scholar]
- Endler J. A. A predator’s view of animal color patterns. Evol. Biol. 11, 319–364 (1978). [Google Scholar]
- Thayer G. H. Concealing-coloration in the animal kingdom: an exposition of the laws of disguise through color and pattern. New York: MacMillan (1909). [Google Scholar]
- Merilaita S. Crypsis through disruptive coloration in an isopod. Proc. R. Soc. Lond. B 265, 1059–1064 (1998). [Google Scholar]
- Endler J. A. Disruptive and cryptic coloration. Proc. R. Soc. Lond.B 273, 2425–2426 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Cuthill I. C., Windsor A. M. M. & Walker H. J. Disruptive contrast in animal camouflage. Proc. R. Soc. Lond. B 273, 2433–2438 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelhorn J., Rowland H. M., Speed M. P. & Ruxton G. D. Masquerade: camouflage without crypsis. Science 327, 51–51 (2010). [DOI] [PubMed] [Google Scholar]
- Brooker R. M., Munday P. L. & Jones G. P. Coral obligate filefish masquerades as branching coral. Coral Reefs 30, 803–803 (2011). [Google Scholar]
- Skelhorn J. Masquerade. Curr. Biol. 25, R643–R644 (2015). [DOI] [PubMed] [Google Scholar]
- Ruxton G. D. Non-visual crypsis: a review of the empirical evidence for camouflage to senses other than vision. Philos. Trans. R. Soc. Lond. B 364, 549–557 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magellan K. I. T. & Swartz E. R. Crypsis in a heterogeneous environment: relationships between changeable polymorphic colour patterns and behaviour in a galaxiid fish. Freshwater Biol. 58, 793–799 (2013). [Google Scholar]
- Merilaita S., Tuomi J. & Jormalainen V. Optimization of cryptic coloration in heterogeneous habitats. Biol. J. Linn. Soc. 67, 151–161 (1999). [Google Scholar]
- Merilaita S., Lyytinen A. & Mappes J. Selection for cryptic coloration in a visually heterogeneous habitat. Proc. R. Soc. Lond. B 268, 1925–1929 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston A. I., Stevens M. & Cuthill I. C. Animal camouflage: compromise or specialise in a two patch-type environment? Behav. Ecol. 18, 769–775 (2007). [Google Scholar]
- Sherratt T. N., Pollitt D. & Wilkinson D. M. The evolution of crypsis in replicating populations of web-based prey. Oikos 116, 449–460 (2007). [Google Scholar]
- Bond A. B. The evolution of color polymorphism: crypticity, searching images, and apostatic selection. Annu. Rev. Ecol. Evol. System. 38, 489–514 (2007). [Google Scholar]
- West-Eberhard M. J. Developmental Plasticity and Evolution. Oxford, UK: Oxford University Press (2003). [Google Scholar]
- Aspengren S., Hedberg D., Skold H. N. & Wallin M. New insights into melanosome transport in vertebrate pigment cells. Inter. Rev. Cell Mol. Biol. 272, 245–302 (2009). [DOI] [PubMed] [Google Scholar]
- Stuart-Fox D. & Moussalli A. Camouflage in color changing animals: trade-offs and constraints. In Animal camouflage: mechanisms and functions (Stevens M. & Merilaita S. Eds), pp. 237–253. Cambridge: Cambridge University Press (2011). [Google Scholar]
- Bergstrom C. A., Whiteley A. R. & Tallmon D. A. The heritable basis and cost of colour plasticity in coastrange sculpins. J. Evol.Biol. 25, 2526–2536 (2012). [DOI] [PubMed] [Google Scholar]
- Ibañez A., Polo-Cavia N., Lopez P. & Martin J. Honest sexual signaling in turtles: experimental evidence of a trade-off between immune response and coloration in red-eared sliders trachemys scripta elegans. Naturwissenschaften 101, 803–811 (2014). [DOI] [PubMed] [Google Scholar]
- Sköld H. N., Aspengren S. & Wallin M. Rapid color change in fish and amphibians – function, regulation, and emerging applications. Pigm. Cell Mel. Res. 26, 29–38 (2013). [DOI] [PubMed] [Google Scholar]
- Wells K. D. The ecology and behavior of amphibians. Chicago: University of Chicago Press (2007). [Google Scholar]
- Kats L. B. & Van Dragt R. G. Background color-matching in the spring peeper, Hyla crucifer. Copeia 1986, 109–115 (1986). [Google Scholar]
- Storfer A., Cross J., Rush V. & Caruso J. Adaptive coloration and gene flow as a constraint to local adaptation in the streamside salamander, Ambystoma barbouri. Evolution 53, 889–898 (1999). [DOI] [PubMed] [Google Scholar]
- Ruxton G. D., Sherratt T. N. & Speed M. P. Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. Oxford, UK: Oxford University Press (2004). [Google Scholar]
- Eastman J. M., Niedzwiecki J. H., Nadler B. P. & Storfer A. Duration and consistency of historical selection are correlated with adaptive trait evolution in the streamside salamander, Ambystoma barbouri. Evolution 63, 2636–2647 (2009). [DOI] [PubMed] [Google Scholar]
- Bagnara J. T. & Hadley M. E. Chromatophores and color change. Englewood Cliffs, NJ: Prentice-Hall (1973). [Google Scholar]
- Nielsen H. I. & Dyck J. Adaptation of the tree frog, Hyla cinerea, to colored backgrounds, and the role of the three chromatophore types. J. Exp.Zool. 205, 79–94 (1978). [Google Scholar]
- King R. B., Hauff S. & Phillips J. B. Physiological color change in the green treefrog: responses to background brightness and temperature. Copeia 1994, 422–432 (1994). [Google Scholar]
- Nery L. E. M. & Lauro Castrucci A. M. Pigment cell signalling for physiological color change. Comp. Biochem. Physiol. A: Physiol. 118, 1135–1144 (1997). [DOI] [PubMed] [Google Scholar]
- Tonosaki Y., Cruijsen P. M., Nishiyama K., Yaginuma H. & Roubos E. W. Low temperature stimulates alpha-melanophore-stimulating hormone secretion and inhibits background adaptation in Xenopus laevis. J. Neuroendocrinol. 16, 894–905 (2004). [DOI] [PubMed] [Google Scholar]
- Thibaudeau G. & Altig R. Coloration of anuran tadpoles (Amphibia): development, dynamics, function, and hypotheses. ISRN Zoology 2012, 16 (2012). [Google Scholar]
- Hanlon R. T., Forsythe J. W. & Joneschild D. E. Crypsis, conspicuousness, mimicry and polyphenism as antipredator defences of foraging octopuses on indo-pacific coral reefs, with a method of quantifying crypsis from video tapes. Biol. J. Linn. Soc. 66, 1–22 (1999). [Google Scholar]
- Fairchild E. A. & Howell W. H. Factors affecting the post-release survival of cultured juvenile Pseudopleuronectes americanus. J. Fish Biol. 65, 69–87 (2004). [Google Scholar]
- Stuart-Fox D., Moussalli A. & Whiting M. J. Predator-specific camouflage in chameleons. Biol. Lett. 4, 326–329 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart-Fox D. & Moussalli A. Camouflage, communication and thermoregulation: lessons from colour changing organisms. Philos. Trans. R. Soc. B 364, 463–470 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum S. A. & Van Buskirk J. Costs and benefits of a predator-induced polyphenism in the gray treefrog Hyla chrysoscelis. Evolution 50, 583–593 (1996). [DOI] [PubMed] [Google Scholar]
- Van Buskirk J. & McCollum S. A. Plasticity and selection explain variation in tadpole phenotype between ponds with different predator composition. Oikos 85, 31–39 (1999). [Google Scholar]
- LaFiandra E. M. & Babbitt K. J. Predator induced phenotypic plasticity in the pinewoods tree frog, Hyla femoralis: necessary cues and the cost of development. Oecologia 138, 350–359 (2004). [DOI] [PubMed] [Google Scholar]
- McIntyre P., Baldwin S. & Flecker A. Effects of behavioral and morphological plasticity on risk of predation in a neotropical tadpole. Oecologia 141, 130–138 (2004). [DOI] [PubMed] [Google Scholar]
- Caldwell J. P. Disruptive selection: a tail color polymorphism in Acris tadpoles in response to differential predation. Can. J. Zool. 60, 2818–2827 (1982). [Google Scholar]
- Van Buskirk J., Anderwald P., Lüpold S., Reinhardt L. & Schuler H. The lure effect, tadpole tail shape, and the target of dragonfly strikes. J. Herp. 37, 420–424 (2003). [Google Scholar]
- Touchon J. C. & Warkentin K. M. Fish and dragonfly nymph predators induce opposite shifts in color and morphology of tadpoles. Oikos 117, 634–640 (2008). [Google Scholar]
- Cooper J. M. & Allen J. A. Selection by wild birds on artificial dimorphic prey on varied backgrounds. Biol. J. Linn. Soc. 51, 433–446 (1994). [Google Scholar]
- Johnsson I. J. & Källman-Eriksson K. Cryptic prey colouration increases search time in brown trout (Salmo trutta): effects of learning and body size. Behav. Ecol. Sociobiol. 62, 1613–1620 (2008). [Google Scholar]
- Kettlewell H. B. Recognition of appropriate backgrounds by the pale and black phases of lepidoptera. Nature 175, 943–944 (1955). [DOI] [PubMed] [Google Scholar]
- Boardman M., Askew R. R. & Cook L. M. Experiments on resting site selection by nocturnal moths. J. Zool. 172, 343–355 (1974). [Google Scholar]
- Kettlewell H. B. D. & Conn D. L. T. Further background-choice experiments on cryptic lepidoptera. J. Zool. 181, 371–376 (1977). [Google Scholar]
- Gillis J. E. Substrate colour-matching cues in the cryptic grasshopper Circotettix rabula rabula (Rehn & Hebard). Anim. Behav. 30, 113–116 (1982). [Google Scholar]
- Sandoval C. P. Differential visual predation on morphs of Timema cristinae (Phasmatodeae: Timemidae) and its consequences for host range. Biol. J. Linn. Soc. 52, 341–356 (1994). [Google Scholar]
- Ahnesjö J. & Forsman A. Differential habitat selection by pygmy grasshopper color morphs; interactive effects of temperature and predator avoidance. Evolut. Ecol. 20, 235–257 (2006). [Google Scholar]
- Eterovick P. C., Oliveira F. F. R. & Tattersall G. J. Threatened tadpoles of Bokermannohyla alvarengai (Anura: Hylidae) choose backgrounds that enhance crypsis potential. Biol. J. Linn. Soc. 101, 437–446 (2010). [Google Scholar]
- Nomura F., De Marco P., Carvalho A. F. A. & Rossa-Feres D. C. Does background colouration affect the behaviour of tadpoles? An experimental approach with an odonate predator. Ethol. Ecol. Evol. 25, 185–198 (2013). [Google Scholar]
- Hoffman E. A. & Blouin M. S. A review of colour and pattern polymorphisms in anurans. Biol. J. Linn. Soc. 70, 633–665 (2000). [Google Scholar]
- Croshaw D. A. Cryptic behavior is independent of dorsal color polymorphism in juvenile northern leopard frogs (Rana pipiens). J. Herp. 39, 125–129 (2005). [Google Scholar]
- Rudh A. & Qvarnström A. Adaptive colouration in amphibians. Sem. Cell Develop. Biol. 24, 553–561 (2013). [DOI] [PubMed] [Google Scholar]
- Johnson J. A. & Brodie E. D. The selective advantage of the defensive posture of the newt, Taricha granulosa. Am. Midl. Nat. 93, 139–148 (1975). [Google Scholar]
- Hensel J. L. & Brodie E. D. An experimental study of aposematic coloration in the salamander Plethodon jordani. Copeia 1976, 59–65 (1976). [Google Scholar]
- Kuchta S. R. Experimental support for aposematic coloration in the salamander Ensatina eschscholtzii xanthoptica: implications for mimicry of pacific newts. Copeia 2005, 265–271 (2005). [Google Scholar]
- Aspengren S., Hedberg D. & Wallin M. Studies of pigment transfer between xenopus laevis melanophores and fibroblasts in vitro and in vivo. Pigm. Cell Mel. Res. 19, 136–145 (2006). [DOI] [PubMed] [Google Scholar]
- Bagnara J. T. & Matsumoto J. Comparative anatomy and physiology of pigment cells in nonmammalian tissues. In Pigmentary system (Nordlund J. J., Boissy R. E., Hearing V. J., King R. A. & Ortonne J. P. Eds), pp. 11–59. Oxford: Oxford University Press (2006). [Google Scholar]
- Duellman W. E. & Trueb L. Biology of amphibians. New York: McGraw-Hill (1986). [Google Scholar]
- Sumner F. B. Quantitative changes in pigmentation, resulting from visual stimuli in fishes and amphibia. Biol. Rev. 15, 351–374 (1940). [Google Scholar]
- Talloen W., Van Dyck H. & Lens L. The cost of melanization: butterfly wing coloration under environmental stress. Evolution 58, 360–366 (2004). [DOI] [PubMed] [Google Scholar]
- Stoehr A. M. Costly melanin ornaments: the importance of taxon? Funct. Ecol. 20, 276–281 (2006). [Google Scholar]
- Bagnara J. T., Taylor J. D. & Hadley M. E. The dermal chromatophore unit. J. Cell Biol. 38, 67–79 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen J. T. The significance of color change in newly metamorphosed american toads (Bufo a. americanus). J. Herpetol. 28, 87–93 (1994). [Google Scholar]
- Wente W. H. & Phillips J. B. Fixed green and brown color morphs and a novel color-changing morph of the pacific tree frog Hyla regilla. Am. Nat. 162, 461–473 (2003). [DOI] [PubMed] [Google Scholar]
- Stegen J. C., Gienger C. M. & Sun L. The control of color change in the pacific tree frog, Hyla regilla. Can. J. Zool. 82, 889–896 (2004). [Google Scholar]
- Fernandez P. J. & Collins J. P. Effect of environment and ontogeny on color pattern variation in arizona tiger salamanders (Ambystoma tigrinum nebulosum Hallowell). Copeia 1988, 928–938 (1988). [Google Scholar]
- Garcia T. S. & Sih A. Color change and color-dependent behavior in response to predation risk in the salamander sister species Ambystoma barbouri and Ambystoma texanum. Oecologia 137, 131–139 (2003). [DOI] [PubMed] [Google Scholar]
- Garcia T. S., Straus R. & Sih A. Temperature and ontogenetic effects on color change in the larval salamander species Ambystoma barbouri and Ambystoma texanum. Can. J. Zool. 81, 710–715 (2003). [Google Scholar]
- Garcia T. S., Stacy J. & Sih A. Larval salamander response to UV radiation and predation risk: color change and microhabitat use. Ecol. Applic. 14, 1055–1064 (2004). [Google Scholar]
- Jablonski N. G. Ultraviolet light-induced neural tube defects in amphibian larvae and their implications for the evolution of melanized pigmentation and declines in amphibian populations. J. Herp. 32, 455–457 (1998). [Google Scholar]
- Lesser M. P., Turtle S. L., Farrell J. H. & Walker C. W. Exposure to ultraviolet radiation (290–400 nm) causes oxidative stress, DNA damage, and expression of p53/p73 in laboratory experiments on embryos of the spotted salamander, Ambystoma maculatum. Physiol. Biochem. Zool. 74, 733–741 (2001). [DOI] [PubMed] [Google Scholar]
- Espanha J., de Vasconcelos M. F. & Eterovick P. C. The role of tadpole coloration against visually oriented predators. Behav. Ecol. Sociobiol. 70, 255–267 (2016). [Google Scholar]
- Kerfoot W. C. & Sih A. Predation: direct and indirect impacts on aquatic communities. Hanover: University Press of New England (1987). [Google Scholar]
- Clarke J. M. & Schluter D. Colour plasticity and background matching in a threespine stickleback species pair. Biol. J. Linn. Soc. 102, 902–914 (2011). [Google Scholar]
- Dimitrova M. & Merilaita S. Prey concealment: visual background complexity and prey contrast distribution. Behav. Ecol. 21, 176–181 (2010). [Google Scholar]
- Kjernsmo K. & Merilaita S. Background choice as an anti-predator strategy: the roles of background matching and visual complexity in the habitat choice of the least killifish. Proc. R. Soc. Lond. B 279, 4192–4198 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shettleworth S. J. Cognition, evolution, and behavior. New York, NY: Oxford University Press (2009). [Google Scholar]
- Shi D. L. & Boucaut J. C. The chronological development of the urodele amphibian Pleurodeles waltl (Michah). Inter. J. Develop. Biol. 39, 427–441 (1995). [PubMed] [Google Scholar]
- Peacor S. D. Behavioural response of bullfrog tadpoles to chemical cues of predation risk are affected by cue age and water source. Hydrobiologia 573, 39–44 (2006). [Google Scholar]
- Gonzalo A., López P. & Martín J. Iberian green frog tadpoles may learn to recognize novel predators from chemical alarm cues of conspecifics. Anim. Behav. 74, 447–453 (2007). [Google Scholar]
- Polo-Cavia N., Gonzalo A., López P. & Martín J. Predator recognition of native but not invasive turtle predators by naïve anuran tadpoles. Anim. Behav. 80, 461–466 (2010). [Google Scholar]
- Polo-Cavia N. & Gomez-Mestre I. Learned recognition of introduced predators determines survival of tadpole prey. Funct. Ecol. 28, 432–439 (2014). [Google Scholar]
- Gomez-Mestre I. & Diaz-Paniagua C. Invasive predatory crayfish do not trigger inducible defences in tadpoles. Proc. R. Soc. Lond. B 278, 3364–3370 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo-Cavia N., Burraco P. & Gomez-Mestre I. Low levels of chemical anthropogenic pollution may threaten amphibians by impairing predator recognition. Aquat. Toxicol. 172, 30–35 (2016). [DOI] [PubMed] [Google Scholar]
- ASIH. Guidelines for use of live amphibians and reptiles in field and laboratory research, 2nd edn. Kansas: Lawrence/Herpetological Animal Care and Use Committee (HACC) of the American Society of Ichthyologists and Herpetologists (2004). [Google Scholar]
- Mayani-Parás F., Kilner R. M., Stoddard M. C., Rodríguez C. & Drummond H. Behaviorally induced camouflage: a new mechanism of avian egg protection. Am. Nat. 186, E91–E97 (2015). [DOI] [PubMed] [Google Scholar]
- Burraco P., Duarte L. J. & Gomez-Mestre I. Predator-induced physiological responses in tadpoles challenged with herbicide pollution. Curr. Zool. 59, 475–484 (2013). [Google Scholar]
- Álvarez D., Cano J. M. & Nicieza A. G. Microgeographic variation in metabolic rate and energy storage of brown trout: countergradient selection or thermal sensitivity? Evolut. Ecol. 20, 345–363 (2006). [Google Scholar]
- Sokal R. R. & Rohlf F. J. Biometry, 3rd edn. New York: WH Freeman (1995). [Google Scholar]