Abstract
The variation in mortality-to-incidence ratios (MIRs) among countries reflects the clinical outcomes and the available interventions for colorectal cancer treatments. The association between MIR of prostate cancer and cancer care disparities among countries is an interesting issue that is rarely investigated. For the present study, cancer incidence and mortality rates were obtained from the GLOBOCAN 2012 database. The rankings and total expenditures on health of various countries were obtained from the World Health Organization (WHO). The association between variables was analyzed by linear regression analyses. In this study, we estimated the role of MIRs from 35 countries that had a prostate cancer incidence greater than 5,000 cases per year. As expected, high prostate cancer incidence and mortality rates were observed in more developed regions, such as Europe and the Americas. However, the MIRs were 2.5 times higher in the less developed regions. Regarding the association between MIR and cancer care disparities, countries with good WHO ranking and high total expenditures on health/gross domestic product (GDP) were significant correlated with low MIR. The MIR variation for prostate cancer correlates with cancer care disparities among countries further support the role of cancer care disparities in clinical outcome.
Prostate cancer is a common malignancy characterized by a generally slower progression than other cancers and is a major cause of cancer deaths in men1,2,3. Moreover, gradual increases in prostate cancer incidence have been reported4. This increased detection is most likely attributable to cancer screenings that measure prostate-specific antigen (PSA) levels1,5. There were 1.4 million cases of prostate cancer and 293,000 deaths worldwide in 20132. However, the incidence varied among countries because of differences in the coverage of PSA screenings; the highest number of screenings occurred in Western countries4,6,7. In contrast, the incidence of prostate cancer is much lower in Asia and Africa8,9. Furthermore, the geographic distribution of prostate cancer incidence in Europe shows that the probability of being diagnosed with prostate cancer is closely associated with prior migrations and settlement histories10. Furthermore, prostate cancer is thought to have a strong ethnic propensity, and there is a higher prevalence among Europeans and African Americans10.
With regard to mortality rates, prostate cancer is a common cause of cancer death in Western countries1,2,3. The age-standardized, 5-year relative survival of patients in Europe was approximately 83.4%, whereas for those under 80 years of age in the United States, this rate was more than 97%11. Current prostate cancer treatments have shown a trend toward personalizing treatments. Future treatment approaches may be guided by cancer DNA sequencing, and personalized drugs could target the weaknesses of certain cancers12. All of these improvements suggest that the health care system may be able to improve cancer screenings and treatments of prostate cancer. Therefore, in countries with better health care systems, the mortality-to-incidence (MIR) ratio should be low.
The purpose of this study was to clarify the association between different factors, including human development, World Health Organization (WHO) ranking, region, total expenditure on health/gross domestic product (GDP; e/GDP), life expectancy, and crude rates of incidence and mortality for prostate cancer. Our results could provide a comprehensive overview of the relationship between MIR and health disparities for various countries.
Materials and Methods
Cancer epidemiological data were obtained from the GLOBOCAN 2012 database, a public database that provides contemporary estimates of the incidence of cancer mortality and prevalence of major cancer types for 184 countries worldwide. The GLOBOCAN 2012 database is maintained by the International Agency for Research on Cancer. The detailed summarized data from GLOBOCAN 2012 were found in an article by Torre LA and colleagues3. The inclusion criterion for the selected countries in this investigation of prostate cancer was an incident case number larger than 5,000 diagnoses; 35 countries were selected. The WHO ranking was obtained from the WHO’s Ranking of the World’s Health Systems, which is maintained by the WHO. The e/GDP and life expectancy of 2012 were obtained from the World Health Statistics 2015, which is the annual compilation of health-related data for its 194 member states.
The MIR was defined as the ratio of the crude rate of mortalities and the crude rate of incidences13; in this study, the percentage of the total number of mortalities and the total number of incidences was used. The associations between the MIR and other factors among various countries were estimated by simple linear regressions. R-squared changes and analysis of variance (ANOVA) were determined using SPSS statistical software version 15.0 (SPSS, Inc., Chicago, IL). P values < 0.05 were considered statistically significant. A scatterplot was generated using Microsoft Excel 2010.
Results
The incidence and mortality rates of prostate cancer were higher in more developed regions than in less developed regions
To understand the global trend of prostate cancer, we analyzed the incidence and mortality rates according to region. The results are summarized in Table 1. Overall, the crude incidence and mortality rates of prostate cancer were 30.8 and 8.6, respectively. Both the incidence and mortality crude rates were higher in more developed regions than in less developed regions (incidence: 122.4 vs. 12.0; mortality: 23.4 vs. 5.6, respectively). Regarding human development levels, the regions with high human development levels had higher crude rates of incidence and mortality of prostate cancer than those with low human development levels (incidence: from 129.0 to 7.3; mortality: from 23.1 to 6.0, respectively). With regard to the WHO regions and continents, the WHO Europe region and Americas region had much higher crude rates of incidence and mortality compared with other regions. With respect to continents, North America had the highest incidence rate, but the highest mortality rate was reported in Europe.
Table 1. Summary of prostate cancer crude rates of incidence, mortality, and mortality-to-incidence ratios according to region.
| Region | Prostate cancer |
||||
|---|---|---|---|---|---|
| Incidence |
Mortality |
MIR(%) | |||
| Number | Crude rate | Number | Crude rate | ||
| World | 1094916 | 30.8 | 307481 | 8.6 | 28.1 |
| Development | |||||
| More developed regions | 741966 | 122.4 | 142014 | 23.4 | 19.1 |
| Less developed regions | 352950 | 12.0 | 165467 | 5.6 | 46.9 |
| Development categories | |||||
| Very high human development | 734128 | 129.0 | 131685 | 23.1 | 17.9 |
| High human development | 195839 | 38.2 | 72623 | 14.2 | 37.1 |
| Medium human development | 115942 | 6.4 | 63739 | 3.5 | 55.0 |
| Low human development | 47809 | 7.3 | 39096 | 6.0 | 81.8 |
| WHO region categories | |||||
| WHO Africa region | 51689 | 11.8 | 37486 | 8.5 | 72.5 |
| WHO Americas region | 412739 | 87.6 | 85425 | 18.1 | 20.7 |
| WHO East Mediterranean region | 18585 | 5.8 | 12141 | 3.8 | 65.3 |
| WHO Europe region | 419915 | 96.1 | 101419 | 23.2 | 24.2 |
| WHO South-East Asia region | 38515 | 4.1 | 24932 | 2.6 | 64.7 |
| WHO Western Pacific region | 153167 | 16.2 | 45977 | 4.9 | 30.0 |
| Continent | |||||
| Africa | 59493 | 11.1 | 42802 | 8.0 | 71.9 |
| Latin America and Caribbean | 152403 | 51.2 | 51313 | 17.2 | 33.7 |
| North America | 260336 | 150.2 | 34112 | 19.7 | 13.1 |
| Asia | 196190 | 9.0 | 82676 | 3.8 | 42.1 |
| Europe | 400364 | 112.0 | 92328 | 25.8 | 23.1 |
| Oceania | 26130 | 138.3 | 4250 | 22.5 | 16.3 |
The mortality-to-incidence ratios for prostate cancer are high in less developed regions
As we know, the MIR demonstrates the related outcome of patients with a certain disease, and we investigated the MIR according to region. The world MIR for prostate cancer was 28.1%. There were higher MIRs of prostate cancer in the WHO Africa region, the East Mediterranean region, and the Southeast Asia region (72.5%, 65.3%, and 64.7%, respectively). With regard to continent, Africa had the highest MIR compared with other regions (71.9%).
World Health Organization ranking and total expenditure on health/GDP were significantly associated with the mortality-to-incidence ratios for prostate cancer
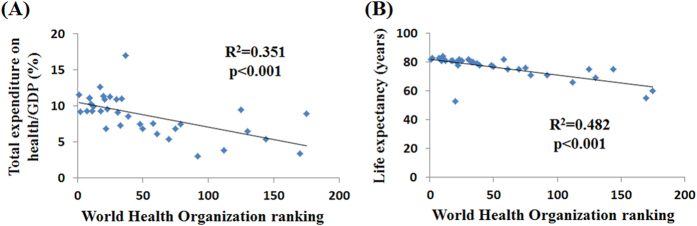
To further compare the differences in epidemiology among countries, we included the countries with more than 5,000 incident cases (Table 2). The information from the WHO rankings, total expenditures on e/GDP, and life expectancies are summarized in Table 2. As expected, the WHO rankings were significantly associated with both the e/GDP and life expectancy (R2 = 0.351, p < 0.001; R2 = 0.482, p < 0.001, respectively, Fig. 1). Among the 35 countries, the country with the highest WHO ranking was France. For the e/GDP, the highest was the United States (17.0%), and the lowest was Indonesia (3.0%). Among all of the countries, the highest crude rate of incidence was in Sweden (244.9), and the highest crude rate of mortality was in Cuba (54.4).
Table 2. Summary of the World Health Organization rankings, total expenditures on health/GDP, life expectancy, and incidence/mortality in crude rates and case numbers of countries with prostate cancer incidences of more than 5,000 cases per year.
| Country | Ranking | Total expenditure on health/GDP (%) | Life expectancy | Incidence | Mortality | MIR(%) | ||
|---|---|---|---|---|---|---|---|---|
| Number | Crude rate | Number | Crude rate | |||||
| Indonesia | 92 | 3.0 | 71 | 13663 | 11.2 | 9191 | 7.5 | 67.3 |
| Nigeria | 170 | 3.4 | 55 | 11944 | 14.2 | 9628 | 11.4 | 80.6 |
| Mexico | 61 | 6.1 | 75 | 14016 | 24.5 | 6367 | 11.1 | 45.4 |
| South African | 175 | 8.9 | 60 | 9957 | 39.6 | 3539 | 14.1 | 35.5 |
| Russian | 130 | 6.5 | 69 | 26885 | 40.7 | 11480 | 17.4 | 42.7 |
| Turkey | 70 | 5.4 | 75 | 12650 | 34.0 | 7231 | 19.5 | 57.2 |
| India | 112 | 3.8 | 66 | 19095 | 2.9 | 12231 | 1.9 | 64.1 |
| China | 144 | 5.4 | 75 | 46745 | 6.6 | 22603 | 3.2 | 48.4 |
| Poland | 50 | 6.8 | 77 | 11029 | 59.7 | 4242 | 23.0 | 38.5 |
| Portugal | 12 | 9.9 | 81 | 6622 | 127.7 | 1582 | 30.5 | 23.9 |
| Colombia | 22 | 6.8 | 78 | 9564 | 40.9 | 2934 | 12.5 | 30.7 |
| Chile | 33 | 7.3 | 80 | 5681 | 66.0 | 2029 | 23.6 | 35.7 |
| Cuba | 39 | 8.6 | 78 | 7931 | 140.2 | 3080 | 54.4 | 38.8 |
| Argentina | 75 | 6.8 | 76 | 11202 | 55.7 | 4489 | 22.3 | 40.1 |
| Brazil | 125 | 9.5 | 75 | 72536 | 74.4 | 17218 | 17.6 | 23.7 |
| Germany | 25 | 11.3 | 81 | 68262 | 169.7 | 12548 | 31.2 | 18.4 |
| France | 1 | 11.6 | 82 | 56841 | 184.0 | 8606 | 27.9 | 15.1 |
| Italy | 2 | 9.2 | 83 | 44525 | 149.0 | 7814 | 26.2 | 17.5 |
| Spain | 7 | 9.3 | 83 | 27853 | 120.5 | 5481 | 23.7 | 19.7 |
| Switzerland | 20 | 11.4 | 53 | 7851 | 206.3 | 1248 | 32.8 | 15.9 |
| Japan | 10 | 10.3 | 84 | 55970 | 90.9 | 11644 | 18.9 | 20.8 |
| Netherlands | 17 | 12.7 | 81 | 13300 | 160.2 | 2650 | 31.9 | 19.9 |
| Sweden | 23 | 9.6 | 82 | 11596 | 244.9 | 2444 | 51.6 | 21.1 |
| South Korea | 58 | 7.6 | 82 | 10351 | 42.7 | 1696 | 7.0 | 16.4 |
| Belgium | 21 | 10.9 | 80 | 9393 | 177.6 | 1913 | 36.2 | 20.4 |
| Czech | 48 | 7.5 | 78 | 6848 | 132.0 | 1268 | 24.4 | 18.5 |
| Ukraine | 79 | 7.5 | 71 | 6637 | 32.1 | 3374 | 16.3 | 50.8 |
| Austria | 9 | 11.1 | 81 | 5833 | 141.6 | 1105 | 26.8 | 18.9 |
| Norway | 11 | 9.3 | 82 | 5789 | 232.9 | 1054 | 42.4 | 18.2 |
| Denmark | 34 | 11.0 | 80 | 5205 | 187.6 | 1316 | 47.4 | 25.3 |
| USA | 37 | 17.0 | 79 | 233159 | 149.5 | 30383 | 19.5 | 13.0 |
| United Kingdom | 18 | 9.3 | 81 | 45406 | 146.7 | 10595 | 34.2 | 23.3 |
| Canada | 30 | 10.9 | 82 | 27087 | 157.4 | 3722 | 21.6 | 13.7 |
| Austria | 9 | 11.1 | 81 | 21966 | 192.2 | 3333 | 29.2 | 15.2 |
| Finland | 31 | 9.1 | 81 | 5366 | 202.2 | 832 | 31.4 | 15.5 |
Figure 1.
The associations of the World Health Organization ranking with (A) the total expenditure on health/GDP and (B) life expectancy among 35 countries included in the analysis of prostate cancer.
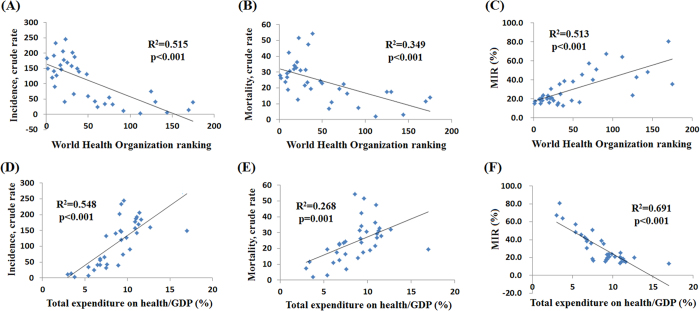
We further correlated the WHO rankings and the e/GDP with the crude rates and MIR for prostate cancer according to country. Countries with better WHO rankings had higher crude rates of incidence and mortality (R2 = 0.515, p < 0.001; R2 = 0.349, p < 0.001, respectively, Fig. 2A and B). The same phenomenon can be found for the correlations of total expenditure on e/GDP with crude rates of incidence and mortality (R2 = 0.548, p < 0.001; R2 = 0.268, p = 0.001, Fig. 2D and E). As for the MIR, the countries with better WHO rankings and e/GDP were associated with favorable MIR (R2 = 0.513, p < 0.001; R2 = 0.691, p < 0.001, respectively, Fig. 2C and F), which means that the WHO ranking and e/GDP were significantly associated with the MIR for prostate cancer.
Figure 2.
Countries with good World Health Organization rankings have high crude rates of (A) incidence and (B) mortality of prostate cancer. Additionally, in those with high total expenditures on health/GDP, the crude rates of (D) incidence and (E) mortality were higher. Higher World Health Organization rankings and total expenditures on health/GDP are associated with favorable MIRs (C) and (F).
Discussion
In this study, we investigated the correlation of MIRs for prostate cancer with cancer care disparities. We used the WHO rankings and e/GDP as indicators of health care disparities among countries. The results suggest that better support of health care expenditures leads to lower MIRs for prostate cancer (Fig. 2). The MIR was calculated with the incidence and mortality rates. This means that besides the dietary, genetic, and environmental contributions of prostate risk, early screenings and advanced surgical and personalized therapy play important roles in improving clinical outcomes which contribute to low MIR1,5,14,15,16. An observation study found that early detection or a lead time bias of more widespread utilization and earlier introduction of PSA testing in America would cause the differences in incidence and stage distributions over time which would influence the survival7. Early detection and appropriate treatments including the advanced surgical intervention equipment and personalized therapies lead to large expenditures in the health care system. This might be the reason that the MIR for prostate cancer is significantly associated with health care disparities between countries. We did not compared the difference of MIR improvement between countries according to the expenditures in the health care system. However, previous studies had shown the advantages of survival rate of developed countries such as America and European countries7,17. It is no doubt that, in recent years, limited improvement of survival rate in prostate cancer was achieved but they still had better MIRs compared with those countries with low expenditures in the health care system which were shown in our results7,17.
Regarding the difference in MIRs between regions and countries, a global study has demonstrated that the incidence trend of prostate cancer has increased in both developed regions and developing regions between 1990 and 20132. Our results suggest that prostate cancer has higher incidence and mortality rates in regions with higher development, such as the Americas and Europe. The MIR may be different with an updated database. A previous study has shown that the MIRs for China, Japan, and Korea in 2008 were 0.42, 0.22, and 0.18, respectively, which are similar to our results18. Similar trends were also shown in a study from the Asia-Pacific region9. These studies indicate that the improvement in the prognosis of prostate cancer was not obvious during these years. However, the high MIR of the African region can be explained by lack of screen, up-staging of disease at the time of diagnosis, the socioeconomic factors, care delivery, and treatment selection that have an African origin8. All of these data support our findings that MIR has a role in prediction of care disparities.
The limitations of our study are that we excluded countries with an incidence less than 5,000 cases per year. Additionally, no further clinical information including stage and PSA screening was analyzed. Furthermore, the use of the WHO rankings and e/GDP to represent the health care disparities of countries is not specific; other factors, such as the national health care systems, disparity in access to cancer care, and insurance coverage, should be analyzed. In this study, crude rate was analyzed. We also investigate the WHO rankings and the e/GDP with the age-standardized rate (ASR) for prostate cancer according to country. Countries with better WHO rankings had higher ASR of incidence and mortality (R2 = 0.328, p < 0.001; R2 = 0.054, p = 0.179, respectively). The correlations of total expenditure on e/GDP with ASR of incidence and mortality showed significance in ASR of incidence (R2 = 0.743, p < 0.001; R2 = 0.068, p = 0.697, respectively). The reason of lack significant association between ASR and WHO ranking or e/GDP needs further investigation.
In this study, we demonstrated that the MIR for prostate cancer is associated with health care disparities. Further investigations with greater detail and focus on the data are needed to support our findings. Moreover, future follow-up studies of the MIRs would be helpful in monitoring improvements in prostate cancer care among countries.
Additional Information
How to cite this article: Chen, S.-L. et al. Prostate Cancer Mortality-To-Incidence Ratios Are Associated with Cancer Care Disparities in 35 Countries. Sci. Rep. 7, 40003; doi: 10.1038/srep40003 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was jointly supported by grants from 105-CCH-IRP-081 (Dr. CJ Chen. Changhua Christian Hospital, Changhua, Taiwan).
Footnotes
Author Contributions Conception and design: Chen S.L.; acquisition of data: Hsieh T.Y., Wu P.R.; analysis and interpretation of data: Kao Y.L., Chen W.J.; drafting of the manuscript: Wang S.C., Ho C.J.; critical revision of the manuscript: Ko J.L., Lee H.; statistical analysis: Chen C.J.; supervision: Sung W.W.
References
- Attard G. et al. Prostate cancer. Lancet 387, 70–82 (2016). [DOI] [PubMed] [Google Scholar]
- Fitzmaurice C. et al. The Global Burden of Cancer 2013. JAMA Oncol 1, 505–527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre L. A. et al. Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108 (2015). [DOI] [PubMed] [Google Scholar]
- Hayes J. H. & Barry M. J. Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA 311, 1143–1149 (2014). [DOI] [PubMed] [Google Scholar]
- Roth J. A., Gulati R., Gore J. L., Cooperberg M. R. & Etzioni R. Economic Analysis of Prostate-Specific Antigen Screening and Selective Treatment Strategies. JAMA Oncol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. E., Taylor Y. J. & Howard D. L. Trends in prostate-specific antigen test use, 2000–2005. Public Health Rep 126, 228–239 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A. et al. Comparison of prostate cancer survival in Germany and the United States: Can differences be attributed to differences in stage distributions? BJU international (2016). [DOI] [PubMed] [Google Scholar]
- McGinley K. F., Tay K. J. & Moul J. W. Prostate cancer in men of African origin. Nat Rev Urol 13, 99–107 (2016). [DOI] [PubMed] [Google Scholar]
- Baade P. D., Youlden D. R., Cramb S. M., Dunn J. & Gardiner R. A. Epidemiology of prostate cancer in the Asia-Pacific region. Prostate Int 1, 47–58 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson K., Wang C. Y. & Wang R. Global prostate cancer incidence and the migration, settlement, and admixture history of the Northern Europeans. Cancer Epidemiol 35, 320–327 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas C. A., Tsodikov A., Ishak-Howard M. & Cooney K. A. Prostate cancer in young men: an important clinical entity. Nat Rev Urol 11, 317–323 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri M. Prostate Cancer Claims for a Personalized Medicine. N Am J Med Sci 7, 436–437 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara V. & Hebert J. R. The colorectal cancer mortality-to-incidence ratio as an indicator of global cancer screening and care. Cancer 121, 1563–1569 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S. et al. Screening for Prostate Cancer Starting at Age 50–54 Years. A Population-based Cohort Study. Eur Urol (2016). [DOI] [PMC free article] [PubMed]
- Gillessen S. et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO (2016). [DOI] [PMC free article] [PubMed]
- Lin P. H., Aronson W. & Freedland S. J. Nutrition, dietary interventions and prostate cancer: the latest evidence. BMC Med 13, 3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr M., Holzel D., Schubert-Fritschle G., Engel J. & Schlesinger-Raab A. Changes in prognostic and therapeutic parameters in prostate cancer from an epidemiological view over 20 years. Oncology research and treatment 38, 8–14 (2015). [DOI] [PubMed] [Google Scholar]
- Ito K. Prostate cancer in Asian men. Nat Rev Urol 11, 197–212 (2014). [DOI] [PubMed] [Google Scholar]