Abstract
PURPOSE OF REVIEW
VWF is a large multi-domain, multimeric glycoprotein that plays an essential role in regulating the balance between blood clotting and bleeding. Aberrant VWF regulation can lead to a spectrum of diseases extending from bleeding disorders (VWD) to aberrant thrombosis (TTP). Understanding the biology of VWF expression and secretion is essential for developing novel targeted therapies for VWF related hemostasis disorders.
RECENT FINDINGS
A number of recent elegant in vitro and in vivo studies will be highlighted including the discovery of intronic splicing in the VWF gene, miRNA regulated VWF gene expression, and syntaxin binding protein and autophagy mediated VWF secretion. Compared with the already established critical role of VWF in VWD and TTP pathophysiology, additional clinical studies have clarified and reinforced the association of increased plasma levels of VWF with an increased risk of stroke, myocardial infarction, venous thrombosis and diabetic thrombotic complications. Moreover, experimental mouse models of ischaemic stroke and myocardial infarction have further support VWF as a potential therapeutic target.
SUMMARY
VWF biosynthesis, maturation, and secretion is a complex process, which mandates tight regulation. Significant progress has been made in our understandings of VWF expression and secretion and its association with thrombotic diseases, contributing to the development of novel targeting VWF drugs for prevention and treatment of deficient and enhanced hemostasis.
Keywords: VWF, Expression, Secretion, Thrombosis
Introduction
VWF is a large plasma glycoprotein that is critical for normal platelet tethering during hemostasis [1]. The VWF gene is located at the tip of the short arm of chromosome 12 (region 12p12-12pter), and contains 52 exons separated by 51 introns [2]. It is approximately 180kb in length [3]. Expression of the VWF gene is mainly in vascular endothelial cells and megakaryocytes [1]. The VWF cDNA translation product is a 2813-residue pre-pro-VWF, which then enters the endoplasmic reticulum, where the signal peptide is proteolytically cleaved [4]. The pro-VWF is glycosylated in the endoplasmic reticulum with N-linked and O-linked oligosaccharides. VWF dimerization subsequently occurs through disulphide bond formation close to its carboxyl terminus. These dimers are transported to the Golgi apparatus, where multimerisation occurs through disulphide bond formation between the amino-termini of adjacent dimers. Multimerisation is promoted by the VWF propeptide. Additional modifications in the Golgi include the removal of the propeptide by the paired dibasic amino acid-cleaving enzyme furin, and the completion of glycosylation. Further multimerisation gives rise to the mature VWF with a molecular weight ranging from 500kDa to 20,000kDa, or more [1]. The majority of newly synthesized VWF multimers undergo basal secretion into the plasma and the sub-endothelial matrix; the rest are stored either in the Weibel-Palade bodies of endothelial cells or in the alpha granules of platelets. These storage granules contain ultra large multimers, whereas plasma VWF is composed of a range of smaller multimers arising in part from proteolytic cleavage [5, 6] (Fig. 1).
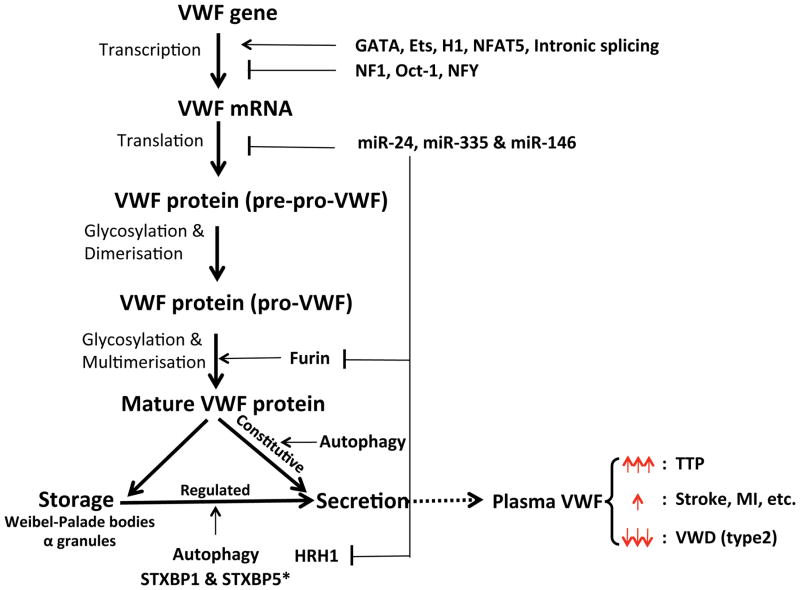
Figure 1.
Regulation of VWF expression & secretion and diseases.
VWF gene expression is tightly regulated in both transcriptional levels (including transcription factors [GATA, Ets, H1, NFAT5, NF1, Oct-1, NFY, etc.] and intronic splicing) and translation levels (miR-24, miR-335 and miR-146). VWF maturation is complex process involved in dimerization, glycoslation, multimerisation and removal of pro-peptide by furin (regulated by miR-24). Partial mature VWF are stored either in the Weibel-Palade bodies of endothelial cells or in the alpha granules of platelets. VWF secretion includes constitutive secretion and regulated secretion, both of which regulated by autophagy. STXBP1 and histamine (histamine receptor HRH1 regulated by miR-24) enhance while STXBP5 inhibits VWF regulated secretion in endothelial cells. However, STXBP5 promotes VWF regulated secretion in platelets. Extremely elevation of VWF (especially ultra-large VWF) leads to TTP while reduced of VWF antigen or function causes some of type 2 VWD. More evidence supports the association between an increase of VWF in plasma with risk of stroke, myocardial infarction (MI) and diabetic thrombosis.
VWF plays a crucial role in maintaining normal hemostasis and contributes to thrombotic disorders following endothelial and platelet dysfunction [7, 8]. In response to blood shear forces, VWF unfolds from its inactive globular conformation into an active string-like form that can specifically recruit platelets [8, 9]. The multimeric size of VWF is a primary determinant of its platelet-tethering function and is proteolytically regulated by the plasma metalloprotease ADAMTS13, which is responsible for the degradation of large, thrombogenic VWF multimers [8, 10, 11]. The importance of ADAMTS13 in maintaining the balance of VWF multimeric size is illustrated by its role in a number of hematologic disorders, including the idiopathic form of thrombotic thrombocytopenic purpura (TTP) and some cases of type 2A von Willebrand disease (VWD). A recent review has updated the current knowledge on VWF and ADAMTS13 in TTP [12]. Although the incidence of both VWD and TTP are relatively low, around 100 per million and 4 per million respectively [13, 14], there is increasing epidemiological and clinical evidence indicating that the incidence of thrombotic diseases, including heart attack and stroke, highly correlates with VWF dysregulation [15, 16]. There are a number of reports demonstrating association between high plasma VWF levels and/or low plasma ADAMTS13 levels with increased risk of thrombotic diseases [17–23]. However, evidence based on clinical trials has not clarified whether increased VWF predisposes to arterial thrombosis_or is just a marker of endothelial damage, the latter being responsible for the thrombosis. This review aims to focus on the advances in regulatory mechanisms involved in VWF expression and secretion. Moreover, we will also analyze the progress of basic and clinical studies and how such regulation of VWF can contribute directly to thrombotic diseases or complications, including stroke, myocardial infarction and diabetes mellitus.
1. Regulation of VWF gene expression
Being a central component of hemostasis and thrombosis, it is understandable that VWF gene expression is restricted to endothelial cells and megakaryocytes [24], key cells involved in directing thrombosis. Previous investigations have demonstrated the existence of regional variations in VWF protein and mRNA levels within the vascular tree [25], suggesting that the VWF gene is differentially regulated in vascular beds of the systemic circulation. Characterization of the mechanism of the endothelial-specific VWF promoter has resulted in the identification of a number of cis-acting elements and trans-acting factors that regulate VWF promoter activity. The transcription factors GATA, Ets, H1 and NFAT5 have been demonstrated to function as activators of transcription [26–29], whereas NF1 and Oct-1 are repressors of transcription [30, 31]. Interestingly, NFY can function both as a repressor and activator of transcription dependent upon the binding site [32]. E4BP4 sequesters negative regulators of VWF transcription, enhancing activated VWF gene expression [33]. Shear stress also enhances VWF promoter activity mediated by a polymorphic GT repeat element [34]. Using a series of elegant in vitro and in vivo experiments, Aird et al demonstrated that the first VWF intron is important for expression, with intronic splicing necessary for endothelial-cell–specific expression of VWF [35, 36]. The focus of VWF gene regulation has been predominantly on the 5′UTR through its promoter and related transcription factors. Our group recently reported that VWF gene expression is also regulated at its 3′UTR though miRNAs [37, 38]. We identified that miR-24 and miR-335 targeted human VWF 3′UTR, while miR-24 and miR-146 target mouse VWF 3′UTR [38]. More interestingly, miR-24 was also found targeting histamine receptor (HRH1) and eNOS, which is known involved Ca2+ and NO mediated VWF secretion[38] (Fig. 1).
2. Regulation of VWF protein secretion
Recently, a number of studies have attempted to elucidate the mechanism by which VWF is released from endothelial cells [6, 39, 40]. Here we provide a brief update on progress in studies of VWF secretion and Weibel-Palade bodies (WPBs) exocytosis. Although the storage of VWF in WPB of endothelial cells has been known for decades, the molecular mechanisms governing WPB docking with plasma membrane and VWF secretion remained poorly understood. Using a combination of pharmacological, genetic, and molecular techniques, Rusu et al dissected a mechanism for VWF secretion from endothelial cells mediated via Gαq/11 and Gα12 that can be triggered during basal or stimulated conditions [41]. In addition, Bierings et al reported the Rab27A effector synaptotagmin-like protein 4-a (Slp4-a) plays a critical role in regulating hormone-evoked WPB exocytosis [42]. Using a nonbiased proteomic screen for targets of Slp4-a, they identify syntaxin-binding protein 1 (STXBP1) and syntaxin-2 and -3 as endogenous Slp4-a binding partners in endothelial cells [43]. Applying co-immunoprecipitations and siRNA techniques, analysis of isolated blood outgrowth endothelial cells from patient carrying mutation in STXBP1, they demonstrate that the Rab27A-Slp4-a complex on WPB promotes exocytosis through an interaction with STXBP1 [43]. Interestingly, STXBP5 has also been shown to differentially regulate exocytosis in endothelial cells and platelets [44, 45]. While STXBP5 facilitates granule release from platelets, it inhibits secretion from the WPB of endothelial cells [46]. Recently, Torisu et al observed another novel type of regulation of VWF release through an observation that WPBs are often in close proximity of autophagosomes [47]. Both in vitro and in vivo inhibition of autophagy led to decreased WPB release, including lower basal levels of VWF and lower response to epinephrine-induced WPB release [47].
3. VWF in diseases beyond VWD and TTP
3.1 VWF and Stroke
Ischemic stroke is a devastating disease with limited therapeutic options. Surprisingly, VWF deficiency in VWF −/− stroke mouse model (middle cerebral artery occlusion) reduced the infarct volumes by ~2-fold [48]. Moreover, infusion of recombinant human ADAMTS13 into a wild-type stroke mouse model also reduced infarct volumes, without cerebral hemorrhage [48]. Previous studies demonstrated that VWF synthesized in ECs is sufficient to support hemostasis in VWF−/− mice, and VWF produced in megakaryocytes/platelets can also contribute to hemostasis in the absence of EC-derived VWF [49]. A more recent study using a mouse stroke model (transient middle cerebral artery occlusion) suggested that platelet-derived VWF plays a crucial role in stroke pathology although it is not essential for normal thrombosis [50]. Moreover, a recent clinical study reported that concurrent FVIII and VWF elevation predicts higher risks of inpatient complications and worse functional outcomes for patients with acute ischemic stroke and suggest that FVIII and VWF levels may serve as clinically useful stroke biomarkers [51]. The Rotterdam Study, a population-based cohort study, demonstrated that low ADAMTS13 activity is associated with increased risk of ischemic stroke and improves the accuracy of risk predictions for ischemic stroke beyond traditional risk factors [52]. Based on these findings, it is suggested that VWF would be a promising target in the prevention and/or treatment of thrombotic diseases such as strokes, and recombinant ADAMTS13 could be considered as a novel therapeutic agent for use against such thrombotic diseases. Glycoprotein (GP)Ib binding to VWF exposed at vascular injury initiates platelet adhesion and contributes to platelet aggregation. GPIb has been suggested as an effective target for antithrombotic therapy in stroke. Anfibatide, a GPIb antagonist derived from snake venom, has demonstrated a protective effect on experimental brain ischemia in mice [53]. Moreover, disruption of GpIba-VWF interactions may restore vessel patency after occlusive thrombosis by specifically disaggregating the external layer of the occlusive thrombi [54].
3.2 VWF and Myocardial Infarction
A recent multicenter and multiethnic study (China, Italy and Scotland) found that plasma levels of active VWF are an independent risk factor for first STEMI in patients, and confirm the presence of VWF abnormalities in patients with STEMI [55]. Consistent with such studies, the prevalence of cardiovascular diseases (CVD) in VWD patients is around half the prevalence of CVD in non-VWD patients [56]. Although low ADAMTS-13 levels have been repeatedly associated with an increased risk of ischemic stroke, results concerning the risk of myocardial infarction are inconclusive. However, a meta-analysis of case studies supports that low ADAMTS-13 levels are associated with an increased risk of myocardial infarction [57]. Using an acute myocardial ischemia/reperfusion (I/R) injury mouse model, VWF(−/−) mice exhibited significantly reduced infarct size while ADAMTS13(−/−) mice exhibited significantly larger infarct size, suggesting a detrimental role for VWF in myocardial I/R injury. Moreover, ADAMTS13(−/−)/VWF(−/−) mice was similar to that in VWF(−/−) mice, suggesting that the exacerbated myocardial I/R injury in the setting of ADAMTS13 deficiency is VWF dependent [58].
3.3 VWF and venous thrombosis
To date, VWF is one of 17 identified genes that has been demonstrated to harbor genetic variations associated with VT risk [59]. Elevated plasma FVIII levels enhance venous thrombus formation and propagation [60]. FXI and VWF-mediated FVIII recruitment induce excess thrombin generation may contribute to the growth of a FVIII-driven venous thrombus [61].
3.4 VWF and diabetes
It is widely accepted that diabetes mellitus (DM) impairs endothelial functions and enhances the production of reactive oxygen species, and causes subsequent vascular impairments and complications. Applying various diabetic mouse models and analysis clinical diabetic samples, we have recently reported that hyperglycemia (type 1 or type 2 diabetes mellitus), regulates VWF through aldose reductase, ROS, and the c-Myc pathway [38]. The increased ROS upregulates c-Myc phosphorylation, which downregulates miR-24, leading to increased expression of VWF, Furin, and HRH1. Therefore, the expression of VWF itself, Furin-dependent VWF maturation, and histamine-regulated VWF secretion, are coordinately upregulated with hyperglycemia. In the BErgamo NEphrologic DIabetes Complications Trial (BENEDICT), it was found that patients with diabetes mellitus, and ADAMTS13 618Ala variant, were associated with less proteolytic activity on VWF, and higher risk of renal and cardiovascular complications [62].
4. In summary
A number of recent exciting advances have been made in elucidating the mechanisms by which VWF is tightly regulated both in expression and secretion from endothelial cells. Studies now strongly support that dysregulation of these critical processes clearly leads to pathological thrombotic conditions. VWF regulation may indeed serve as a powerful therapeutic target in treating thrombotic diseases such as stroke and myocardial infarction.
Conclusion
Von Willebrand factor expression and secretion are increasingly complicated processes involving multiple pathways, and requiring tight control.
VWF gene regulation has previously focused on the 5′UTR through its promoter and related transcription factors, but new findings support that VWF gene expression is also highly regulated at its 3′UTR though miRNAs.
Syntaxin binding proteins, G-proteins and autophagy are involved in regulation of VWF secretion.
Basic and clinical studies indicate VWF contributes directly to stroke, myocardial infarction and diabetic complications, and VWF regulation may serve as a powerful therapeutic target.
Acknowledgments
Financial support and sponsorship
J.H. has received research funding from NIH-NHLBI HL115247, HL122815 and HL117798.
Footnotes
Conflicts of interest
There are no conflicts of interest
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪ ▪ of outstanding interest
- 1.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annual review of biochemistry. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 2.Ginsburg D, Handin RI, Bonthron DT, et al. Human von Willebrand factor (vWF): isolation of complementary DNA (cDNA) clones and chromosomal localization. Science. 1985;228:1401–1406. doi: 10.1126/science.3874428. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri ZM. Structure and function of von Willebrand factor. Thrombosis and haemostasis. 1999;82:576–584. [PubMed] [Google Scholar]
- 4.Bonthron DT, Handin RI, Kaufman RJ, et al. Structure of pre-pro-von Willebrand factor and its expression in heterologous cells. Nature. 1986;324:270–273. doi: 10.1038/324270a0. [DOI] [PubMed] [Google Scholar]
- 5.Moake JL, Turner NA, Stathopoulos NA, et al. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. The Journal of clinical investigation. 1986;78:1456–1461. doi: 10.1172/JCI112736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenting PJ, Christophe OD, Denis CV. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. 2015;125:2019–2028. doi: 10.1182/blood-2014-06-528406. [DOI] [PubMed] [Google Scholar]
- 7.Dayananda KM, Singh I, Mondal N, Neelamegham S. von Willebrand factor self-association on platelet GpIbalpha under hydrodynamic shear: effect on shear-induced platelet activation. Blood. 2010;116:3990–3998. doi: 10.1182/blood-2010-02-269266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawley JT, de Groot R, Xiang Y, et al. Unraveling the scissile bond: how ADAMTS13 recognizes and cleaves von Willebrand factor. Blood. 2011;118:3212–3221. doi: 10.1182/blood-2011-02-306597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dopheide SM, Maxwell MJ, Jackson SP. Shear-dependent tether formation during platelet translocation on von Willebrand factor. Blood. 2002;99:159–167. doi: 10.1182/blood.v99.1.159. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X, Chung D, Takayama TK, et al. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. The Journal of biological chemistry. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 11.Xiang Y, de Groot R, Crawley JT, Lane DA. Mechanism of von Willebrand factor scissile bond cleavage by a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11602–11607. doi: 10.1073/pnas.1018559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng XL. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annual review of medicine. 2015;66:211–225. doi: 10.1146/annurev-med-061813-013241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torok TJ, Holman RC, Chorba TL. Increasing mortality from thrombotic thrombocytopenic purpura in the United States--analysis of national mortality data, 1968–1991. American journal of hematology. 1995;50:84–90. doi: 10.1002/ajh.2830500203. [DOI] [PubMed] [Google Scholar]
- 14.Sadler JE. New concepts in von Willebrand disease. Annual review of medicine. 2005;56:173–191. doi: 10.1146/annurev.med.56.082103.104713. [DOI] [PubMed] [Google Scholar]
- 15.Spiel AO, Gilbert JC, Jilma B. von Willebrand factor in cardiovascular disease: focus on acute coronary syndromes. Circulation. 2008;117:1449–1459. doi: 10.1161/CIRCULATIONAHA.107.722827. [DOI] [PubMed] [Google Scholar]
- 16.De Meyer SF, Stoll G, Wagner DD, Kleinschnitz C. von Willebrand factor: an emerging target in stroke therapy. Stroke; a journal of cerebral circulation. 2012;43:599–606. doi: 10.1161/STROKEAHA.111.628867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miura M, Kaikita K, Matsukawa M, et al. Prognostic value of plasma von Willebrand factor-cleaving protease (ADAMTS13) antigen levels in patients with coronary artery disease. Thrombosis and haemostasis. 2010;103:623–629. doi: 10.1160/TH09-08-0568. [DOI] [PubMed] [Google Scholar]
- 18.Bongers TN, de Bruijne EL, Dippel DW, et al. Lower levels of ADAMTS13 are associated with cardiovascular disease in young patients. Atherosclerosis. 2009;207:250–254. doi: 10.1016/j.atherosclerosis.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Gombos T, Mako V, Cervenak L, et al. Levels of von Willebrand factor antigen and von Willebrand factor cleaving protease (ADAMTS13) activity predict clinical events in chronic heart failure. Thrombosis and haemostasis. 2009;102:573–580. doi: 10.1160/TH09-01-0036. [DOI] [PubMed] [Google Scholar]
- 20.Feys HB, Canciani MT, Peyvandi F, et al. ADAMTS13 activity to antigen ratio in physiological and pathological conditions associated with an increased risk of thrombosis. British journal of haematology. 2007;138:534–540. doi: 10.1111/j.1365-2141.2007.06688.x. [DOI] [PubMed] [Google Scholar]
- 21.Chion CK, Doggen CJ, Crawley JT, et al. ADAMTS13 and von Willebrand factor and the risk of myocardial infarction in men. Blood. 2007;109:1998–2000. doi: 10.1182/blood-2006-07-038166. [DOI] [PubMed] [Google Scholar]
- 22.Bongers TN, de Maat MP, van Goor ML, et al. High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability. Stroke; a journal of cerebral circulation. 2006;37:2672–2677. doi: 10.1161/01.STR.0000244767.39962.f7. [DOI] [PubMed] [Google Scholar]
- 23.Andersson HM, Siegerink B, Luken BM, et al. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood. 2012;119:1555–1560. doi: 10.1182/blood-2011-09-380618. [DOI] [PubMed] [Google Scholar]
- 24.Mancuso DJ, Tuley EA, Westfield LA, et al. Structure of the gene for human von Willebrand factor. The Journal of biological chemistry. 1989;264:19514–19527. [PubMed] [Google Scholar]
- 25.Yamamoto K, de Waard V, Fearns C, Loskutoff DJ. Tissue distribution and regulation of murine von Willebrand factor gene expression in vivo. Blood. 1998;92:2791–2801. [PubMed] [Google Scholar]
- 26.Liu J, Kanki Y, Okada Y, et al. A +220 GATA motif mediates basal but not endotoxin-repressible expression of the von Willebrand factor promoter in Hprt-targeted transgenic mice. Journal of thrombosis and haemostasis : JTH. 2009;7:1384–1392. doi: 10.1111/j.1538-7836.2009.03501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwachtgen JL, Janel N, Barek L, et al. Ets transcription factors bind and transactivate the core promoter of the von Willebrand factor gene. Oncogene. 1997;15:3091–3102. doi: 10.1038/sj.onc.1201502. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Peng Y, Ma Y, Jahroudi N. Histone H1-like protein participates in endothelial cell-specific activation of the von Willebrand factor promoter. Blood. 2004;104:1725–1732. doi: 10.1182/blood-2004-01-0082. [DOI] [PubMed] [Google Scholar]
- 29.Dmitrieva NI, Burg MB. Secretion of von Willebrand factor by endothelial cells links sodium to hypercoagulability and thrombosis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6485–6490. doi: 10.1073/pnas.1404809111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jahroudi N, Ardekani AM, Greenberger JS. An NF1-like protein functions as a repressor of the von Willebrand factor promoter. The Journal of biological chemistry. 1996;271:21413–21421. doi: 10.1074/jbc.271.35.21413. [DOI] [PubMed] [Google Scholar]
- 31.Schwachtgen JL, Remacle JE, Janel N, et al. Oct-1 is involved in the transcriptional repression of the von willebrand factor gene promoter. Blood. 1998;92:1247–1258. [PubMed] [Google Scholar]
- 32.Peng Y, Jahroudi N. The NFY transcription factor functions as a repressor and activator of the von Willebrand factor promoter. Blood. 2002;99:2408–2417. doi: 10.1182/blood.v99.7.2408. [DOI] [PubMed] [Google Scholar]
- 33.Hough C, Cuthbert CD, Notley C, et al. Cell type-specific regulation of von Willebrand factor expression by the E4BP4 transcriptional repressor. Blood. 2005;105:1531–1539. doi: 10.1182/blood-2002-10-3093. [DOI] [PubMed] [Google Scholar]
- 34.Hough C, Cameron CL, Notley CR, et al. Influence of a GT repeat element on shear stress responsiveness of the VWF gene promoter. Journal of thrombosis and haemostasis : JTH. 2008;6:1183–1190. doi: 10.1111/j.1538-7836.2008.03011.x. [DOI] [PubMed] [Google Scholar]
- 35.Ware J. Give me an intron: any intron. Blood. 2013;121:4251–4252. doi: 10.1182/blood-2013-04-494856. [DOI] [PubMed] [Google Scholar]
- 36.Yuan L, Janes L, Beeler D, et al. Role of RNA splicing in mediating lineage-specific expression of the von Willebrand factor gene in the endothelium. Blood. 2013;121:4404–4412. doi: 10.1182/blood-2012-12-473785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grosser T. microRNA represses macromolecule. Blood. 2015;125:3365–3366. doi: 10.1182/blood-2015-04-638353. [DOI] [PubMed] [Google Scholar]
- 38.Xiang Y, Cheng J, Wang D, et al. Hyperglycemia repression of miR-24 coordinately upregulates endothelial cell expression and secretion of von Willebrand factor. Blood. 2015;125:3377–3387. doi: 10.1182/blood-2015-01-620278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadler JE. von Willebrand factor assembly and secretion. Journal of thrombosis and haemostasis : JTH. 2009;7(Suppl 1):24–27. doi: 10.1111/j.1538-7836.2009.03375.x. [DOI] [PubMed] [Google Scholar]
- 40.Nightingale T, Cutler D. The secretion of von Willebrand factor from endothelial cells; an increasingly complicated story. Journal of thrombosis and haemostasis : JTH. 2013;11(Suppl 1):192–201. doi: 10.1111/jth.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rusu L, Andreeva A, Visintine DJ, et al. G protein-dependent basal and evoked endothelial cell vWF secretion. Blood. 2014;123:442–450. doi: 10.1182/blood-2013-03-489351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bierings R, Hellen N, Kiskin N, et al. The interplay between the Rab27A effectors Slp4-a and MyRIP controls hormone-evoked Weibel-Palade body exocytosis. Blood. 2012;120:2757–2767. doi: 10.1182/blood-2012-05-429936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Breevoort D, Snijders AP, Hellen N, et al. STXBP1 promotes Weibel-Palade body exocytosis through its interaction with the Rab27A effector Slp4-a. Blood. 2014;123:3185–3194. doi: 10.1182/blood-2013-10-535831. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Q, Yamakuchi M, Ture S, et al. Syntaxin-binding protein STXBP5 inhibits endothelial exocytosis and promotes platelet secretion. The Journal of clinical investigation. 2014;124:4503–4516. doi: 10.1172/JCI71245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye S, Huang Y, Joshi S, et al. Platelet secretion and hemostasis require syntaxin-binding protein STXBP5. The Journal of clinical investigation. 2014;124:4517–4528. doi: 10.1172/JCI75572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lillicrap D. Syntaxin-binding protein 5 exocytosis regulation: differential role in endothelial cells and platelets. The Journal of clinical investigation. 2014;124:4231–4233. doi: 10.1172/JCI77511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torisu T, Torisu K, Lee IH, et al. Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor. Nature medicine. 2013;19:1281–1287. doi: 10.1038/nm.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao BQ, Chauhan AK, Canault M, et al. von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood. 2009;114:3329–3334. doi: 10.1182/blood-2009-03-213264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanaji S, Fahs SA, Shi Q, et al. Contribution of platelet vs. endothelial VWF to platelet adhesion and hemostasis. Journal of thrombosis and haemostasis : JTH. 2012;10:1646–1652. doi: 10.1111/j.1538-7836.2012.04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verhenne S, Denorme F, Libbrecht S, et al. Platelet-derived VWF is not essential for normal thrombosis and hemostasis but fosters ischemic stroke injury in mice. Blood. 2015;126:1715–1722. doi: 10.1182/blood-2015-03-632901. [DOI] [PubMed] [Google Scholar]
- 51.Samai A, Monlezun D, Shaban A, et al. Von Willebrand factor drives the association between elevated factor VIII and poor outcomes in patients with ischemic stroke. Stroke; a journal of cerebral circulation. 2014;45:2789–2791. doi: 10.1161/STROKEAHA.114.006394. [DOI] [PubMed] [Google Scholar]
- 52.Sonneveld MA, de Maat MP, Portegies ML, et al. Low ADAMTS13 activity is associated with an increased risk of ischemic stroke. Blood. 2015 doi: 10.1182/blood-2015-05-643338. [DOI] [PubMed] [Google Scholar]
- 53.Li TT, Fan ML, Hou SX, et al. A novel snake venom-derived GPIb antagonist, anfibatide, protects mice from acute experimental ischaemic stroke and reperfusion injury. British journal of pharmacology. 2015;172:3904–3916. doi: 10.1111/bph.13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Behot A, Gauberti M, Martinez De Lizarrondo S, et al. GpIbalpha-VWF blockade restores vessel patency by dissolving platelet aggregates formed under very high shear rate in mice. Blood. 2014;123:3354–3363. doi: 10.1182/blood-2013-12-543074. [DOI] [PubMed] [Google Scholar]
- 55.Rutten B, Maseri A, Cianflone D, et al. Plasma levels of active Von Willebrand factor are increased in patients with first ST-segment elevation myocardial infarction: a multicenter and multiethnic study. European heart journal Acute cardiovascular care. 2015;4:64–74. doi: 10.1177/2048872614534388. [DOI] [PubMed] [Google Scholar]
- 56.Seaman CD, Yabes J, Comer DM, Ragni MV. Does deficiency of von Willebrand factor protect against cardiovascular disease? Analysis of a national discharge register Journal of thrombosis and haemostasis : JTH. 2015;13:1999–2003. doi: 10.1111/jth.13142. [DOI] [PubMed] [Google Scholar]
- 57.Maino A, Siegerink B, Lotta LA, et al. Plasma ADAMTS-13 levels and the risk of myocardial infarction: an individual patient data meta-analysis. Journal of thrombosis and haemostasis : JTH. 2015;13:1396–1404. doi: 10.1111/jth.13032. [DOI] [PubMed] [Google Scholar]
- 58.Gandhi C, Motto DG, Jensen M, et al. ADAMTS13 deficiency exacerbates VWF-dependent acute myocardial ischemia/reperfusion injury in mice. Blood. 2012;120:5224–5230. doi: 10.1182/blood-2012-06-440255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morange PE, Suchon P, Tregouet DA. Genetics of Venous Thrombosis: update in 2015. Thrombosis and haemostasis. 2015;114:910–919. doi: 10.1160/TH15-05-0410. [DOI] [PubMed] [Google Scholar]
- 60.Timp JF, Lijfering WM, Flinterman LE, et al. Predictive value of factor VIII levels for recurrent venous thrombosis: results from the MEGA follow-up study. Journal of thrombosis and haemostasis : JTH. 2015;13:1823–1832. doi: 10.1111/jth.13113. [DOI] [PubMed] [Google Scholar]
- 61.Sugita C, Yamashita A, Matsuura Y, et al. Elevated plasma factor VIII enhances venous thrombus formation in rabbits: contribution of factor XI, von Willebrand factor and tissue factor. Thrombosis and haemostasis. 2013;110:62–75. doi: 10.1160/TH13-01-0069. [DOI] [PubMed] [Google Scholar]
- 62.Rurali E, Noris M, Chianca A, et al. ADAMTS13 predicts renal and cardiovascular events in type 2 diabetic patients and response to therapy. Diabetes. 2013;62:3599–3609. doi: 10.2337/db13-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]