Abstract
BACKGROUND
Serum testosterone concentrations decrease as men age, but benefits of raising testosterone levels in older men have not been established.
METHODS
We assigned 790 men 65 years of age or older with a serum testosterone concentration of less than 275 ng per deciliter and symptoms suggesting hypoandrogenism to receive either testosterone gel or placebo gel for 1 year. Each man participated in one or more of three trials — the Sexual Function Trial, the Physical Function Trial, and the Vitality Trial. The primary outcome of each of the individual trials was also evaluated in all participants.
RESULTS
Testosterone treatment increased serum testosterone levels to the mid-normal range for men 19 to 40 years of age. The increase in testosterone levels was associated with significantly increased sexual activity, as assessed by the Psychosexual Daily Questionnaire (P<0.001), as well as significantly increased sexual desire and erectile function. The percentage of men who had an increase of at least 50 m in the 6-minute walking distance did not differ significantly between the two study groups in the Physical Function Trial but did differ significantly when men in all three trials were included (20.5% of men who received testosterone vs. 12.6% of men who received placebo, P=0.003). Testosterone had no significant benefit with respect to vitality, as assessed by the Functional Assessment of Chronic Illness Therapy–Fatigue scale, but men who received testosterone reported slightly better mood and lower severity of depressive symptoms than those who received placebo. The rates of adverse events were similar in the two groups.
CONCLUSIONS
In symptomatic men 65 years of age or older, raising testosterone concentrations for 1 year from moderately low to the mid-normal range for men 19 to 40 years of age had a moderate benefit with respect to sexual function and some benefit with respect to mood and depressive symptoms but no benefit with respect to vitality or walking distance. The number of participants was too few to draw conclusions about the risks of testosterone treatment. (Funded by the National Institutes of Health and others; ClinicalTrials.gov number, NCT00799617.)
Testosterone concentrations in men decrease with increasing age.1,2 Many symptoms and conditions similar to those that are caused by low testosterone levels in men with pituitary or testicular disease become more common with increasing age. Such symptoms include decreases in mobility, sexual function, and energy. These parallels suggest that the lower testosterone levels in older men may contribute to these conditions.
Previous trials of testosterone treatment in men 65 years of age or older, however, have yielded equivocal results. Although testosterone treatment consistently increased muscle mass and decreased fat mass,3,4 effects on physical performance,3,5,6 sexual function,3,6,7 and energy3,6,8 have been inconsistent.
In 2003, an Institute of Medicine panel concluded that there was insufficient evidence that testosterone treatment was beneficial in older men9 and recommended a coordinated set of clinical trials to determine whether testosterone would benefit older men who had low testosterone levels for no known reason other than age and who had clinical conditions to which low testosterone might contribute. The Testosterone Trials were designed to implement that recommendation.10
METHODS
STUDY DESIGN AND OVERSIGHT
The Testosterone Trials are a coordinated set of seven double-blind, placebo-controlled trials that are being conducted at 12 sites.10 To enroll in these trials overall, participants had to qualify for at least one of the three main trials (the Sexual Function Trial, the Physical Function Trial, or the Vitality Trial), but they could participate in more than one if they qualified. Participants were assigned to receive testosterone gel or placebo gel for 1 year. Efficacy was assessed at baseline and at 3, 6, 9, and 12 months. Data on adverse events were collected during the treatment period and for 12 months afterward. This report describes the efficacy results for the three main trials and adverse events in all the participants in these trials.
The protocol and consent forms were approved by the institutional review boards at the University of Pennsylvania and each participating trial site. All participants provided written informed consent. A data and safety monitoring board monitored data in an unblinded fashion every 3 months. The protocol, consent forms, and statistical analysis plan are available with the full text of this article at NEJM.org.
The investigators developed the protocol with assistance from the National Institutes of Health. AbbVie, one of the funders of the trial, donated the testosterone and placebo gels but did not participate in the design or conduct of the trials or in the analysis, review, or reporting of the data before the manuscript was submitted for publication. All the authors participated in the design and conduct of the trials. Trial statisticians performed all data analyses. The first author wrote the first draft of the manuscript, and all the authors contributed to subsequent drafts.
PARTICIPANTS
Participants were recruited principally through mass mailings.11 Respondents were screened first by telephone interview and then during two clinic visits. Eligibility criteria included an age of 65 years or older and serum testosterone levels that averaged less than 275 ng per deciliter. Exclusion criteria were a history of prostate cancer, a risk of all prostate cancer of more than 35% or of high-grade prostate cancer of more than 7% as determined according to the Prostate Cancer Risk Calculator,12 an International Prostate Symptom Score (IPSS; range, 0 to 35, with higher scores indicating more severe symptoms of benign prostatic hyperplasia) of more than 19, conditions known to cause hypogonadism, receipt of medications that alter the testosterone concentration, high cardiovascular risk (myocardial infarction or stroke within the previous 3 months, unstable angina, New York Heart Association class III or IV congestive heart failure, a systolic blood pressure >160 mm Hg, or a diastolic blood pressure >100 mm Hg), severe depression (defined by a score of ≥20 on the Patient Health Questionnaire 9 [PHQ-9; range, 0 to 27, with higher scores indicating greater severity of depressive symptoms]), and conditions that would affect the interpretation of the results.
Inclusion in the Sexual Function Trial required self-reported decreased libido, a score of 20 or less on the sexual-desire domain (range, 0 to 33, with higher scores indicating greater desire) of the Derogatis Interview for Sexual Functioning in Men–II (DISF-M-II),13 and a partner willing to have intercourse twice a month. Inclusion in the Physical Function Trial required self-reported difficulty walking or climbing stairs and a gait speed of less than 1.2 m per second on the 6-minute walk test.14 Men who were not ambulatory or who had disabling neuromuscular or arthritic conditions were excluded. Inclusion in the Vitality Trial required self-reported low vitality and a score of less than 40 on the Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue scale (range, 0 to 52, with higher scores indicating less fatigue).15
STUDY TREATMENT
We assigned participants to testosterone or placebo by means of a minimization technique, with participants assigned to the study treatment that best balanced the balancing factors between groups with 80% probability.16,17 Balancing variables included participation in the main trials, trial site, screening testosterone concentration (≤200 or >200 ng per deciliter), age (≤75 or >75 years), use or nonuse of antidepressants, and use or nonuse of phosphodiesterase type 5 inhibitors.
The testosterone preparation was AndroGel 1% in a pump bottle (AbbVie). The initial dose was 5 g daily. The placebo gel was formulated to have a similar application and appearance. Serum testosterone concentration was measured at months 1, 2, 3, 6, and 9 in a central laboratory (Quest Clinical Trials), and the dose of testosterone gel was adjusted after each measurement in an attempt to keep the concentration within the normal range for young men (19 to 40 years of age). To maintain blinding when the dose was adjusted in a participant receiving testosterone, the dose was changed simultaneously in a participant receiving placebo.
ASSESSMENTS
At the end of the trials, the serum concentrations of total testosterone, free testosterone, dihydrotestosterone, estradiol, and sex hormone–binding globulin were measured in serum samples frozen at −80°C (see the Supplementary Appendix, available at NEJM.org). Steroid assays were performed at the Brigham Research Assay Core Laboratory (Boston) by liquid chromatography with tandem mass spectroscopy, and free testosterone was measured by equilibrium dialysis. All samples from each participant were measured in the same assay run.
Serum prostate-specific antigen (PSA) was measured and a digital rectal examination was performed at months 3 and 12, and PSA was measured at month 18. Detection of a prostate nodule or a confirmed increase in the PSA level by at least 1.0 ng per milliliter above baseline led to referral to the site urologist for consideration of prostate biopsy. The IPSS was determined at months 3 and 12. At every visit, adverse events were recorded and a cardiovascular-event questionnaire (see the protocol) was administered. Cardiovascular events were adjudicated by two cardiologists and two neurologists (see the Supplementary Appendix).
OUTCOMES
Efficacy outcomes were assessed at baseline and after 3, 6, 9, and 12 months of treatment. Dichotomous outcomes were used when a clinically important difference had previously been established. The primary efficacy outcome of each trial and the secondary outcomes of the Physical Function Trial were assessed in all participants; secondary outcomes for the other trials were assessed only in participants in those trials.
The primary outcome of the Sexual Function Trial was the change from baseline in the score for sexual activity (question 4) on the Psycho-sexual Daily Questionnaire (PDQ-Q4; range, 0 to 12, with higher scores indicating a greater number of activities).10,18 Secondary outcomes were changes in the score on the erectile-function domain (range, 0 to 30, with higher scores indicating better function) of the International Index of Erectile Function (IIEF)19 and the sexual-desire domain of the DISF-M-II.13 Details on the assessments in the Sexual Function Trial are provided in the protocol. The primary outcome of the Physical Function Trial was the percentage of men who increased the distance walked in the 6-minute walk test by at least 50 m.10,14 Secondary outcomes were the percentage of men whose score on the physical-function domain (PF-10; range, 0 to 100, with higher scores indicating better function) of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) increased by at least 8 points20 and changes from baseline in the 6-minute walking distance and PF-10 score. The primary outcome of the Vitality Trial was the percentage of men whose score on the FACIT–Fatigue scale increased by at least 4 points10,15; secondary outcomes were the change from baseline in the FACIT–Fatigue, the score on the vitality scale (range, 0 to 100, with higher scores indicating more vitality) of the SF-36,21 scores on the Positive and Negative Affect Schedule (PANAS) scales (range, 5 to 50 for positive affect and for negative affect, with higher scores indicating a greater intensity of the affect),22 and the PHQ-9 depression score.23 Every 3 months, participants were asked about their general impression of the change in sexual desire, walking ability, or energy (depending on the trial) and in overall health.
STATISTICAL ANALYSIS
Participants were evaluated according to the intention-to-treat principle. Each outcome was prespecified. Primary analyses of outcomes at all time points were performed with random-effects models for longitudinal data. Models included visit time as a categorical variable and a single main effect for treatment. For linear models of continuous outcomes, the treatment effect denoted the average difference in response between study groups across all four visits. For logistic models of binary outcomes, the treatment effect was the log odds ratio of a positive versus negative outcome for participants who received testosterone versus those who received placebo, averaged over all visits. Additional fixed effects were the baseline value for each outcome and balancing variables. Random intercepts were included for participant.
We analyzed the three trials as independent studies, without adjusting analyses of the primary outcomes for multiple comparisons. We also did not adjust the analyses of the primary and secondary outcomes within each trial for multiple comparisons, because the correlations among outcomes within a trial were expected to be very high, making such adjustment excessively conservative. Analyses of the primary outcomes that included all participants, however, were adjusted for multiple comparisons; we report the nominal P value only when it was lower than the threshold specified by the multiple-comparisons procedure.24 The sensitivity of results to missing data was assessed with the use of pattern-mixture models25 and shared random-effects models.26 The effect of change in total testosterone level on primary outcomes was assessed with the use of instrumental variables by two-stage residual inclusion,27 with study-group assignment as the instrument and change in testosterone level from baseline as the exposure of interest.
Sample sizes were calculated such that the studies would have 90% power, with the use of a two-sided test at a type I error rate of 0.05,10 to detect the following differences between the placebo group and the testosterone group: 15% versus 30% in the proportion of men with an increase of at least 50 m in the 6-minute walking distance, 20% versus 35% in the proportion of men with an increase of at least 4 points in the FACIT–Fatigue score, and a difference in change of 0.75 in the PDQ-Q4 score. These differences were conservatively based on comparisons between baseline and 12 months. Enrollment targets were 275 men for the Sexual Function Trial, 366 for the Physical Function Trial, and 420 for the Vitality Trial.
RESULTS
PARTICIPANTS AND STUDY TREATMENT
We screened 51,085 men and enrolled 790 who met all the criteria (Fig. S1 in the Supplementary Appendix).11 Relatively few men had a sufficiently low testosterone level to qualify; only 4700 of 21,940 men (21.4%) who had blood sampled qualified by the first measurement and 1490 of 2163 men (68.9%) qualified by the second, for an overall inclusion rate by testosterone level of 14.7%.11
At baseline, the enrollees had unequivocally low serum testosterone concentrations according to criteria for healthy young men (Fig. S2 in the Supplementary Appendix). The participants had relatively high rates of coexisting conditions: 62.9% were obese, 71.6% had hypertension, and 14.7% had a history of myocardial infarction (Table S1 in the Supplementary Appendix). The two study groups, however, had similar rates of these and other coexisting conditions; other baseline characteristics were also similar in the two groups.
Of the 790 men who were enrolled, 705 completed 12 months of study treatment. The characteristics of men who completed 12 months and those who did not complete 12 months did not differ appreciably (Table S2 in the Supplementary Appendix).
Testosterone treatment increased the median testosterone concentration to the mid-normal range for young men and maintained that range during the treatment period (Fig. S2 in the Supplementary Appendix). A total of 91% of men assigned to testosterone maintained a mean testosterone concentration above the lower limit of the normal range from month 3 through month 12. Testosterone treatment also increased levels of free testosterone, estradiol, and dihydrotestosterone but did not increase levels of sex hormone–binding globulin (Fig. S2 in the Supplementary Appendix).
EFFICACY
Sexual Function Trial
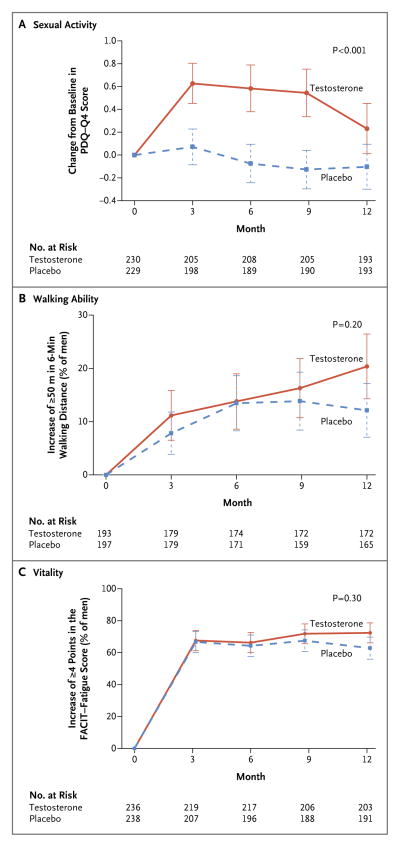
Averaged over all follow-up visits, sexual activity, as determined by the PDQ-Q4 score, increased more with testosterone treatment than with placebo, both among men enrolled in the Sexual Function Trial (treatment effect [the mean difference in the change from baseline between participants assigned to testosterone and those assigned to placebo], 0.58; P<0.001) (Fig. 1A) and among all Testosterone Trials participants (treatment effect, 0.62; P<0.001) (Table 1). A greater increase in testosterone level during treatment was associated with a greater increment in the PDQ-Q4 score (P<0.001 by instrumental variable analysis) (Fig. S3 in the Supplementary Appendix). The response was somewhat less at month 12 (P = 0.08 for the interaction between time and treatment). Testosterone treatment was also associated with increased sexual desire according to the DISF-M-II (treatment effect, 2.93; P<0.001) and increased erectile function according to the IIEF (treatment effect, 2.64; P<0.001) (Table 1). Men in the testosterone group were more likely than those in the placebo group to report that their sexual desire had improved since the beginning of the trial (P<0.001) (Fig. S4 in the Supplementary Appendix).
Figure 1. Primary Outcomes in the Three Main Trials of the Testosterone Trials.
The primary outcome of the Sexual Function Trial (Panel A) was the change from baseline in the score for sexual activity (question 4) on the Psychosexual Daily Questionnaire (PDQ-Q4; range, 0 to 12, with higher scores indicating more activity). The primary outcome of the Physical Function Trial (Panel B) was the percentage of men who had an increase of at least 50 m in the distance walked during the 6-minute walk test. The primary outcome of the Vitality Trial (Panel C) was the percentage of men who had an increase of at least 4 points in the score on the Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue scale (range, 0 to 52, with higher scores indicating less fatigue). P values were calculated with the use of a linear random-effects model for sexual activity and logistic random-effects models for walking ability and vitality. The I bars represent standard deviations.
Table 1.
Sexual Function Trial Outcomes.*
| Cohort and Outcome | No. of Men | Baseline Value | Change from Baseline Value | Treatment Effect (95% CI)† | Effect Size (95% CI)‡ | P Value§ | |||
|---|---|---|---|---|---|---|---|---|---|
| Month 3 | Month 6 | Month 9 | Month 12 | ||||||
| Men enrolled in Sexual Function Trial | |||||||||
| Primary outcome: PDQ-Q4 score¶ | |||||||||
| Testosterone | 230 | 1.4±1.3 | 0.6±1.3 | 0.6±1.5 | 0.5±1.5 | 0.2±1.6 | 0.58 (0.38–0.78) | 0.45 (0.30–0.60) | <0.001 |
| Placebo | 229 | 1.4±1.3 | 0.1±1.1 | −0.1±1.2 | −0.1±1.2 | −0.1±1.4 | |||
| Secondary outcomes | |||||||||
| DISF-M-II sexual desire score|| | |||||||||
| Testosterone | 234 | 11.9±6.7 | 3.5±6.3 | 3.5±6.0 | 4.0±7.4 | 2.6±6.5 | 2.93 (2.13–3.74) | 0.44 (0.32–0.56) | <0.001 |
| Placebo | 236 | 11.6±6.6 | 0.7±5.8 | 0.8±5.6 | 0.9±5.5 | 0.0±5.0 | |||
| IIEF erectile function score** | |||||||||
| Testosterone | 234 | 8.0±8.2 | 3.4±6.1 | 3.3±6.5 | 3.4±6.9 | 3.1±6.9 | 2.64 (1.68–3.61) | 0.32 (0.20–0.44) | <0.001 |
| Placebo | 236 | 7.7±8.2 | 1.0± 5.3 | 0.5±6.1 | 0.5±7.1 | 1.0±6.0 | |||
| All Testosterone Trials participants†† | |||||||||
| PDQ-Q4 score¶ | |||||||||
| Testosterone | 387 | 1.5±1.3 | 0.7±1.3 | 0.6±1.6 | 0.6±1.6 | 0.3±1.7 | 0.62 (0.45–0.79) | 0.45 (0.33–0.58) | <0.001 |
| Placebo | 384 | 1.5±1.4 | 0.0±1.2 | −0.1±1.3 | −0.1±1.3 | −0.1±1.4 | |||
Plus–minus values are means ±SD.
The treatment effect is the mean difference in change from baseline for participants assigned to testosterone versus those assigned to placebo, with adjustment for balancing factors: baseline total testosterone level (≤200 or >200 ng per deciliter), age (≤75 or >75 years), trial site, participation in the main trials, use or nonuse of antidepressants, and use or nonuse of phosphodiesterase type 5 inhibitors.
The effect size is the treatment effect divided by the baseline standard deviation.
The P value for the treatment effect was determined with the use of a linear mixed model with a random effect for participant.
Scores for sexual activity (question 4) on the Psychosexual Daily Questionnaire (PDQ-Q4) range from 0 to 12, with higher scores indicating more activity.
Scores on the sexual-desire domain of the Derogatis Interview for Sexual Functioning in Men–II (DISF-M-II) range from 0 to 33, with higher scores indicating greater desire.
Scores on the erectile-function domain of the International Index of Erectile Function (IIEF) range from 0 to 30, with higher scores indicating better function.
The outcomes for all Testosterone Trials participants are exploratory outcomes.
Physical Function Trial
Among men enrolled in the Physical Function Trial, there were no significant differences between the testosterone group and the placebo group in the percentage of men whose 6-minute walking distance increased by at least 50 m (primary outcome) (odds ratio, 1.42; P = 0.20) (Fig. 1B), the change from baseline in the 6-minute walking distance (mean difference, 4.09 m; P = 0.28) (Table 2), or the percentage of men whose PF-10 score increased by at least 8 points (odds ratio, 1.34; P = 0.15); there was a significant between-group difference in the change from baseline in the PF-10 score (mean difference, 2.75 points; P = 0.03) (Table 2). Among all Testosterone Trials participants, there was a significant between-group difference in all four measures: the percentage of men whose 6-minute walking distance increased by at least 50 m (odds ratio, 1.76; P = 0.003), the change from baseline in the 6-minute walking distance (mean difference, 6.69 m; P = 0.007), the percentage of men whose PF-10 score increased by at least 8 points (odds ratio, 1.50; P = 0.02), and the change from baseline in the PF-10 score (mean difference, 3.06 points; P = 0.002). Men who received testosterone were more likely than those who received placebo to perceive that their walking ability had improved since the beginning of the trial (P = 0.002) (Fig. S4 in the Supplementary Appendix).
Table 2.
Physical Function Trial Outcomes.*
| Cohort and Outcome | No. of Men | Baseline Value | No. of Participants or Change from Baseline Value | Treatment Effect (95% CI)† | Effect Size (95% CI)‡ | P Value§ | |||
|---|---|---|---|---|---|---|---|---|---|
| Month 3 | Month 6 | Month 9 | Month 12 | ||||||
| Men enrolled in Physical Function Trial | |||||||||
| Primary outcome: increase of ≥ 50 m in 6-min walk test — no./total no. (%) | |||||||||
| Testosterone | 191 | 20/179 (11.2) | 24/174 (13.8) | 28/172 (16.3) | 35/172 (20.3) | 1.42 (0.83 to 2.45) | 0.20 | ||
| Placebo | 196 | 14/179 (7.8) | 23/171 (13.5) | 22/159 (13.8) | 20/165 (12.1) | ||||
| Secondary outcomes | |||||||||
| 6-Min walking distance — m | |||||||||
| Testosterone | 191 | 347.7±69.1 | 10.2±35.8 | 8.2±41.5 | 5.3±50.3 | 14.3±45.9 | 4.09 (−3.00 to 11.18) | 0.06 (−0.04 to 0.16) | 0.28 |
| Placebo | 196 | 344.9±68.5 | 4.6±35.2 | 7.8±41.4 | 3.2±52.4 | 5.5±46.4 | |||
| Increase of ≥8 in PF-10 score — no./total no. (%)¶ | |||||||||
| Testosterone | 184 | 77/176 (43.8) | 72/171 (42.1) | 77/172 (44.8) | 66/173 (38.2) | 1.34 (0.90 to 2.00) | 0.15 | ||
| Placebo | 181 | 59/171 (34.5) | 73/159 (45.9) | 60/159 (37.7) | 58/167 (34.7) | ||||
| PF-10 score¶ | |||||||||
| Testosterone | 184 | 65.4±20.0 | 5.6±15.2 | 6.5±16.7 | 5.9±19.4 | 5.8±17.5 | 2.75 (0.20 to 5.29) | 0.13 (0.01 to 0.26) | 0.03 |
| Placebo | 181 | 64.8±21.3 | 4.2±13.7 | 4.8±17.0 | 3.3±18.9 | 2.4±17.3 | |||
| All Testosterone Trials participants|| | |||||||||
| Increase of ≥ 50 m in 6-min walk test — no./total no. (%) | |||||||||
| Testosterone | 392 | 40/368 (10.9) | 52/358 (14.5) | 54/348 (15.5) | 71/346 (20.5) | 1.76 (1.21 to 2.57) | 0.003 | ||
| Placebo | 389 | 25/356 (7.0) | 39/339 (11.5) | 37/320 (11.6) | 41/326 (12.6) | ||||
| 6-Min walking distance — m | |||||||||
| Testosterone | 392 | 387.0±81.7 | 10.9±45.1 | 11.0±40.2 | 6.7±45.1 | 13.6±43.4 | 6.69 (1.80 to 11.57) | 0.08 (0.02 to 0.14) | 0.007 |
| Placebo | 389 | 387.0±83.7 | 1.6±41.9 | 5.7±45.1 | 3.2±47.4 | 6.4±45.8 | |||
| Increase of ≥ 8 in PF-10 score — no./total no. (%)¶ | |||||||||
| Testosterone | 309 | 111/285 (38.9) | 113/281 (40.2) | 115/276 (41.7) | 103/281 (36.7) | 1.50 (1.08 to 2.09) | 0.02 | ||
| Placebo | 305 | 87/275 (31.6) | 103/263 (39.2) | 89/260 (34.2) | 82/272 (30.1) | ||||
| PF-10 score¶ | |||||||||
| Testosterone | 309 | 71.2±20.2 | 5.0±14.7 | 6.1±16.7 | 5.3±18.5 | 4.3±16.9 | 3.06 (1.18 to 4.94) | 0.15 (0.06 to 0.24) | 0.002 |
| Placebo | 305 | 69.7±21.2 | 3.9±12.8 | 3.4±16.2 | 2.3±17.9 | 1.3±16.9 | |||
Plus–minus values are means ±SD.
The treatment effect for dichotomous outcomes is the odds ratio for achieving the outcome versus not achieving the outcome among men assigned to testosterone versus those assigned to placebo. For continuous outcomes, the treatment effect is the mean difference in the outcome among men assigned to testosterone versus those assigned to placebo. All analyses are adjusted for balancing factors: baseline total testosterone level (≤200 or >200 ng per deciliter), age (≤75 or >75 years), trial site, participation in the main trials, use or non-use of antidepressants, and use or nonuse of phosphodiesterase type 5 inhibitors.
For continuous outcomes, the effect size is the treatment effect divided by the baseline standard deviation.
The P value for the treatment effect was determined with the use of a logistic mixed model with a random effect for participant for dichotomous outcomes and a linear mixed model with a random effect for participant for continuous outcomes.
Scores on the physical-function scale (PF-10) of the Medical Outcomes Study 36-Item Short-Form Health Survey range from 0 to 100, with higher scores indicating better function.
The outcomes for all Testosterone Trials participants are exploratory outcomes.
Vitality Trial
Among men enrolled in the Vitality Trial, testosterone treatment showed no significant benefit over placebo with respect to vitality, as determined by an increase of at least 4 points in the FACIT–Fatigue score (primary outcome) (odds ratio, 1.23; P = 0.30) (Fig. 1C). However, there appeared to be a small effect on the change from baseline in the FACIT–Fatigue score that did not reach significance (mean difference, 1.21 points; P=0.06) (Table 3). In addition, a greater increase in testosterone level was associated with a greater increment in the score (P = 0.02 by instrumental variable analysis) (Fig. S3 in the Supplementary Appendix), and the effect of testosterone on the change from baseline in the score in the participants in the three trials combined was significant (P = 0.006). Among participants in the Vitality Trial, there were significant differences between the testosterone group and the placebo group in the SF-36 vitality score (mean difference, 2.41 points; P = 0.03), the PANAS positive affect score (mean difference, 0.47 points; P = 0.04), the PANAS negative affect score (mean difference, −0.49 points; P<0.001), and the PHQ-9 depression score (mean difference, −0.72 points; P = 0.004) (Table 3). The effect sizes (the mean between-group differences in outcome divided by the baseline standard deviations) were all below 0.20. The men who received testosterone were more likely than men who received placebo to report that their energy was better at the end of the trial (P<0.001) (Fig. S4 in the Supplementary Appendix).
Table 3.
Vitality Trial Outcomes.*
| Cohort and Outcome | No. of Men | Baseline Value | No. of Participants or Change from Baseline Value | Treatment Effect (95% CI)† | Effect Size (95% CI)‡ | P Value§ | |||
|---|---|---|---|---|---|---|---|---|---|
| Month 3 | Month 6 | Month 9 | Month 12 | ||||||
| Men enrolled in Vitality Trial | |||||||||
| Primary outcome: increase of ≥4 in FACIT– Fatigue score — no./total no. (%)¶ | |||||||||
| Testosterone | 236 | 148/219 (67.6) | 144/217 (66.4) | 148/206 (71.8) | 147/203 (72.4) | 1.23 (0.83 to 1.84) | 0.30 | ||
| Placebo | 238 | 138/207 (66.7) | 126/196 (64.3) | 127/188 (67.6) | 120/191 (62.8) | ||||
| Secondary outcomes | |||||||||
| FACIT–Fatigue score¶ | |||||||||
| Testosterone | 236 | 31.6±6.4 | 7.7±8.4 | 7.4±9.1 | 8.6±9.1 | 8.0±8.4 | 1.21 (−0.04 to 2.46) | 0.19 (0.01 to 0.38) | 0.06 |
| Placebo | 238 | 31.3±6.4 | 7.2±8.8 | 5.9±9.2 | 7.2±9.2 | 6.7±9.4 | |||
| SF-36 vitality score|| | |||||||||
| Testosterone | 208 | 50.6±13.8 | 7.4±13.6 | 7.2±14.6 | 8.4±14.4 | 8.2±15.3 | 2.41 (0.31 to 4.50) | 0.18 (0.02 to 0.34) | 0.03 |
| Placebo | 196 | 49.4±12.6 | 5.9±11.1 | 4.5±11.2 | 5.7±12.3 | 6.1±13.8 | |||
| PANAS positive affect score** | |||||||||
| Testosterone | 229 | 15.3±3.2 | 0.7±3.2 | 0.9±3.8 | 0.9±3.4 | 0.7±3.9 | 0.47 (0.02 to 0.92) | 0.14 (0.01 to 0.27) | 0.04 |
| Placebo | 234 | 15.4±3.5 | 0.3±3.3 | 0.0±3.3 | 0.4±3.4 | 0.2±3.2 | |||
| PANAS negative affect score** | |||||||||
| Testosterone | 229 | 7.5±2.7 | −0.2±2.5 | −0.4±2.4 | −0.2±2.3 | −0.6±2.1 | −0.49 (−0.79 to −0.19) | −0.18 (−0.29 to −0.06) | <0.001 |
| Placebo | 234 | 7.4±2.8 | 0.3±2.4 | 0.4±2.6 | −0.1±2.6 | −0.1±2.6 | |||
| PHQ-9 depression score†† | |||||||||
| Testosterone | 230 | 6.6±4.0 | −1.3±3.8 | −1.7±3.8 | −1.9±4.0 | −1.8±3.7 | −0.72 (−1.20 to −0.23) | −0.18 (−0.30 to −0.06) | 0.004 |
| Placebo | 234 | 6.6±4.0 | −0.8±3.5 | −0.5±3.7 | −1.2±4.2 | −1.1±3.8 | |||
| All Testosterone Trials participants‡‡ | |||||||||
| Increase of ≥4 in FACIT–Fatigue score — no./total no. (%)¶ | |||||||||
| Testosterone | 394 | 176/351 (50.1) | 181/350 (51.7) | 178/337 (52.8) | 174/333 (52.3) | 1.23 (0.89 to 1.70) | 0.22 | ||
| Placebo | 394 | 166/337 (49.3) | 151/329 (45.9) | 154/317 (48.6) | 152/316 (48.1) | ||||
| FACIT–Fatigue score¶ | |||||||||
| Testosterone | 394 | 37.0±8.6 | 4.7±8.5 | 4.8±8.7 | 5.2±9.1 | 4.7±8.8 | 1.27 (0.37 to 2.16) | 0.15 (0.04 to 0.25) | 0.006 |
| Placebo | 394 | 36.8±8.8 | 4.1±9.0 | 2.8±9.0 | 3.7±9.2 | 3.6±9.5 | |||
Plus–minus values are means ±SD.
The treatment effect for dichotomous outcomes is the odds ratio for achieving the outcome versus not achieving the outcome among men assigned to testosterone versus those assigned to placebo. For continuous outcomes, the treatment effect is the mean difference in the outcome among men assigned to testosterone versus those assigned to placebo. All analyses are adjusted for balancing factors: baseline total testosterone level (≤200 or >200 ng per deciliter), age (≤75 or >75 years), trial site, participation in the main trials, use or nonuse of antidepressants, and use or nonuse of phosphodiesterase type 5 inhibitors.
For continuous outcomes, the effect size is the treatment effect divided by the baseline standard deviation.
The P value for the treatment effect was determined with the use of a logistic mixed model with a random effect for participant for dichotomous outcomes and a linear mixed model with a random effect for participant for continuous outcomes.
Scores on the Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue scale range from 0 to 52, with higher scores indicating less fatigue.
Scores on the vitality scale of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) range from 0 to 100, with higher scores indicating more vitality.
Scores for positive affect and for negative affect on the Positive and Negative Affect Schedule (PANAS) scales range from 5 to 50, with higher scores indicating a greater intensity of the affect.
Scores on the Patient Health Questionnaire 9 (PHQ-9) depression scale range from 0 to 27, with higher scores indicating greater intensity of depressive symptoms.
The outcomes for all Testosterone Trials participants are exploratory outcomes.
All Trials
Sensitivity analyses of the primary outcomes did not suggest that missing values affected any conclusions appreciably (Table S3 in the Supplementary Appendix). We found no significant interactions of treatment with age (P values ranged from 0.45 to 0.78 in the three trials), body-mass index (P values ranged from 0.35 to 0.85), or race (P values ranged from 0.49 to 0.72).
ADVERSE EVENTS
Although more men assigned to testosterone than those assigned to placebo had an increment in the PSA level of 1.0 ng per milliliter or more during the treatment period (23 vs. 8), only 1 man (in the testosterone group) received a diagnosis of prostate cancer during that time. Two men in the testosterone group and 1 in the placebo group received a diagnosis during the subsequent year (Table 4, and Table S4 in the Supplementary Appendix). The change in the IPSS did not differ significantly between the two groups. A hemoglobin level of 17.5 g per deciliter or more was observed in 7 men in the testosterone group and none in the placebo group.
Table 4.
Adverse Events during the First Year (Treatment Period) of the Testosterone Trials.*
| Event | Placebo (N = 394) | Testosterone (N = 394) |
|---|---|---|
| no. of participants | ||
| Prostate-related event | ||
|
| ||
| Increase in PSA level by ≥1.0 ng/ml | 8 | 23 |
|
| ||
| Prostate cancer | 0 | 1 |
|
| ||
| IPSS >19† | 26 | 27 |
|
| ||
| Hemoglobin ≥17.5 g/dl | 0 | 7 |
|
| ||
| Cardiovascular event‡ | ||
|
| ||
| Myocardial infarction (definite or probable) | 1 | 2 |
|
| ||
| Stroke (definite or probable) | 5 | 5 |
|
| ||
| Death from cardiovascular causes | 1 | 0 |
|
| ||
| Myocardial infarction, stroke, or death from cardiovascular causes | 7 | 7 |
|
| ||
| Serious adverse events | ||
|
| ||
| Death | 7 | 3 |
|
| ||
| Hospitalization | 78 | 68 |
|
| ||
| Other§ | 6 | 7 |
PSA denotes prostate-specific antigen.
The International Prostate Symptom Score (IPSS) questionnaire is used to identify symptoms of benign prostatic hyperplasia. Scores range from 0 to 35, with higher scores indicating more severe symptoms. A score of more than 19 indicates moderately severe lower urinary tract symptoms.
Data on cardiovascular adverse events were collected with the use of a specific questionnaire administered at each visit and also identified from the adverse-event log and the form for reporting serious adverse events (see the protocol). Myocardial infarction, stroke, and death from cardiovascular causes were assessed by two adjudicators.
Other serious adverse events were defined as congenital anomaly, disability, a life-threatening event, or an event that may not be immediately life-threatening but is clearly of major clinical significance.
Seven men in each study group were adjudicated to have had major cardiovascular events (myocardial infarction, stroke, or death from cardiovascular causes) during the treatment period and two men in the testosterone group and nine men in the placebo group were adjudicated to have had major cardiovascular events during the subsequent year (Table 4, and Table S4 in the Supplementary Appendix). There was no pattern of a difference in risk with respect to the other cardiovascular adverse events (Table S4 in the Supplementary Appendix). No significant between-group differences were observed in cardiac adverse events defined according to Medical Dictionary for Regulatory Activities classification (Tables S5 and S6 in the Supplementary Appendix).
DISCUSSION
Increasing the serum testosterone concentrations of men 65 years of age or older from moderately low to the mid-normal range for men 19 to 40 years of age had significant effects on all measures of sexual function and some measures of physical function, mood, and depressive symptoms — all to small-to-moderate degrees, consistent with the degree of testosterone deficiency.
Men who received testosterone reported better sexual function, including activity, desire, and erectile function, than those who received placebo. Although the effect sizes were low to moderate, men in the testosterone group were more likely than those in the placebo group to report that their sexual desire had improved, which suggests that this effect was of clinical relevance. The effect of testosterone on erectile function was less than that reported with phosphodiesterase type 5 inhibitors.28
The percentage of men whose 6-minute walking distance increased by at least 50 m did not differ significantly between the two study groups in the Physical Function Trial but did differ significantly when men in all three trials were included, although the effect sizes did not differ markedly (1.42 vs. 1.76). Furthermore, men who received testosterone were more likely than those who received placebo to report that their walking ability was better, which suggests that the effect, although small in magnitude, might be clinically relevant.
Testosterone had no significant benefit with respect to vitality, as assessed by the FACIT–Fatigue scale, except as a continuous outcome when men in all three trials were included. However, testosterone was associated with small but significant benefits with respect to mood and depressive symptoms. Men in the testosterone group were also more likely than those in the placebo group to report that their energy was better.
We observed four cases of prostate cancer, three of which were in men treated with testosterone, and there was no significant difference in urinary symptoms (as assessed by means of the IPSS) between the study groups. The generalizability of these results is limited, however, because we excluded men with a high risk of prostate cancer and men with moderately severe urinary tract symptoms. Furthermore, the sample size was inadequate to assess reliably the effect of testosterone on the risk of these conditions.
Some studies have suggested that testosterone treatment is associated with increased cardiovascular risk,29–32 although others have not.6,33,34 We did not observe a pattern of increased risk, but this trial was too small to exclude other than a large increase.
Our three trials had certain strengths, including enrollment of men with an unequivocally low mean testosterone concentration, adequate sample sizes, a double-blind, placebo-controlled design, an increase in serum testosterone concentration to the normal range for young men, and excellent participant retention. A major limitation, albeit an intentional one, is that the results apply only to men 65 years of age or older whose testosterone levels averaged less than 275 ng per deciliter.
Results of the primary outcomes in our three trials showed that testosterone treatment had a moderate, significant benefit with respect to sexual function but no significant benefit with respect to walking distance (among participants in the Physical Function Trial) or vitality. Testosterone treatment also had a significant benefit with respect to other prespecified outcomes, including walking distance when men in all three trials were included and mood and depressive symptoms. These results, together with those of the other four trials (now completed), should inform decisions about testosterone treatment for men 65 years of age or older whose levels are low for no apparent reason other than age. Such decisions will also require knowing the risks of testosterone treatment, which will necessitate larger and longer trials.
Supplementary Material
Acknowledgments
The Testosterone Trials were supported by a grant (U01 AG030644) from the National Institute on Aging (NIA), National Institutes of Health. The trials also received funding from the National Heart, Lung, and Blood Institute, National Institute of Neurological Disorders and Stroke, and National Institute of Child Health and Human Development. AbbVie (formerly Solvay and Abbott Laboratories) provided funding and donated Andro-Gel and placebo gel. The Boston site was partially supported by a grant (P30-AG013679) from the Boston Claude D. Pepper Older Americans Independence Center. The Yale Field Center was partially supported by a grant (P30-AG021342) from the Yale Claude D. Pepper Older Americans Independence Center and a grant (UL1 TR000142) from the Yale Center for Clinical Investigation. Dr. Cauley was supported by a grant (R01 AG37679) from the NIA. Dr. Gill was the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24-AG021507) and is the recipient of an Academic Leadership Award (K07AG043587), both from the NIA. Dr. Lewis was supported by a grant (DK079626) from the National Institute of Diabetes and Digestive and Kidney Diseases to the University of Alabama at Birmingham Diabetes Research and Training Center. Dr. Resnick was supported by the Intramural Research Program, NIA.
Dr. Snyder reports receiving consulting fees from Watson Laboratories. Dr. Bhasin reports receiving fees for serving on advisory boards from Eli Lilly and Sanofi, consulting fees from AbbVie, and grant support from Regeneron Pharmaceuticals, Eli Lilly, AbbVie, and Novartis; he also reports holding pending patents related to an algorithm for free testosterone determination based on a model of testosterone binding to sex hormone–binding globulin (provisional patent number 61/772,054) and the use of testosterone plus ornithine decarboxylase inhibitor as a prostate-sparing anabolic therapy (provisional patent number 13/679,889). Dr. Cunningham reports receiving fees for serving as an advisor to AbbVie, Apricus Biosciences, Clarus Therapeutics, Endo Pharmaceuticals, Ferring Pharmaceuticals, Eli Lilly, Purdue Pharma, and Repros Therapeutics and grant support from Ardana. Dr. Matsumoto reports receiving consulting fees from AbbVie, Eli Lilly, Endo Pharmaceuticals, and Clarus Therapeutics, study medication from AbbVie, and grant support from GlaxoSmithKline. Dr. Swerdloff reports receiving consulting fees from TesoRx and grant support from Clarus Therapeutics, Eli Lilly, Novartis, and Antares Pharma. Dr. Wang reports receiving fees for serving on an advisory board from TesoRx and grant support from Clarus Therapeutics, Lipocine, and Antares Pharma. Dr. Ensrud reports receiving fees for serving on a data and safety monitoring committee from Merck Sharp & Dohme. Dr. Farrar reports receiving fees for serving on a data and safety monitoring board from Cara Therapeutics, consulting fees from Bayer, Biogen, Mallinckrodt, the Campbell Consortium, Janssen, Daiichi-Sankyo, and Novartis, and grant support from Pfizer and Depomed. Dr. Cella reports receiving consulting fees from Pfizer and being the president of FACIT.org. Dr. Rosen reports receiving grant support from Bayer HealthCare, Eli Lilly, and Besins Healthcare. Dr. Molitch reports receiving consulting fees from AbbVie, Eli Lilly, and Pfizer. Dr. Anton reports receiving fees for serving as a scientific advisor to Reserveage Organics. Dr. Basaria reports receiving consulting fees from Eli Lilly and grant support from AbbVie. Dr. Mohler reports receiving consulting fees from AbbVie and Clarus Therapeutics. Dr. Parsons reports receiving grant support from Actavis. Dr. Ellenberg reports receiving grant support from AbbVie. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Drs. Evan Hadley and Sergei Romashkan of the NIA for their support throughout the trials.
APPENDIX
The authors’ full names and academic degrees are as follows: Peter J. Snyder, M.D., Shalender Bhasin, M.D., Glenn R. Cunningham, M.D., Alvin M. Matsumoto, M.D., Alisa J. Stephens-Shields, Ph.D., Jane A. Cauley, Dr.P.H., Thomas M. Gill, M.D., Elizabeth Barrett-Connor, M.D., Ronald S. Swerdloff, M.D., Christina Wang, M.D., Kristine E. Ensrud, M.D., M.P.H., Cora E. Lewis, M.D., M.S.P.H., John T. Farrar, M.D., Ph.D., David Cella, Ph.D., Raymond C. Rosen, Ph.D., Marco Pahor, M.D., Jill P. Crandall, M.D., Mark E. Molitch, M.D., Denise Cifelli, M.S., Darlene Dougar, M.P.H., Laura Fluharty, M.P.H., Susan M. Resnick, Ph.D., Thomas W. Storer, Ph.D., Stephen Anton, Ph.D., Shehzad Basaria, M.D., Susan J. Diem, M.D., M.P.H., Xiaoling Hou, M.S., Emile R. Mohler III, M.D., J. Kellogg Parsons, M.D., M.H.S., Nanette K. Wenger, M.D., Bret Zeldow, M.S., J. Richard Landis, Ph.D., and Susan S. Ellenberg, Ph.D., for the Testosterone Trials Investigators
The authors’ affiliations are as follows: the Division of Endocrinology, Diabetes, and Metabolism (P.J.S.), the Department of Biostatistics and Epidemiology (A.J.S.-S., J.T.F., X.H., B.Z., J.R.L., S.S.E.), the Center for Clinical Epidemiology and Biostatistics (D. Cifelli, D.D., L.F.), and the Division of Cardiovascular Disease, Section of Vascular Medicine, Department of Medicine (E.R.M.), Perelman School of Medicine, University of Pennsylvania, Philadelphia; Research Program in Men’s Health: Aging and Metabolism, Brigham and Women’s Hospital, Harvard Medical School, Boston (S. Bhasin, T.W.S., S. Basaria), and New England Research Institutes, Watertown (R.C.R.) — both in Massachusetts; the Departments of Medicine and Molecular and Cellular Biology, Division of Diabetes, Endocrinology, and Metabolism, Baylor College of Medicine and Baylor St. Luke’s Medical Center, Houston (G.R.C.); Geriatric Research, Education, and Clinical Center, Department of Veterans Affairs (VA) Puget Sound Health Care System, and the Division of Gerontology and Geriatric Medicine, Department of Internal Medicine, University of Washington School of Medicine — both in Seattle (A.M.M.); the Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh (J.A.C.); the Division of Geriatric Medicine, Yale School of Medicine, New Haven, CT (T.M.G.); the Department of Internal Medicine and Division of Epidemiology, Department of Family Medicine and Public Health, University of California, San Diego, School of Medicine, La Jolla (E.B.-C.), the Division of Endocrinology, Harbor–UCLA Medical Center (R.S.S., C.W.), and Los Angeles Biomedical Research Institute (R.S.S., C.W.), Torrance, and the Department of Urology, Moores Comprehensive Cancer Center, University of California, San Diego (J.K.P.) — all in California; the Department of Medicine, Division of Epidemiology and Community Health, University of Minnesota (K.E.E., S.J.D.), and Minneapolis VA Health Care System (K.E.E.) — both in Minneapolis; the Division of Preventive Medicine, University of Alabama at Birmingham, Birmingham, (C.E.L.); the Department of Medical Social Sciences (D. Cella) and the Division of Endocrinology, Metabolism, and Molecular Medicine (M.E.M.), Feinberg School of Medicine, Northwestern University, Chicago; the Department of Aging and Geriatric Research, University of Florida, Gainesville (M.P., S.A.); the Divisions of Endocrinology and Geriatrics, Albert Einstein College of Medicine, Bronx, NY (J.P.C.); the Laboratory of Behavioral Neuroscience, National Institute on Aging, National Institutes of Health, Baltimore (S.M.R.); and the Division of Cardiology, Emory University School of Medicine, Atlanta (N.K.W.).
Footnotes
The authors’ full names, academic degrees, and affiliations are listed in the Appendix.
References
- 1.Wu FC, Tajar A, Pye SR, et al. Hypo-thalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–45. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 2.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 3.Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–53. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 4.Page S, Amory J, Bowman F, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2004;90:1502–10. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 5.Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66:1090–9. doi: 10.1093/gerona/glr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–50. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 7.Gray PB, Singh AB, Woodhouse LJ, et al. Dose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older men. J Clin Endocrinol Metab. 2005;90:3838–46. doi: 10.1210/jc.2005-0247. [DOI] [PubMed] [Google Scholar]
- 8.Vaughan C, Goldstein FC, Tenover JL. Exogenous testosterone alone or with finasteride does not improve measurements of cognition in healthy older men with low serum testosterone. J Androl. 2007;28:875–82. doi: 10.2164/jandrol.107.002931. [DOI] [PubMed] [Google Scholar]
- 9.Liverman C, Blazer D, editors. Testosterone and aging: clinical research directions. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 10.Snyder PJ, Ellenberg SS, Cunningham GR, et al. The Testosterone Trials: seven coordinated trials of testosterone treatment in elderly men. Clin Trials. 2014;11:362–75. doi: 10.1177/1740774514524032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cauley JA, Fluharty L, Ellenberg SS, et al. Recruitment and screening for the Testosterone Trials. J Gerontol A Biol Sci Med Sci. 2015;70:1105–11. doi: 10.1093/gerona/glv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529–34. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 13.Derogatis LR. The Derogatis Interview for Sexual Functioning (DISF/DISF-SR): an introductory report. J Sex Marital Ther. 1997;23:291–304. doi: 10.1080/00926239708403933. [DOI] [PubMed] [Google Scholar]
- 14.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155:1278–82. doi: 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- 15.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–61. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 16.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15:443–53. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 17.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–15. [PubMed] [Google Scholar]
- 18.Lee KK, Berman N, Alexander GM, Hull L, Swerdloff RS, Wang C. A simple self-report diary for assessing psychosexual function in hypogonadal men. J Androl. 2003;24:688–98. doi: 10.1002/j.1939-4640.2003.tb02728.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosen RC, Cappelleri JC, Gendrano N., III The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14:226–44. doi: 10.1038/sj.ijir.3900857. [DOI] [PubMed] [Google Scholar]
- 20.Ware J, Snow K, Kosinski M. SF-36 Health Survey: manual and interpretation guide. Lincoln, RI: Quality Metric; 2000. [Google Scholar]
- 21.Ware J, Kosinski M. SF-36 Physical and Mental Health Summary Scales: a manual for users of Version 1. 2. Lincoln, RI: Quality Metric; 2005. [Google Scholar]
- 22.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–8. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 25.Little RJA. Pattern-mixture models for multivariate incomplete data. J Am Stat Assoc. 1993;88:125–34. [Google Scholar]
- 26.Follmann D, Wu M. An approximate generalized linear model with random effects for informative missing data. Biometrics. 1995;51:151–68. [PubMed] [Google Scholar]
- 27.Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27:531–43. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller A, Smith L, Parker M, Mulhall JP. Analysis of the efficacy and safety of sildenafil citrate in the geriatric population. BJU Int. 2007;100:117–21. doi: 10.1111/j.1464-410X.2007.06915.x. [DOI] [PubMed] [Google Scholar]
- 29.Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–36. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 30.Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corona G, Maseroli E, Rastrelli G, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014;13:1327–51. doi: 10.1517/14740338.2014.950653. [DOI] [PubMed] [Google Scholar]
- 34.Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706–15. doi: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.