Abstract
Background
Volatile anesthetics have been shown to modify immune cell functions via several mechanisms, some of which have been only partially elucidated. We demonstrated that isoflurane inhibits primary leukocyte integrin lymphocyte function-associated antigen-1 (LFA-1) by binding to the allosteric cavity critical for conformational activation to its high-affinity form. It remains to be determined whether the allosteric inhibition of LFA-1 by isoflurane can be generalized to other anesthetics such as sevoflurane.
Methods
The effects of sevoflurane on the ability of LFA-1 to bind to its counter-ligand, intercellular adhesion molecule-1, was studied in leukocytes by flow cytometry. To examine whether sevoflurane acts directly on LFA-1, we measured ligand-binding using beads coated with purified LFA-1 protein. To distinguish between competitive versus allosteric inhibition, we analyzed the effects of sevoflurane on both wild-type and mutant-locked high-affinity LFA-1. One-way analysis of variance was employed for statistical analysis of the data. Nuclear magnetic resonance spectroscopy was used to identify sevoflurane binding site(s).
Results
Sevoflurane at clinically relevant concentrations inhibited the ligand-binding function of LFA-1 in leukocytes as well as in cell-free assays (P < 0.05). Sevoflurane blocked wild-type but not locked high-affinity LFA-1, thereby demonstrating an allosteric mode of inhibition. Nuclear magnetic resonance spectroscopy revealed that sevoflurane bound to the allosteric cavity, to which LFA-1 allosteric antagonists and isoflurane also bind.
Conclusions
This study suggests that sevoflurane also blocks the activation-dependent conformational changes of LFA-1 to the high-affinity form. The allosteric mode of action exemplified by sevoflurane and isoflurane via LFA-1 might represent one of the underlying mechanisms of anesthetic-mediated immunomodulation.
The volatile anesthetics (VAs) isoflurane and sevoflurane are halogenated ethers currently used to maintain general anesthesia during surgery. VAs are thought to bind to ligand-gated ion channel proteins in the central nervous system (i.e., γ-aminobutyric acid receptor) and to alter the conformational equilibrium between the active and inactive forms.1–3 This leads to the altered excitability of neuronal cells, thus inducing general anesthesia. Accumulating evidence has shown that VAs generate an important array of pleiotropic effects outside the central nervous system, including cardiac protection,4,5 inhibition of platelet aggregation,6 and suppression of leukocyte functions.7,8
The perturbation of leukocyte functionality by VAs is possibly of great clinical relevance to the immunosuppression associated with general anesthesia and surgery.7 One potentially detrimental effect of this is compromised immune function. Because surgical wound infections are established within the first few hours after tissue contamination by bacteria,9 the perturbation of leukocyte functions by VAs during surgery may increase the incidence of postoperative infections.10 It has been suggested that isoflurane might exacerbate postoperative infection in patients suffering from alcoholism, an underlying condition that compromises immune function.11 As one would expect, the increasing clinical necessity to administer anesthetics to patients with immunocompromised conditions (e.g., cancer, aging, human immunodeficiency virus infection, alcoholism, and organ transplantation12) has underscored the need to elucidate the mechanistic basis underlying these immunosuppressive effects. Conversely, surgery and critical illness provoke an acute inflammatory response that can intensify both the initial injury and postoperative morbidity.5 Suppression of the inflammatory response to surgery and acute illness may have a beneficial effect. Therefore, a better understanding of the immunomodulatory effects inherent to VAs is essential in the quest to minimize the secondary effects of these drugs.
Leukocyte adhesion constitutes an essential process in the immune system, one that enables the accumulation of immune cells at sites of infection and inflammation. VAs at clinically relevant concentrations have been shown to affect the ability of leukocytes to adhere to inflamed vascular endothelial cells. Leukocytes treated with isoflurane or sevoflurane exhibited decreased cell adhesion to vascular endothelium ex vivo.13,14 Furthermore, isoflurane anesthesia in mice suppressed infiltration of leukocytes to inflamed lungs15,16 and the inflamed peritoneal space in vivo.17 However, the molecular mechanisms by which leukocyte adhesion and migration are suppressed by VAs remain elusive.
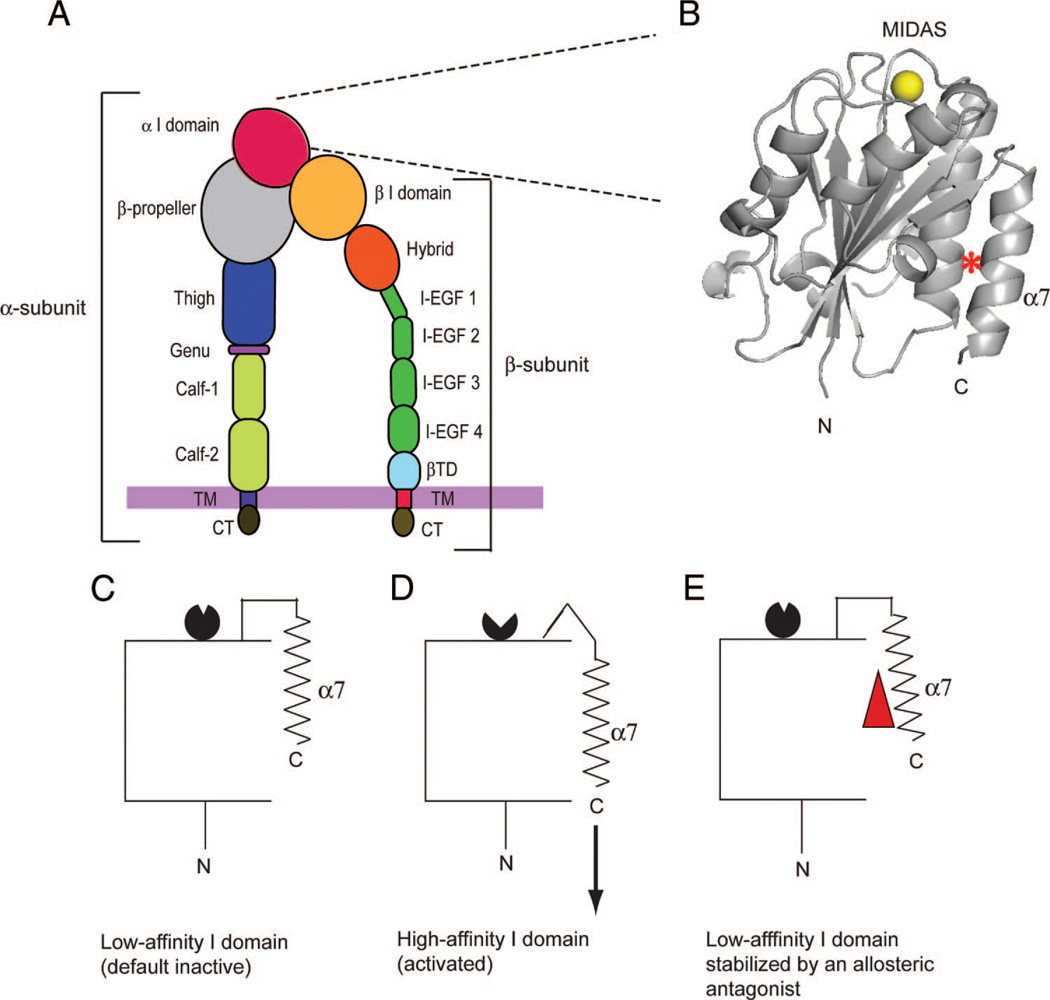
Integrins are α/β heterodimeric cell-adhesion molecules that mediate cell-to-cell and cell-to-matrix interactions. Integrins consist of noncovalently associated α- and β-subunits that contain multiple domains (fig. 1A).18,19 To date, 18 α-and 8 β-subunits have been identified that combine to form at least 24 distinctα/β heterodimers that are expressed across a wide range of cell types. Nine of 18 integrin α subunits contain the α inserted (I) domain, which is located at the most distal part of the extracellular portion in the ternary structure (fig. 1A and B). The α I domain functions as the major ligand-binding domain in those integrins that contain the α I domain, including the predominant leukocyte integrin αLβ2 or lymphocyte function-associated antigen-1 (LFA-1). LFA-1 plays a pivotal role in immune cell trafficking and activation, thereby constituting a critical component in host defense.20,21 LFA-1 is an established therapeutic target for autoimmune diseases such as psoriasis.22 The ability of LFA-1 to bind its ligand intercellular adhesion molecule-1 (ICAM-1) is dynamically regulated by the conformational changes of the α I domain (fig. 1B).18 The I domain adopts a G-protein-like Rossmann fold, in which a central β-sheet is surrounded by 7 α-helices (fig. 1B).18 A magnesium ion (Mg2+) is coordinated at the top of the domain, forming a metal ion-dependent adhesion site to which ICAM-1 binds, whereas N and C termini are located at the bottom of the domain. A conformationally mobile C-terminal α7 helix functions as an allosteric switch (fig. 1C and D). Specifically, a downward shift of the C-terminal α7 helix is allosterically linked to the conformational rearrangements of the metal ion-dependent adhesion site from the low-affinity to the high-affinity configuration, only the latter of which can tightly bind to ICAM-1. Direct inhibitors, such as the therapeutic LFA-1 antibody efalizumab, bind to or near the metal ion-dependent adhesion site, thereby sterically interfering with ligand binding.23 By contrast, small-molecule allosteric LFA-1 inhibitors (termed α I allosteric antagonists) bind to the hydrophobic cavity underneath the α7 helix and thereby interfere with conformational conversion to the high-affinity I domain (fig. 1E).22 We have demonstrated previously that isoflurane directly binds to, and allosterically inhibits, LFA-1.24,25 Using nuclear magnetic resonance (NMR) spectroscopy24 and crystallography,25 we have demonstrated that isoflurane binds to the I domain cavity, to which the α I allosteric antagonists bind, thereby inhibiting LFA-1 in an allosteric manner.
Fig. 1.
Integrin lymphocyte function-associated antigen-1 (LFA-1) domains and activation-dependent conformational changes of the αL inserted (I) domain. (A) Domain organization of integrin LFA-1. Integrin domains in the α- and β-subunits are labeled. A thick purple vertical line represents the plasma membrane. A blow-up of the α I domain is shown. (B) A side view of the LFA-1 I domain structure. A Mg2+ ion at the metal ion-dependent adhesion site (MIDAS) to which the ligand ICAM-1 binds is shown as a yellow sphere. The hydrophobic cavity that contains the allosteric inhibitor- and isoflurane-binding sites is marked with a red asterisk. The N and C termini are labeled. (C–E) Graphic drawings showing the conversion from the low-affinity (C) to the high-affinity (D) α I domain. A downward shift of the C-terminal α7 helix (shown by an arrow in D) is linked to the conversion of the metal ion-dependent adhesion site from the low-affinity (black circle with a small opening; in C) to the high-affinity (back circle with a large opening; in D) configurations. A small-molecule allosteric LFA-1 antagonist (red triangle) binds beneath the α7 helix. It interferes with the downward shift of the α7 helix, thereby suppressing conversion of the metal ion-dependent adhesion to the high-affinity configuration (E). β-TD = β-terminal domain; CT = cytoplasmic domain; I-EGF = integrin-epidermal growth factor-like domain; TM = transmembrane domain.
One of the central questions of anesthetic pharmacology is how structurally similar but still distinct VAs interact with the same allosteric sites on proteins.26 Because sevoflurane shares many structural features with isoflurane, this VA should serve as a good test for generalizing our biochemical and crystallographic findings on isoflurane-integrin interactions to other VAs. In addition, because sevoflurane exhibits immunomodulatory effects,27–29 investigating its potential ability to block LFA-1 is of potential clinical relevance. Here, we study not only whether the allosteric inhibition of LFA-1 by isoflurane can be generalized to another halogenated ether, sevoflurane, but also where sevoflurane binds within the LFA-1 I domain.
Materials and Methods
Cells
Jurkat cells were cultured in RPMI 1640 medium and 10% fetal calf serum at 37°C in 5% CO2. As described previously,30 K562 cells stably transfected to express wild-type (WT) LFA-1 were cultured in RPMI 1640 medium, 10% fetal calf serum, and 4 mg/ml puromycin at 37°C in 5% CO2.
Anesthetic Solution
Saturated isoflurane, sevoflurane, or chloroform solution was prepared by mixing each undiluted drug in HEPES-buffered saline (HBS) in a sealed bottle overnight at room temperature as described previously.31,32 Published data of the saturated solutions’ concentrations were used as reference values (isoflurane, 15.3 mm31; sevoflurane, 11 mm32; chloroform, 66 mm31). Each saturated solution was diluted using a gastight tube to yield the desired anesthetic concentration as described previously.32,33
Measurement of Saturated Anesthetic Solutions by Gas Chromatography/Mass Spectrometry
Saturated anesthetic solutions were slowly sampled with gastight glass Hamilton syringes (Hamilton Company, Reno, NV), and then transferred to fully fill headspace vials. The vials were immediately capped with Teflon-lined caps. Isopropyl alcohol was used for the generation of standard solutions. Known amounts of isoflurane, sevoflurane, and chloroform were weighed in a 50-ml volumetric tube and brought to the required volume with isopropyl alcohol. Concentrations of the standard solutions were 2.1 mg/ml for sevoflurane, 4 mg/ml for isoflurane, and 9.9 mg/ml for chloroform. A series of diluted samples was made from the standard solutions, quickly transferred to headspace vials, filled to the brim, and then stopped with Teflon-lined caps. An HP5890 Series II gas chromatograph (Hewlett Packard, Palo Alto, CA) equipped with a 6′ × 1/8″ SS 10% Carbowax 20M column on 80/100 mesh Chromosorb WHP, a HP7673 autosampler, and a thermal conductivity detector was used to measure the solutions. Helium was used as a carrier gas with a head pressure of 34 psi. The column oven was used at 65°C isothermal, the injector temperature was maintained at 200°C, and the thermal conductivity detector was operated at 200°C. The samples were injected using the autosampler.
Binding of Intercellular Adhesion Molecule-1 to LFA-1 Expressed on Jurkat Cells or K562 Cells
Flow cytometric detection of intercellular adhesion molecule-1 (ICAM-1) binding to LFA-1 on the cell surface was performed as described previously.24,34 In brief, Jurkat cells or K562-stable transfectant cells were harvested and washed once with HBS containing 10 mm EDTA and three times with HBS, and then resuspended in HBS. Cells (5 × 105) in 300 µl HBS were aliquotted to polymerase chain reaction tubes and centrifuged. Cell pellets were given a 150-µl aliquot of HBS and 2 mm MnCl2 containing isoflurane, sevoflurane, or chloroform at 2× final concentration and another 150-µl aliquot of HBS containing 25 µg/ml fluorescein isothiocyanate (FITC)-conjugated goat antihuman immunoglobulin A (IgA) antibody (Pierce, Rockford, IL), and either 10 µg/ml ICAM-1-Fcα fusion protein34,35 or control human IgA. Tubes were immediately capped and incubated for 30 min at room temperature, and unbound ICAM-1-Fcα/anti-IgA-FITC was washed off with HBS and 1 mm MnCl2. Bound ICAM-1 was detected by flow cytometry using a FACScan instrument (BD Biosciences, San Jose, CA). ICAM-1 binding percentage was defined as mean fluorescence intensity at various concentrations of VA divided by mean fluorescence intensity of mock-treated × 100 (%).
ICAM-1 Binding to LFA-1 Protein on Silica Beads
The soluble recombinant extracellular portion of WT or high-affinity (HA) LFA-1 protein was expressed in Chinese hamster ovary cells and purified to a homogeneity as described previously.34 In HA LFA-1, the α I domain is locked in the constitutive HA conformation by an engineered disulfide bond that enforces the downward shift of the C-terminal helix, thereby leading to a 10,000-fold affinity increase.36 To immobilize the LFA-1 proteins on silica beads (Bangs Laboratories, Inc., Fishers, IN), the beads were washed twice with HBS and incubated at 4°C overnight in HBS containing 30 mg/ml soluble WT or HA LFA-1 protein. After blocking nonspecific binding with HBS and 2% bovine serum albumin, LFA-1-coated beads were transferred to 300-µl polymerase chain reaction tubes (Axygen, Union City, CA) at 5 × 105 per tube. After spinning down at 2,200g, supernatant was removed. Bead pellets were given 150-µl aliquots of HBS and 2 mm MnCl2 containing either isoflurane, sevoflurane, or chloroform at 2× final concentration, another 150 µl of HBS containing 25 µg/ml FITC-conjugated goat antihuman IgA antibody, and either 20 µg/ml ICAM-Fcα fusion protein or control human IgA. Tubes were immediately capped and incubated for 15 min at room temperature, and unbound ICAM-1-Fcα/anti-IgA-FITC was washed off with HBS and 1 mm MnCl2. Bound ICAM-1 was detected by flow cytometry using a FACScan instrument. ICAM-1 binding percentage was defined as mean fluorescence intensity at various concentrations of VA/mean fluorescence intensity of mock treated × 100 (%).
NMR Spectroscopy
To study VA interactions with 15N-labeled LFA-1 I domain, NMR spectroscopy was performed as described previously.24 15N-labeled LFA-1 I domain (G128-Y307) protein was expressed as inclusion bodies in Escherichia coli BL21 (DE3) on M9 medium containing 15NH4Cl (Cambridge Isotope Labs, Andover, MA). The I domain was purified to homogeneity as described previously.37 Recombinant I domain was exchanged into a buffer containing 10 mm sodium phosphate, pH 7.2, 10 mm MgSO4, 150 mm NaCl, 0.05% NaN3, and 10% (v/v) 2H2O. The NMR sample contained 600 µl of protein solution at 50 µm concentration. All NMR experiments were acquired at 22°C using a spectrometer (Inova; Varian, Inc., Palo Alto, CA) operating at an 1H frequency of 599.763 MHz and equipped with a triple-resonance, single-axis gradient probe (Varian Inc.). 15N-heteronuclear single quantum coherence experiments38 were acquired with 200 × 1024 points to obtain a spectral width of 2.02 kHz. The chemical shifts were indirectly referenced to a sample of 4,4 dimeththyl-4-silapentane-1-sulfonic acid in sample buffer.39 Previously published chemical shift resonance assignments40,41 were used to identify the amino acid residues of the I domain that were affected by the binding to sevoflurane. Sevoflurane was added in aliquots of undiluted anesthetic through a rubber septum into a screw-cap NMR tube (Wilmad-Labglass, Buena, NJ) via a gas-tight syringe (SGE Analytical, Ringwood, VIC, Australia). The sample was gently mixed by being inverted and allowed to equilibrate for 1 h before the acquisition of each NMR spectrum. A supraclinical concentration of sevoflurane (~9.9 mm) was added to the NMR tubes to saturate the site to obtain accurate NMR data. The concentration of added sevoflurane was determined by dividing the requisite quantity of added anesthetic by the protein solution volume (fixed at 600 µl). This method assumes 100% transfer of VA and is likely to overestimate the final concentration of sevoflurane in the NMR tube. Data were processed using NMRPipe42 and analyzed by Sparky (University of California, San Francisco, San Francisco, CA).
Statistical Analysis
Data were analyzed using a Student’s t test or analysis of variance with Tukey post hoc pairwise comparisons. Statistical significance was defined as P less than 0.05. All statistical calculations were performed using Prism software (version 5; GraphPad Software, La Jolla, CA).
Results
Concentrations of Saturated Isoflurane, Sevoflurane, and Chloroform Solutions
At each measurement, all VAs were eluted in a gas chromatography/ mass spectrometry column at characteristic retention times with a signal-to-noise ratio greater than 10:1 (data not shown). With the use of gas chromatography, we have determined the concentrations of saturated isoflurane, sevoflurane, and chloroform solutions (table 1). Our data are consistent with published reports for isoflurane (15.3mm31), sevoflurane (11 mm32), and chloroform (66 mm31).
Table 1.
Concentrations of Saturated Isoflurane, Sevoflurane, and Chloroform Solutions
| Concentration (mm) | |
|---|---|
| Isoflurane | 13.7 |
| Sevoflurane | 9.9 |
| Chloroform | 59.8 |
Sevoflurane Inhibited LFA-1 on the Cell Surface
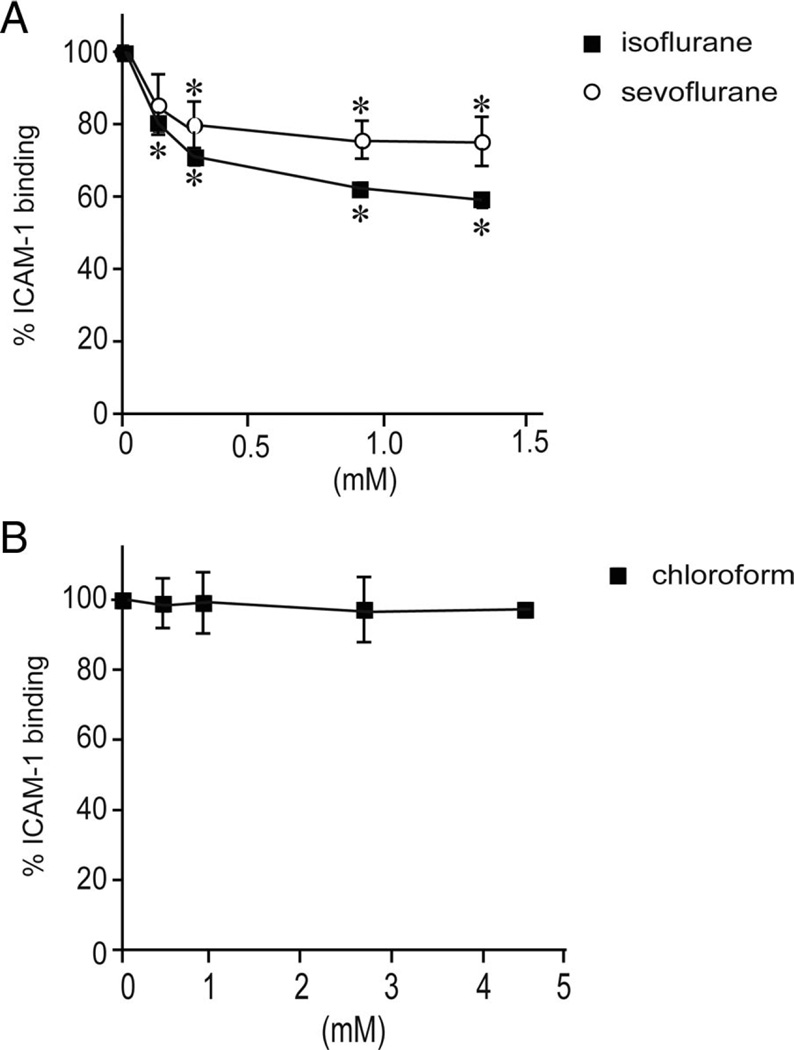
To study the effects of VAs on LFA-1 on the cell surface, we used Jurkat cells, a lymphocyte cell line that expresses endogenous LFA-1 and that has been used extensively for investigating LFA-1 functionality (fig. 2).43–45 Consistent with our previous results,24 isoflurane inhibited ICAM-1 binding to LFA-1 on Jurkat cells stimulated with Mn2+, which is known to directly act on the extracellular portion of LFA-1, thereby inducing conformational changes to up-regulate its affinity.18 We have found that sevoflurane, at clinically relevant concentrations, similarly blocked ICAM-1 binding to Mn2+-stimulated LFA-1 on Jurkat cells (fig. 2A).
Fig. 2.
The effects of volatile anesthetics on intercellular adhesion molecule-1 (ICAM-1) binding to lymphocyte function-associated antigen-1 (LFA-1) on Jurkat cells. (A) Isoflurane and sevoflurane inhibited ICAM-1 binding to LFA-1 on Jurkat cells, as determined by flow cytometry. Data represent the mean ± SEM of three independent experiments and are expressed as a percentage of mock-treated samples. (B) Chloroform did not affect ICAM-1 binding to LFA-1 on Jurkat cells. Data represent the mean ± S.E. of three independent experiments and are expressed as the percentage of mock-treated samples. (A and B) One-way analysis of variance with Tukey post hoc analysis was used to compare the data at different anesthetic concentrations within sevoflurane-isoflurane-, or chloroform-treated samples. *P < 0.05. vs. mock-treated samples.
Sevoflurane Acts Directly on the Extracellular Portion of LFA-1
We hypothesized that, like isoflurane,24 sevoflurane would act directly on LFA-1. However, direct interaction of VAs with the plasma membrane, intracellular proteins, and cytoskeletons could inhibit LFA-1 clustering,46 thereby antagonizing the binding of ICAM-1 to LFA-1 without directly acting on LFA-1. To study the direct action of VAs on LFA-1 and simultaneously exclude the effects on LFA-1 receptor clustering, we have developed a silica bead-based cell-free assay in which we immobilize the recombinant LFA-1 extracellular segment34 on the beads. The LFA-1-coated beads were mixed with ICAM-1/anti-Fc-FITC in PCR microtubes and then incubated for 30 min at room temperature. Samples were subjected to flow cytometry to detect bound ICAM-1. We confirmed that WT LFA-1 immobilized on beads stimulated with Mn2+ showed good ICAM-1 binding.
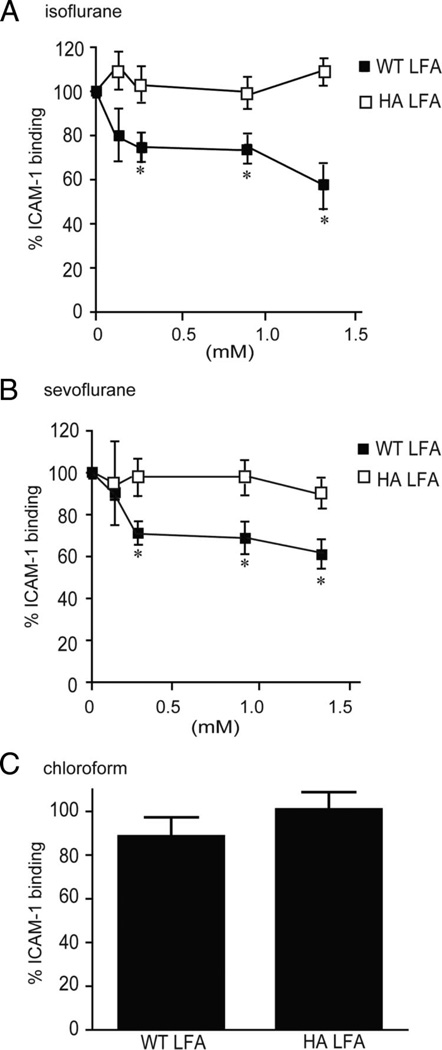
To study the effects of VAs, we added graded amounts of isoflurane or sevoflurane saturated solution, together with ICAM-1/anti-Fc-FITC, to microtubes containing LFA-1-coated beads. The microtubes were immediately capped and incubated for 30 min at room temperature. Samples were then analyzed by flow cytometry. Consistent with our previous results,24 isoflurane inhibited ICAM-1 binding to Mn2+-stimulated WT LFA-1 (fig. 3A). Furthermore, we have demonstrated that sevoflurane blocked ICAM-1 binding to LFA-1 (fig. 3B). These results show that, like isoflurane, sevoflurane acts directly on the extracellular portion of LFA-1.
Fig. 3.
The effects of volatile anesthetics on intercellular adhesion molecule-1 (ICAM-1) binding to lymphocyte function-associated antigen-1 (LFA-1) in cell-free assays. (A–C) Flow cytometry was used to study ICAM-1 binding to wildtype (WT) and high-affinity (HA) LFA-1 extracellular portion proteins on beads in the presence or absence of isoflurane (A), sevoflurane (B), or chloroform (C). Data represent the mean ± SEM of three independent experiments and are expressed as a percentage of mock-treated samples. (A and B) One-way analysis of variance with Tukey post hoc analyses was used to compare the data at different anesthetic concentrations within either WT LFA-1 or HA LFA-1. *P < 0.05 vs. mock-treated WT LFA-1. (C) Percentage of ICAM-1 binding at 4.5 mM chloroform is shown. A Student t test was used to compare mock- and chloroform (4.5 mM)-treated samples within WT LFA-1 or HA LFA-1. No statistical significance was observed.
Sevoflurane Allosterically Inhibits LFA-1 Function
Two potential modes by which inhibitors interfere with the ligand-binding function of the I domain are (1) direct/competitive inhibition of ligand binding at the metal iso-dependent adhesion site (e.g., blocking the antibodies efalizumab23 and AL-5747); and (2) perturbation of the allosteric transition from the low-affinity to the HA conformation (e.g., α I allosteric antagonists22). To distinguish between allosteric and direct inhibition of LFA-1, we used K562 transfectants expressing HA LFA-1 in which the I domain is mutationally locked in a HA conformation. This HA LFA-1 is blocked by direct inhibitors but not by allosteric inhibitors.30,34 We previously demonstrated in an enzyme-linked immunosorbent plate-based assay that isoflurane (up to 1.5 mm) failed to inhibit HA LFA-1 to ICAM-1. Consistent with our previous findings, we have shown in the bead-based assays that isoflurane blocked WT but not HA LFA-1 (fig. 3A; interaction term of two-way analysis of variance; F = 9.355, P = 0.0002).
Despite differences in chemical structure, sevoflurane has also been shown to act as a potent allosteric antagonist to LFA-1. Sevoflurane blocked WT but not HALFA-1 (fig. 3B; interaction term of two-way analysis of variance; F = 5.687, P = 0.0032). These results provide functional evidence that, like isoflurane, sevoflurane allosterically inhibits LFA-1 by perturbing conformational conversion to the active state.
For comparison, we tested the small halocarbon chloroform (trichloromethane). Chloroform is a superannuated VA, the use of which has been supplanted by modern haloethers. In contrast to sevoflurane and isoflurane, chloroform up to 4.5 mm showed no inhibition of LFA-1 on either Jurkat cells (fig. 2B) or beads (fig. 3C). The inability of chloroform to perturb LFA-1/ICAM-1 binding may stem from its diminutive structure, which cannot form any energetically significant interactions with the walls of the anesthetic-binding cavity.
NMR Spectroscopy Identifies the Presence of Sevoflurane Binding to the LFA-1 I Domain
To directly test our hypothesis that sevoflurane binds to the allosteric cavity in the LFA-1 I domain, we sought to map sevoflurane-binding sites in the I domain using heteronuclear 1H, 15N-heteronuclear single quantum coherence-NMR spectroscopy. This is an established technique for identifying small-molecule interaction sites on isotopically labeled biomolecules, including anesthetic interactions with soluble protein domains.48–50 We have previously used this approach to identify a cavity under the C-terminal helix of the I domain as a binding site for isoflurane.24 In these and other published NMR experiments, high concentrations of VAs have been used to saturate the candidate binding site(s).24,48–50 However, because such high anesthetic concentrations may induce nonphysiologic interactions that are not seen at lower anesthetic concentrations, these data were corroborated with our functional data using biochemical or cell biologic assays.
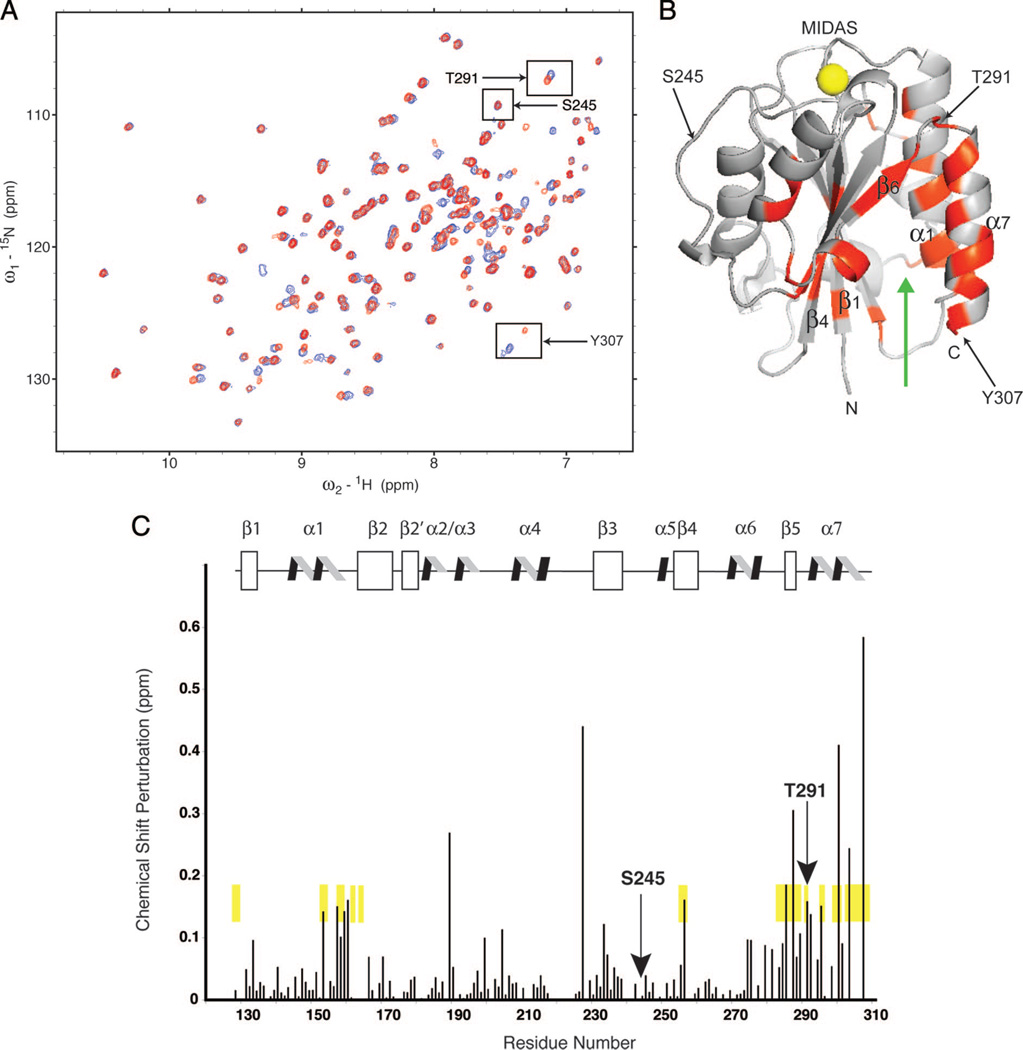
We obtained two sets of heteronuclear single quantum coherence spectra data both in the absence of (blue) and after the addition (red) of ~9.9 mm sevoflurane (fig. 4A). The chemical shift of most resonances, such as Ser245 (inset), remained unchanged (δ less than 0.01 ppm), demonstrating that even at saturation, sevoflurane does not perturb the global protein fold of the I domain. A small subset of resonances, including Thr291 (in the β6-α7 loop) and Tyr307, (in the C-terminal α7 helix), undergo large chemical shift changes (δ more than 0.05 ppm) with the addition of sevoflurane, indicating either direct drug-protein interaction or a modification of the local electronic environment (such as occurs when there are changes in the local structure of the protein).
Fig. 4.
Nuclear magnetic resonance (NMR) spectroscopy to study sevoflurane binding sites in the lymphocyte function-associated antigen-1 (LFA-1) inserted (I) domain. (A) Heteronuclear 1H, 15N single-quantum coherence-NMR spectroscopy was used to map sevoflurane-binding sites in the LFA-1 I domain. An overlay of two heteronuclear single-quantum coherence spectra obtained with 0 mM (blue) and ~9.9 mM sevoflurane (red) is shown. The chemical shift of most resonances, such as Ser245 (inset), remained unchanged (δppm less than 0.01), demonstrating that even at saturation, sevoflurane does not perturb the global fold (structure) of the I domain. A small subset of resonances, including Thr291 in the β6-α7 loop and Tyr307 in the C-terminal α7 helix, undergo large chemical shift changes (δppm greater than 0.05) upon the addition of sevoflurane. (B) Structure of the I domain showing that amide nitrogen residues were affected (δppm greater than 0.05) by the addition of ~9.9 mM sevoflurane. Gray represents residues unperturbed by sevoflurane, whereas red represents residues that met or exceeded the threshold for perturbation. α1 and α7 helices, as well as β1, β4, and β6 strands, are labeled. Residues Thr291, Ser245, and Tyr307 are arrowed and labeled. The yellow spheres represent the Mg2+ ion at the metal ion-dependent adhesion site. The green arrow denotes the cavity that contains the drug-binding sites for the αI allosteric antagonists and isoflurane. (C) Scaled chemical shift perturbation of saturated sevoflurane mapped onto the LFA-1 I domain protein sequence and secondary structure. Chemical shift perturbation was calculated as . A subset of resonances (Asn163, Lys188, Arg221, Ile258, Ile259, Lys268, Phe299, Thr300, Ile306) was excluded from consideration because of overlap, uncertainty in the chemical shift assignment during titration, and/or exchange broadening during drug addition. Unperturbed Ser245 and perturbed Thr291 are indicated with arrows. Secondary structure assignments:β1 (130–137),α1 (144–160),β2 (166–173),β2′ (177–181),α2 (183–188),α3 (192–196),α4 (208–218),β3 (231–238), α5 (249–251), β4 (255–261), α6 (268–277), β5 (286–289), and α7 (293–305). For comparison, residues that shifted upon the addition of the LFA-1 allosteric inhibitor lovastatin are highlighted in yellow.
The distribution of chemical shift perturbations by the binding of sevoflurane was analyzed within the three-dimensional structure (fig. 4B). Sevoflurane did not affect the chemical shifts of the residues near the metal ion-dependent adhesion site, which is the ICAM-1 ligand-binding site that coordinates the Mg2+ ion. This is in agreement with our functional data showing that sevoflurane blocked LFA-1 not in a competitive manner but in an allosteric manner. The pattern of chemical shift perturbations by sevoflurane overlapped well with those by the known allosteric LFA-1 inhibitor lovastatin (fig. 4C). Like lovastatin, sevoflurane most significantly perturbed the chemical shifts at those residues located at a cavity between α1- and α7-helices on one side, and β1- and β4-sheets on the other (fig. 4B and C). This cavity was first identified as the binding site for the α I allosteric antagonists to LFA-1 (e.g., BIRT377,51 LFA703,52 and lovastatin53), and later as the binding site for isoflurane24 (fig. 4B, green arrow). This cavity is an amphipathic pocket with characteristics typical of the anesthetic-binding motif identified in biochemical studies of canonical VA-binding proteins (ion channels) and in structural studies of model anesthetic-binding proteins. These NMR findings, in tandem with our functional data (figs. 2 and 3), support our hypothesis that VAs inhibit LFA-1 function by blocking the obligate motion of the C-terminal helix.
Discussion
In previous investigations using biochemical and structural biological approaches,24,25 we convincingly showed that isoflurane not only directly binds to the hydrophobic cavity of the LFA-1 I domain but also suppresses activation-dependent conformational conversion to the HA form, thereby allosterically inhibiting ICAM-1 binding. Furthermore, our previous crystallographic study25 showed a remarkable parallel between the isoflurane-binding site in the LFA-1 I domain (Protein Data Bank code 3F78) and that in the four-α-helix bundle components of apoferritin (Protein Data Bank code 1XZ3). Specifically, both isoflurane-binding sites constitute an amphiphilic cavity possessing an array of polar and nonpolar interactions. Because apoferritin has been used for modeling membrane-embedded helical bundles of ion channels, wherein the anesthetic binding sites are thought to lie,54 we postulated that the isoflurane-binding site in the LFA-1 I domain might resemble an authentic anesthetic-binding site. Thus, a common underlying mechanism (e.g., the allosteric modulation of protein functionality1) might explain not only the general anesthetic effects induced by VAs in the central nervous system but also the immunomodulatory effects observed in immune cells. Like isoflurane, sevoflurane at clinically relevant concentrations inhibited the ability of LFA-1 to bind to ICAM-1. Our results have revealed remarkable similarities in the modes of action by which sevoflurane and isoflurane inhibit LFA-1. Sevoflurane and isoflurane blocked WT, but not HA LFA-1, in the bead-based cell-free assays, thereby demonstrating that both VAs act directly on the extracellular portion of LFA-1 and inhibit its ability to bind ICAM-1 in an allosteric manner. Furthermore, a comparison of previous24 and present NMR spectrometry results shows that the distributions of chemical shift perturbations induced by isoflurane and sevoflurane are similar. The binding of isoflurane and sevoflurane perturbed the chemical shifts of those residues located near the hydrophobic cavity to which established α I allosteric antagonists22 were found to bind. These antagonists are thought to interfere with the downward shift of the C-terminal helix, thereby stabilizing the low-affinity I domain conformation.22 It is noteworthy that neither isoflurane nor sevoflurane affected the residues at the metal ion-dependent adhesion site to which ICAM-1 binds. Thus, these results strongly support the idea that, like isoflurane, sevoflurane binds to the LFA-1 I domain allosteric cavity, thereby suppressing activation-dependent conversion to the HA conformation.
In the crystal structure of the isoflurane-bound LFA-1 I domain previously determined by our laboratory,25 we observed an array of amphiphilic interactions that helped stabilize the position of isoflurane within the cavity. The trifluoromethyl head of isoflurane formed extensive hydrophobic interactions, whereas the chloride atom and the difluoromethyl fluorine atoms contributed to the polar interactions. In sevoflurane, the chloride atom and difluoromethyl groups involved in the polar interactions in the isoflurane-I domain interaction were replaced by another trifluoromethyl group and monofluoromethyl group, respectively. Although the crystal structure of the sevoflurane-bound LFA-1 I domain remains unresolved, the NMR data obtained in this study strongly suggest that sevoflurane binds to the same cavity to which isoflurane and lovastatin bind. This cavity can accommodate lovastatin, the size of which is 386 Å3. Although isoflurane55 (144 Å3) and sevoflurane55 (154 Å3) are smaller than lovastatin, we believe that the structural flexibility of sevoflurane and isoflurane will allow them to fit into the amphiphilic environment of the I domain allosteric cavity. The smaller sizes of sevoflurane and isoflurane may lead to weaker binding to the cavity than lovastatin, thereby accounting for correspondingly lower LFA-1-blocking potencies versus lovastatin. By contrast, chloroform (90 Å3)55 seems to be too small to span the width of the cavity and form energetically stable interactions with the I domain. This is in good agreement with previous reports that showed the importance of the molecular volumes of VAs in determining their anesthetic potency to ligand-gated ion channels.56,57
Although sevoflurane seems less potent in blocking LFA-1 than isoflurane in the cell-based assays (fig. 2A), both anesthetics showed comparable inhibition in the cell-free assays (fig. 3A and B). Thus, the different potencies of isoflurane and sevoflurane in the cell-based assays might not stem from their different binding affinities to LFA-1. One plausible explanation is that isoflurane and sevoflurane might have different potencies with regard to intracellular signaling and/or cytoskeletal proteins, thereby affecting LFA-1 receptor clustering on the plasma membrane at different levels. It would be of great interest to carry out comparative studies on the affinities of sevoflurane and isoflurane to the αL I domain in cell-free assays as well as their relative impact on LFA-1 receptor clustering on the cell-surface.
Leukocyte integrins, including LFA-1, play a crucial role in host defense, as best illustrated by the genetic disorders leukocyte adhesion deficiency types I and III, which lack expression and/or activation of leukocyte integrins and manifest increased susceptibility to bacterial infections.58 Thus, the suppression of leukocyte integrins by VAs, which we have shown both in the present and in previous studies, is of clinical and biological relevance. Given the importance of leukocyte integrins in mediating the extravasation of immune cells to sites of inflammation,20,21 we believe that the inhibition of leukocyte integrins by sevoflurane and isoflurane accounts, at least in part, for the documented antiinflammatory effects.
In summary, we have demonstrated that sevoflurane, like isoflurane, similarly blocks activation-dependent conformational changes of LFA-1 to its HA form. The allosteric mode of action exemplified by sevoflurane and isoflurane via LFA-1 might represent one of the underlying mechanisms of anesthetic-mediated immunomodulation.
What We Already Know about This Topic
-
❖
Volatile anesthetics modulate immune function and reduce leukocyte adhesion.
-
❖
Isoflurane inhibits a primary leukocyte adhesion signaling molecule (LFA-1), but other anesthetics have not been tested.
What This Article Tells Us That Is New
-
❖
In leukocytes in vitro, sevoflurane, in clinically used concentrations, inhibited ligand binding functions of LFA-1 by binding to its allosteric cavity in a manner similar to that of isoflurane.
Acknowledgments
The authors gratefully acknowledge Whitney Silkworth, M.S., and Elizabeth Tien, M.S. (Technicians, Immune Disease Institute, Boston, Massachusetts), for technical assistance and Hongmin Zhang, Ph.D. (Assistant Professor, University of Hong Kong, Hong Kong Special Administrative Region, China), for structural analysis.
Supported by grants AI063421, HL048675, and CA139444 (to Dr. Shimaoka) from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Eckenhoff RG, Johansson JS. Molecular interactions between inhaled anesthetics and proteins. Pharmacol Rev. 1997;49:343–367. [PubMed] [Google Scholar]
- 2.Franks NP. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 3.Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka K, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mechanisms of cardioprotection by volatile anesthetics. Anesthesiology. 2004;100:707–721. doi: 10.1097/00000542-200403000-00035. [DOI] [PubMed] [Google Scholar]
- 5.Suleiman MS, Zacharowski K, Angelini GD. Inflammatory response and cardioprotection during open-heart surgery: The importance of anaesthetics. Br J Pharmacol. 2008;153:21–33. doi: 10.1038/sj.bjp.0707526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizobe T. Haematological effects of anaesthetics and anaesthesia. Curr Opin Anaesthesiol. 1999;12:437–441. doi: 10.1097/00001503-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 7.McBride WT, Armstrong MA, McBride SJ. Immunomodulation: An important concept in modern anaesthesia. Anaesthesia. 1996;51:465–473. doi: 10.1111/j.1365-2044.1996.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 8.Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22:263–277. doi: 10.1007/s00540-008-0626-2. [DOI] [PubMed] [Google Scholar]
- 9.Miles AA, Miles EM, Burke J. The value and duration of defence reactions of the skin to the primary lodgement of bacteria. Br J Exp Pathol. 1957;38:79–96. [PMC free article] [PubMed] [Google Scholar]
- 10.Greif R, Akça O, Horn EP, Kurz A, Sessler DI. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. Outcomes Research Group. N Engl J Med. 2000;342:161–167. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 11.Von Dossow V, Baur S, Sander M, Tønnesen H, Marks C, Paschen C, Berger G, Spies CD. Propofol increased the interleukin-6 to interleukin-10 ratio more than isoflurane after surgery in long-term alcoholic patients. J Int Med Res. 2007;35:395–405. doi: 10.1177/147323000703500315. [DOI] [PubMed] [Google Scholar]
- 12.Desai DM, Kuo PC. Perioperative management of special populations: Immunocompromised host (cancer, HIV, transplantation) Surg Clin North Am. 2005;85:1267–1282. xi–xii. doi: 10.1016/j.suc.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Möbert J, Zahler S, Becker BF, Conzen PF. Inhibition of neutrophil activation by volatile anesthetics decreases adhesion to cultured human endothelial cells. Anesthesiology. 1999;90:1372–1381. doi: 10.1097/00000542-199905000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Kowalski C, Zahler S, Becker BF, Flaucher A, Conzen PF, Gerlach E, Peter K. Halothane, isoflurane, and sevoflurane reduce postischemic adhesion of neutrophils in the coronary system. Anesthesiology. 1997;86:188–195. doi: 10.1097/00000542-199701000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Shayevitz JR, Rodriguez JL, Gilligan L, Johnson KJ, Tait AR. Volatile anesthetic modulation of lung injury and outcome in a murine model of multiple organ dysfunction syndrome. Shock. 1995;4:61–67. doi: 10.1097/00024382-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Reutershan J, Chang D, Hayes JK, Ley K. Protective effects of isoflurane pretreatment in endotoxin-induced lung injury. Anesthesiology. 2006;104:511–517. doi: 10.1097/00000542-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Chiang N, Schwab JM, Fredman G, Kasuga K, Gelman S, Serhan CN. Anesthetics impact the resolution of inflammation. PLoS One. 2008;3:e1879. doi: 10.1371/journal.pone.0001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimaoka M, Takagi J, Springer TA. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct. 2002;31:485–516. doi: 10.1146/annurev.biophys.31.101101.140922. [DOI] [PubMed] [Google Scholar]
- 19.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 20.Dustin ML, Springer TA. In: Lymphocyte function associated-1 (LFA-1, CD11a/CD18), Guidebook to the Extracellular Matrix and Adhesion Proteins. 2nd. Kreis T, Vale R, editors. New York: Sambrook and Tooze; 1999. pp. 228–232. [Google Scholar]
- 21.Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci. 2009;122:215–225. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 22.Shimaoka M, Springer TA. Therapeutic antagonists and the conformational regulation of integrin function. Nat Rev Drug Discov. 2003;2:703–716. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Wang H, Peng B, Zhang M, Zhang D, Hou S, Guo Y, Ding J. Efalizumab binding to the LFA-1 alphaL I domain blocks ICAM-1 binding via steric hindrance. Proc Natl Acad Sci USA. 2009;106:4349–4354. doi: 10.1073/pnas.0810844106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuki K, Astrof NS, Bracken C, Yoo R, Silkworth W, Soriano SG, Shimaoka M. The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J. 2008;22:4109–4116. doi: 10.1096/fj.08-113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Astrof NS, Liu JH, Wang JH, Shimaoka M. Crystal structure of isoflurane bound to integrin LFA-1 supports a unified mechanism of volatile anesthetic action in the immune and central nervous systems. FASEB J. 2009;23:2735–2740. doi: 10.1096/fj.09-129908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckenhoff RG. Promiscuous ligands and attractive cavities: How do the inhaled anesthetics work? Mol Interv. 2001;1:258–268. [PubMed] [Google Scholar]
- 27.Steurer M, Schläpfer M, Z’graggen BR, Booy C, Reyes L, Spahn DR, Beck-Schimmer B. The volatile anaesthetic sevoflurane attenuates lipopolysaccharide-induced injury in alveolar macrophages. Clin Exp Immunol. 2009;155:224–230. doi: 10.1111/j.1365-2249.2008.03807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HT, Chen SW, Doetschman TC, Deng C, D’Agati VD, Kim M. Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway. Am J Physiol Renal Physiol. 2008;295:F128–F136. doi: 10.1152/ajprenal.00577.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidani Y, Taniguchi T, Kanakura H, Takemoto Y, Tsuda K, Yamamoto K. Sevoflurane pretreatment inhibits endotoxin-induced shock in rats. Anesth Analg. 2005;101:1152–1156. doi: 10.1213/01.ane.0000167768.55939.e1. [DOI] [PubMed] [Google Scholar]
- 30.Lu C, Shimaoka M, Ferzly M, Oxvig C, Takagi J, Springer TA. An isolated, surface-expressed I domain of the integrin αLβ2 is sufficient for strong adhesive function when locked in the open conformation with a disulfide bond. Proc Natl Acad Sci USA. 2001;98:2387–2392. doi: 10.1073/pnas.041606398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKenzie D, Franks NP, Lieb WR. Actions of general anaesthetics on a neuronal nicotinic acetylcholine receptor in isolated identified neurones of Lymnaea stagnalis. Br J Pharmacol. 1995;115:275–282. doi: 10.1111/j.1476-5381.1995.tb15874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watts MT, Escarzaga M, Williams CH. Gas chromatographic headspace analysis of sevoflurane in blood. J Chromatogr. 1992;577:289–298. doi: 10.1016/0378-4347(92)80250-t. [DOI] [PubMed] [Google Scholar]
- 33.Stevens R, Rüsch D, Solt K, Raines DE, Davies PA. Modulation of human 5-hydroxytryptamine type 3AB receptors by volatile anesthetics and n-alcohols. J Pharmacol Exp Ther. 2005;314:338–345. doi: 10.1124/jpet.105.085076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimaoka M, Salas A, Yang W, Weitz-Schmidt G, Springer TA. Small molecule integrin antagonists that bind to the β2 subunit I-like domain and activate signals in one direction and block them in another. Immunity. 2003;19:391–402. doi: 10.1016/s1074-7613(03)00238-3. [DOI] [PubMed] [Google Scholar]
- 35.Lu C, Shimaoka M, Zang Q, Takagi J, Springer TA. Locking in alternate conformations of the integrin αLβ2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc Natl Acad Sci USA. 2001;98:2393–2398. doi: 10.1073/pnas.041618598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimaoka M, Xiao T, Liu JH, Yang Y, Dong Y, Jun CD, McCormack A, Zhang R, Joachimiak A, Takagi J, Wang JH, Springer TA. Structures of the αL I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell. 2003;112:99–111. doi: 10.1016/s0092-8674(02)01257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimaoka M, Lu C, Palframan RT, von Andrian UH, McCormack A, Takagi J, Springer TA. Reversibly locking a protein fold in an active conformation with a disulfide bond: Integrin αL I domains with high affinity and antagonist activity in vivo. Proc Natl Acad Sci USA. 2001;98:6009–6014. doi: 10.1073/pnas.101130498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schleucher J, Schwendinger M, Sattler M, Schmidt P, Schedletzky O, Glaser SJ, Sørensen OW, Griesinger C. A general enhancement scheme in heteronuclear multidimensional NMR employing pulsed field gradients. J Biomol NMR. 1994;4:301–306. doi: 10.1007/BF00175254. [DOI] [PubMed] [Google Scholar]
- 39.Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, Oldfield E, Markley JL, Sykes BD. 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J Biomol NMR. 1995;6:135–140. doi: 10.1007/BF00211777. [DOI] [PubMed] [Google Scholar]
- 40.Kriwacki RW, Legge GB, Hommel U, Ramage P, Chung J, Tennant LL, Wright PE, Dyson HJ. Assignment of 1H, 13C and 15N resonances of the I-domain of human leukocyte function associated antigen-1. J Biomol NMR. 2000;16:271–272. doi: 10.1023/a:1008358912334. [DOI] [PubMed] [Google Scholar]
- 41.Zimmerman T, Oyarzabal J, Sebastián ES, Majumdar S, Tejo BA, Siahaan TJ, Blanco FJ. ICAM-1 peptide inhibitors of T-cell adhesion bind to the allosteric site of LFA-1. An NMR characterization. Chem Biol Drug Des. 2007;70:347–353. doi: 10.1111/j.1747-0285.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 42.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 43.Weber C, Lu CF, Casasnovas JM, Springer TA. Role of αLβ2 integrin avidity in transendothelial chemotaxis of mononuclear cells. J Immunol. 1997;159:3968–3975. [PubMed] [Google Scholar]
- 44.Shamri R, Grabovsky V, Feigelson SW, Dwir O, Van Kooyk Y, Alon R. Chemokine stimulation of lymphocyte α4 integrin avidity but not of leukocyte function-associated antigen- I avidity to endothelial ligands under shear flow requires cholesterol membrane rafts. J Biol Chem. 2002;277:40027–40035. doi: 10.1074/jbc.M206806200. [DOI] [PubMed] [Google Scholar]
- 45.Katagiri K, Hattori M, Minato N, Irie S, Takatsu K, Kinashi T. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol Cell Biol. 2000;20:1956–1969. doi: 10.1128/mcb.20.6.1956-1969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carman CV, Springer TA. Integrin avidity regulation: Are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Liu JH, Yang W, Springer T, Shimaoka M, Wang JH. Structural basis of activation-dependent binding of ligand-mimetic antibody AL-57 to integrin LFA-1. Proc Natl Acad Sci USA. 2009;106:18345–18350. doi: 10.1073/pnas.0909301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manderson GA, Johansson JS. Role of aromatic side chains in the binding of volatile general anesthetics to a four-alpha-helix bundle. Biochemistry. 2002;41:4080–4087. doi: 10.1021/bi0160718. [DOI] [PubMed] [Google Scholar]
- 49.Yonkunas MJ, Xu Y, Tang P. Anesthetic interaction with ketosteroid isomerase: Insights from molecular dynamics simulations. Biophys J. 2005;89:2350–2356. doi: 10.1529/biophysj.105.063396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang M, Tao YX, He F, Zhang M, Levine CF, Mao P, Tao F, Chou CL, Sadegh-Nasseri S, Johns RA. Synaptic PDZ domain- mediated protein interactions are disrupted by inhalational anesthetics. J Biol Chem. 2003;278:36669–36675. doi: 10.1074/jbc.M303520200. [DOI] [PubMed] [Google Scholar]
- 51.Last-Barney K, Davidson W, Cardozo M, Frye LL, Grygon CA, Hopkins JL, Jeanfavre DD, Pav S, Qian C, Stevenson JM, Tong L, Zindell R, Kelly TA. Binding site elucidation of hydantoin-based antagonists of LFA-1 using multidisciplinary technologies: Evidence for the allosteric inhibition of a protein-protein interaction. J Am Chem Soc. 2001;123:5643–5650. doi: 10.1021/ja0104249. [DOI] [PubMed] [Google Scholar]
- 52.Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 53.Kallen J, Welzenbach K, Ramage P, Geyl D, Kriwacki R, Legge G, Cottens S, Weitz-Schmidt G, Hommel U. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J Mol Biol. 1999;292:1–9. doi: 10.1006/jmbi.1999.3047. [DOI] [PubMed] [Google Scholar]
- 54.Liu R, Loll PJ, Eckenhoff RG. Structural basis for high-affinity volatile anesthetic binding in a natural 4-helix bundle protein. FASEB J. 2005;19:567–576. doi: 10.1096/fj.04-3171com. [DOI] [PubMed] [Google Scholar]
- 55.Jenkins A, Greenblatt EP, Faulkner HJ, Bertaccini E, Light A, Lin A, Andreasen A, Viner A, Trudell JR, Harrison NL. Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J Neurosci. 2001;21:RC136. doi: 10.1523/JNEUROSCI.21-06-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevens RJ, Rüsch D, Davies PA, Raines DE. Molecular properties important for inhaled anesthetic action on human 5-HT3A receptors. Anesth Analg. 2005;100:1696–1703. doi: 10.1213/01.ANE.0000151720.36988.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kash TL, Jenkins A, Harrison NL. Molecular volume determines the activity of the halogenated alkane bromoform at wild-type and mutant GABA(A) receptors. Brain Res. 2003;960:36–41. doi: 10.1016/s0006-8993(02)03748-4. [DOI] [PubMed] [Google Scholar]
- 58.Etzioni A. Defects in the leukocyte adhesion cascade. Clin Rev Allergy Immunol. 2010;38:54–60. doi: 10.1007/s12016-009-8132-3. [DOI] [PubMed] [Google Scholar]