Abstract
This review intended to provide an overview of the effects of dental materials, used in dentin-pulp complex and dental pulp regeneration, on angiogenesis processes during regenerative endodontic procedures. An electronic search was performed in PubMed and MEDLINE databases via OVID using the keywords mentioned in the PubMed and MeSH headings for English language published articles from January 2005–April 2014 that evaluated the angiogenic properties of different dental materials used in regenerative endodontic procedures. Of the articles identified in an initial search, only 40 articles met the inclusion criteria set for this review. Vital pulp therapy materials might have positive effects on angiogenesis events, while most of the canal irrigating solutions and antibiotic pastes have anti-angiogenic activity except for EDTA. Future clinical studies will be helpful in defining the mechanisms of action for dental materials that promote or inhibit angiogenesis events at applied areas.
Keywords: Angiogenesis, Dentin-pulp complex, Regeneration, Stem cells
INTRODUCTION
Regenerative endodontic has gained widespread interest in recent years as it is attempting to fill the root canal space with living tissues instead of artificial materials1). Most of dental materials release inorganic trace elements2–5), and most of inorganic trace elements regulate angiogenesis6,7). In the field of regenerative endodontics the most important goal is to provide a suitable environment for the regeneration of healthy tissue and restore the lost biological tissues8). Based on the treatment site, this field is divided into two distinct categories of dentin-pulp complex regeneration and dental pulp regeneration1,8). The dentin-pulp complex regenerative procedures or vital pulp therapies include the direct pulp capping, indirect pulp capping, and pulpotomy. Direct pulp capping is defined as covering an exposed dental pulp with a protective agent and indirect pulp capping is referred to the application of a protective agent, on a thin layer of dentin over the nearly exposed dental pulp4). The other treatment in vital pulp therapies is the pulpotomy. It is defined as the surgical removal of inflamed coronal part of the dental pulp in the exposed pulpal tissue to save the remaining healthy tissue5). In dentin-pulp complex regeneration, clinicians attempt to provide an effective pulp capping with appropriate sealing ability5), and maintain the vitality of irritated pulp tissues and promote the formation of a dentinal bridge8–10) and other tissues including neural cells11). In these procedures, the progenitor dental pulp stem cells (DPSCs) are migrated, recruited, and differentiated into odontoblast-like cells, which have the ability to produce reparative dentin9,12). Although, this issue seems very simple in theory, in reality the whole process is possible if the homeostasis of pulp is reestablished8). In other words, restorations of the vascular network, through up- or down regulation of pro- or anti-angiogenic growth factors, is a key determinant component that guides the regenerative procedure toward survival or necrosis of pulp tissue13).
The second field of regenerative endodontics deals with regeneration of dental pulp tissue in necrotic teeth14,15). In this procedure the treatment is initiated with the complete removal of necrotic dental pulp tissue, which is referred to as pulpectomy, by instrumentation and irrigation that is followed by disinfecting the root canal space16). After canal preparation, the regenerative treatment, the revascularization process, begins with instrumentation of periapical tissue to cause bleeding into the canal space. The blood clot formed inside the canal provides a provisional matrix scaffold for the recruited stem cells from apical papilla17). It is also demonstrated that complete disinfection plays a key role in the successful treatment outcomes18,19).
Beside the stem cells derived from apical papilla, other investigators have used tissue engineered DPSCs for transplantation into the empty canal20). In this treatment protocol, the establishment of a functional vascular network in transplanted tissue is the challenging goal for a successful result21). The formation of this vascular structure is possible through angiogenesis, which is defined as the formation of new blood vessels from pre-existing vasculature22). In addition to regeneration of dental pulp tissue, apexogenesis and apexification are other endodontic procedures that are performed in immature permanent teeth23). Apexogenesis is the procedure that enables the immature permanent teeth to continue root end development, while the apexification provides a calcified barrier at the end of immature root by biocompatible material next to periapical tissue23). It has been reported that the revascularization process occurs through the angiogenesis events derived from the periapical tissues that grow into the engineered pulp tissue. Furthermore, the immature teeth with open apices are the best candidates for these regenerative procedures24).
These facts emphasize angiogenesis as an important factor involved in homeostasis of dentin-pulp complex and dental pulp regeneration25). In addition, angiogenesis has a pivotal role in dentinal and dental pulp tissues’ regenerative and reparative procedures1,9). The present study attempts to review the effects of dental materials and treatment modalities used in dentin-pulp complex and dental pulp regeneration on angiogenesis. The possible influences of dental procedures and materials on angiogenesis events are reviewed.
MATERIALS AND METHODS
The review purpose
In this review, the effects of dental treatments and materials used in direct or indirect pulp capping, pulpotomy, pulpectomy, apexogenesis, and apexification were overviewed on dental pulp tissue regeneration and revascularization. The main aspect of this review is to evaluate the possible influence of dental procedures and materials used in regenerative procedures, which can either promote or inhibit angiogenesis, during regeneration or revascularization.
Inclusion and exclusion criteria
The inclusion criteria were: 1) studies accepted and published in English language between January 2005–April 2014; 2) the scientific in-vivo, ex-vivo, or in-vitro articles, reviews, systematic reviews, case reports, and clinical trials with controlled study design; 3) studies that had evaluated the effect dental materials used in regenerative endodontics, direct or indirect pulp capping, pulpotomy, pulpectomy, apexogenesis, and apexification treatments on angiogenesis processes occurring in the applied area.
The exclusion criteria were: 1) studies that were published before January 2005 or after April 2014; 2) studies that did not evaluate the direct angiogenic potentials of the dental materials used in regenerative endodontics, direct or indirect pulp capping, pulpotomy, pulpectomy, apexogenesis, and apexification treatments; 3) studies that mainly focused on other aspects of dental materials, which have no effect on angiogenesis process.
Search methodology
An electronic search was performed in PubMed and MEDLINE databases via OVID using the keywords mentioned in the PubMed and MeSH headings for English language published articles from January 2005–April 2014 that evaluate the angiogenic properties of different dental materials used in regenerative endodontic procedures.
Search strategy
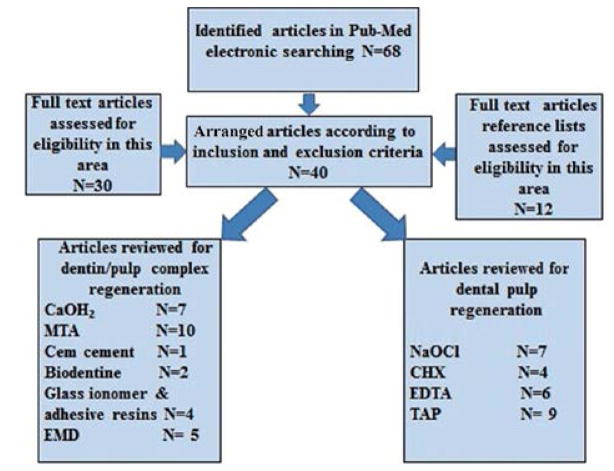
The electronic searching key words in PubMed and MEDLINE databases included: angiogenesis, pulp stem cells, regenerative endodontics, direct or indirect pulp capping, pulpotomy, pulpectomy, apexogenesis, apexification treatments and dental materials including calcium hydroxide, mineral trioxide aggregate, Bioaggregate, Cem cement, Biodentine, glass ionomer, adhesive resin, and enamel matrix derivatives in evaluation of dental pulp tissue regeneration and revascularization. Some of the most relevant article’s full texts and reference lists were evaluated for eligibility. A flowchart of mentioned activities is presented in Fig. 1 to clarify the number of relevant articles used for this review.
Fig. 1.
A flowchart of search strategy to identify English articles from January 2005–April 2014 based on inclusion and exclusion criteria in this review study.
THE EFFECT OF DENTAL TREATMENTS AND MATERIALS ON ANGIOGENESIS IN DENTIN-PULP COMPLEX REGENERATION
Direct or indirect pulp capping and pulpotomy
1. Calcium hydroxide
The introduction of vital pulp therapies including direct or indirect pulp capping date back to 1939 by Zander26). An ideal pulp capping material should provide easy handling, infection control, good sealing ability, and induce dentinal bridge formation27). Among several materials, calcium hydroxide (Ca(OH)2) was one of the most common material used in pulp capping28). Schröder indicated that Ca(OH)2 can induce a limited necrotic zone on the surface of pulp tissue at the application sites28). Due to its alkalinity, Ca(OH)2 has antibacterial activity and stimulates dentin formation29).
The effect of Ca(OH)2 on dentin-pulp complex regeneration has been evaluated by several authors. Ji et al. indicated that Ca(OH)2 increases the recruitment, migration, proliferation, and mineralization of DPSCs, and periodontal ligament stem cells (PDLSCs) through the expression of STRO-1 and CD146 markers30). Sangwan et al. reviewed the mechanisms of action of Ca(OH)2 in tertiary dentinogenesis31). They indicated that the regenerative effects of Ca(OH)2 are due to calcium ion release and the high pH value31). Calcium ions promote the migration of pulp progenitor cells, increase the synthesis of biomolecules such as fibronectin and bone morphogenic proteins (BMPs), and participate in mineralization31).
The alkaline pH can present antibacterial and anti-inflammatory effects, activate transforming growth factor β (TGF-β), increase the activity of alkaline phosphatase (ALP), and enhance the dissolution of dentine extracellular matrix (ECM)31).
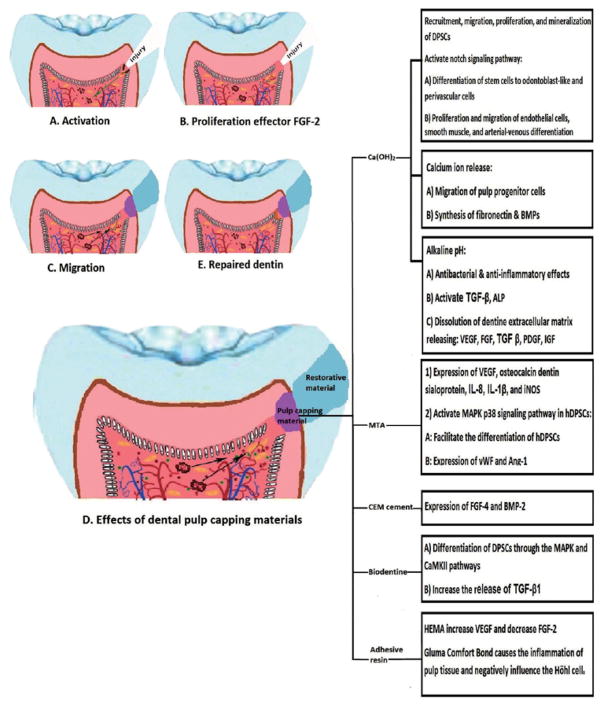
The pro-angiogenic effects of Ca(OH)2 is mainly attributed to the release of growth factors preserved in the dentin matrix including TGF-β, platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and insulin-like growth factor (IGF)31) (Fig. 2B). This issue was also discussed by Chun et al. where they demonstrated that chelating agents such as ethylenediaminetetraacetic acid (EDTA) can dissolve the dentin matrix and release pro-angiogenic factors, and promote the regenerative process32). Roberts-Clark and Smith demonstrated that in addition to PDGF and FGF-2, dentine matrix also contains vascular endothelial growth factor (VEGF)33), which is one of the most important factors in angiogenesis events34). Løvschall et al. introduced another mechanism that Ca(OH)2 can influence angiogenesis process35). They suggested that this pulp capping material can activate notch signaling pathway, which is implicated in cell-cell interactions involved in many regenerative processes such as angiogenesis36). In dental pulp tissue, this signaling pathway is activated due to injury, and participates in differentiation of stem cells to odontoblast-like and perivascular cells35). Previously, Iso et al. reported that notch signaling plays an active role in angiogenesis including the proliferation and migration of endothelial cells, smooth muscle, and arterial-venous differentiation36) (Fig. 2D).
Fig. 2. Schematic presentation of dentin-pulp complex regeneration.
A: deep injury imposed to the pulp tissue, which activates regenerative processed; B: Recruitment of pulp stem cells and expression of angiogenic factors such as FGF-2, which are responsible for proliferation of endothelial cells; C: Migration of proliferated endothelial cells to the injured area; D: The angiogenic effects of different pulp capping materials in the regenerative processes; E: Differentiation of stem cells to odontoblast-like cells and secretion of reparative dentin. Please note the vascular structure returning to its normal architecture at the end of the regeneration process.
2. MTA
Mineral trioxide aggregate (MTA) is another commonly used dental material in pulp capping treatments with more advantages over Ca(OH)237). MTA has been recently optimized in many aspects including the setting time3) and calcium ion release38). Al-Hezaimi et al. compared the regenerative effects of Portland cement and MTA with Ca(OH)2 and indicated that the thickness of reparative dentin was thicker in MTA and Portland cement, while the quality was not different37). Cavalcanti et al. showed that MTA, Ca(OH)2, and Single Bond adhesive system can increase the release of IL-8, while the release of interleukin-1β (IL-1β) was only increased in samples treated with MTA39). IL-1β is a pro-inflammatory cytokine which is secreted after tissue injury and induces the release of chemokines such as IL-8, which is considered a pro-angiogenic factor40). Similar results were noticed by Ferreira et al., which evaluated the effect of pulpotomy agents including Ca(OH)2, MTA, adhesive resin, and formocresol on dental pulp tissue fibroblasts41). These authors reported that MTA was the only pulpotomy material which increased the release of IL-1β and IL-8 by fibroblasts. Calcium hydroxide only stimulated the release of IL-1β, while adhesive resin and formocresol could increase IL-8 levels41) (Fig. 2D).
Zhang et al. reported that MTA had a greater potential for expression of TGF-β1 in rat dental pulp 42). Paranjpe et al. tissue compared with Ca(OH)2 demonstrated that MTA had positive effects on angiogenesis and differentiation of dental pulp cells when it was placed in direct contact with dental pulp43). These authors reported that MTA, as a direct pulp capping agent, can induce the expression of VEGF, osteocalcin and dentin sialoprotein43,44).
Zhang et al. evaluated the effect of MTA and Ca(OH)2 on expression of inducible nitric oxide synthase (iNOS) in rat dental pulp tissue45). These pulp capping materials increased the expression of iNOS 3 days after their application45). Ziche et al. indicated that NO plays an important role in regulating the angiogenesis process through the enhancement of endothelial cells proliferation and migration46). Huang et al. indicated that MTA could activate mitogen-activated protein kinase (MAPK), specifically the p38 signaling pathway in hDPSCs47). The in vitro culture of hDPSCs with MTA facilitated their differentiation, and also increased the expression of angiogenic factors including von Willebrand factor (vWF) and angiopoietin-1 (Ang-1)47) (Fig. 2D).
3. CEM cement
Calcium-enriched mixture (CEM) cement is one of the pulp capping agents, which has osteogenic, cementogenic and dentinogenic functions48). Asgary et al. compared the ability of CEM and MTA as capping agents. They concluded that CEM could increase the expression of FGF-4 and bone morphogenic protein 2 (BMP-2), while MTA positively affected the expression of TGF-β1 in pulp tissue48) (Fig. 2D).
4. Biodentine
Biodentine (Septodont, Saint-Maur-des-Fossès, France) is a tricalcium silicate-based cement, which has been recently introduced as a pulp capping material49). Luo et al. reported that the induction effect of Biodentine cement on differentiation of DPSCs is through the mitogen-activated protein kinase (MAPK) and calcium/calmodulin-dependent protein kinase II (CaMKII) pathways49). The angiogenic effect of this cement was evaluated by Laurent et al. who suggested that Biodentine can induce early mineralization in dental pulp due to an increase in release of TGF-β1, a pro-angiogenic factor produced by pulp cells50) (Fig. 2D).
5. Glass ionomer and adhesive resins
Lutfi et al. showed that glass ionomer cement (GIC) as a lining material can act similar to Ca(OH)2 in inducing proliferative activity in dental pulp of exfoliated deciduous teeth51). Dammaschke et al. compared the effect of dentine adhesive Gluma Comfort Bond (GCB) and Ca(OH)2 on the proliferation of pulp cells and concluded that GCB, due to its missing antibacterial efficacy and foreign body reactions, causes the inflammation of pulp tissue. Direct contact of GCB with dental pulp could increase the number of fibroblasts and endothelial cells in granulation tissue and negatively influence the Höhl cells52) (Fig. 2D).
Adhesive systems have also been used as dental pulp capping agents. Tran-Hung et al. evaluated the effect of HEMA on secretion of pro-angiogenic factors in dental pulp after mechanical injury53). They indicated that HEMA can increase the level of VEGF, decrease the expression of FGF-2, and has no effect on platelet derived growth factor-AB (PDGF-AB) in mechanically injured human dental pulp tissue53). Similar results were reported by Mantellini et al. who demonstrated that HEMA or SingleBond adhesive resin could increase the expression of pro-angiogenetic factor VEGF in mouse odontoblast-like cells (MDPC-23) and macrophages, while this up-regulation was not observed in the undifferentiated mouse pulp cells and gingival fibroblasts13) (Fig. 2D). However, other investigators demonstrated that in case of mechanically exposed dental pulp, the application of dentine adhesive systems cannot induce the formation of an acceptable tertiary dentine bridge at applied area54).
6. Enamel matrix derivative (EMD)
Guven et al. demonstrated that enamel matrix derivative (EMD) can also be used as a pulp capping material55). These authors showed that EMD was more capable of inducing the differentiation and proliferation of human tooth germ stem cells (hTGSCs) compared with calcium hydroxide-containing cement (DYCAL) and mineral trioxide aggregate (MTA)55). Even the EMD-coated DYCAL was shown to be less toxic, which emphasizes the biocompatible nature of EMD55). Yuan et al. demonstrated that EMD can exhibit angiogenic effects by presenting chemotactic effect on human umbilical vein endothelial cells (HUVEC) in vitro56). These authors reported that more endothelial cells and new blood vessels were detected in cell cultures treated with EMD than in control group56). Several authors have indicated that EMD has angiogenic activity at the applied sites57), and it can stimulate periodontal cells to produce VEGF58). However, Darwish et al. suggested that enamel matrix derivative, could be preferable material for periodontium, while it is not suitable for dentin-pulp complex regeneration59). Olsson et al. reported similar results by comparing the effect of calcium hydroxide and Emdogain Gel (Biora AB, Malmö, Sweden), consisting of a enamel matrix derivative (EMD) in a propylene glycol alginate (PGA) vehicle, on the postoperative symptoms of the experimentally exposed human dental pulps60). It was shown that the application of Emdogain Gel to the exposed dental pulp causes more inflammation with no effective formation of hard tissue barrier compared with samples subjected to calcium hydroxide application60).
THE EFFECT OF DENTAL TREATMENTS AND MATERIALS ON ANGIOGENESIS IN DENTAL PULP REGENERATION AND REVASCULARIZATION
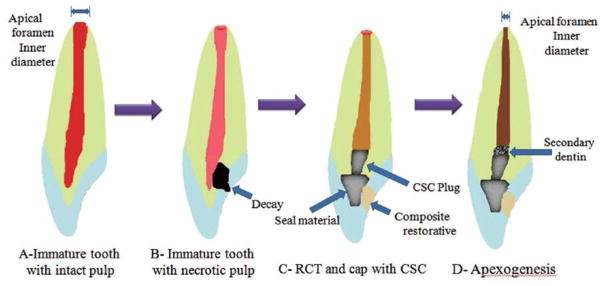
The summit goal of the regenerative endodontic procedures is to provide a suitable environment for the regeneration of healthy tissue to restore the lost biological tissues61). The challenge, however, is to create pulp tissue in a vacant canal, which is exactly identical to the pulpal tissue before necrosis and can function properly8,9). This issue is one of the main differences between regenerative attempts and the conventional treatments including apexification. In conventional modalities, the treatment is ended with obturation of empty canal rather than giving it a chance to be filled with biological tissue. The expected clinical and radiographic outcomes of regenerative procedures are the resolution of apical periodontitis, continuity of root development including the length and thickness, and normal responding of regenerated pulp tissue to different pulp tests62,63) (Fig. 3C).
Fig. 3. Schematic presentation of dental pulp regeneration processes in an immature tooth.
A: Immature tooth with intact pulp; B: Necrotic dental pulp and accumulation of stem cells of the apical papilla (SCAP) in periapical area; C: Mechanical provocation of bleeding from the periapical site to transport the SCAPs into the empty canal space and establishment of a coronal seal by MTA cement and composite resin materials; D: Formation of pulp-like tissue in apical and mid third of root, which contributes to the maturation of apical third and apical foramen closure by the formation of a cementum-like tissue.
According to current status, previous authors presented a general protocol for pulp regeneration or root revascularization procedures. However, some modifications and improvements have been suggested as well. The overall steps in these procedures included: 1) canal irrigation and disinfection; 2) blood clot formation; and 3) coronal seal to provide appropriate environment for regeneration61,64,65) (Fig. 3). In the following section, the effects of canal irrigating solutions and disinfecting agents on angiogenesis are discussed.
NaOCl
The first step in dental pulp regeneration is the complete disinfection of root canal system. At first, root canal space should be irrigated with sodium hypochloride (NaOCl). NaOCl has proteolytic and antimicrobial activities which dissolves the organic debris and eliminate microorganisms inside the dental canal66). Bennett et al. evaluated the effect of different antiseptic or antimicrobial agents including 5% mafenide acetate (Sulfamylon solution), 10% povidone with 1% free iodine (Betadine), 0.25% sodium hypochlorite (“half-strength” Dakin), 3% hydrogen peroxide, and 0.25% acetic acid on wound healing. It was indicated that 0.25% sodium hypochlorite and sulfamylon could significantly increase neodermal thickness and sulfamylon was the most effective agent on promoting angiogenesis67). However, the dilution of sodium hypochlorite can decrease the disinfecting efficiency of this solution. Kozol et al. also suggested that NaOCl has toxic effects on cells and did not recommend it for use in open wounds68).
Jaimes et al. indicated that HOCl can inhibit nitric oxide production during inflammation and suggested that this effect might attribute to dysregulation of vascular events and negatively affect the interactions between leukocytes and endothelial cells69). Alkahtani et al. reported that NaOCl solution has toxic effects on human bone marrow mesenchymal stem cells (MSCs)70). Martin et al. also reported that high concentrations of NaOCl can drastically effect the survival and differentiation of stem cells of the apical papilla (SCAPs) and significantly reduce the expression of dentin sialophosphorprotein (DSPP)71). These authors suggested using lower concentrations like 1.5% and using 17% EDTA after NaOCl irrigation reduced the NaOCl negative effects, and increased the survival rate of SCAPs and expression of DSPP71). Trevino et al. also acclaimed that irrigation with NaOCl can lower the survival rate of SCAPs, while inclusion of EDTA to the irrigation protocol was beneficial and increased the number of viable cells72).
Chlorhexidine gluconate
The other disinfecting endodontic solution used in regenerative procedures is 2% chlorhexidine gluconate (CHX)73). The effect of CHX on DPSCs was measured and no viable cell was detected in samples irrigated with 2% CHX72). Ring et al. reported that 2% CHX, as well as 6% NaOCl, showed cytotoxic effects on DPSCs due to negative influence on their attachment to root canal wall surface74).
EDTA
EDTA is an endodontic irrigating solution with chelating activity, which is suggested to be added to canal irrigation protocol as a final rinse for smear layer removal or in combination with NaOCl and CHX solutions72). Ring et al. demonstrated that the absence or presence of smear layer is not as effective on activity of DPSCs74). However, the addition of EDTA to other rinsing solutions can increase the viability of DPSCs72), and positively affect stem cell’s attachment to root canal wall74). Authors showed that the time of irrigation with EDTA should be 1 min, while after 3 min the microhardness of dentin can significantly reduce75).
Pang et al. also noted that EDTA can induce DPSCs cell attachment and odontoblastic/osteoblastic differentiation76). Similar to other investigators Galler et al. recommend the usage of EDTA for canal irrigation77). In addition to favorable effect of EDTA on other solutions, it was indicated that EDTA can stimulate the release of pro-angiogenic growth factors in dentin matrix including TGF-β, VEGF, FGF-2, PDGF, and BMP-233,78,79).
Triple antibiotic paste (TAP)
TAP is a disinfecting regimen containing three antibiotic pastes including: ciprofloxacin, metronidazole, and minocycline used for complete elimination of microorganisms inside necrotic root canal in regenerative procedures80). Bottino et al. indicated that the scaffolds containing 5%wt ciprofloxacin or 5 and 25%wt metronidazole were safe for hDPSCs, and only 25%wt ciprofloxacin had cytotoxic effects on pulp stem cells81). Bezwada et al. evaluated the intrinsic cytotoxicity of five fluoroquinolone antibiotics including: ciprofloxacin, levofloxacin, ofloxacin, moxifloxacin, and gatifloxacin on human corneal endothelial cells. These authors reported that among these antibiotics, ciprofloxacin showed the highest concentration- and time-dependent cytotoxicity, while levofloxacin had the lowest value82). Galley et al. indicated that ciprofloxacin can alter the inflammatory responses in endothelial cells. It was suggested that ciprofloxacin could decrease the expression of inflammatory cytokine IL-6, and increase the expression of the IL-8, a pro-angiogenic cytokine83). Michalska et al. indicated that metronidazole plus clindamycin had anti-agiogenic activity and could strongly interact with pro-angiogenic factors like FGF-2 and fibirin concentration and viscosity84).
Jung et al. demonstrated that minocycline has anti-angiogenic activity due to suppression of the hypoxia-induced vascular endothelial growth factor (VEGF) expression85). These authors also reported that minocycline can inhibit the activity of matrix metalloproteinase (MMPs)85). Li et al. showed that minocycline can accelerate breakdown of hypoxia-inducible factor-1 (HIF-1) and inhibit hypoxia-induced neovasculogenesis86). Yao et al. reported that minocycline inhibits the migration of human aortic smooth muscle cell (HASMCs) by down-regulating PI3K/Akt pathway87). Although Arslan et al. recommended the passive ultrasonic irrigation (PUI) with 1% NaOCl to be useful for removal of the triple antibiotic paste88), but the complete removal may not be achieved.
CONCLUSIONS
The evaluation of angiogenesis events in regenerative endodontic field is quite a new approach in this era. The present review focused on possible influences of dental treatments and materials on angiogenesis during regenerative endodontic procedures, and the following outcomes can be drawn.
In dentin-pulp complex regeneration, the local angiogenesis events occur at the site of necrotic or injured tissues. Two distinct roles can be considered for pulpal local angiogenesis: 1) the blood supplying the inflammatory phase which should bring the immune cells and components, and the nutrition required for both inflammatory and regeneration phases; 2) the recruitment of perivascular stem cells which in addition to local stem cells can be differentiated into odontoblast-like cells for the production of reparative dentin.
The dentin matrix might play an important role as a reservoir of intrinsic growth factors. These factors can be released due to changes imposed on dentin structure and also in response to the treatments and materials used on dentin surfaces. In light of this, the pro-or anti-angiogenic properties of dental materials used for dentin-pulp complex regeneration should be considered as one of the important characteristics for selecting an ideal dental pulp capping material. This issue was also raised by Tziafas et al.27) besides the traditional criteria such as easy handling, infection control, good sealing ability, and exploiting endogenous signaling for dentinal bridge formation. These authors also mentioned the use of exogenous signaling molecules in new pulp capping strategies9).
In dental pulp regeneration procedures, most of the materials including irrigating solutions or antibiotic pastes have anti-angiogenic effects. However, EDTA solution has pro-angiogenic activity among other solutions. The residual antibiotic paste inside the canal can delay or jeopardize the angiogenesis process. It has been suggested that in future studies, other more biocompatible agents such as levofloxacin, instead of ciprofloxacin, to be evaluated for regenerative procedures.
Future studies regarding the angiogenic properties of dental materials will lead to a better understanding of their mechanism of action in angiogenesis events at applied area. In addition, the enrichment of these materials with pro-or anti-angiogenic factors can be considered as target goal for regulation and establishment of balanced angiogenesis events. This balanced neoangiogenesis can promote the healing process of dentin-pulp complex on one side and prevent pulp tissue necrosis on the other. The additional biomolecules can enhance the biocompatibility of dental materials as well as their treatment outcomes in regeneration procedures.
Concerning the regenerative endodontics, it should be mentioned that the trend of restoring lost tissue with biological tissues, rather than replacing with synthetic materials, is appreciable and promising. However, the similarity of the structure and functions of restored tissues to the primary tissue must be taken into consideration.
Acknowledgments
This publication is dedicated to the memory of Dr. Hajar Afsar Lajevardi, a legendry Iranian pediatrician (1953–2015) who passed away during the writing of the series of articles regarding the role of angiogenesis in regenerative dentistry. She helped the authors of these article series. We will never forget Dr. H Afsar Lajevardi’s kindness and support. She was the only clinician-scientist with a particular focus on infectious diseases of children in Iran. She established Dr. H. A. Lajevardi foundation, with great mission in angiogenesis and regenerative medicine for the next generation of scientists. This work was supported in part by P30 EY016665, and an unrestricted departmental award from Research to Prevent Blindness. Dr. Sheibani is a recipient of a research award from the Retina Research Foundation.
References
- 1.Saghiri MA, Asatourian A, Sheibani N. Angiogenesis in regenerative dentistry. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:122. doi: 10.1016/j.oooo.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saghiri MA, Orangi J, Asatourian A, Sheibani N. Functional role of inorganic trace elements in angiogenesis Part II:(Cr, Si, Zn, Cu, and S) Crit Rev Oncol Hematol. 2015;96:143–155. doi: 10.1016/j.critrevonc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Saghiri MA, Asgar K, Lotfi M, Garcia-Godoy F. Nanomodification of mineral trioxide aggregate for enhanced physiochemical properties. Int Endod J. 2012;45:979–988. doi: 10.1111/j.1365-2591.2012.02056.x. [DOI] [PubMed] [Google Scholar]
- 4.Saghiri MA, Lotfi M, Aghili H. Dental cement composition. 8, 668, 770. US Patent. 2014
- 5.Saghiri MA, Lotfi M, Saghiri AM, Vosoughhosseini S, Fatemi A, Shiezadeh V, Ranjkesh B. Effect of pH on sealing ability of white mineral trioxide aggregate as a root-end filling material. J Endod. 2008;34:1226–1229. doi: 10.1016/j.joen.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Saghiri MA, Asatourian A, Orangi J, Sheibani N. Functional role of inorganic trace elements in angiogenesis Part I:(N, Fe, Se, P, Au, and Ca) Crit Rev Oncol Hematol. 2015;96:129–142. doi: 10.1016/j.critrevonc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Saghiri MA, Orangi J, Asatourian A, Sorenson CM, Sheibani N. Functional role of inorganic trace elements in angiogenesis Part III:(Ti, Li, Ce, As, Hg, Va, Nb and Pb) Crit Rev Oncol Hematol. 2016;98:290–301. doi: 10.1016/j.critrevonc.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon S, Goldberg M. The Dent Pulp. Springer; 2014. Regenerative endodontics: regeneration or repair? pp. 267–276. [Google Scholar]
- 9.Saghiri MA, Asatourian A, Sorenson CM, Sheibani N. Role of angiogenesis in endodontics: Contributions of stem cells and proangiogenic and antiangiogenic factors to dental pulp regeneration. J Endod. 2015;41:797–803. doi: 10.1016/j.joen.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumsha T, Hovland E. Considerations and treatment of direct and indirect pulp-capping. Dent Clin North Am. 1985;29:251–259. [PubMed] [Google Scholar]
- 11.Saghiri MA, Asgar K, Daliri M, Lotfi M, Delvarani A, Mehrvarzfar P, Karamifar K. Morphological behavior and attachment of p19 neural cells to root-end filling materials. Scanning. 2010;32:369–374. doi: 10.1002/sca.20209. [DOI] [PubMed] [Google Scholar]
- 12.Ida-Yonemochi H, Nakatomi M, Ohshima H. Establishment of in vitro culture system for evaluating dentin-pulp complex regeneration with special reference to the differentiation capacity of BrdU label-retaining dental pulp cells. Histochem Cell Biol. 2014;142:323–333. doi: 10.1007/s00418-014-1200-7. [DOI] [PubMed] [Google Scholar]
- 13.Mantellini MG, Botero T, Yaman P, Dennison JB, Hanks CT, Nör JE. Adhesive resin and the hydrophilic monomer HEMA induce VEGF expression on dental pulp cells and macrophages. Dent Mater. 2006;22:434–440. doi: 10.1016/j.dental.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 14.Thibodeau B, Trope M. Pulp revascularization of a necrotic infected immature permanent tooth: case report and review of the literature. Pediatr Dent. 2007;29:47–50. [PubMed] [Google Scholar]
- 15.Lenzi R, Trope M. Revitalization procedures in two traumatized incisors with different biological outcomes. J Endod. 2012;38:411–414. doi: 10.1016/j.joen.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Keswani D, Pandey R. Revascularization of an immature tooth with a necrotic pulp using platelet-rich fibrin: a case report. Int Endod J. 2013;46:1096–1104. doi: 10.1111/iej.12107. [DOI] [PubMed] [Google Scholar]
- 17.Nagata JY, Figueiredo de Almeida Gomes BP, Rocha Lima TF, Murakami LS, de Faria DE, Campos GR, de Souza-Filho FJ, de Soares AJ. Traumatized immature teeth treated with 2 protocols of pulp revascularization. J Endod. 2014;40:606–612. doi: 10.1016/j.joen.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 18.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196–200. doi: 10.1097/00004770-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi N, Yamauchi S, Nagaoka H, Duggan D, Zhong S, Lee SM, Teixeira FB, Yamauchi M. Tissue engineering strategies for immature teeth with apical periodontitis. J Endod. 2011;37:390–397. doi: 10.1016/j.joen.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Langer RS, Vacanti JP. Tissue engineering: the challenges ahead. Sci Am. 1999;280:86–89. doi: 10.1038/scientificamerican0499-86. [DOI] [PubMed] [Google Scholar]
- 21.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Polverini PJ. Angiogenesis in health and disease: insights into basic mechanisms and therapeutic opportunities. J Dent Educ. 2002;66:962–975. [PubMed] [Google Scholar]
- 23.Rafter M. Apexification: a review. Dent Traumatol. 2005;21:1–8. doi: 10.1111/j.1600-9657.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Godoy F, Murray PE. Recommendations for using regenerative endodontic procedures in permanent immature traumatized teeth. Dent Traumatol. 2012;28:33–41. doi: 10.1111/j.1600-9657.2011.01044.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711–718. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- 26.Zander H. Reaction of the pulp to calcium hydroxide. J Dent Res. 1939;18:373–379. [Google Scholar]
- 27.Tziafas D, Smith A, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77–92. doi: 10.1016/s0300-5712(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 28.Schröder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res. 1985;64:541–548. doi: 10.1177/002203458506400407. [DOI] [PubMed] [Google Scholar]
- 29.Nosrat I, Nosrat C. Reparative hard tissue formation following calcium hydroxide application after partial pulpotomy in cariously exposed pupls of permanent teeth. Int Endod J. 1998;31:221–226. doi: 10.1046/j.1365-2591.1998.00147.x. [DOI] [PubMed] [Google Scholar]
- 30.Ji YM, Jeon SH, Park JY, Chung JH, Choung YH, Choung PH. Dental stem cell therapy with calcium hydroxide in dental pulp capping. Tissue Eng Part A. 2010;16:1823–1833. doi: 10.1089/ten.TEA.2009.0054. [DOI] [PubMed] [Google Scholar]
- 31.Sangwan P, Sangwan A, Duhan J, Rohilla A. Tertiary dentinogenesis with calcium hydroxide: A review of proposed mechanisms. Int Endod J. 2013;46:3–19. doi: 10.1111/j.1365-2591.2012.02101.x. [DOI] [PubMed] [Google Scholar]
- 32.Chun SY, Lee HJ, Choi YA, Kim KM, Baek SH, Park HS, Kim JY, Ahn JM, Cho JY, Cho DW, Shin HI, Park EK. Analysis of the soluble human tooth proteome and its ability to induce dentin/tooth regeneration. Tissue Eng Part A. 2010;17:181–191. doi: 10.1089/ten.TEA.2010.0121. [DOI] [PubMed] [Google Scholar]
- 33.Roberts-Clark D, Smith A. Angiogenic growth factors in human dentine matrix. Arch Oral Biol. 2000;45:1013–1016. doi: 10.1016/s0003-9969(00)00075-3. [DOI] [PubMed] [Google Scholar]
- 34.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 35.Løvschall H, Tummers M, Thesleff I, Füchtbauer EM, Poulsen K. Activation of the Notch signaling pathway in response to pulp capping of rat molars. Eur J Oral Sci. 2005;113:312–317. doi: 10.1111/j.1600-0722.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 36.Iso T, Hamamori Y, Kedes L. Notch signaling in vascular development. Arterioscler Thromb Vasc Biol. 2003;23:543–553. doi: 10.1161/01.ATV.0000060892.81529.8F. [DOI] [PubMed] [Google Scholar]
- 37.Al-Hezaimi K, Salameh Z, Al-Fouzan K, Al Rejaie M, Tay FR. Histomorphometric and micro-computed tomography analysis of pulpal response to three different pulp capping materials. J Endod. 2011;37:507–512. doi: 10.1016/j.joen.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Saghiri MA, Asatourian A, Orangi J, Lotfi M, Soukup JW, Garcia-Godoy F, Sheibani N. Effect of particle size on calcium release and elevation of pH of endodontic cements. Dent Traumatol. 2015;31:196–201. doi: 10.1111/edt.12160. [DOI] [PubMed] [Google Scholar]
- 39.Cavalcanti BN, Rode SdM, França CM, Marques MM. Pulp capping materials exert an effect on the secretion of IL-1β and IL-8 by migrating human neutrophils. Braz Oral Res. 2011;25:13–18. doi: 10.1590/s1806-83242011000100003. [DOI] [PubMed] [Google Scholar]
- 40.Norrby K. Mast cells and angiogenesis. APMIS. 2002;110:355–371. doi: 10.1034/j.1600-0463.2002.100501.x. [DOI] [PubMed] [Google Scholar]
- 41.Ferreira DCD, Brito DG, Cavalcanti BN. Cytokine production from human primary teeth pulp fibroblasts stimulated by different pulpotomy agents. J Dent Child. 2009;76:194–198. [PubMed] [Google Scholar]
- 42.Zhang X, Yao Y, Kang H, Dong P. Comparison of expression of transforming growth factor-β1 in rat dental pulp during direct pulp capping with 2 capping agents. Shanghai J Stomatol. 2014;23:154–159. [PubMed] [Google Scholar]
- 43.Paranjpe A, Smoot T, Zhang H, Johnson JD. Direct contact with mineral trioxide aggregate activates and differentiates human dental pulp cells. J Endod. 2011;37:1691–1695. doi: 10.1016/j.joen.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paranjpe A, Zhang H, Johnson JD. Effects of mineral trioxide aggregate on human dental pulp cells after pulp-capping procedures. J Endod. 2010;36:1042–1047. doi: 10.1016/j.joen.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Lin Y, Zhao Y. An experimental study of expression changes of inducible nitric oxide synthase in rat dental pulp during direct pulp capping with two capping agents. Hua Xi Kou Qiang Yi Xue Za Zhi. 2011;29:420–423. [PubMed] [Google Scholar]
- 46.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang SC, Wu BC, Kao CT, Huang TH, Hung CJ, Shie MY. Role of the p38 pathway in mineral trioxide aggregate-induced cell viability and angiogenesis-related proteins of dental pulp cell in vitro. Int Endod J. 2014;48:236–245. doi: 10.1111/iej.12305. [DOI] [PubMed] [Google Scholar]
- 48.Asgary S, Nazarian H, Khojasteh A, Shokouhinejad N. Gene expression and cytokine release during odontogenic differentiation of human dental pulp stem cells induced by 2 endodontic biomaterials. J Endod. 2014;40:387–392. doi: 10.1016/j.joen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 49.Luo Z, Kohli MR, Yu Q, Kim S, Qu T, He Wx. Biodentine induces human dental pulp stem cell differentiation through mitogen-activated protein kinase and calcium-/calmodulin-dependent protein kinase II pathways. J Endod. 2014;40:937–942. doi: 10.1016/j.joen.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 50.Laurent P, Camps J, About I. BiodentineTM induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int Endod J. 2012;45:439–448. doi: 10.1111/j.1365-2591.2011.01995.x. [DOI] [PubMed] [Google Scholar]
- 51.Lutfi A, Kannan T, Fazliah M, Jamaruddin M, Saidi J. Proliferative activity of cells from remaining dental pulp in response to treatment with dental materials. Aust Dent J. 2010;55:79–85. doi: 10.1111/j.1834-7819.2009.01185.x. [DOI] [PubMed] [Google Scholar]
- 52.Dammaschke T, Stratmann U, Fischer R-J, Sagheri D, Schäfer E. Proliferation of rat molar pulp cells after direct pulp capping with dentine adhesive and calcium hydroxide. Clin Oral Investig. 2011;15:577–587. doi: 10.1007/s00784-010-0409-7. [DOI] [PubMed] [Google Scholar]
- 53.Tran-Hung L, Laurent P, Camps J, About I. Quantification of angiogenic growth factors released by human dental cells after injury. Arch Oral Biol. 2008;53:9–13. doi: 10.1016/j.archoralbio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Koliniotou-Koumpia E, Tziafas D. Pulpal responses following direct pulp capping of healthy dog teeth with dentine adhesive systems. J Dent. 2005;33:639–647. doi: 10.1016/j.jdent.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Guven EP, Yalvac ME, Sahin F, Yazici MM, Rizvanov AA, Bayirli G. Effect of dental materials calcium hydroxide-containing cement, mineral trioxide aggregate, and enamel matrix derivative on proliferation and differentiation of human tooth germ stem cells. J Endod. 2011;37:650–656. doi: 10.1016/j.joen.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Yuan K, Chen CL, Lin MT. Enamel matrix derivative exhibits angiogenic effect in vitro and in a murine model. J Clin Periodontol. 2003;30:732–738. doi: 10.1034/j.1600-051x.2003.00413.x. [DOI] [PubMed] [Google Scholar]
- 57.Kauvar AS, Thoma DS, Carnes DL, Cochran DL. In vivo angiogenic activity of enamel matrix derivative. J Periodontol. 2010;81:1196–1201. doi: 10.1902/jop.2010.090441. [DOI] [PubMed] [Google Scholar]
- 58.Schlueter SR, Carnes DL, Jr, Cochran DL. In vitro effects of enamel matrix derivative on microvascular cells. J Periodontol. 2007;78:141–151. doi: 10.1902/jop.2007.060111. [DOI] [PubMed] [Google Scholar]
- 59.Darwish SS, El Meguid SHA, Wahba NA, Mohamed AA, Chrzanowski W, Neel EAA. Root maturation and dentin-pulp response to enamel matrix derivative in pulpotomized permanent teeth. J Tissue Eng. 2014;5 doi: 10.1177/2041731414521707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olsson H, Davies J, Holst K, Schröder U, Petersson K. Dental pulp capping: effect of Emdogain Gel on experimentally exposed human pulps. Int Endod J. 2005;38:186–194. doi: 10.1111/j.1365-2591.2004.00932.x. [DOI] [PubMed] [Google Scholar]
- 61.Kumar H, Al-Ali M, Parashos P, Manton DJ. Management of 2 teeth diagnosed with dens invaginatus with regenerative endodontics and apexification in the same patient: a case report and review. J Endod. 2014;40:725–731. doi: 10.1016/j.joen.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 62.Law AS. Considerations for regeneration procedures. J Endod. 2013;39:S44–S56. doi: 10.1016/j.joen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 63.Geisler TM. Clinical considerations for regenerative endodontic procedures. Dent Clin North Am. 2012;56:603–626. doi: 10.1016/j.cden.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Hargreaves KM, Geisler T, Henry M, Wang Y. Regeneration potential of the young permanent tooth: what does the future hold? J Endod. 2008;34:S51–S56. doi: 10.1016/j.joen.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 65.Hargreaves KM, Diogenes A, Teixeira FB. Treatment options: biological basis of regenerative endodontic procedures. J Endod. 2013;39:S30–S43. doi: 10.1016/j.joen.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rutberg M, Spangberg E, Spangberg L. Evaluation of enhanced vascular permeability of vendodontic medicaments in vivo. J Endod. 1977;3:347–351. doi: 10.1016/S0099-2399(77)80064-2. [DOI] [PubMed] [Google Scholar]
- 67.Bennett LL, Rosenblum RS, Perlov C, Davidson JM, Barton RM, Nanney LB. An in vivo comparison of topical agents on wound repair. Plast Reconstr Surg. 2001;108:675–687. doi: 10.1097/00006534-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 68.Kozol RA, Gillies C, Elgebaly SA. Effects of sodium hypochlorite (Dakin’s solution) on cells of the wound module. Arch Surg. 1988;123:420–423. doi: 10.1001/archsurg.1988.01400280026004. [DOI] [PubMed] [Google Scholar]
- 69.Jaimes EA, Sweeney C, Raij L. Effects of the reactive oxygen species hydrogen peroxide and hypochlorite on endothelial nitric oxide production. Hypertension. 2001;38:877–883. [PubMed] [Google Scholar]
- 70.AlKahtani A, Alkahtany SM, Mahmood A, Elsafadi MA, Aldahmash AM, Anil S. Cytotoxicity of QMixTM endodontic irrigating solution on human bone marrow mesenchymal stem cells. BMC Oral Health. 2014;14:27. doi: 10.1186/1472-6831-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin DE, De Almeida JFA, Henry MA, Khaing ZZ, Schmidt CE, Teixeira FB, Diogenes A. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod. 2014;40:51–55. doi: 10.1016/j.joen.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 72.Trevino EG, Patwardhan AN, Henry MA, Perry G, Dybdal-Hargreaves N, Hargreaves KM, Diogenes A. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod. 2011;37:1109–1115. doi: 10.1016/j.joen.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 73.Reynolds K, Johnson J, Cohenca N. Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: a case report. Int Endod J. 2009;42:84–92. doi: 10.1111/j.1365-2591.2008.01467.x. [DOI] [PubMed] [Google Scholar]
- 74.Ring KC, Murray PE, Namerow KN, Kuttler S, Garcia-Godoy F. The comparison of the effect of endodontic irrigation on cell adherence to root canal dentin. J Endod. 2008;34:1474–1479. doi: 10.1016/j.joen.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Saghiri MA, Delvarani A, Mehrvarzfar P, Malganji G, Lotfi M, Dadresanfar B, Saghiri AM, Dadvand S. A study of the relation between erosion and microhardness of root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e29–34. doi: 10.1016/j.tripleo.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 76.Pang NS, Lee SJ, Kim E, Shin DM, Cho SW, Park W, Zhang X, Jung IY. Effect of EDTA on attachment and differentiation of dental pulp stem cells. J Endod. 2014;40:811–817. doi: 10.1016/j.joen.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 77.Galler KM, D’Souza RN, Federlin M, Cavender AC, Hartgerink JD, Hecker S, Schmalz G. Dentin conditioning codetermines cell fate in regenerative endodontics. J Endod. 2011;37:1536–1541. doi: 10.1016/j.joen.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 78.Zhao S, Sloan A, Murray P, Lumley P, Smith A. Ultrastructural localisation of TGF-β exposure in dentine by chemical treatment. Histochem J. 2000;32:489–494. doi: 10.1023/a:1004100518245. [DOI] [PubMed] [Google Scholar]
- 79.Begue-Kirn C, Smith AJ, Ruch JV, Wozney JM, Purchio A, Hartmann D, Lesot H. Effects of dentin proteins, transforming growth factor beta 1 (TGF beta 1) and bone morphogenetic protein 2 (BMP2) on the differentiation of odontoblast in vitro. Int J Dev Biol. 1992;36:491–503. [PubMed] [Google Scholar]
- 80.Windley W, III, Teixeira F, Levin L, Sigurdsson A, Trope M. Disinfection of immature teeth with a triple antibiotic paste. J Endod. 2005;31:439–443. doi: 10.1097/01.don.0000148143.80283.ea. [DOI] [PubMed] [Google Scholar]
- 81.Bottino M, Kamocki K, Yassen GH, Platt JA, Vail MM, Ehrlich Y, Spolnik KJ, Gregory RL. Bioactive nanofibrous scaffolds for regenerative endodontics. J Dent Res. 2013;92:963–969. doi: 10.1177/0022034513505770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bezwada P, Clark LA, Schneider S. Intrinsic cytotoxic effects of fluoroquinolones on human corneal keratocytes and endothelial cells. Curr Med Res Opin. 2007;24:419–424. doi: 10.1185/030079908x261005. [DOI] [PubMed] [Google Scholar]
- 83.Galley HF, Dhillon JK, Paterson RL, Webster NR. Effect of ciprofloxacin on the activation of the transcription factors nuclear factor B, activator protein-1 and nuclear factor interleukin-6, and interleukin-6 and interleukin-8 mRNA expression in a human endothelial cell line. Clin Sci (London) 2000;99:405–410. [PubMed] [Google Scholar]
- 84.Michalska M, Palatyńska-Ulatowska A, Palatyński A, Mirowski M, Kaplińska K, Nawrot-Modranka J, Lazarenkow A. Influence of antibiotic therapy on the level of selected angiogenic factors in patients with benign gynecologic tumors-preliminary report. Pharmazie. 2011;66:619–622. [PubMed] [Google Scholar]
- 85.Jung HJ, Seo I, Jha BK, Suh SI, Suh MH, Baek WK. Minocycline inhibits angiogenesis in vitro through the translational suppression of HIF-1α. Arch Biochem Biophys. 2014;545:74–82. doi: 10.1016/j.abb.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 86.Li CH, Liao PL, Yang YT, Huang SH, Lin CH, Cheng YW, Kang JJ. Minocycline accelerates hypoxia-inducible factor-1 alpha degradation and inhibits hypoxia-induced neovasculogenesis through prolyl hydroxylase, von Hippel–Lindau-dependent pathway. Arch Toxicol. 2014;88:659–671. doi: 10.1007/s00204-013-1175-5. [DOI] [PubMed] [Google Scholar]
- 87.Yao JS, Shen F, Young WL, Yang GY. Comparison of doxycycline and minocycline in the inhibition of VEGF-induced smooth muscle cell migration. Neurochem Int. 2007;50:524–530. doi: 10.1016/j.neuint.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arslan H, Capar I, Saygili G, Uysal B, Gok T, Ertas H, Topcuoglu HS. Efficacy of various irrigation protocols on the removal of triple antibiotic paste. Int Endod J. 2014;47:594–599. doi: 10.1111/iej.12194. [DOI] [PubMed] [Google Scholar]