Abstract
BACKGROUND
Interaction between maternal obesity, intrauterine environment and adverse clinical outcomes of newborns has been described.
METHODS
Using statewide birth certificate data, this retrospective, matched-control cohort study compared paired birth weights and complications of infants born to women before and after Roux-en-Y gastric bypass surgery (RYGB) and to matched obese non-operated women in several different groups. Women who had given birth to a child before and after RYGB (group 1; n = 295 matches) and women with pregnancies after RYGB (group 2; n = 764 matches) were matched to non-operated women based on age, body mass index (BMI) prior to both pregnancy and RYGB, mother’s race, year of mother/s birth, date of infant births and birth order. In addition, birth weights of 13 143 live births before and/or after RYGB of their mothers (n = 5819) were compared (group 3).
RESULTS
Odds ratios (ORs) for having a large-for-gestational-age (LGA) neonate were significantly less after RYGB than for non-surgical mothers: ORs for groups 1 and 2 were 0.19 (0.08–0.38) and 0.33 (0.21–0.51), respectively. In contrast, ORs in all three groups for risk of having a small for gestational age (SGA) neonate were greater for RYGB mothers compared to non-surgical mothers (ORs were 2.16 (1.00–5.04); 2.16 (1.43–3.32); and 2.25 (1.89–2.69), respectively). Neonatal complications were not different for group 1 RYGB and non-surgical women for the first pregnancy following RYGB. Pregnancy-induced hypertension and gestational diabetes were significantly lower for the first pregnancy of mothers following RYGB compared to matched pregnancies of non-surgical mothers.
CONCLUSION
Women who had undergone RYGB not only had lower risk for having an LGA neonate compared to BMI-matched mothers, but also had significantly higher risk for delivering an SGA neonate following RYGB. RYGB women were less likely than non-operated women to have pregnancy-related hypertension and diabetes.
INTRODUCTION
Long term, sequelae of obesity include an increased risk for female infertility, maternal and perinatal pregnancy complications such as miscarriage, cesarean section (C-section), gestational diabetes, hypertension and fetal macrosomia.1–6 Increased pregnancy-related health risks are especially apparent among severely obese women.5,6 Maternal overweight and obesity and/or excess weight gain during pregnancy have also been associated with increased obesity and metabolic risk in offspring.7–12
Severely obese women who have undergone bariatric surgery represent an ideal population to appraise whether or not pre-pregnancy voluntary weight loss reduces maternal pregnancy complications, macrosomia and other fetal complications. Bariatric surgery results in significant and sustained weight loss;13,14 however, during the period of major weight loss (within the first 12 to 18 months following surgery) and perhaps thereafter, food intake restriction and/or malabsorption may inhibit maternal nutrient intake and compromise fetal growth.4,15 In addition, offspring of mothers exposed to malnutrition have increased risk of cardiovascular disease and type 2 diabetes.16 Therefore, greater understanding of the benefits and risks associated with pregnancy following bariatric surgery is clinically important. It is especially relevant in light of the increasing number of women who have become pregnant after having undergone bariatric surgery, as bariatric surgery is increasing in popularity.17–19 Approximately 80% of all bariatric surgeries are performed on women;20,21 and a significant percentage undertaken during the female’s reproductive years.4
This study builds upon previously reported investigations of pregnancy and bariatric surgery, which have employed wide variation in methodological approaches.22–33 Using a large cohort of post Roux-en-Y gastric bypass surgery (post-RYGB) women and a unique and representative population-based, non-surgical matched cohort, the aim of this study was to further test the association between mothers’ body mass index (BMI), the newborns’ gestational age and birth weight and pregnancy complications before and following RYGB.
MATERIALS AND METHODS
Study subjects and groups
Two primary study populations were included in this study, surgical patients and non-surgical subjects. The surgical cohort consisted of a consecutive series of 5819 female Utah residents who underwent RYGB between 1979 and 2011 (performed by six bariatric surgeons representing a single Utah surgical practice, Rocky Mountain Associated Physicians, Inc.) and their live births (n = 13 112). These surgical patients were linked with the Utah Population Database (UPDB), which holds Utah records for nearly eight million individuals connected from various sources, including genealogy records, inpatient hospital and ambulatory surgery records, driver’s license records and birth and death certificates.34 Once linked, births of all surgical women were ascertained both before and after RYGB for the purposes of matching and statistical analyses. Non-surgical severely obese controls were selected from Utah females (n = 525 653) and their live births (n =1 071 767) and whose data were also part of the UPDB (see Supplementary Figure 1).
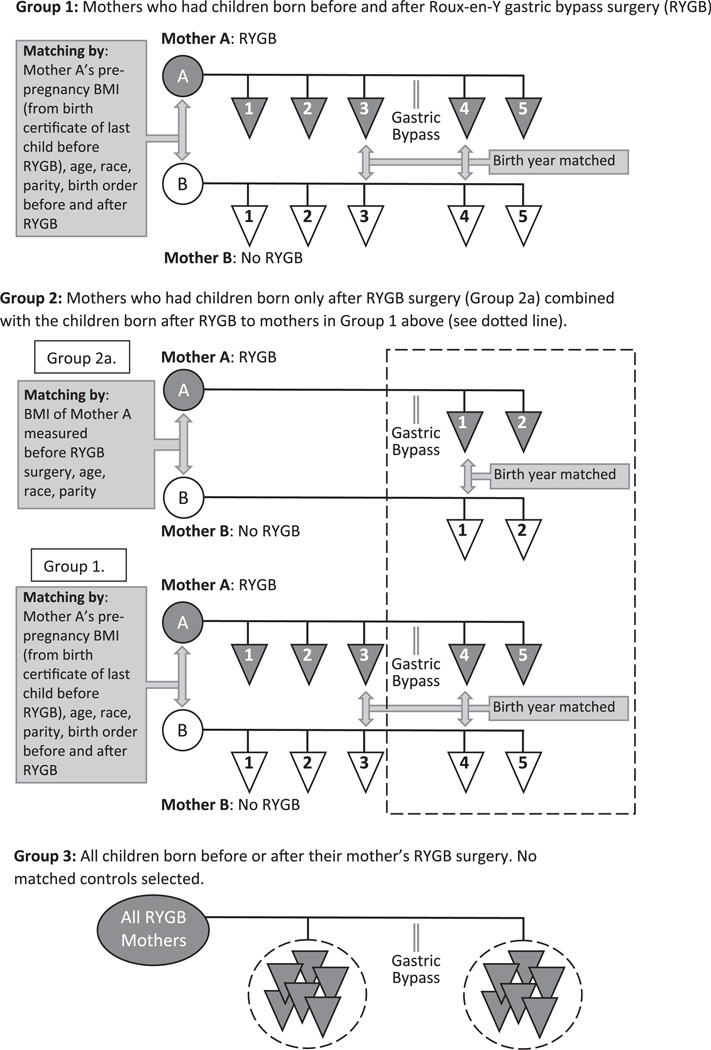
A primary study group was defined using extensive matching criteria on both pre- and post-surgical variables (Figure 1). This matching allowed control for the age, race, BMI, and parity of the mother and the birth order and birth year of the newborns in order to prevent confounding of the results. Because of the strict matching criteria, this group was limited in sample size. We then added a second group with matched women who had newborns only after surgery. Finally, we added a third group who had newborns either before or after RYGB surgery without any matched women to further increase the sample size and to compare with the other two groups. Group 1 consisted of RYGB mothers who had births both before and after RYGB. Using the UPDB birth certificate records, non-surgery women and their respective fertility data were matched one-to-one to these RYGB mothers and births. The following matching criteria were used: mother’s birth year; mother’s race (white/non-white); birth year for the last neonate born before the mother’s RYGB and birth year for the first neonate born after the mother’s RYGB; birth order for the two deliveries; total parity; birth multiplicity (that is, singletons and twins); and pre-pregnancy BMI (kg m−2) on the birth certificate of the RYGB mother for the birth just prior to her RYGB. Categories used for matching pre-pregnancy BMI were: 18.5–24.9, 25.0–29.9, 30.0–34.9, 35.0–39.9, 40–49.9 and ⩾ 50. Group 1 had the advantage of allowing comparisons of neonates of RYGB mothers before and after RYGB with neonates of control mothers and also paired comparisons of neonates born to the same mother before and after their mothers’ RYGB.
Figure 1.
Schematic description of groups 1, 2 and 3 used for study analysis. For groups 1 and 2, matching schemes are also depicted.
Study group 2 included all of the mothers from group 1 plus mothers who only had a pregnancy after RYGB and where a matched mother was available (groups 2a and 1; Figure 1). Group 2 allowed a greater number of post-surgical newborns to be included. Birth certificate data of the mother and her live birth associated with the first pregnancy that occurred after her RYGB were matched with the data of a non-surgery mother and her neonate as was done for group 1. Since group 2a had their first birth following RYGB, there was no pre-surgical birth certificate to obtain a presurgical BMI (kg m−2) to be used for matching. Therefore, we used the mother’s BMI measured just prior to her RYGB at the surgeon’s office for matching with the non-surgical mother’s pre-pregnancy birth certificate BMI.
Group 3 did not involve matching, thereby greatly increasing the sample size (Figure 1). Rather, this group included all live births of all RYGB women that had occurred prior to their surgery compared to all live births of all RYGB women following their RYGB.
Data extraction
Pre-pregnancy height and weight was not reported on birth certificates in Utah prior to 1989 and as a result, non-surgical women could only be selected from births occurring after 1989. When the RYGB mother’s newborn was a twin or triplet, all newborns in the set of multiple births were used, but they were required to match to corresponding non-surgery multiple births. The initial matching attempt of RYGB- to non-surgical-related subjects, resulted in 97% of RYGB women in group 1 successfully matched for categories other than age. Another 158 women did not match within ± 1 year, but were matched after relaxing the age criteria to ± 3 years. In addition to age, changes in criteria included combining the two BMI groups of 40–49.9 and ⩾50 (four additional matches) and, grouping parity and birth order into one group if ⩾5 (six additional matches).
Following the matching of patients and their births with non-surgery mothers and their births, pregnancy-related information and complications were extracted from the respective birth certificates. Data on birth weight, gestational age at birth, Apgar scores at 1 and 5 min, C-section deliveries, use of forceps or vacuum pump deliveries, chronic hypertension, pregnancy-induced hypertension, pre-existing type 2 diabetes and gestational diabetes were obtained for all pregnancies/births. Additional maternal information extracted from the birth certificate included: self-reported weight gain during pregnancy, smoker (yes or no) and self-reported maternal height and weight prior to becoming pregnant. Neonate complications included respiratory complications, sepsis or infection, congenital anomalies, birth injury, jaundice, feeding difficulties and intraventricular hemorrhage. Two sets of criteria were used to clinically evaluate the birth weight of newborns and are described in the Supplementary Material.35,36
Statistical analysis
A t-test was used to assess how well the RYGB subgroups were matched with the non-surgical groups (that is, age of mothers, birth year of babies, and mothers’ BMI) and the variables that were used for matching were presented as means ± s.d. A chi-square test was used to compare frequency differences between the RYGB and non-surgical women with regard to maternal race (white/non-white), ethnic group (Hispanic: yes/no) and smoking. For matched analyses of groups 1 and 2, conditional logistic regression was used to determine the odds ratios (ORs) and 95% confidence intervals for birth weight differences between the two exposure groups (with and without adjustment for gestational age at birth), gestational age at birth and pregnancy complications. Covariates in the model included concordance of sex of the neonate and, when comparing the post-surgical variables, the study group differences of the pre-surgical neonate. For group 3, which did not involve matching, unconditional logistic regression, adjusted for sex of neonate, mother’s age at delivery, number of previously born children (that is, birth order), mother’s race and repeated measures for multiple pregnancies was used to test for birth weight and gestational age at birth. Significance level was set at P < 0.05 and the study data were analyzed using SAS 9.3 (SAS, Inc., Cary, NC, USA).
RESULTS
Table 1 details the number of matched mothers and live births for groups 1 and 2 and non-matched RYGB mothers and live births for group 3. When comparing the neonates born to matched surgical and non-surgical women in group 1 before RYGB, there were no differences in OR for birth weight or gestational weeks categories (Table 2). However in group 1, the first births following RYGB surgery were significantly less likely to be born >4000 g (OR 0.28, 95% CI 0.11–0.61; P = 0.003) and had a lower risk to be born large for gestational age (LGA; OR 0.19, 95% CI 0.08–0.38; P < 0.0001) compared to neonates of non-surgical mothers. There was also a trend for neonates born following RYGB to have a greater risk for being born small for gestational age (SGA; OR 2.16, 95% CI 1.00– 5.04; P = 0.059) or born with a birth weight < 2500 g (OR 1.69, 95% CI 0.81–3.70; P = 0.17). Because there is greater risk of complications for neonates born with a weight <1500 g compared with 1500–2500 g, we reran the birth weight analyses using these two subgroups. However, the ORs were similar in the two subgroups, the number of neonates <1500 g was small, and the results were consistent with the combined group ORs shown in Table 2. Group 1 RYGB mothers had significantly greater risk for having a forceps or vacuum delivery and pregnancy-induced hypertension compared to non-surgical mothers for the pregnancy prior to surgery (OR 2.54, 95% CI 1.30–5.26; P = 0.005) and (OR 2.2, 95% CI 1.14–4.50; P = 0.016). However, for the first pregnancy following RYGB, the surgical mothers demonstrated significantly lower pregnancy-induced hypertension (OR 0.31, 95% CI 0.14–0.65; P = 0.0009) and gestational diabetes (OR 0.33, 95% CI 0.13–0.77; P = 0.005).
Table 1.
Maternal and pregnancy-related characteristics for groups 1–3
| Variables | Group 1 |
Group 2 |
Group 3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before RYGB surgery |
After RYGB surgery |
|||||||||||
| RYGB women |
Non- surgery women |
P-value | RYGB women |
Non- surgery women |
P-value | RYGB women |
Non- surgery women |
P-value | RYGB women before surgery |
RYGB women after surgery |
P-value | |
| Mothers (N = 295) Neonates (N = 295) |
Mothers (N = 295) Neonates (N = 295) |
Mothers (N = 295) Neonates (N = 295) |
Mothers (N = 295) Neonates (N = 295) |
Mothers (N = 764) Neonates (N = 764) |
Mothers (N = 764) Neonates (N = 764) |
Mothers (N = 4931) Neonates (N = 10447) |
Mothers (N = 1574) Neonates (H = 2666) |
|||||
| Maternal age at delivery, years | 25.5 ± 4.2 | 25.6 ± 4.2 | 0.53 | 31.0 ± 4.2 | 31.0 ± 4.2 | 0.53 | 27.6 ± 5.2 | 27.5 ± 5.2 | 0.19 | 26.4 ± 5.2 | 30.4 ± 5.3 | < 0.0001 |
| Maternal BMI, pre-pregnancy, kg m−2 | 36.1 ± 6.5 | 35.1 ± 6.2 | < 0.0001 | 27.6 ± 5.5 | 36.7 ± 7.7 | < 0.0001 | 28.6 ± 5.7 | 40.0 ± 6.8 | < 0.0001 | 34.7 ± 7.4, N = 3465a | 29.5 + 6.2, N = 2209a | < 0.0001 |
| Maternal race, white, n (%) | 278 (94) | 278 (94) | NA | 278 (94) | 278 (94) | NA | 730 (96) | 730 (96) | NA | 9940 (96.6) | 2469 (95.4) | 0.004 |
| Maternal, Hispanic, yes; n (%) | 32 (11) | 32 (11) | NA | 32 (11) | 32 (11) | NA | 59(8) | 59(8) | NA | 560 (5.4) | 149 (5.8) | 0.53 |
| Years between delivery and surgery | 2.8 ± 2.3 | 2.7 ± 2.4 | NA | 2.6 ± 1.5 | 2.7 ± 1.8 | NA | 4.2 ± 3.2 | 4.2 ± 3.2 | NA | 11.1 ± 7.3 | 4.8 ± 3.7 | NA |
| Total parity, n | 3.3 ±1.2 | 3.3 ± 1.2 | NA | 3.3 ± 1.2 | 3.3 ± 1.2 | NA | 2.5 ± 1.3 | 2.5 ±1.3 | NA | 3.8 ± 1.7 | 3.2 ± 1.6 | < 0.0001 |
| Sets of multiple births, n (%) | 1 ( < 1), 1 twin set |
1 (< 1, 1 twin set |
NA | 6 (2), 6 twin sets |
6 (2), 6 twin sets |
NA | 6 (< 1), 6 twin sets |
6 (< 1), 6 twin sets |
NA | 146b (2.8) | 71c (5.5) | < 0.0001 |
| Birth order of pregnancy just before surgery, n | 1.94 ± 1.06 | 1.96 ± 1.14 | 0.94 | 2.99 ± 1.07 | 3.02 ± 1.16 | 0.94 | 1 | 1 | NA | 2.64 ± 1.62 | 2.56 ± 1.51 | NA |
| Apgar 1 min | 7.6 ± 1.3 | 7.6 ± 1.4 | 0.62 | 7.6 ± 1.5 | 7.7 ± 1.4 | 0.53 | 7.7 ± 1.4 | 7.4 ± 1.7 | 0.0001 | 7.5 ± 1.4 | 7.6 ± 1.3 | 0.009 |
| Apgar 5 min | 8.8 ± 0.8 | 8.8 ± 0.6 | 0.60 | 8.7 ± 1.0 | 8.8 ± 0.7 | 0.32 | 8.8 ± 0.9 | 8.7 ± 1.0 | 0.025 | 8.8 ± 0.9 | 8.8 ± 0.8 | 0.18 |
| Maternal weight gain, pounds | 28.8 ± 16.9 | 22.9 ± 15.4 | < 0.0001 | 27.4 ± 15.3 | 22.0 ± 15.7 | < 0.0001 | 28.8 ± 16.0 | 21.7 ± 15.6 | < 0.0001 | 27.7 ± 17.9, N=3738a | 27.5 + 15.4, N = 2202a | 0.64 |
| Smoke, yes; n (%) | 18 (6.1) | 27 (9.2) | 0.59 | 20 (6.8) | 33 (11.2) | 0.86 | 62 (8.1) | 70 (9.2) | 0.88 | 313 (3.0) | 194 (7.5) | < 0.0001 |
Abbreviations: BMI, body mass index; NA, not applicable; RYGB, Roux-en-Y gastric bypass surgery. See Materials and Methods section for a description of what statistical model was used for each group to calculate P-values.
Birth certificates included maternal pre-pregnancy BMI and weight gain only after 1989. As a result, the total N for mothers with birth certificate recorded BMI is less than the total N for mothers identified in the table heading.
142 Twin sets, 3 triplet sets and 1 quadruplet set.
68 Twin sets, 1 triplet set and 2 quadruplet sets.
Table 2.
Group 1: birth outcomes and complications of Roux-en-Y gastric bypass mothers, matched control mothers and their newborns
| Outcomes | Births before RYGB | Births after RYGB | ||||||
|---|---|---|---|---|---|---|---|---|
| RYGB, n = 295 | Non-operated, n = 295 | Adjusted ORa (95% CI) | P-value | RYGB, n = 295 | Non-operated, n = 295 | Adjusted OR3 (95% CI) | P-value | |
| Gestational age, weeks | 38.82 ± 2.16 | 39.16 ± 1.86 | — | 0.040b | 38.07 ± 2.23 | 38.27 ± 2.36 | — | 0.25b |
| > 42 Weeks, n (%) | 20 (6.8) | 23 (7.8) | 0.96(0.51–1.77) | 0.88 | 8 (2.7) | 16(5.4) | 0.58 (0.22–1.45) | 0.25 |
| 37–41 Weeks, n (%) | 250 (84.8) | 259 (87.7) | 1 | — | 246 (83.4) | 241 (81.7) | 1 | — |
| < 37 Weeks, n (%) | 25 (8.5) | 13 (4.4) | 1.84 (0.92–3.84) | 0.091 | 41 (13.9) | 38 (12.9) | 0.96(0.53–1.76) | 0.90 |
| Birth weight, g | 3392 ± 572 | 3452 ± 486 | — | 0.18b | 310 ± 548 | 3380 ± 623 | — | < 0.0001b |
| >4000 g, n (%) | 29 (9.8) | 36(12.2) | 0.73 (0.42–1.26) | 0.27 | 10 (3.4) | 34(11.5) | 0.28 (0.11–0.61) | 0.003 |
| 2500–4000 g, n (%) | 252 (85.4) | 251 (85.1) | 1 | — | 255 (86.4) | 244 (82.7) | 1 | — |
| < 2500 g, n (%) | 14 (4.7) | 8 (2.7) | 2.20 (0.87–6.26) | 0.11 | 30 (10.2) | 17(5.8) | 1.69 (0.81–3.70) | 0.17 |
| LGA (⩾90th %), n (%) | 35 (11.9) | 32 (10.8) | 1.34 (0.76–2.39) | 0.31 | 10 (3.4) | 48 (16.3) | 0.19 (0.08–0.38) | < 0.0001 |
| AGA (11–89%), n (%) | 244 (82.7) | 246 (83.4) | 1 | — | 259 (87.8) | 235 (79.7) | 1 | — |
| SGA (⩽10th %), n (%) | 16 (5.4) | 17(5.7) | 0.81 (0.37–1.72) | 0.58 | 26 (8.8) | 12(4.1) | 2.16(1.00–5.04) | 0.059 |
| Cesarean section deliveries, n (%) | 91 (30.8) | 91 (30.8) | 1.0 (0.68–1.47) | 1.0 | 106 (35.9) | 122 (41.4) | 0.79 (0.55–1.12) | 0.19 |
| Use of forceps or vacuum pump, n (%) | 44 (14.9) | 24(8.1) | 2.54(1.30–5.26) | 0.005 | 12(4.1) | 9(3.1) | 1.38 (0.50–3.94) | 0.65 |
| Pregnancy-induced hypertension, n (%) | 41 (13.9) | 24(8.1) | 2.2 (1.14–4.50) | 0.016 | 13 (4.4) | 35 (11.9) | 0.31 (0.14–0.65) | 0.0009 |
| Chronic hypertension, n (%) | 8 (2.7) | 7 (2.4) | 1.2 (0.34–4.2) | 1.0 | 5(1.7) | 14(4.7) | 0.31 (0.07–0.99) | 0.049 |
| Gestational diabetes, n (%) | 10 (3.4) | 16(5.4) | 0.60 (0.23–1.46) | 0.31 | 10 (3.4) | 26 (8.8) | 0.33 (0.13–0.77) | 0.005 |
| Pre-existing diabetes, n (%); non-insulin using | 5 (1.7) | 2 (0.7) | 0.25 (0.41–26.3) | 0.45 | 2 (0.7) | 3(0.1) | 0.67 (0.06–5.8) | 1.0 |
Abbreviations: AGA, appropriate for gestational age; CI, confidence interval; LGA, large for gestational age; OR, odds ratio; RYGB, Roux-en-Y gastric bypass surgery; SGA, small for gestational age.
See Materials and Methods section for the conditional models used.
Paired t-test.
Post-RYGB pregnancies in group 2 were significantly less likely to extend beyond 42 weeks gestation compared to pregnancies of non-surgical mothers (Table 3; OR 0.53, 95% CI 0.30–0.91; P = 0.024). Pre-term births were not different between the two groups. In addition to a significantly smaller mean birth weight for neonates of surgical mothers compared to non-surgical born neonates (3092 ± 568 vs 3292 ± 696 g; P < 0.0001), neonates born to surgical mothers also had a significantly lower risk for a birth weight >4000 g or being born LGA (P < 0.0001). However, the risk for having an SGA birth was significantly greater for the neonates born to RYGB surgical mothers compared to non-surgical born neonates (OR 2.16, 95% CI 1.43–3.32; P = 0.0003).
Table 3.
Group 2: birth weight and gestational age of infants from Roux-en-Y gastric bypass mothers’ first neonate born after surgery, compared to infants born to matched non-surgical mothers
| Neonatal outcomes | Births after RYGB |
|||
|---|---|---|---|---|
| RYGB, n = 764a | Non-operated, n = 764a | Adjusted ORb (95% CI) | P-value | |
| Gestational age, weeks | 38.38 ± 2.34 | 38.31± 2.92 | 0.057b | |
| > 42 Weeks, n (%) | 15 (2.0) | 27 (3.5) | 0.53 (0.30–0.91) | 0.024 |
| 37–41 Weeks, n (%) | 650 (85.4) | 626 (82.3) | 1 | — |
| < 37 Weeks, n (%) | 96 (12.6) | 108 (14.2) | 0.81 (0.59–1.13) | 0.22 |
| Birth weight, g | 3092 ± 568 | 3292 ± 696 | < 0.0001b | |
| > 4000g, n (%) | 30 (3.9) | 83 (10.9) | 0.33 (0.21–0.52) | < 0.0001 |
| 2500–4000 g, n (%) | 652 (85.4) | 611 (80.0) | 1 | — |
| < 2500, n (%) | 82 (10.7) | 70 (9.1) | 1.13 (0.78–1.64) | 0.51 |
| LGA (⩾90th %), n (%) | 33 (4.3) | 99 (13.0) | 0.33 (0.21–0.51) | < 0.0001 |
| AGA (11–89%), n (%) | 636 (83.6) | 619 (81.3) | 1 | — |
| SGA (⩽10th %), n | 92 (12.1) | 43 (5.7) | 2.16 (1.43–3.32) | 0.0003 |
Abbreviations: AGA, appropriate for gestational age; CI, confidence interval; LGA, large for gestational age; OR, odds ratio; RYGB, Roux-en-Y gastric bypass surgery; SGA, small for gestational age.
Gestational age data were missing for three neonates born to RYGB mothers and three neonates born to non-surgical mothers. As a result, the total N for gestational age data was 761.
Paired t-test.
Group 3 results (Table 4), contrasting neonates born before surgery to those born following RYGB, showed that while neonates born after RYGB were at a significantly lower risk for gestational age >42 weeks (OR 0.23, 95% CI 0.19–0.28; P < 0.0001) compared to pre-surgical neonates, the post-surgery neonate deliveries were at a significantly greater risk to occur < 37 weeks (OR 1.93, 95% CI 1.62–2.31; P < 0.0001) compared to pre-surgical deliveries. The post-surgical neonates were significantly less likely to weigh > 4000 g than the pre-surgical neonates compared to the referent neonate weight of 2500–4000 g (OR 0.19, 95% CI 0.15–0.24; P < 0.0001). However, there was a greater risk for post-surgery neonates to have low birth weights (< 2500) than the pre-RYGB surgical neonates when compared to the before and following surgery referent weight neonates (OR 2.63, 95% CI 2.17–3.18; P < 0.0001).
Table 4.
Group 3: birth weight and gestational age of all neonates born before or after surgery of all Roux-en-Y gastric bypass mothers; odds ratios of outcomes after surgery compared with before surgery
| Neonatal outcomes | RYGB before, n = 10 447 | RYGB after, n = 2666 | Adjusted ORa (95% CI) | P-value |
|---|---|---|---|---|
| Gestational age, weeks | 39.15 ± 2.22 | 38.26 ± 2.44 | < 0.0001b | |
| > 42 Weeks, n (%) | 1875 (18.0) | 127 (4.8) | 0.23 (0.19–0.28) | < 0.0001 |
| 37–41 Weeks, n (%) | 8021 (76.8) | 2232 (83.7) | 1 | — |
| < 37 Weeks, n (%) | 551 (5.3) | 307 (11.5) | 1.93 (1.62–2.31) | < 0.0001 |
| Birth weight, g | 3482 ± 598 | 3067 ± 592 | < 0.0001b | |
| > 4000g, n (%) | 1676 (16.0) | 95 (3.6) | 0.19 (0.15–0.24) | < 0.0001 |
| 2500–4000 g, n (%) | 8309 (79.5) | 2201 (82.6) | 1 | — |
| < 2500g, n (%) | 462 (4.4) | 370 (13.9) | 2.63 (2.17–3.18) | < 0.0001 |
| LGA (90th %), n (%) | 1892 (17.9) | 268 (1.7) | 0.22 (0.18–0.27) | < 0.0001 |
| AGA (11–89%), n (%) | 8121 (76.8) | 2232 (83.7) | 1 | — |
| SGA (10th %), n (%) | 551 (5.3) | 307 (11.5) | 2.25 (1.89–2.69) | < 0.0001 |
Abbreviations: AGA, appropriate for gestational age; CI, confidence interval; LGA, large for gestational age; OR, odds ratio; RYGB, Roux-en-Y gastric bypass surgery; SGA, small for gestational age. No repeated measures adjustment for multiple pregnancies due to small sample size.
Logistic regression adjusted for sex of neonate, mother’s age at delivery, number of previously born children (i.e., birth order), mother’s race (white or non-white) and repeated measures for multiple pregnancies.
Paired t-test.
We investigated further possible underlying mechanisms for increased incidence of SGA neonates born to post-RYGB mothers in group 3, by assessing pregnancy weight gain for all women in all groups, stratifying by LGA, AGA and SGA live births (Table 5). Although mothers giving birth to LGA neonates prior to their RYGB had significantly greater weight gain compared to weight gain of pre-RYGB mothers giving birth to AGA neonates (P = 0.0008), no other associations were significant. In groups 1 and 2, pregnancy weight gain in mothers after RYGB was lower when giving birth to SGA neonates compared to pregnancy weight gain of post-RYGB surgery mothers having AGA neonates, but these differences were not significant (P = 0.07 and P = 0.08, respectively). We also tested for the relationship of the RYGB mothers’ pre-pregnancy BMI on the LGA, AGA and SGA status of their newborns. There were no significant differences among the pre-pregnancy BMI for either pre-surgery or post-surgery LGA vs AGA or SGA vs AGA.
Table 5.
Pregnancy weight gain for RYGB women stratified by LGA, AGA and SGA live births
| Newborn classification | Group 1: RYGB women who had pregnancies both before and after RYGB surgery |
Group 2: all pregnancies following RYGB surgery |
Group 3: all RYGB women who had pregnancies before or after RYGB surgery |
||
|---|---|---|---|---|---|
| Weight gain before RYGB surgery, lbs N = 295 |
Weight gain after RYGB surgery, lbs N = 295 |
Weight gain after RYGB surgery, lbs N = 764 |
Weight gain before RYGB surgery, lbs N = 3808 |
Weight gain after RYGB surgery, lbs N = 2270 |
|
| Mothers giving birth to LGA neonatesa |
38.1 ± 2.8, P = 0.0008 | 32.8 ± 4.9, P = 0.31 | 26.1 ± 2.7, P = 0.26 | 30.9 ± 0.8, P < 0.0001 | 26.9 ± 2.0, P = 0.51 |
| Mothers giving birth to AGA neonates |
27.8 ± 1.1 | 27.7 ± 0.9 | 29.3 ± 0.6 | 27.4 ± 0.4 | 28.3 ± 0.4 |
| Mothers giving birth to SGA neonatesa |
22.7 ± 4.2, P = 0.23 | 22.0 ± 3.0, P = 0.07 | 26.1 ± 1.7, P = 0.08 | 25.7 ± 1.4, P = 0.23 | 27.3±1.1, P = 0.39 |
Abbreviations: AGA, appropriate for gestational age; LGA, large for gestational age; RYGB, Roux-en-Y gastric bypass surgery; SGA, small for gestational age.
P-values from tests comparing either LGA or SGA neonates vs AGA neonates.
A separate analysis to remove possible selection biases was conducted where only the infants born before and after to the same RYGB mother of group 1 were compared (that is, no matched control mothers included). If the same mother in group 1 had a high-birth-weight baby (> 4000 g) or an LGA baby for her pregnancy just prior to her RYGB then the ORs for her first neonate following RYGB being > 4000 g or LGA were 0.25, 95% CI 0.09– 0.57; P = 0.002 and 0.17, 95% CI 0.06–0.41; P = 0.003, respectively (data not shown). Further, for group 1 RYGB mothers who had an SGA or low-birth-weight baby (< 2500 g) for the pre-RYGB pregnancy, the OR for their first neonate following RYGB being < 2500 g or SGA was 2.90, 95% CI 1.35–6.92; P =0.010 and 1.70, 95% CI 0.87–3.47; P = 0.13, respectively.
Although the number of C-sections were fewer in the post-RYGB surgical mothers compared with the matched controls, the difference was not significant (OR 0.79, 95% CI 0.55–1.12; Table 2). One- and five-minute Apgar scores did not differ between RYGB and non-surgical deliveries for group 1 either pre-surgery or post-surgery (Table 1). However, significantly better 1- and 5-min Apgar scores were seen in group 2 for the newborns of post-RYGB mothers compared to newborns of non-surgery mothers (7.7 ± 1.4 vs 7.4 ± 1.7, P < 0.001 and 8.8 ± 0.9.4 vs 8.7 ± 1.0, P < 0.025 for Apgar 1 and 5 min, respectively). The 1-min Apgar score was significantly worse in group 3 for the newborns of RYGB surgical mothers (7.5 ± 1.4 vs 7.6 ± 1.3; P = 0.009).
There were no significant differences in neonatal complications for the first pregnancy of mothers following RYGB compared to matched pregnancies of the non-surgery mothers for group 1 (Supplementary Table 1). The change in fetal deaths (before and after RYGB) among the RYGB and non-surgery groups for group 1 did not differ. Prior to RYGB, there were two and three fetal deaths among the RYGB and non-operated groups, respectively, and following RYGB, there were one and two fetal deaths among the RYGB and non-operated groups, respectively (Supplementary Table 1).
Group 1 fetal deaths are also discussed in the Supplementary Material.
DISCUSSION
In view of the increased number of bariatric surgical procedures now undertaken in the US, with nearly 80% of all surgeries performed on females, there is an important clinical need to understand potential benefits and risks of pregnancy to women and their children following bariatric surgery. This study of women who had undergone RYGB found that following surgery the risk of giving birth to a LGA neonate is significantly lower when compared to neonates born to matched, non-operated mothers. However, we also found that post-RYGB women were at a greater risk to deliver an SGA neonate, even though women post-RYGB had a greater pregnancy weight gain. The study also demonstrated that mothers who had RYGB were significantly less likely to have pregnancy-induced hypertension or gestational diabetes and that there were no differences in neonatal-related complications for their first pregnancy following RYGB compared to neonates born to the matched non-surgical mothers.
In a recently published systematic review and meta-analysis of 45 studies comparing pre-pregnancy normal-weight mothers to pre-pregnancy obese mothers, there was a reported increased risk for LGA in the obese mothers (OR 2.08, 95% CI 1.95–2.33), with similar ORs for high body weight.11 The incidence of LGA for live births in the US in 2008 was 6.6%.37 The incidence of LGA reported among the Utah RYGB patients prior to their having had surgery was 11.9% (35/295; LGA neonates/total neonates), and is somewhat less than the 16.4% LGA births reported by Getahun et al.,38 in a longitudinal study of over 12 000 live births born to obese women.
In addition to maternal complications related to LGA, infants born with the diagnosis of LGA are at a greater risk for a wide variety of comorbidities,39 including an increased metabolic risk profile in childhood,11,12,40 during adolescence41,42 and into adulthood.43 Thompson et al., tracking the National Growth and Health Study population to adulthood, reported children with reported obesity onset prior to age 12 years were 11 to 30 times more likely to present with obesity as adults. In addition to increased obesity risk, the overweight/obese National Growth and Health Study children had a greater incidence of hypertension, hyperlipidemia and metabolic syndrome as adults.44
Studies have reported that even a minimal reduction in an obese woman’s BMI may result in improved health status as well as lower risk for pregnancy-related complications,23,45 and that reduction in pre-pregnancy BMI can reduce the risk for LGA.38,46 A longitudinal retrospective study by Getahun et al. examined the first two consecutive singleton live births (n = 146 227) to determine the association between pre-pregnancy BMI and LGA. When a mother’s first pre-pregnancy BMI was in the obese range and subsequently reduced to the overweight or normal pre-pregnancy BMI for the second pregnancy, the overall risk of her having a birth that was LGA was reduced.38
If minimal weight reduction has been shown to improve pregnancy-related outcomes, then it should follow that weight loss from bariatric surgery would also result in reduced pregnancy complications for the mother and the newborn. We found a significantly lower risk (P < 0.0001) for high-birth-weight neonates (that is, >4000 g) and for LGA neonates comparing pregnancies of women who had undergone RYGB with matched pregnancies of non-operated women (groups 1 and 2), and when outcomes for live birth weights were compared between pregnancies that occurred before and after RYGB (group 3). This represents a 67– 84% reduction in risk for LGA births among the post-RYGB mothers, robust to our several different approaches to select matched mothers. A study by Kjaer et al.22 compared singleton deliveries following bariatric surgery (n = 355 women with at least one live birth following surgery; 83.5% RYGB surgical procedures) to non-bariatric surgical women, matched for pre-pregnancy BMI, maternal age and date of delivery. They found a 69% reduction in LGA risk.
Interestingly, in group 1 of our study, there were no significant differences in neonatal-related complications (listed in Supplementary Table 1) between the first babies born to mothers following their RYGB compared to the babies of non-operated mothers. There was a significantly lower risk, however, for a mother developing hypertension or gestational diabetes during her first pregnancy following RYGB compared to the pregnancies of non-surgical mothers.
We also found a greater risk for SGA births for post-RYGB pregnancies significant for groups 2 and 3 and borderline significant for group 1 (P = 0.054). The ORs for SGA of 2.20, 2.16 and 2.25 between post-surgical neonates and BMI-matched non-surgical neonates for groups 1, 2 and 3, respectively, of the Utah study are very similar to the OR of 2.3 reported by Kjaer et al.22 who compared the first pregnancy following bariatric surgery of 339 women BMI-matched to non-surgery mothers. SGA birth has been shown to be associated with a greater future risk for both diabetes and the metabolic syndrome for these babies.47,48 Many SGA births appear to be associated with intrauterine growth restriction, a condition that results from the fetus failing to receive adequate nutrients and oxygen for appropriate growth processes.49
RYGB results in an anatomical bypassing of all but a small pouch of the stomach, the entire duodenum and the proximal part of the jejunum resulting in the potential risk for nutritional deficiencies of the mother and the fetus. However, there were no significant differences between pregnancy weight gain of mothers who had SGA neonates compared to pregnancy weight gain of mothers delivering AGA babies. Long-term outcomes of SGA-born neonates after bariatric surgery have not been described. However, a study by Smith et al.50 that followed 111 siblings (age 2.5–26 years) who were born before and following maternal bariatric surgery (biliopancreatic diversion, a malabsorptive procedure) reported the children born following the surgery had a more favorable metabolic risk when compared to the children born before surgery. Further, Guenard et al.51 analyzed the impact of maternal weight loss resulting from biliopancreatic diversion by analyzing differential methylation in glucoregulatory genes (that is, potential pathways involved with improved cardiometabolic processes) and markers for insulin resistance between offspring born before and after their mothers biliopancreatic diversion (n = 25 before and 25 after surgery; ages 2–25 years). The after-surgery sib had lower HOMA-IR, insulin and blood pressure compared to before-surgery sibs, with over representation of physiologically favorable gene expression changes in glucoregulatory, inflammatory and vascular disease pathways.51 Finally, a recent meta-analysis of 45 studies contrasted pre-pregnancy underweight, normal weight and overweight/obesity of women with SGA and LGA.11 Overweight/obese pre-pregnancy increased the risk of LGA and high body weight, whereas pre-pregnancy underweight was reported to increase the risk for SGA as well as low body weight. However, the likelihood of post-RYGB women reaching a BMI considered to be underweight is minimal. However protein malnutrition, and micronutrient and vitamin deficiencies have been described in women after RYGB,52–55 especially iron-deficiency anemia in pre-menopausal women. These deficiencies occur while their BMI remains in the obesity or overweight range. Whether the risk of compromised nutritional status in pregnant overweight and/or obese post-RYGB mothers is comparable to that of underweight or normal weight malnourished, unoperated mothers is not known. However, it is reassuring that neonatal complications (including death) did not significantly differ between the RYGB and the non-operated groups.
Although Apgar scores were similar and not significant between the RYGB and non-surgery women in group 1, group 2 showed a significant improvement in babies born after RYGB surgery compared with non-surgery women and group 3 showed a significant improvement for the 1-min score following RYGB. The larger numbers in groups 2 and 3 enabled small differences to become significant, and it is not clear if any of the Apgar score differences in any of the groups are clinically meaningful.
A limitation of this study is that the maternal pre-pregnancy BMI obtained from birth certificates may be self-reported, and therefore may be less than the actual pre-pregnancy BMI. However, this potential bias may be equally operative for both RYGB and non-surgery women in group 1. Likewise, the same bias is likely to exist in group 2 because the RYGB women with measured pre-pregnancy weights were matched to non-surgery women who only had a reported pre-pregnancy BMI. The clinical variables of the patients and subjects were also self-reported and limited to birth certificate extraction (that is, recorded by the delivering physician, nurse or allied health professional). We have no reason to believe that this limitation would be differential with respect to a history of RBYB surgery.
The lack of a significant improvement in C-section rates for post-surgical deliveries may have been influenced by hesitancy to allow vaginal births after a prior C-section has been performed. Inclusion of twins and triplets in the study, who might be expected to have much lower birth weights, might alter our results. However, the number of multiple births was very small, the multiple births were matched to other multiple births, and these matched pairs were analyzed with the conditional model. Exclusion of the few multiple births had no effect on the results. Finally, we had no biological markers of metabolic disease in the mother and/or in their offspring, which would have indicated if an LGA or SGA birth had important consequences.
To our knowledge, this study represents the first study to compare pregnancy outcomes in RYGB women and matched non-RYGB women using both the pregnancy closest to and before surgery and the first pregnancy after surgery. In addition, this study is larger than most previous studies, with a high statistical power to detect differences in pregnancy outcomes before and following surgery (group 3). The use of the UPDB to provide matching between RYGB patients and population-based, non-surgical subjects and their pregnancies (that is, 525 653 mothers and 1 071 767 live births) is a strength of this investigation.
In conclusion, following RYGB, women are at a significantly reduced risk for having an LGA live birth. The short- and long-term clinical benefits of this reduced LGA risk are likely to be substantial. However, post-RYGB mothers are also at a significantly greater risk to deliver an SGA neonate. The increased risk for SGA delivery may raise clinical concerns related to potential surgery-related nutritional deficiencies for the mother and the developing fetus. Women in childbearing age after bariatric surgery should be cared for by multidisciplinary teams to ensure optimal nutritional status prior to conception and during pregnancy, and that there is appropriate weight gain during pregnancy. Further research investigating underlying mechanisms that may account for the increased SGA risk following RYGB as well as clinical surveillance of development and health outcomes of children born to RYGB mothers is warranted.
Supplementary Material
Acknowledgments
This study was supported by NIH R01-DK-55006, M01-RR00064, NIH R01-AG-022095 and by the Huntsman Cancer Institute. Partial support for all datasets within the Utah Population Database was provided by the Huntsman Cancer Institute.
TDA received partial funding through the Huntsman Fellowship—Advancing Community Cancer Prevention, Intermountain Research and Medical Foundation and Intermountain Healthcare Corporation, Inc.
Footnotes
CONFLICT OF INTEREST
All other authors declare no conflict of interest.
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
REFERENCES
- 1.Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5:247–250. doi: 10.1097/00001648-199403000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod. 2004;19:720–726. doi: 10.1093/humrep/deh277. [DOI] [PubMed] [Google Scholar]
- 3.Oveson P, Rasmussen S, Kesmodel U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol. 2011;118:305–312. doi: 10.1097/AOG.0b013e3182245d49. [DOI] [PubMed] [Google Scholar]
- 4.Kjaer MM, Nilas L. Pregnancy after bariatric surgery—a review of benefits and risks. Acta Obstet Gynecol Scand. 2012;92:264–271. doi: 10.1111/aogs.12035. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, et al. Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol. 2004;190:1091–1097. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 6.Kumari AS. Pregnancy outcome in women with morbid obesity. Int J Gynaecol Obstet. 2001;73:101–107. doi: 10.1016/s0020-7292(00)00391-x. [DOI] [PubMed] [Google Scholar]
- 7.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:3290–3296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 8.Sridhar SB, Darbinian J, Ehrlich SF, Markman MA, Gunderson EP, Ferrara A, et al. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am J Obstet Gynecol. 2014;211:e1–e8. doi: 10.1016/j.ajog.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai M, Beall M, Ross MG. Developmental origins of obesity: programmed adipogenesis. Curr Diab Rep. 2013;13:27–33. doi: 10.1007/s11892-012-0344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rkhzay-Jaf J, O’Dowd JF, Stocker CJ. Maternal obesity and the fetal origins of the metabolic syndrome. Curr Cardiovasc Risk Rep. 2012;6:487–495. doi: 10.1007/s12170-012-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Z, Han S, Zhu J, Sun XM, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard R, Steegers EAP, Duijts L, Felix JF, Hofman A, Franco OH, et al. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension. 2014;63:683–691. doi: 10.1161/HYPERTENSIONAHA.113.02671. [DOI] [PubMed] [Google Scholar]
- 13.Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308:1122–1131. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 15.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21:S1–S27. doi: 10.1002/oby.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venna SR, Geetha S, Leary SD, Saperia J, Fisher DJ, Kumaran K, et al. Relationships of maternal and pateranal birthweight to features of the metabolic syndrome in the adult offsporing: an intergenerational study in South India. Diabetologia. 2007;50:43–54. doi: 10.1007/s00125-006-0516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trus TL, Pope GD, Finlayson SR. National trends in utilization and outcomes of bariatric surgery. Surg Endosc. 2005;19:616–620. doi: 10.1007/s00464-004-8827-8. [DOI] [PubMed] [Google Scholar]
- 18.Davis MM, Slish K, Chao C, Cabana MD. National trends in bariatric surgery, 1996–2002. Arch Surg. 2006;141:71–74. doi: 10.1001/archsurg.141.1.71. discussion 5. [DOI] [PubMed] [Google Scholar]
- 19.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19:1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 20.Birkmeyer NJ, Gu N. Race, socioeconomic status, and the use of bariatric surgery in Michigan. Obes Surg. 2012;22:259–265. doi: 10.1007/s11695-010-0210-3. [DOI] [PubMed] [Google Scholar]
- 21.Farinholt GN, Carr AD, Chang EJ, Ali MR. A call to arms: obese men with more severe comorbid disease and underutilization of bariatric operations. Surg Endosc. 2013;27:4556–4563. doi: 10.1007/s00464-013-3122-1. [DOI] [PubMed] [Google Scholar]
- 22.Kjaer MM, Lauenborg J, Breum M, Nilas L. The risk of adverse pregnancy outcome after bariatric surgery: a nationwide register-based matched cohort study. Am J Obstet Gynecol. 2013;208:e1–e5. doi: 10.1016/j.ajog.2013.02.046. [DOI] [PubMed] [Google Scholar]
- 23.Aricha-Tamir B, Weintraub AY, Levi I, Sheiner E. Downsizing pregnancy complications: a study of paired pregnancy outcomes before and after bariatric surgery. Surg Obes Relat Dis. 2012;8:434–439. doi: 10.1016/j.soard.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Weintraub AY, Levy A, Levi I, Mazor M, Wiznitzer A, Sheiner E. Effect of bariatric surgery on pregnancy outcomes. Int J Gynaecol Obstet. 2008;103:246–251. doi: 10.1016/j.ijgo.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Marceau P, Kaufman D, Biron S, Hould FS, Lebel S, Marceau S, et al. Outcome of pregnancies after biliopancreatic diversion. Obes Surg. 2004;14:318–324. doi: 10.1381/096089204322917819. [DOI] [PubMed] [Google Scholar]
- 26.Lesco J, Peaceman A. Pregnancy outcomes in women after bariatric surgery compared with obese and morbidly obese controls. Obstet Gynecol. 2012;119:547–554. doi: 10.1097/AOG.0b013e318239060e. [DOI] [PubMed] [Google Scholar]
- 27.Santulli P, Mandelbrot L, Facchiano E, Dussaux C, Ceccaldi PF, Ledoux S, et al. Obstetrical and neonatal outcomes of pregnancies following gastric bypass surgery: a retrospective cohort study in a French referral centre. Obes Surg. 2010;20:1501–1508. doi: 10.1007/s11695-010-0260-6. [DOI] [PubMed] [Google Scholar]
- 28.Sheiner E, Levy A, Silverberg D, Menes TS, Levy I, Katz M, et al. Pregnancy after bariatric surgery is not associated with adverse perinatal outcome. Am J Obstet Gynecol. 2004;190:1335–1340. doi: 10.1016/j.ajog.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Burke AE, Bennett WL, Jamshidi RM, Gilson MM, Clark JM, Segal JB, et al. Reduced incidence of gestational diabetes with bariatric surgery. J Am Coll Surg. 2010;211:169–175. doi: 10.1016/j.jamcollsurg.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Bennett WL, Gilson MM, Jamshidi RM, Burke AE, Segal JB, Steele KE, et al. Impact of bariatric surgery on hypertensive disorders in pregnancy: retrospective analysis of insurance claims data. BMJ. 2010;340:c1662. doi: 10.1136/bmj.c1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norgaard LN, Gjerris AC, Kirkegaard I, Beriac JF, Tabor A. Danish Fetal Medicine Researh Group Fetal growth in pregnancies conceived after gastric bypass surgery in relation to surgery-to-conception interval: a Danish national cohort study. PLoS One. 2014;9:e90317. doi: 10.1371/journal.pone.0090317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magdaleno R, Jr, Pereira BG, Chaim EA, Turato ER. Pregnancy after bariatric surgery: a current view of maernal, obstetrical and perinatal challenges. Arch Gynecol Obstet. 2012;285:559–566. doi: 10.1007/s00404-011-2187-0. [DOI] [PubMed] [Google Scholar]
- 33.Maggard MA, Yermilov I, Li Z, Maglione M, Newberry S, Suttorp M, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286–2296. doi: 10.1001/jama.2008.641. [DOI] [PubMed] [Google Scholar]
- 34.Wylie JE, Mineau GP. Biomedical databases: protecting privacy and promoting research. Trends Biotechnol. 2003;21:113–116. doi: 10.1016/S0167-7799(02)00039-2. [DOI] [PubMed] [Google Scholar]
- 35.Yerushalmy J. The classification of newborn infants by birth weight and gestational age. J Pediatrics. 1967;71:164–172. doi: 10.1016/s0022-3476(67)80067-2. [DOI] [PubMed] [Google Scholar]
- 36.Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatrics. 1967;71:2. doi: 10.1016/s0022-3476(67)80066-0. [DOI] [PubMed] [Google Scholar]
- 37.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: final data for 2006. Natl Vital Stat Rep. 2006;55:1–101. [PubMed] [Google Scholar]
- 38.Getahun D, Ananth MR, Peltier MR, Salihu HM, Scorza WE. Changes in prepregnancy body mass index between the first and second pregnancies and risk for large-for-gestational-age birth. Am J Obstet Gynecol. 2007;196:530e1–538ee. doi: 10.1016/j.ajog.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 39.Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the United States: determinants, outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188:1372–1378. doi: 10.1067/mob.2003.302. [DOI] [PubMed] [Google Scholar]
- 40.Zhang S, Rattanatray L, Morrison JL, Nicholas LM, Lie S, McMillen C. Maternal obesity and the early origins of childhood obesity: weighing up the benefits and costs of maternal weight loss in the periconceptional period for the offspring. Exp Diab Res. 2011;2011:585749. doi: 10.1155/2011/585749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorenson HT, Sabroe S, Rothman KJ, Gillman M, Fischer P, Sorensen TI. Relation between weight and length at birth and body mass index in young adulthood: cohort study. BMJ. 1997;315:1137. doi: 10.1136/bmj.315.7116.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seidman DS, Laor A, Gale R, Stevenson DK, Danon YL. A longitudinal study of birth weight and being overweight in late adolescence. Am J Dis Child. 1991;145:782–785. [PubMed] [Google Scholar]
- 43.Drake A, Reynolds RM. Impact of maternal obesity on offspring obesity and car-diometabolic disease risk. Reproduction. 2010;140:387–398. doi: 10.1530/REP-10-0077. [DOI] [PubMed] [Google Scholar]
- 44.Thompson RR, Obarzanek E, Franko DL, Barton BA, Morrison J, Bird FM, et al. Childhood overweight and cardiovascular disease risk factors: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2007;150:18–25. doi: 10.1016/j.jpeds.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobson HN, Burke BS, Smith CAB, Reid DE. Effect of weight reduction in obese pregnant women on pregnancy, labor, and delivery, and on the condition of the infant at birth. Am J Obstet Gynecol. 1962;83:1609–1616. doi: 10.1016/0002-9378(62)90178-3. [DOI] [PubMed] [Google Scholar]
- 46.Thangaratinam S, Rogozi SE, Jolly K, Glinkowski S, Duda W, Borowiack E, et al. Interventions to reduce or prevent obesity in pregnant women: a systematic review. Natl Inst Technol. 2012;16:1–191. doi: 10.3310/hta16310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maes T. Fetal origins of insulin resistance and the metabolic syndrome: a key role for adipose tissue? Diabetes Metab. 2010;36:11–20. doi: 10.1016/j.diabet.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 48.McCowan L, Horgan RP. Risk factors for small gestational age infants. Best Pract Res Clin Obstet Gynaecol. 2009;23:779–793. doi: 10.1016/j.bpobgyn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Rochester Uo. [accessed 22 April 2014];Small for gestational age. URMC Health Encyclopedia [Internet] 2014 http://www.urmc.rochester.edu/Encyclopedia/Content.aspx?ContentTypeID=90&ContentID= P02411] [Google Scholar]
- 50.Smith J, Cianflone K, Biron S, Hould FS, Lebel S, Marceau S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab. 2009;94:4275–4283. doi: 10.1210/jc.2009-0709. [DOI] [PubMed] [Google Scholar]
- 51.Guenard F, Deshaies Y, Cianflone K, Kral JG, Marceau P, Vohl MC. Differential methylation in glucoregulatory genes of offspring born before vs after maternal gastrointestinal bypass surgery. Proc Natl Acad Sci. 2013;110:11439–11444. doi: 10.1073/pnas.1216959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obenwanne KM, Fredrickson KA, Mathiason MA, LKallies KJ, Farnen JP, Kothari SN. Incidence, treatment, and outcomes of iron deficiency after laparoscopic Roux-en-Y gastric bypass: a 10-year analysis. J Am Coll Surg. 2013;218:246–252. doi: 10.1016/j.jamcollsurg.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 53.Blume CA, NBoni CC, Casagrande DS, Rizzolli J, Padoin AV, Mottin CC. Nutritional profile of patients before and after Roux-en-Y gastric bypass: 3-year follow-up. Obes Surg. 2012;22:1676–1685. doi: 10.1007/s11695-012-0696-y. [DOI] [PubMed] [Google Scholar]
- 54.Gletsu-Miller N, Broderius M, Frediani JK, Zhao VM, Griffith DP, Davis SSJ, et al. Incidence and prevalence of copper deficiency following Roux-en-Y gastric bypass surgery. Int J Obes (Lond) 2012;36:328–335. doi: 10.1038/ijo.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91:4223–4231. doi: 10.1210/jc.2006-0557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.