Abstract
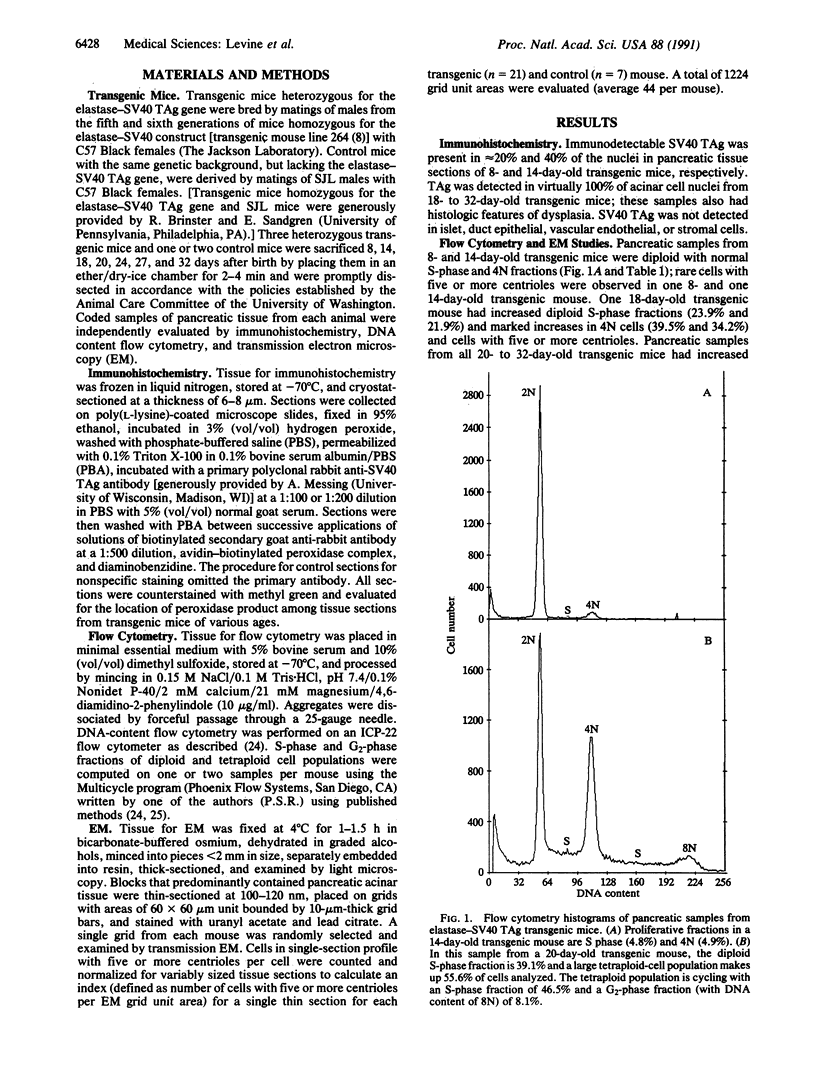
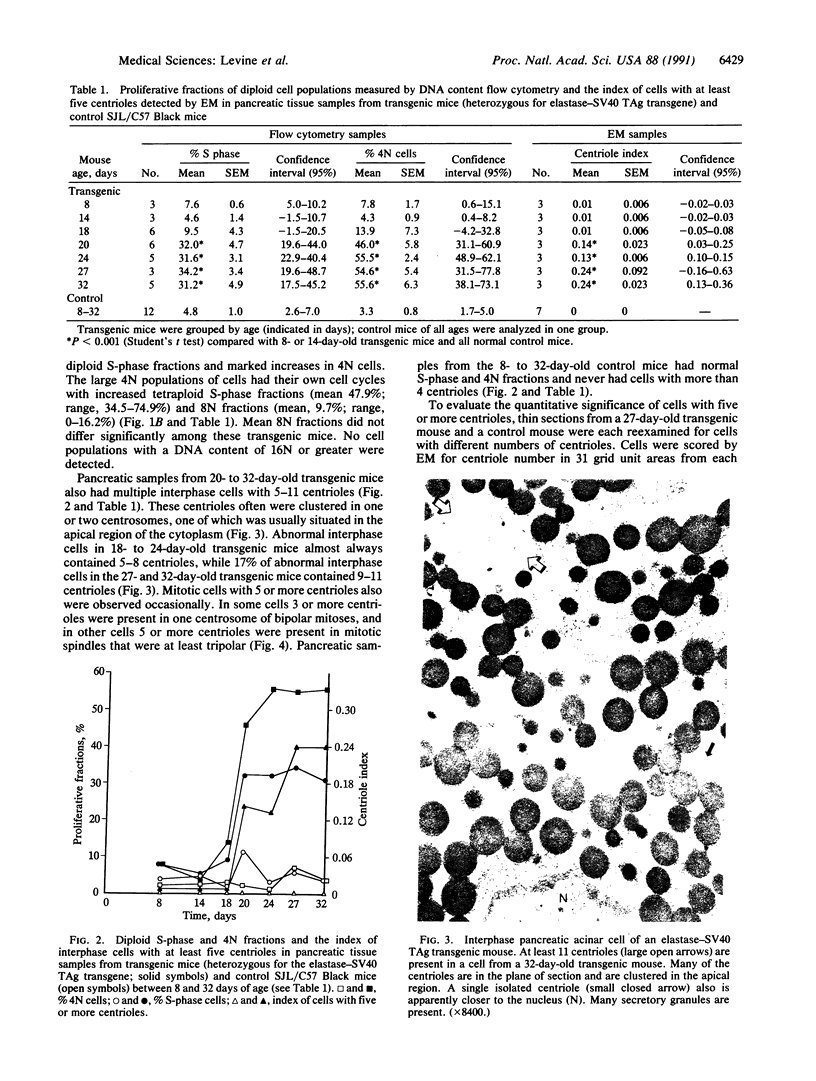
The development of pancreatic cancer in transgenic mice expressing the simian virus 40 tumor antigen placed under controlling regions of the elastase I gene is characterized by the sequential appearance of tetraploid and then multiple aneuploid cell populations. Pancreatic tissues from such transgenic mice were studied between 8 and 32 days of age. Virtually 100% of acinar cell nuclei had immunohistochemically detectable tumor antigen by 18 days. Tetraploid cells were demonstrated by DNA content flow cytometry by 20 days and were associated with the appearance of interphase cells that had 5-11 centrioles per cell in single thin sections of pancreatic tissue examined by electron microscopy. Mitotic cells also were observed that had 5 or more centrioles per cell that were incorporated into the poles of bipolar or at least tripolar spindle apparatuses. These observations indicate that formation of the tetraploid intermediate in the diploid----tetraploid----aneuploid sequence of pancreatic tumor formation in elastase-simian virus 40 tumor antigen transgenic mice is accompanied by the development of cells with 5 or more centrioles that can be incorporated into the poles of abnormal mitotic spindles. We speculate that cells with more than 4 centrioles are predisposed to the formation of multipolar mitoses that may yield daughter cells with chromosomal gains and losses, resulting in the subsequent development of aneuploid tumors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum P., Yip C., Goetsch L., Byers B. A yeast gene essential for regulation of spindle pole duplication. Mol Cell Biol. 1988 Dec;8(12):5386–5397. doi: 10.1128/mcb.8.12.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Jarvis D. L., Maxwell S. A. SV40 T-antigen as a dual oncogene: structure and function of the plasma membrane-associated population. Ann N Y Acad Sci. 1989;567:104–121. doi: 10.1111/j.1749-6632.1989.tb16463.x. [DOI] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Duplication of spindle plaques and integration of the yeast cell cycle. Cold Spring Harb Symp Quant Biol. 1974;38:123–131. doi: 10.1101/sqb.1974.038.01.016. [DOI] [PubMed] [Google Scholar]
- Creanor J., Mitchison J. M. Continued DNA synthesis after a mitotic block in the double mutant cut1 cdc11 of the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1990 Jul;96(Pt 3):435–438. doi: 10.1242/jcs.96.3.435. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Friedlander M. L., Hedley D. W., Taylor I. W. Clinical and biological significance of aneuploidy in human tumours. J Clin Pathol. 1984 Sep;37(9):961–974. doi: 10.1136/jcp.37.9.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D. L., Hafezi S., Zhang T., Doxsey S. J. Centrosome duplication continues in cycloheximide-treated Xenopus blastulae in the absence of a detectable cell cycle. J Cell Biol. 1990 Jun;110(6):2033–2042. doi: 10.1083/jcb.110.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Smith D. Altered fidelity of mitotic chromosome transmission in cell cycle mutants of S. cerevisiae. Genetics. 1985 Jul;110(3):381–395. doi: 10.1093/genetics/110.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Keryer G., Ris H., Borisy G. G. Centriole distribution during tripolar mitosis in Chinese hamster ovary cells. J Cell Biol. 1984 Jun;98(6):2222–2229. doi: 10.1083/jcb.98.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985 Apr;45(4):1437–1443. [PubMed] [Google Scholar]
- Laffin J., Fogleman D., Lehman J. M. Correlation of DNA content, p53, T antigen, and V antigen in simian virus 40-infected human diploid cells. Cytometry. 1989 Mar;10(2):205–213. doi: 10.1002/cyto.990100212. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Benchimol S. p53: oncogene or anti-oncogene? Genes Dev. 1990 Jan;4(1):1–8. doi: 10.1101/gad.4.1.1. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Bradley M. K. The simian virus 40 large T antigen. A lot packed into a little. Mol Biol Med. 1987 Apr;4(2):63–80. [PubMed] [Google Scholar]
- Loeb L. A., Cheng K. C. Errors in DNA synthesis: a source of spontaneous mutations. Mutat Res. 1990 May;238(3):297–304. doi: 10.1016/0165-1110(90)90021-3. [DOI] [PubMed] [Google Scholar]
- Maxwell S. A., Ames S. K., Sawai E. T., Decker G. L., Cook R. G., Butel J. S. Simian virus 40 large T antigen and p53 are microtubule-associated proteins in transformed cells. Cell Growth Differ. 1991 Feb;2(2):115–127. [PubMed] [Google Scholar]
- Maxwell S. A., Santos M., Wong C., Rasmussen G., Butel J. S. Solubilization of SV40 plasma-membrane-associated large tumor antigen using single-phase concentrations of 1-butanol. Mol Carcinog. 1989;2(6):322–335. doi: 10.1002/mc.2940020607. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Hammer R. E., Messing A., Palmiter R. D., Brinster R. L. Pancreatic neoplasia induced by SV40 T-antigen expression in acinar cells of transgenic mice. Science. 1987 Oct 9;238(4824):188–193. doi: 10.1126/science.2821617. [DOI] [PubMed] [Google Scholar]
- Petersen S. E., Friedrich U. A comparison between flow cytometric ploidy investigation and chromosome analysis of 32 human colorectal tumors. Cytometry. 1986 Jul;7(4):307–312. doi: 10.1002/cyto.990070403. [DOI] [PubMed] [Google Scholar]
- Rabinovitch P. S., Reid B. J., Haggitt R. C., Norwood T. H., Rubin C. E. Progression to cancer in Barrett's esophagus is associated with genomic instability. Lab Invest. 1989 Jan;60(1):65–71. [PubMed] [Google Scholar]
- Raff J. W., Glover D. M. Nuclear and cytoplasmic mitotic cycles continue in Drosophila embryos in which DNA synthesis is inhibited with aphidicolin. J Cell Biol. 1988 Dec;107(6 Pt 1):2009–2019. doi: 10.1083/jcb.107.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B. J., Haggitt R. C., Rubin C. E., Rabinovitch P. S. Barrett's esophagus. Correlation between flow cytometry and histology in detection of patients at risk for adenocarcinoma. Gastroenterology. 1987 Jul;93(1):1–11. [PubMed] [Google Scholar]
- Remvikos Y., Muleris M., Vielh P., Salmon R. J., Dutrillaux B. DNA content and genetic evolution of human colorectal adenocarcinoma. A study by flow cytometry and cytogenetic analysis. Int J Cancer. 1988 Oct 15;42(4):539–543. doi: 10.1002/ijc.2910420411. [DOI] [PubMed] [Google Scholar]
- Ring D., Hubble R., Kirschner M. Mitosis in a cell with multiple centrioles. J Cell Biol. 1982 Sep;94(3):549–556. doi: 10.1083/jcb.94.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C., Kuriyama R., Nishizawa K. Microtubule-organizing centers abnormal in number, structure, and nucleating activity in x-irradiated mammalian cells. J Cell Biol. 1983 Mar;96(3):776–782. doi: 10.1083/jcb.96.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Shackney S. E., Smith C. A., Miller B. W., Burholt D. R., Murtha K., Giles H. R., Ketterer D. M., Pollice A. A. Model for the genetic evolution of human solid tumors. Cancer Res. 1989 Jun 15;49(12):3344–3354. [PubMed] [Google Scholar]
- Stürzbecher H. W., Maimets T., Chumakov P., Brain R., Addison C., Simanis V., Rudge K., Philp R., Grimaldi M., Court W. p53 interacts with p34cdc2 in mammalian cells: implications for cell cycle control and oncogenesis. Oncogene. 1990 Jun;5(6):795–781. [PubMed] [Google Scholar]
- Sunkel C. E., Glover D. M. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988 Jan;89(Pt 1):25–38. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- Tribukait B., Gustafson H., Esposti P. Ploidy and proliferation in human bladder tumors as measured by flow-cytofluorometric DNA-analysis and its relations to histopathology and cytology. Cancer. 1979 May;43(5):1742–1751. doi: 10.1002/1097-0142(197905)43:5<1742::aid-cncr2820430525>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Uzawa S., Samejima I., Hirano T., Tanaka K., Yanagida M. The fission yeast cut1+ gene regulates spindle pole body duplication and has homology to the budding yeast ESP1 gene. Cell. 1990 Sep 7;62(5):913–925. doi: 10.1016/0092-8674(90)90266-h. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- Weinert T. A., Hartwell L. H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988 Jul 15;241(4863):317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- Yunis J. J. The chromosomal basis of human neoplasia. Science. 1983 Jul 15;221(4607):227–236. doi: 10.1126/science.6336310. [DOI] [PubMed] [Google Scholar]