Abstract
Background
Sensitive diagnostic tools are necessary for the detection of Mycobacterium suricattae infection in meerkats (Suricata suricatta) in order to more clearly understand the epidemiology of tuberculosis and the ecological consequences of the disease in this species. We therefore aimed to develop a cytokine release assay to measure antigen-specific cell-mediated immune responses of meerkats.
Results
Enzyme-linked immunosorbent assays (ELISAs) were evaluated for the detection of interferon-gamma (IFN-γ) and IFN-γ inducible protein 10 (IP-10) in meerkat plasma. An IP-10 ELISA was selected to measure the release of this cytokine in whole blood in response to Bovigam® PC-HP Stimulating Antigen, a commercial peptide pool of M. bovis antigens. Using this protocol, captive meerkats with no known M. suricattae exposure (n = 10) were tested and results were used to define a diagnostic cut off value (mean plus 2 standard deviations). This IP-10 release assay (IPRA) was then evaluated in free-living meerkats with known M. suricattae exposure, categorized as having either a low, moderate or high risk of infection with this pathogen. In each category, respectively, 24.7%, 27.3% and 82.4% of animals tested IPRA-positive. The odds of an animal testing positive was 14.0 times greater for animals with a high risk of M. suricattae infection compared to animals with a low risk.
Conclusion
These results support the use of this assay as a measure of M. suricattae exposure in meerkat populations. Ongoing longitudinal studies aim to evaluate the value of the IPRA as a diagnostic test of M. suricattae infection in individual animals.
Electronic supplementary material
The online version of this article (doi:10.1186/s12917-016-0927-x) contains supplementary material, which is available to authorized users.
Keywords: Cytokine, Diagnosis, IP-10, Meerkat, Mycobacterium suricattae, Tuberculosis
Background
In the Kalahari Desert of South Africa, tuberculosis (TB) caused by Mycobacterium suricattae results in morbidity and mortality in meerkats [1, 2]. In this species, TB presents as disseminated disease primarily affecting the spleen, liver, lungs and head lymph nodes and a characteristic clinical finding is swelling of the sub-mandibular lymph nodes [1] (Fig. 1). Clinical disease often progresses to mortality resulting in changes in population ecology through group-level extinctions [3].
Fig. 1.

Meerkats with pathology typical of tuberculosis caused by Mycobacterium suricattae. a A meerkat displaying swelling of the submandibular lymph node. These lesions typically present at post mortem as granulomatous hyperplasia. b A meerkat with a draining sinus tract following necrosis and abscessation of the submandibular lymph node
Improved diagnostic tests for TB in meerkats are necessary to advance the understanding of disease epidemiology and may allow the development of an effective TB control strategy. Serological assays for TB have previously been shown to have low sensitivity in this species [4]; however, assays detecting cell-mediated immune responses have, to date, not been evaluated [5]. In other species, such as cattle, in vitro tests of cell-mediated immunity (CMI) offer the most sensitive methods for TB diagnosis by detecting the release of interferon-gamma (IFN-γ) in response to mycobacterial antigens [6, 7]. Moreover, an alternative diagnostic marker of CMI, IFN-γ-inducible protein 10 (IP-10), has been shown to improve diagnostic sensitivity in humans and buffaloes (Syncerus caffer) [8, 9]. The specificity of such assays can be optimised by using highly specific antigens such as 6 kDa early secretory antigenic target (ESAT-6) and 10 kDa culture filtrate protein (CFP-10) [10, 11]. However, the genes encoding these proteins have been deleted from M. suricattae and they are therefore unlikely to be suitable diagnostic antigens for this pathogen [2]. As an alternative, the commercially available Bovigam® PC-HP peptide pool, which contains ESAT-6 and CFP-10 peptides, includes antigens derived from the gene Rv3615c and an additional 3 genes, and could be useful for the detection of M. suricattae infection [11].
The purposes of this study were, therefore, to develop enzyme-linked immunosorbent assays (ELISAs) for the measurement of IFN-γ and IP-10 in meerkat plasma, evaluate the diagnostic utility of the PC-HP peptide pool in this species, and assess the test performance of an optimised diagnostic assay for M. suricattae infection in a population of free-living meerkats.
Materials and methods
Animals
Captive meerkats with no known history of M. suricattae exposure served as an uninfected control group for the development of the cytokine release assay. These animals (n = 10) were housed in two groups on a natural soil substrate in an enriched environment in enclosures (6 × 10 meters) at Giraffe House, a wildlife awareness centre situated in Stellenbosch, South Africa. They were fed eggs and day-old chicks or chicken meat daily and had free access to clean water.
Animals with known exposure to M. suricattae were opportunistically sampled from a population of free-living meerkats, which have been habituated to the presence of researchers [12], from the Kuruman River Reserve, Northern Cape, South Africa (26°58’S, 21°49’E). Between September 2014 and February 2015, animals from this population were sampled from 12 social groups. Hereafter, animals were classified according to their presumed infection risk: low risk (category 1), comprising animals from social groups with no known history of TB; intermediate risk (category 2), comprising animals from social groups with either one or two known deaths due to TB in the preceding two years; and high risk (category 3), comprising meerkats from social groups with more than two deaths due to TB in the preceding two years.
Permission to perform the study was obtained from the University of Stellenbosch Animal Ethics committee (Reference no. SU-ACUM14-00042). Permits to conduct animal research were obtained from the Northern Cape Department of Environment and Nature Conservation (Permit no. FAUNA 194/2014) and the National Department of Agriculture, Forestry and Fisheries (Reference no. 12/11/1/7/3).
Blood collection and processing
Meerkats were captured by hand and placed in cotton bags or caught in nets and physically restrained with towels prior to induction and maintenance of anaesthesia with isoflurane (Safeline Pharmaceuticals Ltd, Roodepoort, SA) via facemask. Using a 25G needle and syringe, 2 ml blood was collected from the jugular vein and transferred to a heparinised blood tube (Greiner Bio-one, Kremsmünster, Austria). Animals were monitored after completion of the procedure and returned to their natural environment once fully recovered.
Aliquots of whole blood (150 μl) were transferred to each of three 1.5 ml microcentrifuge tubes containing, respectively, 15 μl phosphate buffered saline (PBS); 15 μl PC-HP peptide solution (Prionics AG, Schlieren, Switzerland), prepared according to the manufacturer’s instructions; and 15 μl pokeweed mitogen (PWM) solution in PBS (final concentration 50 μg/ml). Tubes were thoroughly mixed, incubated at 37 °C for 24 hours and centrifuged at 1300 × g for 6 minutes, after which the plasma was harvested and stored at - 80 °C.
ELISA protocol
All ELISAs described below were performed according to the following protocol. Capture antibody (Table 1) in PBS (50 μl) was used to coat wells of 96-well MaxiSorp polystyrene ELISA plates (Thermo Fisher Scientific, Massachusetts, USA) which were incubated at 4 °C overnight. All subsequent steps were performed at room temperature. Plates were washed three times with wash buffer (WB) consisting of 0.05% Tween-20 (Sigma-Aldrich, Missouri, USA) in PBS and blocked with 200 μl/well blocking buffer (BB) comprising WB with 0.1% bovine serum albumin (Roche, Basel, Switzerland). After 1 h incubation, plates were washed again. For IFN-ɣ assays, 25 μl of each plasma sample was incubated with 25 μl BB and for IP-10 assays, 12.5 μl of each plasma sample with 37.5 μl BB. After 2 h, plates were washed and incubated for 1 h with 50 μl/well biotinylated detection antibody (Table 1) diluted in BB. After washing, plates were incubated for an additional 1 h with 50 μl/well streptavidin-horseradish peroxidase (R&D systems, Minnesota, USA) diluted 1:200 in BB. After a final wash step, plates were incubated for 20 minutes with 50 μl/well tetramethylbenzidine (TMB) substrate solution (BD Pharmingen, New Jersey, USA) after which sulphuric acid (2 M; 25 μl/well) was added to stop the colour reaction. The optical density (OD) of each well was measured at 450 nm using an LT-4000 Microplate Reader (Labtech, Uckfield, UK).
Table 1.
Capture and detection antibodies screened for use in enzyme-linked immunosorbent assays for the detection of meerkat interferon-gamma (IFN-ɣ) and IFN-γ inducible protein 10 (IP-10)
| Cytokine | Species | Manufacturer | Antibody catalogue no. | |
|---|---|---|---|---|
| Capture | Detection | |||
| IFN-ɣ | Human | BD Pharmingen, New Jersey, USA | 551221 | 554550 |
| Equine | Mabtech, Nacka Strand, Sweden | 3117-1H-6 | 3117-1H-6 | |
| Feline | R&D Systems Minnesota, USA | DY764 | DY764 | |
| Feline | AbD Serotech, Kidlington, UK | MCA2140 | N/A | |
| Feline | Kingfisher Biotech, Minnesota, USA | PB0281F-100 | PBB0283F-050 | |
| IP-10 | Human | Peprotech, London, UK | 900-K39 | 500-P93Bt |
| Equine | Kingfisher biotech | PB0418E-100 | PBB0423E-050 | |
| Bovine | Kingfisher biotech | PB0385B-100 | PBB0393B-050 | |
Antibody selection and ELISA optimisation
Selected anti-IFN-ɣ and anti-IP-10 antibodies (Table 1) were screened for potential reactivity to these cytokines in meerkat plasma as follows. Plasma from PWM-stimulated blood of 7 randomly selected animals was pooled and assayed using ELISAs comprising all possible combinations of selected capture and detection antibodies for either IFN-ɣ or IP-10. All antibodies were used at concentrations recommended by the manufacturer (Table 1). The antibody combination that resulted in the greatest OD values for each analyte was then selected and a dilution series of these antibodies was used in a checkerboard titration to assay pooled plasma samples from both PWM-stimulated and unstimulated blood. The optimal ELISA conditions were defined as the concentrations of capture and detection antibodies which resulted in the greatest relative OD difference derived from these samples.
Cytokine release assay
Plasma samples, derived from whole blood which was processed as described above, were assayed in duplicate using the optimised ELISA. M. suricattae-specific cytokine release was defined as the OD obtained for the PC-HP-stimulated sample minus that for the sample co-incubated with PBS (ODHP-Nil). The PWM-stimulated plasma sample was used as a positive control for whole blood cytokine release and animals that showed greater cytokine release in whole blood co-incubated with PBS than in response to PWM stimulation (ODPWM-nil < 0) were excluded from the study. Using the results from uninfected control animals, a diagnostic cut off value for this assay was calculated as the mean of all ODHP-Nil values plus 2 standard deviations (SD).
Statistical analysis
Using the Wilcoxon signed rank test, the release of IP-10 in blood co-incubated with PBS, PC-HP and PWM was compared for animals from the uninfected control group as well as those from the M. suricattae-exposed group. Data were analysed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, USA). The performance of the cytokine release assay was evaluated in the free-living M. suricattae-exposed meerkat population by calculating the odds of an animal testing positive in each of the three infection risk categories defined above. These values were compared to that of category 1 using a chi-squared analysis.
Results
Development of a meerkat cytokine release assay
Of the ELISAs evaluated for the detection of meerkat IFN-γ (Table 1), the assay comprising anti-human capture (2 μg/ml) and detection (2 μg/ml) antibodies resulted in the greatest OD value for PWM-stimulated samples, i.e. OD = 0.076. Of the IP-10 ELISAs (Table 1), the assay consisting of anti-bovine capture antibody (0.5 μg/ml) and anti-human detection antibody (0.25 μg/ml) resulted in the greatest ELISA signal, i.e. OD = 0.45. Hereafter, the IP-10 assay was selected for further analysis of PC-HP-stimulations and recombinant human IP-10 protein (Peprotech) was used as a positive control for the assay. Meerkats in the uninfected control group showed no significant difference in IP-10 release in blood incubated with PC-HP peptides and blood co-incubated with PBS (Fig. 2a). However, there was a significant difference in the release of this cytokine in PWM-stimulated blood and blood co-incubated with PBS (Fig. 2a). Using the IP-10 release assay (IPRA) test results (ODHP-Nil) from this group, a diagnostic cut off value was calculated as 0.038 (mean ODHP-Nil + 2SD). This value was then used to classify meerkats from the low, intermediate and high infection risk categories as either IPRA-positive or -negative.
Fig. 2.
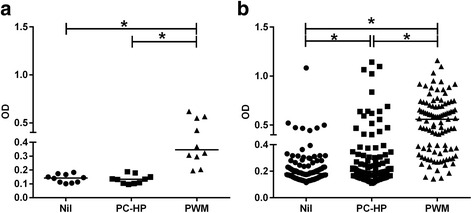
IP-10 release in whole blood incubated at 37 °C for 24 h with phosphate buffered saline (Nil), Bovigam PC-HP peptides and pokeweed mitogen (PWM), from a Mycobacterium suricattae-unexposed meerkats (n = 10), and b M. suricattae-exposed meerkats (n = 101). Significant differences between treatment conditions are indicated (Wilcoxon signed rank test, *p < 0.005)
Evaluation of test performance
A total of 108 M. suricattae-exposed meerkats were tested: 79 from social groups with no known history of TB (category 1), 11 from social groups with one or two deaths due to TB (category 2), and 18 from social groups with 3 to 7 deaths (category 3). For these animals, the ODHP (median 0.20, interquartile range 0.16–0.32), and the ODPWM (0.50, 0.32–0.69), were both significantly greater than the ODNil (0.19, 0.15–0.27) (Fig. 2b). The median ODPWM-Nil value was 0.29 (range,−0.36 to 0.84) and 6/79, 0/11 and 1/18 animals from categories 1, 2 and 3, respectively, were excluded from further analysis on the basis of their ODPWM-Nil result being less than zero. Of these 7 cases, 5 had ODNil values in the highest quartile of all samples.
For the remaining 101 meerkats, 35 animals had values greater than the diagnostic cut off (ODHP-Nil > 0.038) (Table 2) with 24.7% testing positive in category 1, 27.3% in category 2 and 82.4% in category 3. The odds of an animal testing IPRA-positive was 14.0 times greater for animals with a high risk of M. suricattae infection (category 3) compared to animals with a low risk (category 1) (95% CI: 3.6–54.3, p < 0.0001).
Table 2.
Analysis of IP-10 release assay (IPRA) results for 101 opportunistically sampled meerkats with a low, intermediate and high risk of infection with M. suricattae
| Category | Infection risk (n) | Positive (%) | ORa (95% confidence interval) | P |
|---|---|---|---|---|
| 1 | Low (73) | 18 (24.7) | 1 | - |
| 2 | Medium (11) | 3 (27.3) | 1.13 (0.27–4.70) | 0.87 |
| 3 | High (17) | 14 (82.4) | 14 (3.61–54.34) | <0.0001 |
| Total | 101 | 35 (34.7) |
aOR, Odds ratio of an animal in a particular category testing positive, relative to category 1
Discussion
We have developed a novel diagnostic assay for M. suricattae infection in meerkats which measures antigen-specific IP-10 release in whole blood incubated with Bovigam® PC-HP peptides. All meerkats with no known exposure to M. suricattae (n = 10) tested negative with the IPRA, while 35 out of 101 (34.6%) animals with known exposure to this pathogen tested positive. Although M. suricattae infection in these individuals was not confirmed, the odds of a meerkat testing positive were significantly greater for animals with a high risk of infection when compared to those with a low risk of infection, supporting the validity of the IPRA.
IP-10 has been shown to be a useful diagnostic biomarker for detection of M. tuberculosis infection in humans [9] and M. bovis infection in African buffaloes [11]. In both these species, IP-10 is produced in greater amounts than IFN-γ in antigen-stimulated whole blood and has been shown to be a more sensitive marker of mycobacterial infection [8, 9, 13]. In the present study, despite the use of ELISA antibodies produced against distantly related species, measurement of IP-10 proved to be a useful marker of immune activation. While this indicates the sensitivity of the ELISA for meerkat IP-10, it may, in part, reflect the abundance of this molecule in antigen-stimulated samples. Moreover, the similarity between the amino acid sequences of cattle, horses, cats and humans is significantly greater for IP-10 than for IFN-γ (data not shown) and this may explain the increased potential for cross-species reactivity of the anti-IP-10 antibodies. For these reasons, IP-10 may also be a useful diagnostic target in other species.
In order to define a diagnostic cut off value for the IPRA, we tested 10 captive meerkats with no known exposure to M. suricattae. These animals showed no significant difference in their IP-10 responses to PBS and PC-HP peptides indicating that their selection as uninfected controls was appropriate. Moreover, a threshold value of ODHP-Nil > 0.038 classified all control animals as test-negative. Such a low threshold will increase the sensitivity of the assay, thereby reducing the number of false negative test results; however, this could be at the expense of specificity [14]. In part, this low cut off value resulted from plasma samples being diluted 1:4 in the present study and a lower dilution factor might improve test accuracy. However, this was not tested in the present study because of limited sample volumes.
In contrast to the control animals, 35 of 101 M. suricattae-exposed meerkats displayed significant IP-10 responses to the PC-HP peptides, confirming the antigenicity of this peptide pool for these animals. However, it is currently unclear which components of the PC-HP antigens might be responsible for this immune sensitization. The genetic region of difference 1 (RD1), a variant of which is deleted from the M. suricattae genome [15], encodes both ESAT-6 and CFP-10 and is also required for the secretion of Esx-1 substrate protein C (espC) which is encoded by the gene Rv3615c [16]. While Rv3615c is present in the M. suricattae genome (pers. comm., Anzaan Dippenaar), it is possible that this component of the PC-HP peptide pool, in addition to ESAT-6/CFP-10, would have a limited diagnostic contribution to detection of M. suricattae-infected animals. Nonetheless, the use of the PC-HP reagent is supported by the fact that the odds of an animal testing IPRA-positive were significantly greater for meerkats with the greatest risk of M. suricattae infection. Moreover, our results suggest that the PC-HP peptides might be useful for diagnostic testing of species infected with related RD1-deleted strains, i.e. M. microti, M. mungi and the dassie bacillus [5].
In both the captive and free-living populations, the median ODPWM was significantly higher than the values for either ODNil or ODHP, indicating that PWM is an appropriate mitogen in this species. Seven animals were excluded from our analysis based on negative ODPWM-Nil values; however, in 5 of these cases, the exclusion criterion was met as a result of unusually high ODNil values, not failure to respond to PWM. Similar spontaneous release of IP-10 has previously been seen in cattle [17], although the mechanism for this phenomenon is not currently understood. In the present study, although the positive control was intended to confirm the viability of cytokine production in blood samples, it additionally served to identify samples where high ODNil values would have affected the test interpretation. In such cases, repeat sampling of the animal would be recommended.
Conclusion
The measurement of PC-HP induced IP-10 release is a useful biomarker of the risk of M. suricattae infection in meerkat populations. Moreover, while no M. suricattae-unexposed meerkats tested IPRA-positive, numerous animals from the exposed population with known cases of TB did, suggesting that the assay shows promise as a specific test for individual animals. Ongoing work which includes longitudinal testing and confirmation of infection status seeks to confirm the assay’s utility in individual meerkats. This IPRA will have use in further clarifying the epidemiology of M. suricattae infection in meerkats and test results may be used to inform management strategies for infected populations.
Acknowledgements
We are grateful to Tim Clutton-Brock and Marta Manser for permission to work with the Kalahari Meerkat Project data. We thank Dave Gaynor, site manager for the Kalahari Research Trust for providing access to habituated animals and field facilities, and Tim Vink for assistance with the database. We are grateful to the Northern Cape Department of Environment and Nature Conservation for permission to carry out research and to the owners of farms neighbouring the Kuruman River Reserve for allowing us to work on their land. We thank Sky Bischoff-Mattson, and Lyndsay Marris, the field managers at the time of the study. We also thank Stephen Smith and Werner Fourie from the Giraffe House for blood collection of the meerkat-uninfected control group.
Funding
The work was funded by the Biotechnology and Biological Sciences Research Council (BB/F016891/1), the National Research Foundation of South Africa (SARChI in Animal TB) and the Friends of the Kalahari Meerkat Project, friends.kalahari-meerkats.com. Additional funding came from the Royal Veterinary College (publication reference PPH_01295).
Availability of data and materials
All data used in this study is available in Additional File 1.
Authors’ contributions
CC performed all laboratory work during the experimental development of ELISAs, performed statistical data analysis and co-authored the first draft of the manuscript; SJP captured and sampled free-living meerkats, performed laboratory analysis on these samples, performed statistical data analysis and co-authored the first draft of the manuscript; JAD and PDvH contributed to data analysis and revision of the manuscript; MAM and SDCP conceived and designed the study and contributed to the revision of the manuscript. All the authors have read and approved the final manuscript.
Competing interests
The authors declares that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
Ethical approval to perform the study was obtained from the University of Stellenbosch Animal Ethics Committee (Reference no. SU-ACUM14-00042).
Abbreviations
- BB
Blocking buffer
- CFP-10
10 kDa culture filtrate protein
- CMI
Cell-mediated immunity
- ELISA
Enzyme-linked immunosorbent assays
- ESAT-6
6 kDa early secretory antigenic target
- espC
Esx-1 substrate protein C
- IFN-ɣ
Interferon-gamma
- IP-10
Interferon-gamma inducible protein 10
- IPRA
IP-10 release assay
- OD
Optical density
- PBS
Phosphate buffered saline
- PWM
Pokeweed mitogen
- RD
Region of difference
- SD
Standard deviation
- TB
Tuberculosis
- TMB
Tetramethylbenzidine
- WB
Wash buffer
Additional file
IP-10 release in whole blood from Mycobacterium suricattae-unexposed meerkats (Control Group) and meerkats with a low risk (Category 1), moderate risk (Category 2) and high risk (Category 3) of infection with this pathogen. Blood was incubated at 37 °C for 24 h with phosphate buffered saline (Nil), Bovigam PC-HP peptides (HP) and pokeweed mitogen (PWM). The HP-specific (HP-Nil) and PWM-specific (PWM-Nil) IP-10 release was calculated by subtracting the Nil value from the HP and PWM values, respectively. (XLSX 21 kb)
Contributor Information
Charlene Clarke, Email: cclarke@sun.ac.za.
Stuart James Patterson, Email: spatterson@rvc.ac.uk.
Julian Ashley Drewe, Email: jdrewe@rvc.ac.uk.
Paul David van Helden, Email: pvh@sun.ac.za.
Michele Ann Miller, Email: miller@sun.ac.za.
Sven David Charles Parsons, Email: sparsons@sun.ac.za.
References
- 1.Drewe JA, Foote AK, Sutcliffe RL, Pearce GP. Pathology of Mycobacterium bovis infection in wild meerkats (Suricata suricatta) J Comp Pathol. 2009;140:12–24. doi: 10.1016/j.jcpa.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Parsons SDC, Drewe JA. Gey van Pittius NC, Warren RM, van Helden PD. Novel cause of tuberculosis in meerkats, South Africa. Emerg Infect Dis. 2013;19:2004–7. doi: 10.3201/eid1912.130268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drewe JA. Who infects whom? Social networks and tuberculosis transmission in wild meerkats. Proc R Soc B Biol Sci. 2010;277:633–42. doi: 10.1098/rspb.2009.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drewe JA, Dean GS, Michel AL, Lyashchenko KP, Greenwald R, Pearce GP. Accuracy of three diagnostic tests for determining Mycobacterium bovis infection status in live-sampled wild meerkats (Suricata suricatta) J Vet Diagn Invest. 2009;21:31–9. doi: 10.1177/104063870902100105. [DOI] [PubMed] [Google Scholar]
- 5.Clarke C, van Helden P, Miller M, Parsons S. Animal-adapted members of the Mycobacterium tuberculosis complex endemic to the southern African subregion. J S Afr Vet Assoc. 2016;87(1):a1322. doi: 10.4102/jsava.v87i1.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, Clifton-Hadley RS. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Res Vet Sci. 2006;81:190–210. doi: 10.1016/j.rvsc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Schiller I, Oesch B, Vordermeier HM, Palmer MV, Harris BN, Orloski KA, et al. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound Emerg Dis. 2010;57:205–20. doi: 10.1111/j.1865-1682.2010.01148.x. [DOI] [PubMed] [Google Scholar]
- 8.Goosen WJ, Cooper D, Miller MA, van Helden PD, Parsons SDC. IP-10 is a sensitive biomarker of antigen recognition in whole-blood stimulation assays used for the diagnosis of Mycobacterium bovis infection in African buffaloes (Syncerus caffer) Clin Vaccine Immunol CVI. 2015;22:974–8. doi: 10.1128/CVI.00324-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruhwald M, Bjerregaard-Andersen M, Rabna P, Eugen-Olsen J, Ravn P. IP-10, MCP-1, MCP-2, MCP-3, and IL-1RA hold promise as biomarkers for infection with M. tuberculosis in a whole blood based T-cell assay. BMC Res Notes. 2009;2:19. doi: 10.1186/1756-0500-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bass KE, Nonnecke BJ, Palmer MV, Thacker TC, Hardegger R, Schroeder B, et al. Clinical and diagnostic developments of a gamma interferon release assay for use in bovine tuberculosis control programs. Clin Vaccine Immunol CVI. 2013;20:1827–35. doi: 10.1128/CVI.00519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goosen WJ, Cooper D, Warren RM, Miller MA, van Helden PD, Parsons SDC. The evaluation of candidate biomarkers of cell-mediated immunity for the diagnosis of Mycobacterium bovis infection in African buffaloes (Syncerus caffer) Vet Immunol Immunopathol. 2014;162:198–202. doi: 10.1016/j.vetimm.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Clutton-Brock TH, Gaynor D, Kansky R, MacColl AD, McIlrath G, Chadwick P, et al. Costs of cooperative behaviour in suricates (Suricata suricatta) Proc R Soc B Biol Sci. 1998;265:185–90. doi: 10.1098/rspb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chegou NN, Heyckendorf J, Walzl G, Lange C, Ruhwald M. Beyond the IFN-γ horizon: biomarkers for immunodiagnosis of infection with Mycobacterium tuberculosis. Eur Respir J. 2014;43:1472–86. doi: 10.1183/09031936.00151413. [DOI] [PubMed] [Google Scholar]
- 14.Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K. False-negative results in screening programs: systematic review of impact and implications. Health Technol Assess. 2000;4:1–120. [PubMed] [Google Scholar]
- 15.Dippenaar A, Parsons SDC, Sampson SL, van der Merwe RG, Drewe JA, Abdallah AM, et al. Whole genome sequence analysis of Mycobacterium suricattae. Tuberculosis. 2015;95:682–8. doi: 10.1016/j.tube.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Millington KA, Fortune SM, Low J, Garces A, Hingley-Wilson SM, Wickremasinghe M, et al. Rv3615c is a highly immunodominant RD1 (Region of Difference 1)-dependent secreted antigen specific for Mycobacterium tuberculosis infection. Proc Natl Acad Sci U. S. A. 2011;108:5730–5. doi: 10.1073/pnas.1015153108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsons SDC, McGill K, Doyle MB, Goosen WJ, van Helden PD, Gormley E. Antigen-specific IP-10 release is a sensitive biomarker of Mycobacterium bovis infection in cattle. PLoS One. 2016;11(5):e0155440. doi: 10.1371/journal.pone.0155440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study is available in Additional File 1.


