Abstract
Background
Metastatic melanoma is an aggressive form of skin cancer with a high mortality rate and the fastest growing global incidence rate of all malignancies. The introduction of BRAF/MEK inhibitor combinations has yielded significant increases in PFS and OS for melanoma. However, at present, no direct comparisons between different BRAF/MEK combinations have been conducted. In light of this, an indirect treatment comparison was performed between two BRAF/MEK inhibitor combination therapies for metastatic melanoma, dabrafenib plus trametinib and vemurafenib plus cobimetinib, in order to understand the relative efficacy and toxicity profiles of these therapies.
Methods
A systematic literature search identified two randomized trials as suitable for indirect comparison: the coBRIM trial of vemurafenib plus cobimetinib versus vemurafenib and the COMBI-v trial of dabrafenib plus trametinib versus vemurafenib. The comparison followed the method of Bucher et al. and analyzed both efficacy (overall survival [OS], progression-free survival [PFS], and overall response rate [ORR]) and safety outcomes (adverse events [AEs]).
Results
The indirect comparison revealed similar efficacy outcomes between both therapies, with no statistically significant difference between therapies for OS (hazard ratio [HR] 0.94, 95% confidence interval [CI] 0.68 − 1.30), PFS (HR 1.05, 95% CI 0.79 − 1.40), or ORR (risk ratio [RR] 0.90, 95% CI 0.74 − 1.10). Dabrafenib plus trametinib differed significantly from vemurafenib plus cobimetinib with regard to the incidence of treatment-related AE (RR 0.92, 95% CI 0.87 − 0.97), any AE grade ≥3 (RR 0.71, 95% CI 0.60 − 0.85) or dose interruption/modification (RR 0.77, 95% CI 0.60 − 0.99). Several categories of AEs occurred significantly more frequently with vemurafenib plus cobimetinib, while some occurred significantly more frequently with dabrafenib plus trametinib. For severe AEs (grade 3 or above), four occurred significantly more frequently with vemurafenib plus cobimetinib and no severe AE occurred significantly more frequently with dabrafenib plus trametinib.
Conclusions
This indirect treatment comparison suggested that dabrafenib plus trametinib had comparable efficacy to vemurafenib plus cobimetinib but was associated with reduced adverse events.
Keywords: Dabrafenib, Trametinib, Vemurafenib, Cobimetinib, Metastatic melanoma, Indirect treatment comparison
Background
Metastatic melanoma is an uncommon but aggressive form of skin cancer, with a high mortality rate [1, 2]. Although melanoma represents less than 5% of all diagnosed skin cancers, the World Health Organization has indicated that its incidence is increasing faster than any other type of malignancy, mainly due to the general population’s increasing exposure to ultraviolet light [3–5]. Estimates put new diagnoses of melanoma at 132,000 globally in 2015 [3, 5]. The year-on-year increase in global incidence of melanoma is estimated to be between 3 and 7%; based on these estimates, thus a doubling in the incidence of melanoma occurs every 10–20 years [3].
Up to 70% of patients diagnosed with melanoma and approximately 50% of patients with the advanced form of melanoma possess a mutation in the BRAF gene, leading to aberrant activation of the mitogen-activated protein kinase (MAPK) pathway, a well-documented cancer pathway [6–9]. Patients with distant metastases and a BRAF mutation have significantly reduced median overall survival (OS) when compared with patients with distant metastases and BRAF wild-type [10]. These attributes have provided the impetus for significant drug development efforts that target BRAF-mutated metastatic melanoma.
The introduction of BRAF inhibitors such as vemurafenib and dabrafenib have yielded significantly improved outcomes in patients with metastatic melanoma with either BRAF V600E or V600K mutations [11, 12]. However, BRAF inhibitors have substantial therapeutic disadvantages. Acquired resistance to such inhibitors frequently develops due to reactivation of the MAPK pathway. This reactivation occurs primarily through three mechanisms: mutations in the upstream RAS proteins, mutant BRAF amplification, and alternative splicing mechanisms [9, 13]. This acquired resistance limits the median progression-free survival (PFS) and OS achievable with BRAF inhibitors to 6–8 months [14, 15]. In addition, the use of BRAF inhibitors may result in the development of secondary skin cancer, further limiting the therapeutic benefit of this monotherapy [11, 13, 16–20].
The addition of a MEK inhibitor along with a BRAF inhibitor can combat the BRAF inhibitor-related resistance and side effects that occur during monotherapy. This combination therapy has demonstrated an increase in median PFS and OS, along with a decrease in the incidence of BRAF-inhibited induced skin tumors [10, 16, 21]. The 2015 United States and European guidelines recommend the use of dabrafenib plus trametinib for metastatic melanoma patients with a BRAF V600 mutation [22, 23]. More recently, the Food and Drug Administration in the United States and European Medicines Agency have approved vemurafenib plus cobimetinib as a combination therapy for patients with BRAF V600E or V600K mutation-positive unresectable or metastatic melanoma [24, 25].
In the absence of evidence from head-to-head trials providing a direct comparison of treatments, health technology assessment agencies require an indirect comparison to help them in their evaluations. In addition, these types of comparisons can inform therapeutic decisions. Srivastava et al. in 2015 published an indirect treatment comparison (ITC) of dabrafenib versus vemurafenib, showing that both dabrafenib and trametinib monotherapies demonstrated comparable PFS and OS, and different tolerability and safety profiles, when indirectly compared with vemurafenib [26].
The objective of this study was to conduct an ITC between two common BRAF/MEK inhibitor combinations, dabrafenib plus trametinib and vemurafenib plus cobimetinib, in patients with metastatic melanoma without prior therapy for the metastatic disease stage in order to further understand the therapeutic and tolerability profile of these therapies.
Methods
A systematic literature review was conducted to identify published studies that would permit an ITC between dabrafenib plus trametinib and vemurafenib plus cobimetinib. From the literature review, two studies were identified that would support an ITC of dabrafenib plus trametinib versus vemurafenib plus cobimetinib using vemurafenib as a common comparator: COMBI-v, an international, open-label, randomized, Phase 3 trial of dabrafenib plus trametinib versus vemurafenib monotherapy in previously untreated patients with unresectable stage IIIC or IV melanoma with BRAF V600E or V600K mutations [27] and coBRIM, an international, multicenter, randomized Phase 3 trial of cobimetinib plus vemurafenib versus vemurafenib plus placebo in previously untreated patients with advanced BRAF-mutated melanoma [21] (Fig. 1). In both trials, vemurafenib 960 mg orally twice daily was administered in the control arm.
Fig. 1.
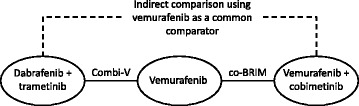
Network diagram for the indirect comparison of trametinib with vemurafenib
The ITC was conducted using the methodology described by Bucher et al. [28]. The method outlined by Bucher et al. in 1997 relies on the principle that the log of the effect size measured for drug A versus drug B is equal to the difference of the log effect size measures for drug A versus drug C and drug B versus drug C [28]. This holds true for both dichotomous outcomes, where risk ratios (RRs) and odds ratios can be used as the effect size measure, and time-to-event outcomes, where hazard ratios (HRs) can be used as the effect size measure. The principal assumption of the model proposed by Bucher et al. is that the relative efficacy of a treatment is the same in all trials included in the indirect comparison, meaning that if A and B are compared in two or more trials, then the effect size of A versus B is similar across all the trials [28]. Further, this method assumes independence between pairwise comparisons, i.e., the comparison of A versus B is independent of the comparison of B versus C [28].
The trials included in the ITC were qualitatively assessed for their patient population in terms of disease characteristics, disease stage, severity of disease, and patient characteristics. Additional subgroup analyses were conducted in cases where a difference in a patient baseline characteristic between the two trial populations was considered potentially clinically meaningful.
The efficacy outcomes that were assessed were overall response rate (ORR), PFS, and OS. The primary efficacy outcome was OS in the COMBI-v trial and PFS in the coBRIM trial. The secondary efficacy outcomes were PFS and ORR in the COMBI-v trial and ORR and OS in the coBRIM trial. Safety was assessed as a secondary endpoint in both of the trials. Multiple data sources including both published and unpublished sources were utilized to retrieve efficacy and safety data for the COMBI-v and coBRIM trials (Table 1).
Table 1.
Sources and data cut-offs used in primary and additional ITCs
| Outcome | COMBI-v | coBRIM | ||
|---|---|---|---|---|
| Data cut-off | Data source | Data cut-off | Data source | |
| Efficacy outcomes | ||||
| OS | Primary Analysis —March 2015 Additional analysis (without crossover)—April 2014 Additional analysis (LDH subgroups)—March 2015 |
Robert 2015b [29] Robert 2015a [27] Novartis Pharmaceuticals Corporation, unpublished observations |
August 2015 | Atkinson 2015 [30] |
| PFS | Primary analysis—March 2015 | Robert 2015b [29] | January 2015 | Larkin 2015c [32] |
| Additional analysis LDH subgroups—April 2014 |
Novartis Pharmaceuticals Corporation, unpublished observations | |||
| ORR | April 2014 | Robert 2015a [27] | January 2015 | Larkin 2015c [32] |
| General adverse events | ||||
| Any AE, any SAE, discontinuation due to AE, AE leading to death, any grade ≥3 AE | March 2015 | Robert 2015b [29] and Novartis Pharmaceuticals Corporation, unpublished observations |
September 2014 | EMA label [33, 34] |
| Any treatment-related AE, any dose interruptions/modifications | April 2014 | Robert 2015a [27] | ||
| Specific adverse events | ||||
| All specific adverse events except those highlighted in the row below | March 2015 | Robert 2015b [29] and Novartis Pharmaceuticals Corporation, unpublished observations |
September 2014 | EMA label |
| Keratocanthoma | May 2014 | Larkin 2014 [21] | ||
| CuSCC—all grades | April 2014 | |||
| Chills—all grades grade 3 to 5: alopecia, nausea, pyrexia, vomiting | March 2015 | May 2014 | EMA label | |
AE Adverse event, CuSS cutaneous squamous cell carcinoma, D + T dabrafenib plus trametinib, ECOG Eastern Cooperative Oncology Group, EMA European Medicines Agency, ITC Indirect treatment comparison, LDH lactate dehydrogenase, OS Overall survival, ORR Overall response rate, PFS Progression-free survival, SAE Severe adverse event, V vemurafenib, V + C vemurafenib plus cobimetinib
The primary ITC for OS and PFS was based on the most recent data cut-off dates; March 2015 for COMBI-v [29] and August 2015 for coBRIM [30] (Table 1). The primary ITC for ORR was based on the April 2014 data cut-off for COMBI-v and the January 2015 data cut-off for coBRIM (Table 1). Of note, crossover was permitted in COMBI-v, following the recommendation by the Independent Data Monitoring Committee (IDMC) based on the planned interim results, whereas no crossover was permitted in coBRIM. The interim analysis was conducted using COMBI-v at the cut-off date of April 2014. To assess whether the crossover might have confounded OS results of the primary ITC, an additional ITC for OS was conducted using the COMBI-v interim data cut-off of April 2014, the point at which no patients had crossed over, and the August 2015 cut-off for coBRIM.
Two other additional ITCs were conducted for OS and PFS in two subgroup populations, i.e., patients with normal and elevated lactate dehydrogenase (LDH) levels, to assess the impact of any variation in the baseline LDH levels on the primary ITC results.
The effect sizes for indirect comparisons were calculated using the methodologies proposed by Bucher et al. [28]. The 95% confidence interval (CI) values and p values for the effect sizes were calculated using Cochran-Mantel-Haenszel statistics. All calculations were conducted using STATA® software (version 11).
Results
The baseline epidemiological and disease characteristics of the patient cohorts in the COMBI-v and coBRIM studies have been reported previously and are summarized in Table 2 [21, 27]. Baseline patient characteristics, including known prognostic factors, were generally well balanced in all the treatment arms of both studies, except that slightly more patients in the coBRIM trial had elevated serum LDH at baseline. Thirty-three percent of patients presented with elevated LDH levels (dabrafenib plus trametinib [34%] and vemurafenib [32%]) in the COMBI-v trial, while the coBRIM trial had 46% of patients with elevated LDH levels (vemurafenib plus cobimetinib [46%] and vemurafenib [43%]).
Table 2.
Baseline characteristics of patients in the COMBI-v and coBRIM studies
| COMBI-v | coBRIM | |||
|---|---|---|---|---|
| D + T | V | V + placebo | V + C | |
| Intent-to-treat population | 352 | 352 | 248 | 247 |
| Age, median (range), yr | 55(18–91) | 54(18–88) | 55(25–85) | 56(23–88) |
| Male sex, no. (%) | 208(59) | 180(51) | 140(56) | 146(59) |
| ECOG score, no./total no. (%) | ||||
| 0 | 248/350(71) | 248/352(70) | 164/244(67) | 184/243(76) |
| 1 | 102/350(29) | 14/352(30) | 80/244(33) | 58/243(24) |
| 2 | 0/350 | 0/352 | 0/244 | 1/243(<1) |
| Metastatic status, no./total no. (%) | ||||
| M0 | 14/351(4) | 26/351(7) | 13(5) | 21(9) |
| M1a | 55/351(16) | 50/351(14) | 40(16) | 40(16) |
| M1b | 61/351(17) | 67/351(19) | 42(17) | 40(16) |
| M1c | 221/351(63) | 208/351(59) | 153(62) | 146(59) |
| Elevated LDH, no. /total no. (%) | 118/351(34) | 114/352(32) | 104/242(43) | 112/242(46) |
| BRAF mutation, no. /total no. (%) | ||||
| V600E | 312/346(90) | 317/351(90) | 174/206(84) | 170/194(88) |
| V600K | 34/346(10) | 34 /351(10) | 32/206(16) | 24/194(12) |
D + T dabrafenib plus trametinib, ECOG Eastern Cooperative Oncology Group, LDH lactate dehydrogenase, V vemurafenib, V + C vemurafenib plus cobimetinib
Efficacy
In the primary ITC, the HR (for OS and PFS) or RR (for ORR) for dabrafenib plus trametinib versus vemurafenib plus cobimetinib was statistically non-significant (Table 3). For OS and PFS, a HR of 0.94 (95% CI 0.68 − 1.30; p = 0.7227) and 1.05 (95% CI 0.79 − 1.40; p = 0.730), respectively, was observed, while ORR had a RR of 0.90 (95% CI 0.74 − 1.10; p = 0.3029) (Table 3). These p values and CIs for efficacy outcomes suggested comparable efficacy profiles for the two combination therapies.
Table 3.
Comparison of efficacy for dabrafenib plus trametinib versus vemurafenib plus cobimetinib
| Outcome | COMBI-v | coBRIM | ITC results | |||||
|---|---|---|---|---|---|---|---|---|
| D + T | V | V | V + C | HR/RRa | LCI | UCI | p value | |
| Overall survival, median (95% CI), months | 25.6(22.6 − NR) | 18.0(15.6 − 20.7) | 17.4(15.0 − 19.8) | 22.3(20.3 − NR) | 0.94 | 0.68 | 1.30 | 0.7227 |
| Progression-free survival, median (95% CI), months | 12.6(10.7 − 15.5) | 7.3(5.8 − 7.8) | 7.2(5.6 – 7.5) | 12.3(9.5 – 13.4) | 1.05 | 0.79 | 1.40 | 0.7300 |
| Overall response rate, no./total no. (%) | 226/352(64%) | 180/352(51%) | 124/248(70%) | 172/247(50%) | 0.90 | 0.74 | 1.10 | 0.3029 |
aFor D + T versus V + C; HR is the output for time-to-event outcomes, i.e., overall survival and progression-free survival; RR is the output for overall response rate
CI confidence interval, D + T dabrafenib plus trametinib, HR hazard ratio, NR not reached, RR risk ratio, LCI lower 95% CI, UCI upper 95% CI, V vemurafenib, V + C vemurafenib plus cobimetinib
To determine if the crossover might have confounded the results of the primary ITC, the additional analysis was conducted using pre-crossover data for COMBI-v (i.e., April 2014 data cut-off) and the August 2015 cut-off for coBRIM. Similar results were shown, i.e., no significant difference in HR between the two combination therapies (HR [95% CI] 0.99 [0.69, 1.41]).
Two other additional subgroup analyses were conducted for OS and PFS outcomes for two subgroup populations, namely, patients with normal and elevated LDH levels at baseline. The ITC results did not show significant differences between dabrafenib plus trametinib and vemurafenib plus cobimetinib for either subgroup in terms of OS (normal LDH levels, HR = 0.95 [95% CI 0.58 − 1.54]; elevated LDH levels, HR = 1.05 [95% CI 0.67 − 1.65]) and PFS (normal LDH levels, HR = 1.05 [95% CI 0.67 − 1.65]; elevated LDH levels, HR = 1.23 [95% CI 0.81 − 1.87]).
Safety
Based on the ITC, the overall toxicity profile of dabrafenib plus trametinib appeared to be better than that of vemurafenib plus cobimetinib. The incidence of any treatment-related adverse event (AE) (RR 0.92, 95% CI 0.87 − 0.97; p = 0.0015), incidence of any AE of grade ≥3 (RR 0.71; 95% CI 0.60 − 0.85; p = 0.0002), as well as the incidences of dose interruption or dose modification (RR 0.77, 95% CI 0.60 − 0.99; p = 0.0471) were all significantly lower with dabrafenib plus trametinib when compared with vemurafenib plus cobimetinib (Table 4). There were no significant differences between dabrafenib plus trametinib and vemurafenib plus cobimetinib with regard to the incidences of any AE (RR 0.98, 95% CI 0.96 − 1.01; p = 0.3078), any serious AE (RR 0.84; 95% CI 0.60 − 1.16; p = 0.2835), or any AE leading to death or the rate of discontinuation due to an AE (RR 0.62; 95% CI 0.33 − 1.16; p = 0.135) (Table 4).
Table 4.
Comparison of general AEs for dabrafenib plus trametinib versus vemurafenib plus cobimetinib
| Incidence, number (%) | ITC results | |||||||
|---|---|---|---|---|---|---|---|---|
| COMBI-v | coBRIM | |||||||
| AE type | D + T (n = 350) | V (n = 349) | V (n = 246) | V + C (n = 247) | RR | LCI | UCI | p value |
| Any AE | 345(98.6) | 345(98.9) | 240(97.6) | 244(98.8) | 0.98 | 0.96 | 1.01 | 0.3078 |
| Any serious AE | 151(43.1) | 136(39.0) | 64(26.0) | 85(34.4) | 0.84 | 0.60 | 1.16 | 0.2835 |
| Any treatment-related AE | 320(91.4) | 342(98.0) | 232(94.3) | 237(96.0) | 0.92 | 0.87 | 0.97 | 0.0015 |
| AE leading to death | 4(1.1) | 4(1.2) | 3(1.2) | 5(2.0) | 0.60 | 0.08 | 4.35 | 0.6137 |
| Any grade ≥3 AE | 199 (56.9) | 232 (66.5) | 146 (59.4) | 176 (71.3) | 0.71 | 0.60 | 0.85 | 0.0002 |
| Any dose interruptions/modifications | 192(54.9) | 197(56.5) | 87(35.4) | 110(44.5) | 0.77 | 0.60 | 1.00 | 0.0471 |
| Discontinuation due to AE | 55(15.7) | 48(13.8) | 20(8.1) | 37(15.0) | 0.62 | 0.33 | 1.16 | 0.1350 |
AE adverse event, CI confidence interval, D + T dabrafenib plus trametinib, ITC indirect treatment comparison, RR risk ratio, LCI lower 95% CI, UCI upper 95% CI, V vemurafenib, V + C vemurafenib plus cobimetinib
With regard to individual AEs, some AEs occurred at a significantly higher incidence with vemurafenib plus cobimetinib compared with dabrafenib plus trametinib, including (in alphabetical order) alopecia, arthralgia, blurred vision, increased blood creatinine, diarrhea, dry skin, dysgeusia, increased alanine transaminase (ALT) and aspartate transaminase (AST), keratosis pliaris, nausea, photosensitivity reaction, pruritus, rash, rash maculopapular, skin papilloma, and sun burn (Table 5). Some AEs occurred more frequently with dabrafenib plus trametinib compared with vemurafenib plus cobimetinib: chills, constipation, cough, and pyrexia.
Table 5.
Comparison of individual AEs of any grade for dabrafenib plus trametinib versus vemurafenib plus cobimetinib
| Incidence, number (%) | ITC results | |||||||
|---|---|---|---|---|---|---|---|---|
| COMBI-v | coBRIM | |||||||
| AE type | D + T (n = 350) | V (n = 349) | V (n = 246) | V + C (n = 247) | RR | LCI | UCI | p value |
| Abdominal pain | 39(11.1) | 32(9.2) | 19(7.7) | 25(10.1) | 0.93 | 0.45 | 1.91 | 0.8378 |
| Alopecia | 23(6.6) | 136(39) | 73(29.7) | 37(15.0) | 0.33 | 0.19 | 0.58 | 0.0001 |
| Anemia | 26(7.4) | 21(6.0) | 20(8.1) | 32(13.0) | 0.77 | 0.36 | 1.67 | 0.5147 |
| Arthralgia | 93(26.6) | 182(52.2) | 99(40.2) | 89(36.0) | 0.57 | 0.42 | 0.77 | 0.0003 |
| Asthenia | 61(17.4) | 58(16.6) | 40(16.3) | 43(17.4) | 0.98 | 0.59 | 1.63 | 0.9368 |
| Blurred vision | 17(4.9) | 18(5.2) | 6(2.4) | 25(10.1) | 0.23 | 0.08 | 0.67 | 0.0075 |
| Increased blood creatinine | 15(4.3) | 37(10.6) | 20(8.1) | 34(13.8) | 0.24 | 0.11 | 0.52 | 0.0003 |
| Chills | 116(33.1) | 28(8.0) | 12(5.0)a | 20(7.9)# | 2.63 | 1.19 | 5.82 | 0.0167 |
| Chorioretinopathy | 2(<1) | 1(<1) | 1(<1) | 31(12.6) | 0.06 | 0.00 | 1.45 | 0.0843 |
| Constipation | 54(15.4) | 25(7.2) | 26(10.6) | 24(9.7) | 2.34 | 1.17 | 4.68 | 0.0160 |
| Cough | 77(22.0) | 40(11.5) | 30(12.2) | 19(7.7) | 3.04 | 1.59 | 5.83 | 0.0008 |
| Cutaneous squamous cell carcinoma | 5(1.4) | 63(18.1) | 27(11.3)a | 7(2.8)# | 0.32 | 0.10 | 1.09 | 0.0685 |
| Decreased appetite | 44(12.6) | 70(20.1) | 50(20.3) | 46(18.6) | 0.68 | 0.42 | 1.13 | 0.1361 |
| Dermatitis acneiform | 23(6.6) | 20(5.7) | 22(8.9) | 34(13.8) | 0.75 | 0.34 | 1.61 | 0.4539 |
| Diarrhea | 120(34.3) | 136(39.0) | 82(33.3) | 150(60.7) | 0.48 | 0.36 | 0.64 | <0.0001 |
| Dry skin | 33(9.4) | 67(19.2) | 39(15.9) | 35(14.2) | 0.55 | 0.31 | 0.97 | 0.0407 |
| Dysgeusia | 23(6.6) | 47(13.5) | 26(10.6) | 37(15.0) | 0.34 | 0.18 | 0.67 | 0.0018 |
| Edema peripheral | 48(13.7) | 42(12.0) | 28(11.4) | 31(12.6) | 1.03 | 0.56 | 1.91 | 0.9165 |
| Erythema | 35(10.0) | 42(12.0) | 33(13.4) | 24(9.7) | 1.15 | 0.60 | 2.20 | 0.6795 |
| Fatigue | 110(31.4) | 117(33.5) | 80(32.5) | 85(34.4) | 0.89 | 0.64 | 1.23 | 0.4697 |
| Headache | 112(32.0) | 84(24.1) | 39(15.9) | 41(16.6) | 1.27 | 0.80 | 2.03 | 0.3172 |
| Hyperkeratosis | 18(5.1) | 89(25.5) | 75(30.5) | 27(10.9) | 0.56 | 0.30 | 1.06 | 0.0734 |
| Hypertension | 103(29.4) | 82(23.5) | 19(7.7) | 37(15.0) | 0.65 | 0.36 | 1.15 | 0.1399 |
| Increased ALT | 49(14.0) | 61(17.5) | 44(17.9) | 65(26.3) | 0.54 | 0.34 | 0.88 | 0.013 |
| Increased AST | 42(12.0) | 46(13.2) | 31(12.6) | 60(24.3) | 0.47 | 0.27 | 0.82 | 0.008 |
| Increased blood ALP | 26(7.4) | 30(8.6) | 22(8.9) | 36(14.6) | 0.53 | 0.26 | 1.08 | 0.0799 |
| Increased blood CPK | 10(2.9) | 2(<1) | 7(2.9) | 80(32.4) | 0.44 | 0.08 | 2.37 | 0.3378 |
| Increased GGT | 38(10.9) | 33(9.5) | 44(17.9) | 54(21.9) | 0.94 | 0.53 | 1.66 | 0.8292 |
| Keratocanthoma | 2(<1) | 35(10.0) | 20(8.4)a | 2(<1)# | 0.61 | 0.08 | 4.58 | 0.6269 |
| Keratosis pilaris | 4(1.1) | 48(13.8) | 26(10.6) | 8(3.2) | 0.27 | 0.08 | 0.97 | 0.0442 |
| Myalgia | 66(18.8) | 56(16.1) | 30(12.2) | 28(11.3) | 1.26 | 0.71 | 2.26 | 0.4298 |
| Nausea | 126(36.0) | 130(37.3) | 62(25.2) | 102(41.3) | 0.59 | 0.43 | 0.82 | 0.0015 |
| Pain in extremity | 45(12.9) | 44(12.6) | 35(14.2) | 24(9.7) | 1.49 | 0.80 | 2.79 | 0.2079 |
| Photosensitivity reaction | 15(4.3) | 81(23.2) | 93(37.8) | 118(47.8) | 0.15 | 0.08 | 0.26 | <0.0001 |
| Pruritus | 36(10.3) | 78(22.4) | 46(18.7) | 48(19.4) | 0.44 | 0.26 | 0.74 | 0.0020 |
| Pyrexia | 193(55.1) | 74(21.2) | 56(22.8) | 69(27.9) | 2.12 | 1.45 | 3.09 | 0.0001 |
| Rash | 84(24.0) | 150(43.0) | 94(38.2) | 98(39.7) | 0.54 | 0.39 | 0.74 | 0.0001 |
| Rash maculopapular | 13(3.7) | 28(8.0) | 38(15.5) | 38(15.4) | 0.46 | 0.22 | 1.00 | 0.0490 |
| SCC of skin | 2(<1) | 21(6.0) | 31(12.6) | 8(3.2) | 0.37 | 0.07 | 1.88 | 0.2310 |
| Skin papilloma | 8(2.3) | 82(23.5) | 29(11.8) | 12(4.9) | 0.24 | 0.09 | 0.62 | 0.0033 |
| Sun burn | 3(<1) | 51(14.6) | 43(17.5) | 34(13.8) | 0.07 | 0.02 | 0.25 | <0.0001 |
| Vomiting | 107(30.6) | 55(15.8) | 31(12.6) | 60(24.3) | 1.01 | 0.62 | 1.64 | 0.9798 |
a N = 239; # N = 254
AE adverse event, ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, CI confidence interval, CPK creatine phosphokinase, GGT gamma-glutamyltransferase, LCI lower 95% CI, RR risk ratio, SCC squamous cell carcinoma, UCI upper 95% CI
Focusing on individual AEs that were graded as severe (grade 3 or above), the incidence was in many cases similar between dabrafenib plus trametinib and vemurafenib plus cobimetinib (Table 6). A few AEs occurred more frequently with vemurafenib plus cobimetinib than with dabrafenib plus trametinib: increased ALT, increased AST, rash maculopapular, and rash.
Table 6.
Comparison of individual AEs of >grade 3 for dabrafenib plus trametinib versus vemurafenib plus cobimetinib
| Incidence (% patients) | ITC results | |||||||
|---|---|---|---|---|---|---|---|---|
| COMBI-v | coBRIM | |||||||
| AE type | D + T (n = 350) | V (n = 349) | V (n = 239) | V + T (n = 254) | RR | LCI | UCI | p value |
| Alopecia | 0(0) | 1(<1) | 1(<1) | 1(<1) | 0.35 | 0.01 | 24.22 | 0.6295 |
| Anemia | 7(2.0) | 4(1.2) | 6(2.4) | 4(1.6) | 2.63 | 0.46 | 15.10 | 0.2787 |
| Arthralgia | 3(<1) | 15(4.3) | 12(4.9) | 6(2.4) | 0.40 | 0.08 | 1.91 | 0.2513 |
| Asthenia | 5(1.4) | 4(1.2) | 3(1.2) | 5(2.0) | 0.75 | 0.11 | 5.17 | 0.7711 |
| Cutaneous squamous cell carcinoma | 5(1.4) | 62(17.8) | 27(11.3) | 6(2.4) | 0.38 | 0.11 | 1.34 | 0.1337 |
| Dermatitis acneiform | 0(0) | 4(1.2) | 3(1.2) | 6(2.4) | 0.06 | 0.00 | 1.40 | 0.0792 |
| Diarrhea | 4(1.1) | 2(<1) | 2(<1) | 16(6.5) | 0.25 | 0.03 | 2.34 | 0.2242 |
| Fatigue | 4(1.1) | 7(2.0) | 7(2.9) | 10(4.1) | 0.40 | 0.09 | 1.88 | 0.2459 |
| Hyperkeratosis | 0(0) | 2(<1) | 6(2.4) | 0(0) | 2.60 | 0.04 | 169.50 | 0.6534 |
| Hypertension | 54(15.4) | 33(9.5) | 6(2.4) | 11(4.5) | 0.89 | 0.31 | 2.58 | 0.8353 |
| Increased ALT | 9(2.6) | 15(4.3) | 15(6.1) | 28(11.3) | 0.32 | 0.12 | 0.88 | 0.0280 |
| Increased AST | 5(1.4) | 9(2.6) | 5(2.0) | 21(8.5) | 0.13 | 0.03 | 0.56 | 0.0062 |
| Increased blood ALP | 7(2.0) | 5(1.4) | 4(1.6) | 10(4.1) | 0.56 | 0.11 | 2.82 | 0.4826 |
| Increased blood CPK | 6(1.7) | 1(<1) | 0(0) | 28(11.3) | 0.11 | 0.00 | 3.49 | 0.2076 |
| Increased GGT | 19(5.4) | 17(4.9) | 25(10.2) | 32(13.0) | 0.87 | 0.39 | 1.96 | 0.7436 |
| Keratocanthoma | 2(<1) | 35(10.0) | 20(8.1) | 3(1.2) | 0.38 | 0.06 | 2.44 | 0.3091 |
| Maculopapular rash | 2(<1) | 13(3.7) | 13(5.3) | 17(6.9) | 0.12 | 0.02 | 0.61 | 0.0105 |
| Myalgia | 0(0) | 4(1.2) | 6(2.4) | 1(<1) | 0.67 | 0.02 | 24.45 | 0.8258 |
| Nausea | 1(<1) | 1(<1) | 2(<1) | 2(<1) | 0.53 | 0.02 | 11.65 | 0.6871 |
| Pain in extremity | 4(1.1) | 2(<1) | 6(2.4) | 3(1.2) | 4.00 | 0.45 | 35.40 | 0.2121 |
| Photosensitivity reaction | 0(0) | 1(<1) | 0(0) | 7(2.8) | 0.02 | 0.00 | 1.62 | 0.0820 |
| Pyrexia | 16(4.6) | 2(<1) | 0(0) | 4(1.6) | 0.94 | 0.04 | 24.60 | 0.9712 |
| Rash | 3(<1) | 30(8.6) | 14(5.7) | 13(5.3) | 0.11 | 0.03 | 0.43 | 0.0017 |
| SCC of skin | 2(<1) | 20(5.7) | 31(12.6) | 7(2.8) | 0.44 | 0.08 | 2.32 | 0.3349 |
| Vomiting | 4(1.1) | 3(<1) | 3(1.3) | 3(1.2) | 0.94 | 0.09 | 9.60 | 0.9598 |
AE adverse event, ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, CI confidence interval, CPK creatine phosphokinase, GGT gamma-glutamyltransferase, LCI lower 95% CI, RR risk ratio, SCC squamous cell carcinoma, UCI upper 95% CI
Discussion
We applied an established methodology [28] to a simple network based on two trials that were similar in terms of patient populations and trial protocol. The comparison indicated that dabrafenib plus trametinib had comparable efficacy as vemurafenib plus cobimetinib in patients with BRAF-mutated metastatic melanoma, with no statistically significant difference in ORR, PFS, and OS. Comparison of the two combination therapies in terms of AEs found that dabrafenib plus trametinib was associated with a better safety profile and a lower occurrence of AEs. A wide variety of individual AEs occurred more frequently with vemurafenib plus cobimetinib, while fewer occurred more frequently with dabrafenib plus trametinib. When individual severe AEs (grade 3 or above) were compared between treatments, a few occurred more frequently with vemurafenib plus cobimetinib compared with dabrafenib plus trametinib (p < 0.05), and no severe AE was observed to occur more frequently with dabrafenib plus trametinib.
The time-to-event outcomes including OS and PFS were evaluated using the most recent data cut-offs of both COMBI-v and coBRIM with different follow-up duration. Specifically, the duration of follow-up was 19 months for dabrafenib plus trametinib and 15 months for vemurafenib in COMBI-v, and 20.6 months for vemurafenib plus cobimetinib and 16.6 months for vemurafenib in coBRIM. Based on the proportional hazards assumption, it can be assumed that variation in the follow-up times between the COMBI-v and coBRIM trials had no significant effect on the results.
The COMBI-v trial allowed crossover following IDMC recommendation, which might have been a confounding factor for the primary ITC. An additional ITC that was conducted using pre-crossover data for COMBI-v (April 2014 data cut-off) and the August 2015 cut-off for coBRIM showed no difference in OS between the two combination therapies, suggesting minimal impact of crossover on the primary ITC.
There was some variation in baseline LDH level between two trials, i.e., COMBI-v had slightly lower proportion of patients with elevated LDH than coBRIM (i.e., COMBI-v: 34% with dabrafenib plus trametinib and 32% with vemurafenib; coBRIM: 46% with vemurafenib plus cobimetinib and 43% with vemurafenib). The additional analyses conducted for OS and PFS outcomes in two subgroup populations, namely patients with normal and elevated LDH levels, showed similar results as the primary ITC in overall population. Specifically, there were no significant differences between dabrafenib plus trametinib and vemurafenib plus cobimetinib for OS and PFS within the subgroup populations defined according to the baseline LDH levels, suggesting that the overall results were not confounded by variation in the distribution of baseline LDH levels.
The advantage of following the method outlined by Bucher et al. [28] is that the randomization of the individual studies is partially retained. Nonetheless, the evidence provided by an ITC is not as strong as that provided by a direct randomized head-to-head trial between the two treatments; the evidence should be considered with this in mind and interpreted with caution. Additionally, concern has been expressed about the suitability of using ITC to compare safety data, due to their non-dichotomous nature. However, it should be noted that health technology agencies such as the French Haute Autorité de Santé and the Agency for Healthcare Research and Quality recommend the use of Bucher’s ITC method for both efficacy and safety outcomes. Additionally, one study has been published using this method when indirectly comparing safety outcomes [31].
Consideration should also be given to the fact that while the ITC indicates a better safety profile of dabrafenib plus trametinib in terms of the frequency of adverse events, this may not translate into a real-world patient experience, where, for example, certain grade 2 adverse events may have a greater impact on a patient’s quality life than a grade 3-elevated AST/ALT. The safety results presented in this manuscript should therefore be interpreted with caution until data from a direct head-to-head trial can provide further insights for physicians. Additionally, COMBI-v and co BRIM trials had different levels of dose interruptions/modifications this may have impacted on the severity of toxicity profiles experienced in both studies.
Conclusions
In conclusion, in the absence of direct, head-to-head treatment comparisons, ITCs such as the one conducted in this study provided useful information for physicians when evaluating available options of BRAF/MEK inhibitor combinations in order to choose the most suitable treatment for patients, albeit with an understanding of the limitations of such an analysis. The ITC that compared dabrafenib plus trametinib with vemurafenib plus cobimetinib suggested similar efficacies between two combination therapies but reduced adverse events associated with dabrafenib plus trametinib.
Acknowledgements
Editorial support was provided by Nick Rusbridge and Peter Gray of PAREXEL, which received payment from Novartis Pharmaceuticals Corporation in connection with the conduct of this study and the preparation of the manuscript.
Funding
The performance of the study and the writing of the manuscript were funded by Novartis Pharmaceuticals Corporation.
Availability of data and materials
The datasets used during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
AA and LC designed the systematic literature review and indirect treatment comparison. JG, SK, and AA performed the systematic literature review and indirect treatment comparison. AD, LC, and AA interpreted the results of the indirect treatment comparison. AD and LC developed the manuscript. All authors read and approved the final manuscript.
Competing interests
AD has served on advisory boards to, and received research funding from, Novartis Pharmaceuticals Corp., GSK, Pfizer Inc., Merck & Co. Inc., and Roche Pharmaceuticals, and has served on an advisory board to Oncosec Inc., of which he holds stock options. LC is an employee of Novartis Pharmaceuticals Corporation and holds the stock options of Novartis. JG, SK, and AA are employees of PAREXEL, which received payment from Novartis Pharmaceuticals Corporation in connection with the conduct of this study and preparation of the manuscript.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- AE
Adverse event
- ALT
Alanine transaminase
- AST
Aspartate aminotransferase
- CI
Confidence interval
- GGT
Gamma-glutamyltransferase
- HR
Hazard ratio
- ITC
Indirect treatment comparison
- LDH
Lactate dehydrogenase
- MAPK
Mitogen-activated protein kinase
- ORR
Overall response rate
- OS
Overall survival
- PFS
Progression-free survival
- RR
Risk ratio
References
- 1.Algazi AP, Soon CW, Daud AI. Treatment of cutaneous melanoma: current approaches and future prospects. Cancer Manag Res. 2010;2:197–211. doi: 10.2147/CMR.S6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finn L, Markovic SN, Joseph RW. Therapy for metastatic melanoma: the past, present, and future. BMC Med. 2012;10:23. doi: 10.1186/1741-7015-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150:179–85. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Melanoma Skin Cancer. 2016. http://www.cancer.org/cancer/skincancer-melanoma/. Accessed 20 Jul 2016.
- 5.Erdmann F, Lortet-Tieulent J, Schüz J, Zeeb H, Greinert R, Breitbart EW, et al. International trends in the incidence of malignant melanoma 1953–2008—are recent generations at higher or lower risk? Int J Cancer. 2013;132:385–400. doi: 10.1002/ijc.27616. [DOI] [PubMed] [Google Scholar]
- 6.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 7.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 8.Lin K, Baritaki S, Militello L, Malaponte G, Bevelacqua Y, Bonavida B. The role of B-RAF mutations in melanoma and the induction of EMT via dysregulation of the NF-κB/Snail/RKIP/PTEN circuit. Genes Cancer. 2010;1:409–20. doi: 10.1177/1947601910373795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nissan MH, Solit DB. The “SWOT” of BRAF inhibition in melanoma: RAF inhibitors, MEK inhibitors or both? Curr Oncol Rep. 2011;13:479–87. doi: 10.1007/s11912-011-0198-4. [DOI] [PubMed] [Google Scholar]
- 10.Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239–46. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 11.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 13.Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–15. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi H, Hugo W, Kong X, Hong A, Koya RC, Moriceau G, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Allen EM, Wagle N, Sucker A, Treacy DJ, Johannessen CM, Goetz EM, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4:94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert C, Arnault JP, Mateus C. RAF inhibition and induction of cutaneous squamous cell carcinoma. Curr Opin Oncol. 2011;23:177–82. doi: 10.1097/CCO.0b013e3283436e8c. [DOI] [PubMed] [Google Scholar]
- 18.Carnahan J, Beltran PJ, Babij C, Le Q, Rose MJ, Vonderfecht S, et al. Selective and potent Raf inhibitors paradoxically stimulate normal cell proliferation and tumor growth. Mol Cancer Ther. 2010;9:2399–410. doi: 10.1158/1535-7163.MCT-10-0181. [DOI] [PubMed] [Google Scholar]
- 19.Oberholzer PA, Kee D, Dziunycz P, Sucker A, Kamsukom N, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol. 2012;30:316–21. doi: 10.1200/JCO.2011.36.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boussemart L, Routier E, Mateus C, Opletalova K, Sebille G, Kamsu-Kom N, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691–7. doi: 10.1093/annonc/mdt015. [DOI] [PubMed] [Google Scholar]
- 21.Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–76. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 22.Dummer R, Hauschild A, Lindenblatt N, Pentheroudakis G, Keilholz U. ESMO Guidelines Committee. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v126–32. doi: 10.1093/annonc/mdv297. [DOI] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network. NCCN Guidelines Version 2.2015 - Melanoma. https://www.nccn.org/professionals/physician_gls/PDF/melanoma.pdf. Accessed 20 Jul 2016.
- 24.European Medicines Agency. EPAR summary for the public. Cotellic, cobimetinib. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/003960/WC500198615.pdf. Accessed 20 Jul 2016.
- 25.Food US, Administration D. FDA approves Cotellic as part of combination treatment for advanced melanoma. 2015. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm471934.htm. Accessed 20 Jul 2016.
- 26.Srivastava K, Amonkar MM, Ahuja A, Stapelkamp C, Swann S, Casey M, et al. Systematic review and indirect treatment comparison of dabrafenib and trametinib versus other treatments used in previously untreated metastatic melanoma patients. J Clin Exper Dermatol Res. 2015;6:294. doi: 10.4172/2155-9554.10000294. [DOI] [Google Scholar]
- 27.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–9. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 28.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–91. doi: 10.1016/S0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 29.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroyakovskiy D, et al. Two-year estimate of overall survival in COMBI-v, a randomized, open-label, phase 3 study comparing the combination of dabrafenib and trametinib vs vemurafenib as first-line therapy in patients with unresectable or metastatic BRAF V600E/K mutation-positive cutaneous melanoma. European Cancer Congress, 25–29 September, 2015b, abstract 3301.
- 30.Atkinson V, Larkin J, McArthur G, Ribas A, Ascierto P, et al. (2015b) Improved overall survival (OS) with cobimetinib (COBI) + vemurafenib (V) in advanced BRAF-mutated melanoma and biomarker correlates of efficacy. Presented at the annual meeting of the Society for Melanoma Research held November 6–9, 2015 in Boston, Massachusetts.
- 31.Tang J, Zhang H, Yan J, Shao R. (2015) Indirect comparison of the efficacy and safety of gefitinib and cetuximab-based therapy in patients with advanced non-small-cell lung cancer. Mol Clin Oncol. 3(1): 145–150. [DOI] [PMC free article] [PubMed]
- 32.Larkin JMG, Yan Y, McArthur GA, Ascierto PA, Liszkay G, et al. (2015c) Update of progression-free survival (PFS) and correlative biomarker analysis from coBRIM: Phase III study of cobimetinib (cobi) plus vemurafenib (vem) in advanced BRAF-mutated melanoma. J Clin Oncol. 33(15) suppl; abstr 9006.
- 33.European Medicines Agency . Cotellic, INN-Cobimetinib - Label. 2015. [Google Scholar]
- 34.Dréno B, Ascierto PA, McArthur GA, Ribas A, Eng S, Hsu JJ, et al. Adverse event (AE) incidence rates with cobimetinib (C) plus vemurafenib (V) treatment: extended follow-up (f/u) of the phase III coBRIM study. American Society of Clinical Oncology Annual Meeting, Chicago, IL, USA, 3–7 June 2016, abstract 9533. http://meetinglibrary.asco.org/content/165055-176. Accessed 20 July 2016.32.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during and/or analyzed during the current study are available from the corresponding author on reasonable request.


