Abstract
Introduction
Operable oesophagogastric adenocarcinoma management in the UK includes three cycles of neoadjuvant chemotherapy (NAC) followed by resection. Determination of oxygen uptake at the anaerobic threshold (AT) with cardiopulmonary exercise testing (CPET) is used to objectively measure cardiorespiratory reserve. Oxygen uptake at AT predicts perioperative risk, with low values associated with increased morbidity. Previous studies indicate NAC may have a detrimental impact on cardiorespiratory reserve.
Methods
CPET was completed by 30 patients before and after a standardised NAC protocol. The ventilatory AT was determined using the V-slope method, and the peak oxygen uptake and ventilatory equivalents for carbon dioxide measured. Median AT before and after chemotherapy was compared using a paired Student’s t-test.
Results
Median oxygen uptake at AT pre- and post-NAC was 13.9±3.1 ml/kg/min and 11.5±2.0 ml/kg/min, respectively. The mean decrease was 2.4 ml/kg/min (95% confidence interval [CI] 1.3–3.85; p<0.001). Median peak oxygen delivery also decreased by 2.17 ml/kg/min (95% CI 1.02–3.84; p=0.001) after NAC. Ventilatory equivalents were unchanged.
Conclusions
This reduction in AT objectively quantifies a decrease in cardiorespiratory reserve after NAC. Patients with lower cardiorespiratory reserve have increased postoperative morbidity and mortality. Preventing this decrease in cardiorespiratory reserve during chemotherapy, or optimising the timing of surgical resection after recovery of AT, may allow perioperative risk-reduction.
Keywords: Exercise test, Neoadjuvant chemotherapy, gastrointestinal neoplasms, Oesophagectomy, Preoperative care
Based on the results of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial,1 the current management in the UK of advanced but resectable oesophagogastric adenocarcinoma combines surgical resection and perioperative chemotherapy. Perioperative chemotherapy improves survival over surgery alone by 13%, but it is not without potential toxicity; in the MAGIC trial, only 41% of patients completed all six planned cycles of chemotherapy.1,2 In our centre, 86% of patients complete the preoperative component of MAGIC chemotherapy and 43% complete all six cycles. Twenty-six per cent of patients who complete preoperative chemotherapy do not commence postoperative treatment (unpublished audit data). Among the side effects of chemotherapy, fatigue is almost universally reported.
Cardiorespiratory reserve is important to patients undergoing major surgery. The metabolic stress incurred during major surgery and in the perioperative period requires a protracted increase in oxygen delivery.3 The cardiopulmonary exercise test (CPET), alongside estimation of the lactate threshold/anaerobic threshold (AT), is employed during preoperative assessment before major surgery. The AT provides an objective measure of physical fitness and is associated with postoperative survival and morbidity for a variety of populations undergoing major non-cardiac and cancer surgery.4-6 Older et al originally described the association between a low AT (<11 ml/kg/min) and surviving cardiopulmonary and surgical complications after non-cardiac surgery.6 More recently, AT has been shown to be an independent predictor of complications and length of stay in other major surgeries,4 and to predict mortality and length of stay after hepatobiliary surgery.7 Wilson et al demonstrated that AT and ventilatory equivalent for carbon dioxide (VeVCO2) was associated with all-cause mortality after elective intra-abdominal surgery and could be used to identify high-risk patients.5
The evidence base for CPET before oesophagogastric cancer resection is limited and does not yet show a strong correlation between CPET measurement and postoperative complications.8,9 The most recent UK publication exploring the ability of CPET to predict postoperative cardiopulmonary complications in these patients showed a trend towards patients with a lower AT developing more cardiopulmonary complications.9 However, this was a small, single-centre study of a heterogeneous group, and many patients with existing cardiorespiratory comorbidities were deemed unfit for surgery and managed nonoperatively. Patients with comorbidities had a significantly lower mean AT than the resected group, at 8.6 versus 10.8 ml/kg/min.9
The Northern Oesophagogastric Cancer Unit, based at The Royal Victoria Infirmary, Newcastle-upon-Tyne, UK, is a large tertiary referral unit that received 659 new patient referrals and performed 159 surgical resections for malignant disease in 2013. Coordinated multidisciplinary team (MDT) management is essential to the success of the unit. Patients deemed to have potentially curable disease at initial presentation are seen in a staging clinic and complete tumour staging is carried out in accordance with national guidelines. This includes endoscopic ultrasound examination, computed tomography/positron emission tomography, staging laparoscopy and ultrasound scanning of the neck. A health and fitness assessment, including CPET, is also performed. This informs the MDT discussion and treatment planning.
As part of a service evaluation project, we performed CPET at two time points within our MDT pathway to appropriately design and optimise the timing of CPET for our patients. We analysed the data as part of a service evaluation and improvement project within our unit. Hypothesising that perioperative chemotherapy may have a deleterious effect on physical fitness and cardiorespiratory reserve, we compared the results from CPET at baseline and after neoadjuvant chemotherapy (NAC).
Methods
Between November 2012 and April 2014, all patients with oesophagogastric adenocarcinomas deemed by the MDT suitable for perioperative chemotherapy and surgical resection were included in this retrospective analysis. All patients had undergone paired CPET testing at baseline as part of their fitness assessment and after the preoperative component of chemotherapy as part of our service evaluation. There was no randomisation; selection for a second CPET test was based on the availability of patients and CPET slots. The decision to perform a second CPET test was not related to poor performance or any perceived deterioration in health post-NAC.
Staging and surgical data were obtained retrospectively from the prospectively collected Northern Oesophagogastric Unit database. Preoperative chemotherapy data was retrospectively obtained from patient notes. CPET measurements for each patient were derived from the CPET database.
Chemotherapy
Patients with T3 and/or N1 resectable oesophagogastric adenocarcinoma were considered for perioperative ECX chemotherapy. This consisted of three preoperative and three postoperative cycles of intravenous epirubicin 50 mg/m2 body-surface area and cisplatin 60 mg/m2 on day 1, and a continuous intravenous infusion of fluorouracil 200 mg/m2/day for 21 days, as per the MAGIC Trial.1 To be suitable for chemotherapy, all patients had World Health Organization Performance status 0 or 1, and adequate renal, haematological and cardiac function. All patients had a restaging computed tomography scan post-chemotherapy and were rediscussed in the MDT to assess operability.
Cardiopulmonary exercise testing
CPET was performed in accordance with the American Thoracic Society/American College of Chest Physicians guidelines for cardiopulmonary exercise testing.10 Patients performed a symptom-limited continuous ramped test using a cycle ergometer (Lode, Groningen, the Netherlands), while metabolic gas analysis was performed using a metabolic cart (Scott Medical, Plumsteadville, PA, USA). In addition, 12-lead electrocardiography, heart rate monitoring and pulse oximetry were recorded throughout the test (Welch Allyn, Skaneateles Falls, NY, USA). Each test was conducted using an individualised ramp protocol.10 Tests were performed at baseline as part of standard cancer staging and after completion of preoperative chemotherapy.
The data derived from the CPET was recorded by the clinician responsible for their interpretation. The amount of oxygen extracted from the gases (VO2) was calculated as ml/min and indexed to body weight as ml/kg/min. This was measured both at peak exercise (VO2peak) and at the ventilatory anaerobic threshold (VAT). Ventilatory equivalents for carbon dioxide (VCO2) were measured at VAT. Ventilatory AT was derived from the V-Slope breakpoint between VO2 and VCO2,11 and by confirming the increase in Ve/VO2 and the plateau of Ve/VCO2. The same investigator interpreted all tests and all data were analysed using BreezeSuite version 6.2 (MGC Diagnostics Corporation, Saint Paul, MN, USA).
The primary measured outcome was VO2 at AT (ml/kg/min) before and after preoperative chemotherapy. We also considered the VO2peak and VE/VCO2 before and after chemotherapy.
Statistical analysis
Median values for the baseline tests were compared to those for our whole patient population using a paired Student’s t-test to exclude inclusion bias. CPET results before and after neoadjuvant chemotherapy were compared on a paired Student’s t-test. All analyses were carried out using SPSS Statistics version 21 (IBM, Armonk, NY, USA). P values <0.05 were deemed significant and 95% confidence intervals (CIs) were determined.
Results
Between November 2012 and April 2014, 30 patients with operable oesophagogastric adenocarcinomas underwent preoperative ECX chemotherapy, at a median of three cycles, and had paired CPET at baseline and post-chemotherapy. The median patients age was 67.8 years, and 24 were male. Ten patients stopped treatment early, all as a result of toxicity. The most commonly observed grade 3/4 toxicity was neutropenia, in 17% of patients, followed by diarrhoea in 13%, fatigue in 10%, neutropenic sepsis in 10%, thromboembolism in 7% (one axillary vein thrombus and one ulnar artery embolus) and emesis in 3%. The median time (range) from the final oral chemotherapy tablet to postchemotherapy CPET was 30 (6–78) days.
Baseline CPET measurements were compared with those of our entire oesophagogastric population over the same time period, with no significant differences found between the groups (Table 1). The two groups were managed identically with NAC and surgery, and had comparable baseline descriptive characteristics. The CPET data were normally distributed.
Table 1.
Baseline characteristics of our study population (before NAC) and the reference population. All patients were managed with the same perioperative chemotherapy regimen and surgery for operable oesophagogastric cancer. The reference population had CPET prior to NAC. The study group had CPET pre- and post-chemotherapy CPET.
| Reference population (n=140) | Study group (n=31) | |
|---|---|---|
| Age (years) | 66 (10.1) | 67.8 (7.9) |
| BMI (kg/m2) | 25.9 | 27.8 |
| VO2 at AT (ml/kg/min) | 13.0 (3.8) | 13.8 (4.3) |
| VO2 at AT (ml/min) | 1002 (389) | 819 (340) |
| VO2peak (ml/kg/min) | 18.0 (4.8) | 16.8 (5.1) |
| VO2peak (ml/min) | 1341 (509) | 1186 (331) |
| Median Ve/VCO2 at AT | 30 | 30 |
All values median (SD) unless otherwise stated
BMI, body mass index; VO2 at AT, oxygen uptake measured at ventilatory anaerobic threshold; VO2peak, peak oxygen uptake during testing; Ve/VCO2 at AT, ventilatory equivalents for carbon dioxide measured at ventilatory anaerobic threshold
There was a significant change in VO2 measured at AT and at peak exercise between baseline and post-chemotherapy CPET (see Table 2). VAT decreased from 13.8 ml/kg/min to 11.3 ml/kg/min (95% CI 1.30–3.85; p<0.001). VO2peak decreased from 16.8 to 14.7 ml/kg/min (95% CI 1.02–3.84; p=0.001) (see Figure 1).
Table 2.
CPET at baseline and post-NAC
| Baseline | Post-NAC | P value | 95% CI | |
|---|---|---|---|---|
| Median VO2 at AT (ml/kg/min) | 13.8 | 11.3 | <0.001 | 1.30 to 3.85 |
| Median VO2 at AT (ml/min) | 902 | 768 | 0.012 | 32.0 to 235.3 |
| Median VO2peak (ml/kg/min) | 16.8 | 14.7 | 0.001 | 1.02 to 3.84 |
| Median VO2peak (ml/min) | 1186 | 1026 | 0.001 | 71.0 to 248.7 |
| Median Ve/VCO2 at AT | 30 | 31 | 0.33 | -2.70 to -0.21 |
| Median Hb (g/L) | 136.5 | 120.5 | 0.72 | -1.25 to 27.5 |
Hb, haemoglobin concentration; VO2 at AT, oxygen uptake measured at ventilatory anaerobic threshold; VO2peak, peak oxygen uptake during testing; Ve/VCO2 at AT, ventilatory equivalents for carbon dioxide measured at ventilatory anaerobic threshold
Figure 1.
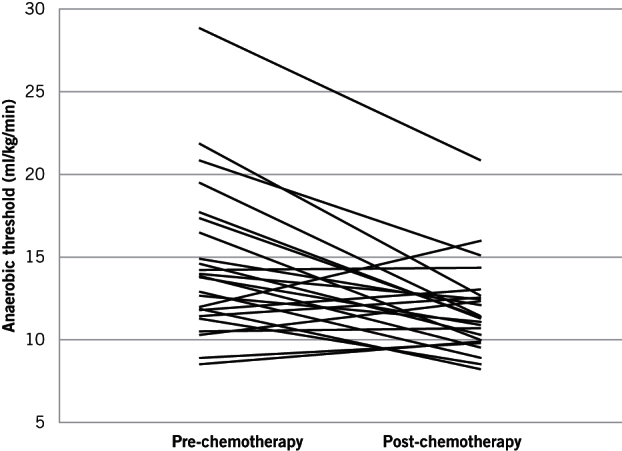
Ladder plot showing individual pairs of anaerobic thresholds at baseline and post-NAC
Discussion
Our results support the hypothesis that, alongside the known toxic effects of chemotherapy, there is a measurable reduction in cardiorespiratory reserve (fitness), quantified here by CPET. These results are consistent with the only other published data in patients receiving preoperative chemotherapy for oesophagogastric cancers.12
Jack et al conducted a prospective study between 2007 and 2009 in 39 patients with resectable oesophagogastric cancers. They reported a mean reduction in the measured VO2 at lactate threshold of 2.19 ml/kg/min (95% CI 1.47–2.91) 4 weeks after neoadjuvant chemotherapy. VO2peak was also significantly reduced after neoadjuvant chemotherapy (2.5 ml/kg/min (95% CI 0.44–4.07 ). Kaplan-Meier analysis suggested that a lower level of fitness (VO2 at AT) was associated with decreased 1-year survival in patients who completed both full preoperative chemotherapy and surgery.12 The authors postulated whether higher baseline fitness was required to offset effectively the dual insult of chemotherapy and surgery. It was also observed that the 50 patients who did not complete all their cycles of preoperative chemotherapy had a lower baseline VAT and VO2peak than those who did complete chemotherapy. However, there was no association between baseline VO2 at AT and 1-year survival in this group.
A similar study investigating the effects on fitness of neoadjuvant chemoradiotherapy before rectal cancer resection demonstrated a reduction in VO2 at AT and VO2peak of 1.5 and 1.4 ml/kg/min, respectively (p<0.001).13
CPET measurements have also been published in women with treated breast cancer.14 In a study of 222 patients, VO2peak was lower in matched groups who had completed surgery and a course of either neoadjuvant or adjuvant chemotherapy compared with those tested in the first 2 weeks of their adjuvant chemotherapy. The group who had surgery alone had a lower VO2peak than those managed with surgery and adjuvant chemotherapy. VO2 at AT was not significantly different across the groups (p=0.21). VO2peak and VO2 at AT were lowest following surgery and postoperative adjuvant chemotherapy.14
Dual modality treatment with perioperative chemotherapy and surgery for operable oesophagogastric adenocarcinomas offers the best chance of cure. However, it results in substantial morbidity, as evidenced by only 40% of patients completing all planned treatment. Our data demonstrates that preoperative chemotherapy resulted in a decline in fitness, as measured by AT. The interpretation of these preliminary results should not, on the basis of this study, be extrapolated beyond this statement; however, potentially important clinical implications arise from our findings. Reduced fitness (cardiorespiratory reserve) may alter the ability of an individual to respond appropriately of the perioperative and postoperative physiological insults. In other major surgeries, a low AT is predictive of increased morbidity.4,6,15 A reduction in AT and cardiopulmonary reserve below the pre-chemotherapy baseline may move patients into a higher perioperative risk category. This may also be the case for those undergoing surgery for oesophagogastric cancers, and we may therefore be able to identify a group of patients who could benefit from prehabilitation to maintain or improve fitness during chemotherapy. Perhaps we should adjust the timing of surgery to allow recovery of baseline fitness. Moreover, intervention to maintain fitness during treatment may increase the number of patients able to complete the entire planned chemotherapy and surgery programme and, in turn, improve survival. This would be a useful focus for future studies.
As demonstrated in Figure 1, not all patients experienced a decline in fitness; however, the group effect was a reduction in median VAT and VO2peak, despite a measured increase in fitness in six patients. This increase in fitness may partially be explained by improved nutrition following commencement of NAC, alongside improvements in dysphagia and/or the insertion of adjuncts such as feeding jejunostomies prior to NAC. As no dysphagia scores were maintained, this is, however, only a hypothesis and further studies comparing quantitative measurements of nutritional status and fitness measures are required. Although there was a reduction in haemoglobin concentration between the pre- and post-NAC measurements, this did not reach statistical significance.
CPET is an integrated test of all the homeostatic mechanisms involved in oxygen delivery, including respiratory, cardiovascular and circulatory systems, as well as muscle oxygen extraction and oxygen utilisation at the cellular level. As such, any attempt to elicit the mechanisms that might alter oxygen homeostasis and result in the earlier use of anaerobic metabolism (AT) after chemotherapy is outside the scope of this investigation. However, it should be noted that analysis of patient comorbidities, via clinical histories, in our group confirmed that these did not alter during testing. Similarly, there was no significant cancer disease progression that would confound the CPET results. Analysis of haemoglobin concentrations before and after neoadjuvant chemotherapy showed a reduction in haemoglobin measurement between the two time points that did not reach statistical significance. There is little evidence to illuminate the clinical effect of decreased haemoglobin in this context. Published work from our institution suggests that each g/dl increase in haemoglobin in patients with chronic haematological disorders receiving blood transfusions results in a mean increase in VO2 of 0.39 ml/kg/min.16
Our study has a number of limitations. It is observational and retrospectively examines data on a small proportion of the patients treated in our unit. Chemotherapy data has been collected retrospectively and is therefore reliant on the accurate recording of clinical information. In contrast, CPET data, the principle outcome measure, was derived from the original test data and is thus robust. True randomisation of testing was not observed and yet our cohort have the same characteristics as our local operable population. Despite the limitations, our results concur with other recently published data.12 Furthermore, our management of patients via MDT meeting reviews remained consistent throughout the study and all patients were treated by the same oncology and surgical teams using a consistent chemotherapy regimen and surgical approach. CPET was performed in a single testing clinic using standardised protocols and equipment.
We have not included postoperative data in this analysis, since this is beyond the power of this small, retrospective, observational sample, and we do not believe that further conclusions can be drawn from this data. We hypothesise that the observed reduction in fitness was caused by a multi-system effect of chemotherapy, including bone marrow suppression. However, we acknowledge that there are many factors involved that cannot be elucidated with our data.
Conclusions
In conclusion, this study objectively demonstrates a significant reduction in fitness following preoperative chemotherapy for oesophagogastric cancer when using a robust objective measurement. Prospective evaluation of ‘prehabilitation’ or ‘fitness maintenance’ exercise training before and during preoperative chemotherapy may help to maintain cardiorespiratory reserve and have an impact upon survival, and is therefore worthy of prospective evaluation in further studies.
Acknowledgement
Our thanks are extended to the data managers of the Northern Oesophagogastric Cancer Unit database and to our pre-assessment clinic for facilitating this work.
Contributions to the writing of this paper were as follows: RCFS, study design, data collection, analysis and interpretation, writing all drafts, decision to submit; MN, data collection, analysis and interpretation, report writing; SMG, writing report final drafts, decision to submit; and KS, study design, data collection, analysis and interpretation, report writing all drafts, decision to submit.
References
- 1.Cunningham D, Allum WH, Stenning SP et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; : 11–20. [DOI] [PubMed] [Google Scholar]
- 2.Sjoquist KM, Burmeister BH, Smithers BM et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011; : 681–692. [DOI] [PubMed] [Google Scholar]
- 3.Older P, Smith R, Courtney P et al. Preoperative evaluation of cardiac failure and ischemia in elderly patients by cardiopulmonary exercise testing. Chest 1993; : 701–704. [DOI] [PubMed] [Google Scholar]
- 4.Snowden CP, Prentis JM, Anderson HL et al. Submaximal Cardiopulmonary Exercise Testing predicts Mortality and Hospital Length of Stay in patients undergoing major surgery. Ann Surg 2010; : 535–541. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RJ, Davies S, Yates D et al. Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br J Anaesth 2010; : 297–303. [DOI] [PubMed] [Google Scholar]
- 6.Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest 1999; : 355–362. [DOI] [PubMed] [Google Scholar]
- 7.Snowden CP, Prentis J, Jacques B et al. Cardiorespiratory fitness predicts mortality and hospital length of stay after major elective surgery in older people. Ann Surg 2013; : 999–1004. [DOI] [PubMed] [Google Scholar]
- 8.Forshaw MJ, Strauss DC, Davies AR et al. Is cardiopulmonary exercise testing a useful test before esophagectomy? Ann Thorac Surg 2008; : 294–299. [DOI] [PubMed] [Google Scholar]
- 9.Moyes LH, McCaffer CJ, Carter RC et al. Cardiopulmonary exercise testing as a predictor of complications in oesophagogastric cancer surgery. Ann R Coll Surg Engl 2013; : 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisman IM. ATS/ACCP Statement on Cardiopulmonary exercise testing. American Journal of Respiratory and Critical Care Medicine 2003; : 211–277. [DOI] [PubMed] [Google Scholar]
- 11.Beaver WL, Wasserman K, Whipp BJ. A new method of detecting anaerobic threshold by gas exchange. Journal of Applied Physiology 1996; : 2,020–2,027. [DOI] [PubMed] [Google Scholar]
- 12.Jack S, West MA, Raw D et al. The effect of neoadjuvant chemotherapy on physical fitness and survival in patients undergoing oesophagogastric cancer surgery. Eur J Surg Oncol 2014; : 1,313–1,320. [DOI] [PubMed] [Google Scholar]
- 13.West MA, Loughney L, Barben CP et al. The effects of neoadjuvant chemoradiotherapy on physical fitness and morbidity in rectal cancer surgery patients. Eur J Surg Oncol 2014; : 1,421–1,428. [DOI] [PubMed] [Google Scholar]
- 14.Klassen O, Schmidt ME, Scharhag-Rosenberger F et al. Cardiorespiratory fitness in breast cancer patients undergoing adjuvant therapy. Acta Oncologica 2014; : 1,356–1,365. [DOI] [PubMed] [Google Scholar]
- 15.West MA, Lythgoe D, Barben CP et al. Cardiopulmonary exercise variables are associated with postoperative morbidity after major colonic surgery: a prospective blinded observational study. Br J Anaesth 2014; : 665–671. [DOI] [PubMed] [Google Scholar]
- 16.Wright SE, Pearce B, Snowden CP et al. Cardiopulmonary exercise testing before and after blood transfusion: a prospective clinical study. Br J Anaesth 2014; : 91–96. [DOI] [PubMed] [Google Scholar]


