Abstract Abstract
We conducted an international study to evaluate practices in the diagnosis and management of patients with chronic thromboembolic pulmonary hypertension (CTEPH) globally across different regions. Between August and October 2012, CTEPH-treating physicians completed a 15-minute online questionnaire and provided patient record data for their 2–5 most recent patients with CTEPH. Overall, 496 physicians (Europe: 260; United States: 152; Argentina: 52; Japan: 32) completed the questionnaire and provided patient record data for 1,748 patients. The proportion of physicians who described themselves as working in or affiliated with a specialized pulmonary hypertension (PH) center ranged from 38% in France and Italy to 83% in the United States. A large proportion of patients did not undergo ventilation/perfusion scanning (46%–67%) or right heart catheterization (24%–57%) for the diagnosis of CTEPH. Referral rates for pulmonary endarterectomy evaluation ranged from 25% in Japan to 44% in Europe, with higher referral rates in PH centers; the main reasons for lack of referral were that surgery was not considered unless medical treatment was failing and patient refusal. Other variations in management included greater use of phosphodiesterase 5 inhibitors in the United States than in Europe and Japan and greater use of combination treatment in the United States than in Europe. Physicians’ perceptions of their treatment strategy were generally consistent with patient record data. Results from this study, which includes a global aspect of CTEPH care, demonstrate not only regional differences in CTEPH management but, more importantly, considerable nonadherence to the diagnosis and treatment guidelines for CTEPH, even in PH centers.
Keywords: chronic thromboembolic pulmonary hypertension, pulmonary arterial hypertension–specific therapies, diagnosis, clinical practice, guidelines
Chronic thromboembolic pulmonary hypertension (CTEPH) is a disease of obstructive pulmonary artery remodeling as a consequence of major vessel thromboembolism.1 A ventilation/perfusion (V/Q) scan is recommended in the workup of all patients with pulmonary hypertension (PH) to screen for CTEPH.1 Diagnosis of CTEPH is challenging for several reasons, including nonspecific presenting symptoms, which may occur late in the progression of the disease, and a lack of prior pulmonary embolism in a high percentage of patients.2
Once CTEPH is diagnosed, pulmonary endarterectomy (PEA) is the gold-standard treatment for these patients and is potentially curative.1,3 However, between 20% and 40% of these patients are considered to have inoperable CTEPH,4 and approximately 30% may have residual PH after PEA (defined as mean pulmonary artery pressure of ≥30 mmHg at 3-month follow-up after surgery).5 Balloon pulmonary angioplasty (BPA) is an emerging treatment option for patients with inoperable or persistent CTEPH.3 However, the role of BPA in CTEPH has not been investigated in randomized trials, and further studies are warranted; current guidelines state that it should not replace PEA for the treatment of CTEPH.3 At the time this study was initiated, there were no medical therapies approved for CTEPH. Therefore, apart from referring patients for PEA, physicians were restricted to using a range of drugs approved for pulmonary arterial hypertension (PAH) only.
Complex and evolving treatment options for CTEPH warrant examination of current management approaches to identify differences in clinical practice between regions and to inform the need for change where necessary, particularly since a recent survey has suggested that practice patterns among PAH experts diverge from consensus recommendations and differ by practice location.6 Therefore, we conducted a large, physician-based international study to determine differences in the diagnosis and management of patients with CTEPH across different countries and regions worldwide, using a quantitative online questionnaire. The objectives of the study were (1) to assess the diagnosis and management of CTEPH in different countries and regions by analyzing differences in referral patterns, diagnostic procedures, and use of PAH-specific drug therapies; (2) to explore CTEPH-treating physicians’ approaches toward the management of CTEPH; and (3) to determine the accuracy of physicians’ perceptions regarding the diagnosis and management of their patients, by comparing questionnaire results with physicians’ patient medical records. The study captured responses from a variety of clinical settings, including designated specialist PH centers as well as non-PH centers. A parallel study evaluated the diagnosis and management of patients with PAH, with results reported in a separate article.7
Methods
The study was conducted in five European countries (France, Germany, Italy, Spain, and the United Kingdom, between August 31 and September 30, 2012), the United States (between August 31 and September 28, 2012), Argentina (between September 11 and October 19, 2012), and Japan (between September 7 and October 18, 2012). Bayer Pharma initiated the study in collaboration with Ipsos Healthcare. The study had two components: a retrospective patient chart review and a physician questionnaire. The retrospective chart review collected the current and historical data at the time the online patient record was completed by the physician. Subsequent follow-up for changes in treatment or outcomes was not carried out. The physician perception questionnaire focused on physician experience and satisfaction with current medical and surgical treatments for CTEPH. The same physicians were enrolled for both parts of the study; they provided data from their patients’ records and completed the questionnaire.
Physician selection criteria
Physicians previously registered with Medefield, a market research panel provider, with an appropriate specialty (cardiology, pulmonology, or rheumatology) were recruited to the study if they met all the following criteria: they were actively involved in decisions for PAH-specific drug therapy in patients with CTEPH; they were treating at least 5 (United States), 3 (Europe), or 2 (Argentina and Japan) patients with CTEPH and had personally initiated PAH-specific treatment in at least 1 of these patients; they had experience in managing CTEPH for at least 2 years; and they received no funding from any pharmaceutical company other than in the context of clinical trials. Physicians recruited from the United States had to have self-reported board certification in cardiology, pulmonology, or rheumatology. Depending on the country, internal medicine physicians were also eligible to take part in the study if they specialized in cardiology, pulmonology, or rheumatology.
Physicians were asked whether they worked in a PH center and to provide the center’s name. A PH center was defined on the basis of the 2009 European Society of Cardiology/European Respiratory Society guidelines1 as follows: manages a minimum of 50 patients with PAH or CTEPH; receives at least 2 new patient referrals per month; performs at least 20 vasoreactivity tests per year; participates in clinical research on PH, including phase 2 and phase 3 clinical trials; contains a multidisciplinary team (including cardiologists, pulmonologists, radiologists, specialist nursing staff, and adequate on-call service); and has direct connections and quick access to other medical programs (specialists for connective-tissue disease, PEA services, lung transplantation, and congenital heart disease in adults). The definition of a PH center allowed for the inclusion of PEA centers. While US physicians were self-defined with respect to their association with PH centers, the relations of European physicians to recognized specialist centers were confirmed with country lists of recognized specialist centers. Physicians from Argentina and Japan were asked whether they worked for an institution with a department that specialized in the treatment of PH. For Argentinian physicians, verification was performed against country lists, whereas Japanese physicians were self-defined. Physicians whose place of work was not affiliated with a PH center were classified as non-PH-center respondents.
Data acquisition
Physicians were asked to provide patient records of their last consecutive 2, 3, or 5 patients (2 patients for Argentina or Japan, 3 patients for Europe, and 5 patients for the United States). The number of patient records requested for the different countries/regions was based on a prescreening questionnaire that revealed that the average caseload was higher in Europe and the United States than in Argentina and Japan (Table S1). Patients had to meet the following criteria: have a diagnosis of CTEPH, be at least 18 years of age, and currently be receiving treatment with PAH-specific drug therapies. Patients were not eligible for inclusion in the study if they were participating in a clinical trial, excluding postmarketing clinical trials. Patient record data were collected in a deidentified manner; patient consent was therefore not required. For the physician perception questionnaire, physicians were required to complete a 15-minute online survey.
Statistical analyses
A feasibility assessment was carried out to specify a realistic target physician sample size for each country (50 for Argentina, France, Germany, Italy, Spain, and the United Kingdom; 150 for the United States; and 30 for Japan), with a particular focus on recruiting pulmonologists and cardiologists. Patient record data are reported as proportions by category, according to the categorical responses to the questions listed in the figure legends. Patient record data were weighted on the physician’s self-reported caseloads of qualifying patients at a country level. No additional weighting—for example to account for varying prevalence rates between countries—was applied to the data.
Physician perception questionnaire data are reported as proportions for questions with categorical responses, and as mean values ± the standard error of the mean and median values for numerical responses. Data from the perception questionnaire were collected as absolute patient numbers. Therefore, there was an implicit weighting in these data toward physicians with higher patient caseloads, and no adjustment was applied to these data.
Data are presented by country or by region. In the latter case, data from the five European countries were pooled.
Statistical significance was tested with 2-tailed probability tests at the 95% confidence level. Bonferroni corrections were applied for multiple comparisons between countries for the same category, and χ2 tests were used to test the distribution of New York Heart Association functional class (NYHA FC). Statistical testing was not performed in regions with fewer than 30 patients (termed “small base”). All statistical testing was performed as post-hoc analyses.
Results
Physician sample
A total of 496 physicians met the inclusion criteria and agreed to participate in the study. Tables 1 and S1 report details of the physicians participating in the study. The proportion of physicians practicing in a hospital-based setting ranged from 31% in the United States to 100% in the United Kingdom, Italy, and Japan (Table 1). The proportion of physicians who described themselves as working in or affiliated with a specialist PH center ranged from 38% in France and Italy to 83% in the United States (Table 1).
Table 1.
Physician sample
| Characteristic | UK | FR | DE | IT | ES | US | AR | JP |
|---|---|---|---|---|---|---|---|---|
| No. of physicians | 50 | 52 | 51 | 50 | 57 | 152 | 52 | 32 |
| Specialty, % | ||||||||
| Cardiology | 54 | 56 | 39 | 50 | 37 | 53 | 62 | 81 |
| Pulmonology | 40 | 40 | 49 | 28 | 46 | 39 | 38 | 16 |
| Rheumatology | 6 | 0 | 6 | 10 | 9 | 3 | NA | NA |
| Internal medicinea | 0 | 4 | 6 | 12 | 9 | 5 | NA | 3 |
| Cardiology | 0 | 4 | 0 | 6 | 4 | 4 | NA | 3 |
| Pulmonology | 0 | 0 | 4 | 6 | 4 | 0 | NA | 0 |
| Rheumatology | 0 | 0 | 2 | 0 | 2 | 1 | NA | NA |
| Setting, % | ||||||||
| Hospital | 100 | 90 | 88 | 100 | 98 | 31 | 87 | 100 |
| Office | 0 | 10 | 12 | 0 | 2 | 69 | 13 | NA |
| Affiliation, % | ||||||||
| Working in a specialized PH centerb | 32c | 38c | 47c | 22c | 37c | 49d | 15c | 31d |
| Affiliated with a PH center | 26 | NA | 16 | 16 | 37 | 34 | NA | NA |
| Not affiliated with a PH center (non-PH center) | 42 | 62 | 37 | 62 | 26 | 18 | 81 | 62 |
| Don’t know | NA | NA | NA | NA | NA | NA | 4 | 6 |
In cases where the physician type is not involved in the management of PH in their country, “NA” is used. AR: Argentina; DE: Germany; ES: Spain; FR: France; IT: Italy; JP: Japan; NA: not applicable; PH: pulmonary hypertension; UK: United Kingdom; US: United States.
Internal medicine physicians specializing in cardiology, pulmonology, or rheumatology.
Defined on the basis of the European Society of Cardiology/European Respiratory Society 2009 guidelines;1 in Argentina and Japan, institution with a department specialized in treating PH.
Verified recognized specialized PH center.
Self-defined specialized PH center.
Results from patients’ medical records
Patient characteristics
Physicians provided medical records for a total of 1,748 patients: 780 from Europe, 760 from the United States, 139 from Argentina, and 69 from Japan. Retrospective analysis of patients’ records showed that at diagnosis, the majority of patients were classified as NYHA FC 3/4, demonstrating significant functional limitation, although there were statistically significant variations between regions in patient distribution across NYHA classes (Fig. 1).
Figure 1.
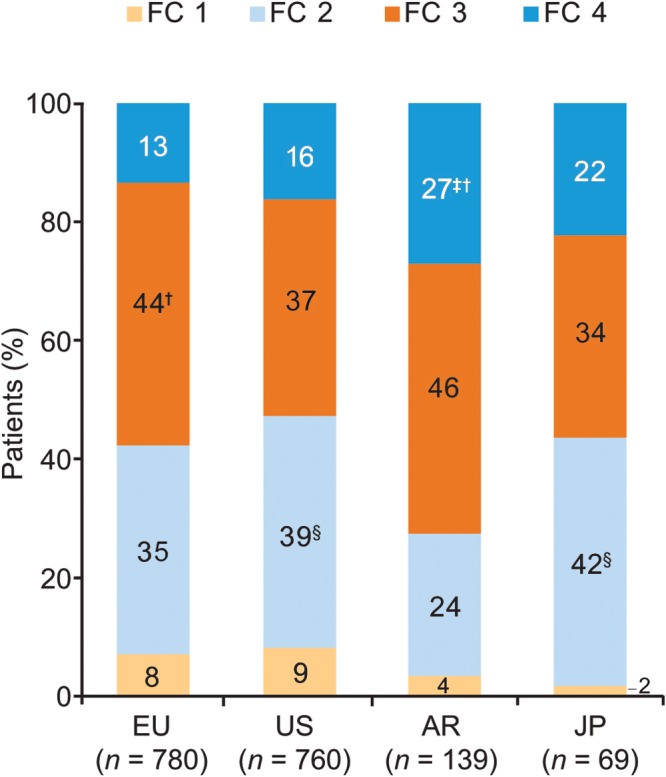
Patients’ New York Heart Association functional class at diagnosis (patient record data). Responses to the study question “What was this patient’s New York Heart Association functional class at time of diagnosis?” Probability test pairwise comparisons (with Bonferroni correction) showed significant (P < 0.05) differences versus the corresponding functional class in †the US, ‡Europe, or §Argentina. AR: Argentina; EU: Europe; FC: functional class; JP: Japan; US: United States.
Diagnosis of CTEPH
Physicians reported that patients with CTEPH were mostly referred to them by pulmonologists, cardiologists, or internal medicine physicians for evaluation and/or treatment (Fig. 2A). A large proportion of patients were referred without a diagnosis, from either a primary care physician (PCP) or another specialist (51% in Europe, 42% in the United States, 40% in Argentina, and 36% in Japan).
Figure 2.
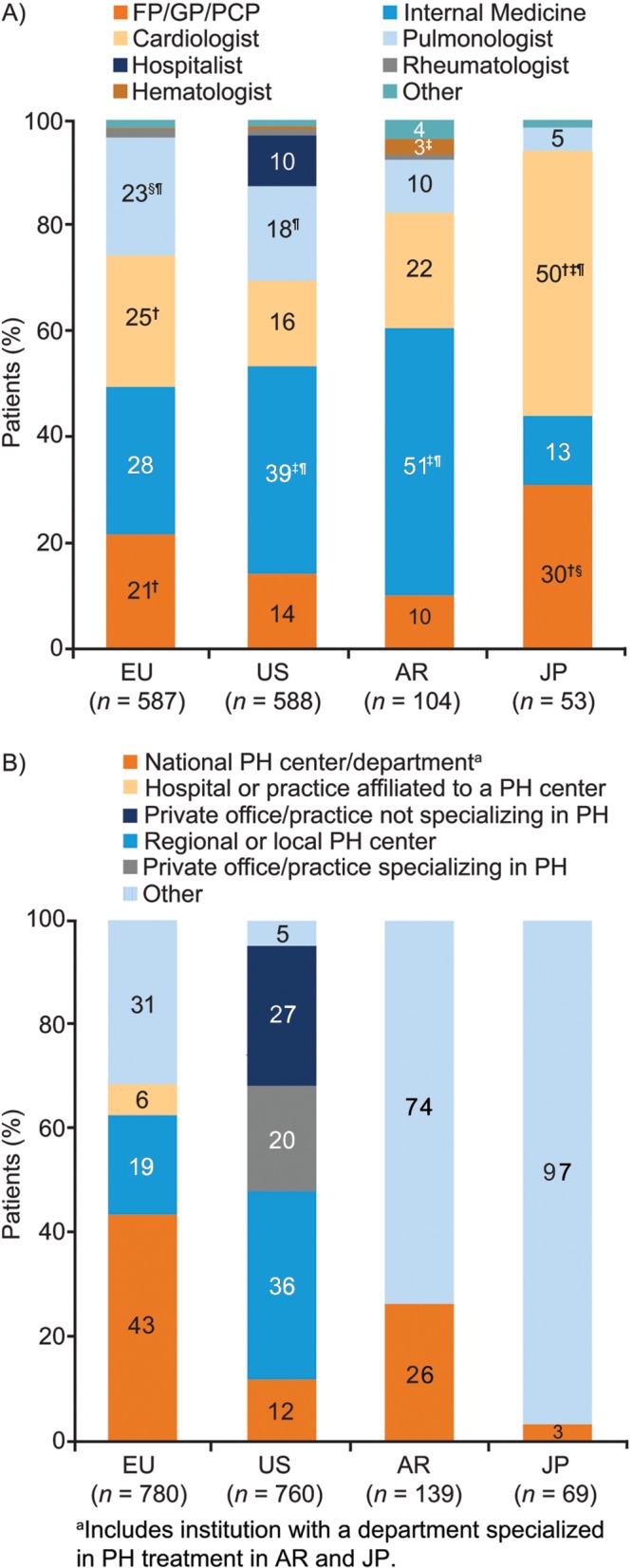
Referral and diagnosis (patient record data). A, Responses to the study question “What was the specialty of the referring physician?” Probability test pairwise comparisons were significantly (P < 0.05) different versus the corresponding category in †the US, ‡Europe, §Argentina, or ¶Japan. All significances are Bonferroni corrected for multiple comparisons. B, Responses to the study question “Which of the provided facility types best describes where this patient received his/her CTEPH diagnosis?” The “other” category was classified as follows: Europe: 61% academic/teaching hospital, 27% general hospital, 9% office, 4% private hospital; Argentina: 39% academic/teaching hospital, 27% private hospital, 23% general hospital, 11% office; Japan: 70% public hospital, 13% private hospital, 9% national hospital, 9% other. AR: Argentina; CTEPH: chronic thromboembolic pulmonary hypertension; EU: Europe; FP: family practitioner: GP: general practitioner; JP: Japan; PCP: primary care physician; PH: pulmonary hypertension; US: United States.
The type of clinical facility at which CTEPH was diagnosed varied between regions (Fig. 2B). CTEPH was diagnosed in a national PH center for 43% of patients in Europe, 12% in the United States, 26% in Argentina, and 3% in Japan. In the United States, 47% of patients received their diagnosis in a private office/practice, while in Japan, 70% of patients in the “other” location received their diagnosis at a public hospital.
Echocardiography was the most commonly used diagnostic technique (81%–98% of patients across all regions in both PH centers and non-PH centers), while the use of right heart catheterization (RHC; 43%–76%), computed tomography (CT) angiography (29%–89%), lung function test (41%–73%), and chest X-ray (51%–77%) varied widely (Table 2). Only 33%–54% of patients underwent a V/Q scan during their diagnostic workup. In Europe, a significantly greater proportion of patients underwent V/Q scanning in PH centers than in non-PH centers, whereas in the United States, there was no significant difference in the use of V/Q scanning between PH centers and non-PH centers (Table 2). Of 1,748 patients, 326 (19%) received echocardiography in the absence of RHC or a V/Q scan, and 30 (2%) received echocardiography alone, during diagnostic assessment for CTEPH.
Table 2.
Procedures performed to establish CTEPH diagnosis (patient record data)
| Europe | United States | Argentina | Japan | |||||
|---|---|---|---|---|---|---|---|---|
| Procedures performed, % | PH center (n = 276) | Non-PH center (n = 504) | PH center (n = 370) | Non-PH center (n = 390) | PH center (n = 19)a | Non-PH center (n = 120) | PH center (n = 22)a | Non-PH center (n = 47) |
| Echocardiography | 89 | 94b | 81 | 90b | 96 | 95b | 96 | 98 |
| Electrocardiogram | 79b,c | 84b,c | 54 | 60 | 88 | 90b,c | 77 | 95b,c |
| RHC | 76b | 66d | 63d | 67d | 53 | 43 | 67 | 70d |
| CT angiography | 71b | 68b | 54 | 62b | 29 | 58 | 69 | 89b,c,d |
| Lung function test | 66b,d | 73b,c,d | 52 | 56 | 47 | 41 | 60 | 68d |
| Chest X-ray | 62 | 69b | 51 | 59 | 76 | 64 | 63 | 77b |
| V/Q scan | 52c,e | 40 | 45 | 42 | 33 | 46 | 54 | 46b |
| Cardiopulmonary exercise test | 37f | 39b,f | 28 | 29f | 43 | 24 | 14 | 7 |
| Abdominal ultrasound scan | 20b,c,d | 17b,c,d | 5 | 4 | 0 | 4 | 0 | 15b,c,d |
| Conventional pulmonary angiography | 20c,e | 11 | 12 | 7 | 8 | 15 | 57 | 43b,c,d,e,g |
| Left heart catheterization | 19d | 22d | 23d | 24d | 4 | 5 | 30 | 42d,g |
| MRI angiography | 16c,d,e | 7c | 12c | 2 | 12 | 1 | 0 | 15c,d |
| Coronary angiography | 15d | 12d | 12d | 8 | 12 | 2 | 34 | 40b,c,d,e,g |
| Other | 2 | 2 | <1 | 1 | 0 | 7b,c | 0 | 0 |
Responses to the study question “Which of the listed diagnostic procedures were performed to diagnose this patient with CTEPH, either ordered by yourself, or if known, by another physician?” Columns add up to more than 100% because more than 1 diagnostic procedure was used in patients to establish a diagnosis. Footnotes b–g highlight significant differences from probability test pairwise comparisons across rows. CTEPH: chronic thromboembolic pulmonary hypertension; CT: computed tomography; MRI: magnetic resonance imaging; PH: pulmonary hypertension; RHC: right heart catheterization; US: United States; V/Q: ventilation/perfusion.
Significance testing was not performed because of the small base.
P < 0.05 versus US PH center.
P < 0.05 versus US non-PH center.
P < 0.05 versus non-PH center in Argentina.
P < 0.05 versus non-PH center in Europe.
P < 0.05 versus non-PH center in Japan.
P < 0.05 versus PH center in Europe.
PEA evaluation
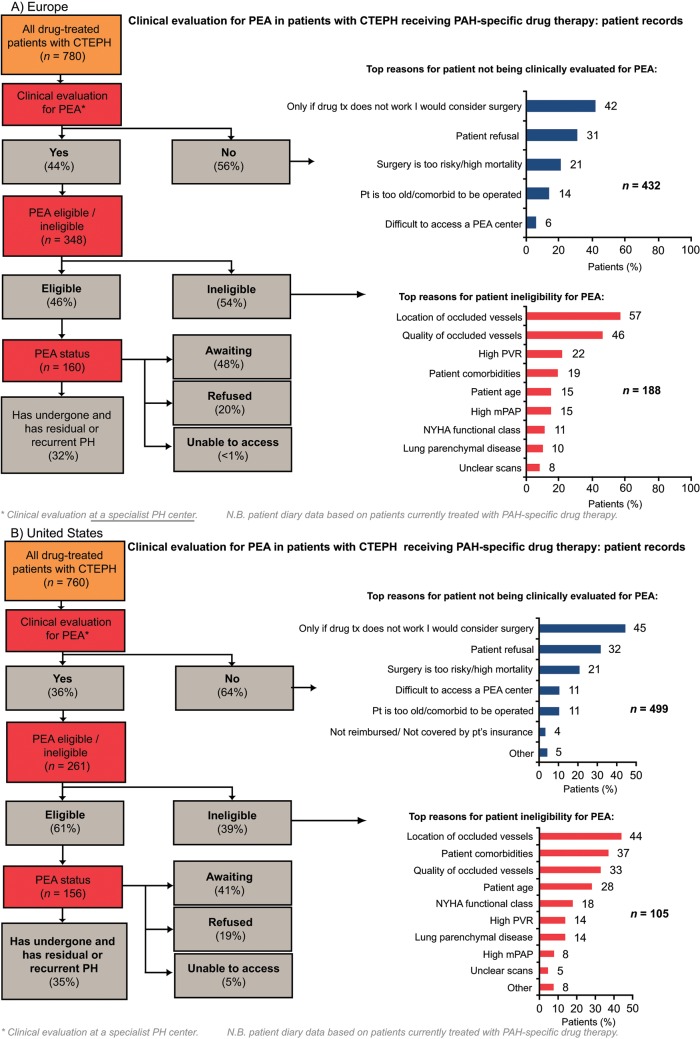
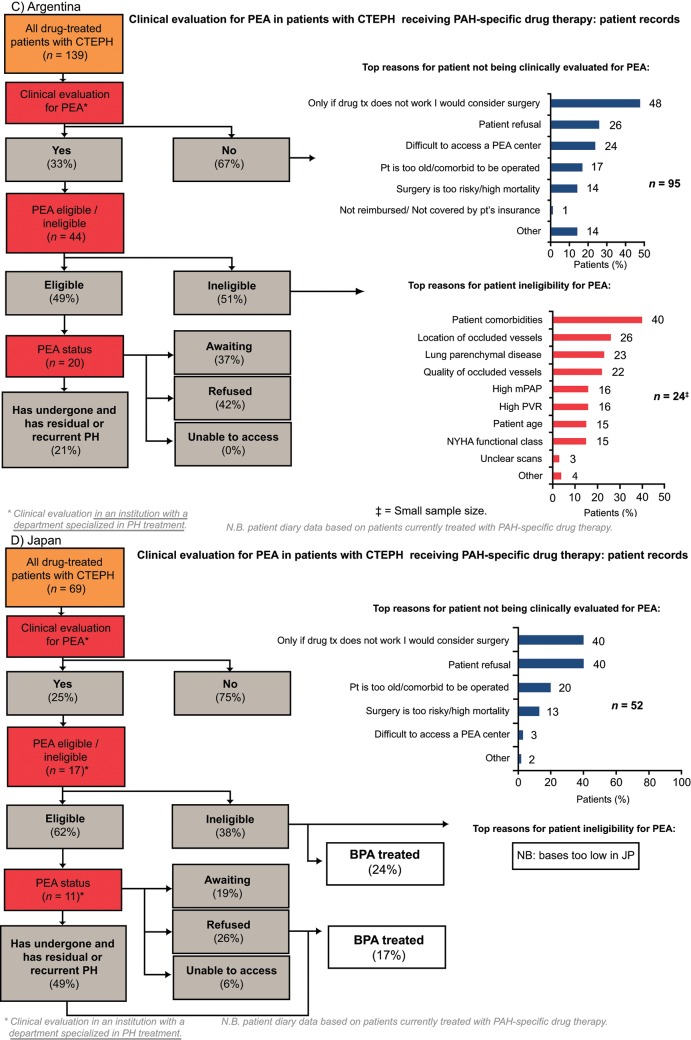
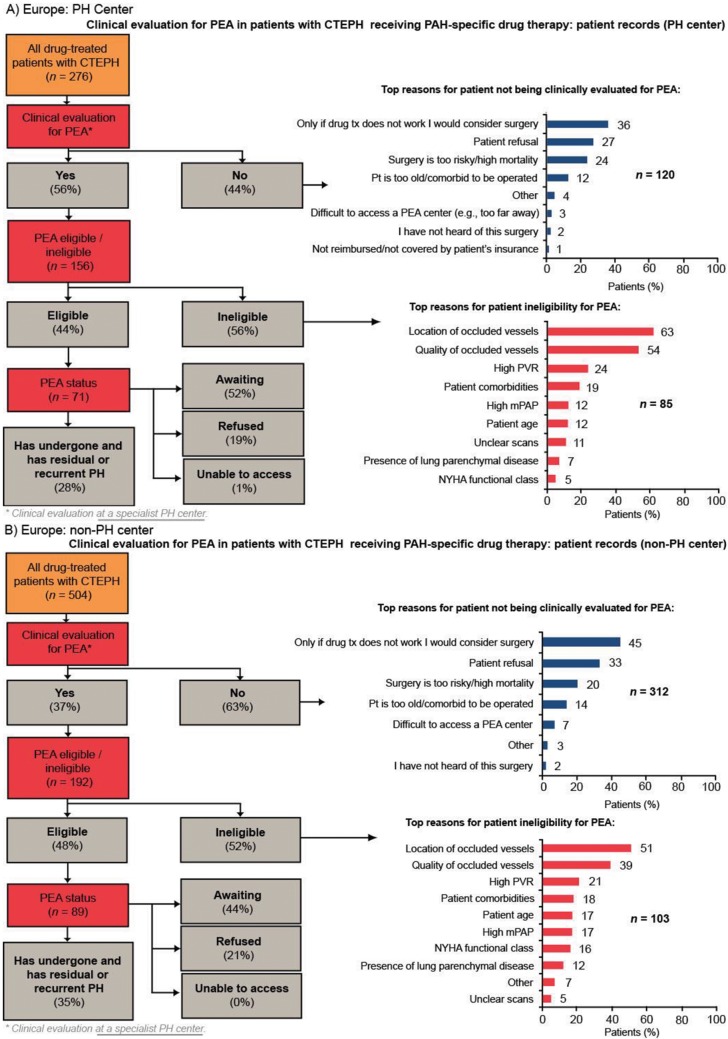
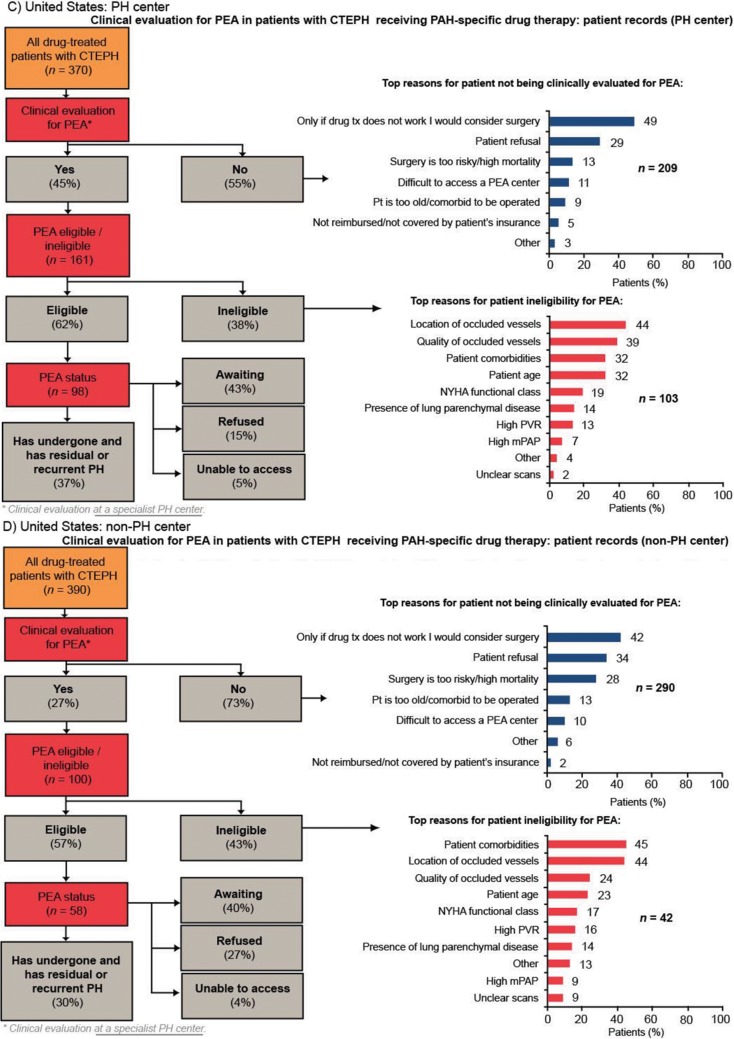
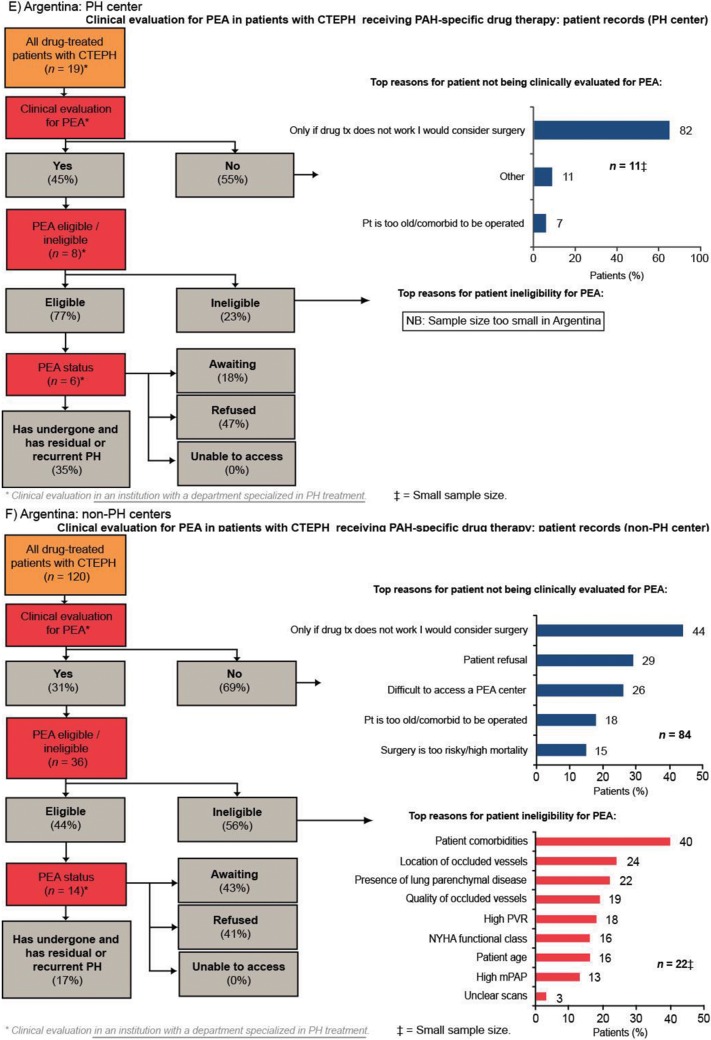
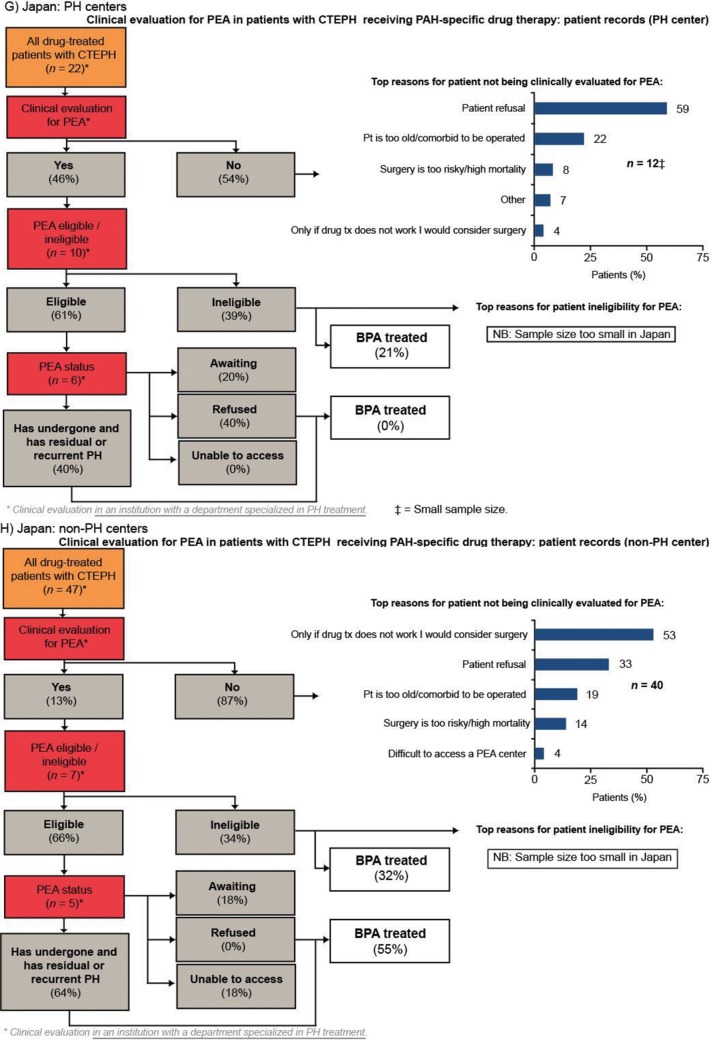
Of patients who were evaluated for PEA, more than 90% were evaluated by a PEA surgeon. Europe had the highest rate of PEA evaluation across the four regions, with 44% of patients being evaluated for PEA. In the United States and Argentina, approximately one-third of patients were evaluated for PEA. Japan had the lowest rate of PEA evaluation across the four regions (25% of patients; Fig. 3). In all four regions, the rate of PEA evaluation was higher in PH centers than in non-PH centers; the rate of PEA evaluation in PH centers in Europe, the United States, Argentina, and Japan was 56%, 45%, 45%, and 46%, respectively, compared with 37%, 27%, 31%, and 13%, respectively, in non-PH centers (Fig. S1). The two most common reasons for not evaluating patients for PEA across all the regions studied were surgery being considered an option only if drug therapy failed and patient refusal (Fig. 3). Patient refusal was most prevalent in Japan (40%), a country where difficulty accessing a PEA center was infrequently stated (3%). In contrast, lack of access to a PEA center was most prevalent in Argentina (24% vs. 6% in Europe and 11% in the United States).
Figure 3.
Clinical evaluation for PEA in patients with CTEPH receiving PAH-specific drug therapy (patient record data). BPA: balloon pulmonary angioplasty; CTEPH: chronic thromboembolic pulmonary hypertension; JP: Japan; mPAP: mean pulmonary artery pressure; NYHA: New York Heart Association; PAH: pulmonary arterial hypertension; PEA: pulmonary endarterectomy; PH: pulmonary hypertension; pt: patient; PVR: pulmonary vascular resistance.
The main diagnostic procedures on which PEA eligibility was based were RHC (63%–81%) and CT pulmonary angiography (65%–75%; Table 3). Conventional pulmonary angiography was used to determine eligibility in only 29%–39% of patients in Europe, the United States, and Argentina, compared with 81% in Japan. Magnetic resonance imaging pulmonary angiography was used more frequently in Europe. In Europe and the United States, the location of occluded vessels (57% and 44% of patients, respectively) and the quantity of occluded vessels (46% and 33% of patients, respectively) were major reasons for PEA ineligibility (Fig. 3). In addition, patient comorbidities were common reasons for PEA ineligibility in the United States (37% of patients) and Argentina (40%).
Table 3.
Procedures to evaluate PEA eligibility (patient record data)
| Proportion of patients | Europe (n = 348) |
United States (n = 261) |
Argentina (n = 44) |
Japan (n = 17) |
|---|---|---|---|---|
| RHC | 81a | 81a | 63 | 81 |
| CT pulmonary angiography | 65 | 69 | 67 | 75 |
| V/Q scan | 46 | 52 | 54 | 47 |
| Conventional pulmonary angiography | 39 | 33 | 29 | 81 |
| MRI pulmonary angiography | 24a | 14 | 6 | 8 |
| Others | 0 | 0 | 1 | 13 |
Responses to the study question “Which procedures were used to evaluate this patient’s clinical eligibility for PEA? Please select all that apply.” Data are the percentage of patients for which these procedures were performed. This question was asked only for patients clinically evaluated in a specialist pulmonary hypertension center (Europe/United States) or department (Argentina/Japan). Probability test pairwise comparisons across rows. CT: computed tomography; MRI: magnetic resonance imaging; PEA: pulmonary endarterectomy; RHC: right heart catheterization; V/Q: ventilation/perfusion.
P < 0.05 versus Argentina.
Management of CTEPH with PAH-specific drug therapies
In comparisons of PAH-specific drug therapy use for CTEPH between different regions, prostanoid analogs were used more often in Japan (55% vs. 17%–31% across other regions), and endothelin receptor antagonist (ERA) use was lowest in Argentina (19% vs. 46%–56% across other regions; Fig. 4A). Across the regions, more than 50% of patients were using a phosphodiesterase 5 inhibitor (PDE-5i; 52%–86%). Prostanoids tended to be used as an additional therapy with oral treatments. For example, in Europe, prostanoids were used in only 10% of patients as first-line therapy and in 62% of patients as third-line therapy (P < 0.05; data not shown).
Figure 4.
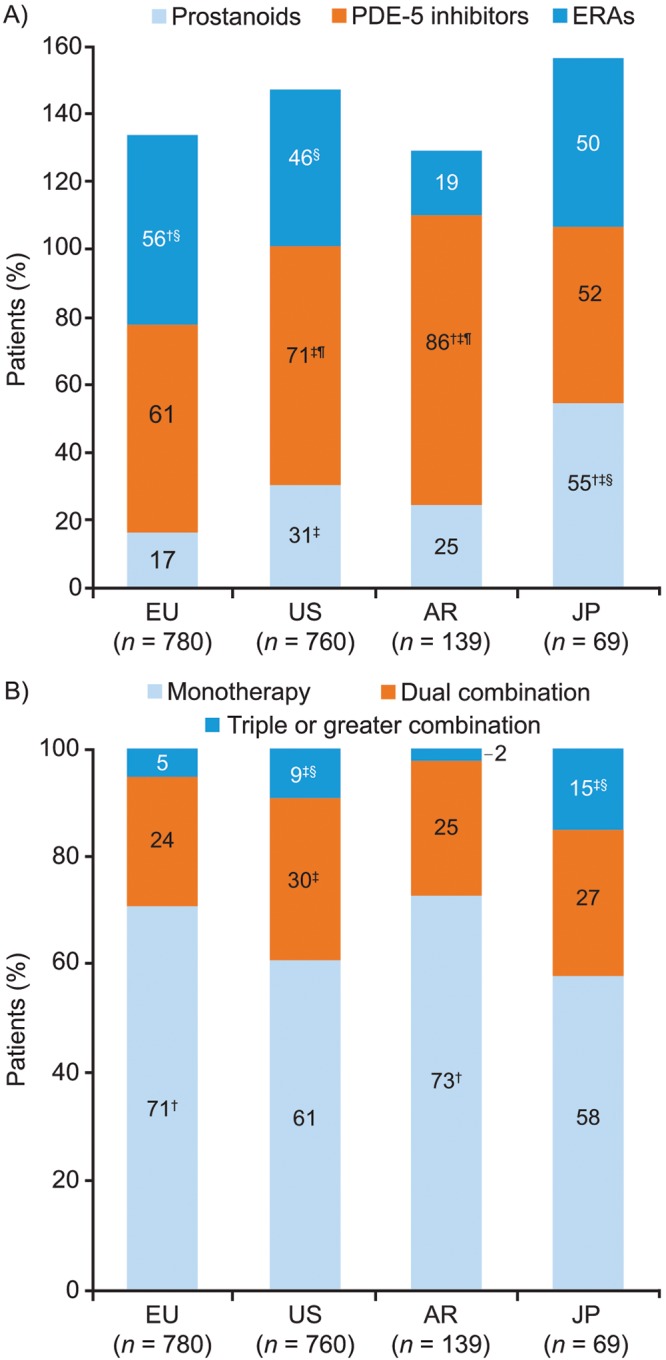
PAH-specific drug therapies (patient record data). Response to the study question “Please select from the options listed which PAH-specific drugs this patient is currently receiving?” A, Drug category; B, Monotherapy, dual combination, and triple or greater therapy. Probability test pairwise comparisons (with Bonferroni correction) were significantly (P < 0.05) different versus the corresponding category in †the US, ‡Europe, §Argentina, or ¶Japan. AR: Argentina; ERAs: endothelin receptor antagonists; EU: Europe; JP: Japan; PAH: pulmonary arterial hypertension; PDE-5: phosphodiesterase 5; US: United States.
While the majority of patients received monotherapy (58%–73%), a significant proportion received dual therapy (24%–30%) or triple or greater combination therapy (2%–15%; Fig. 4B). Monotherapy with PDE-5is was most commonly used in Europe, the United States, and Argentina (36%, 36%, and 60%, respectively), whereas in Japan, PDE-5i monotherapy usage was significantly lower (P < 0.05) and there was no single dominant treatment regimen (Fig. 5). ERA monotherapy was used less frequently in the United States (15%) and Argentina (8%) than in Europe (30%; P < 0.05 for both the United States and Argentina vs. Europe). The use of combination therapy was more frequently reported in the United States and Japan than in Europe (P < 0.05 for both the United States and Japan vs. Europe) or Argentina (significance testing was not performed because of the small base). In Europe, there was little difference in treatment practices between PH centers and non-PH centers, whereas in the United States the use of prostanoid monotherapy was significantly more common in PH centers than in non-PH centers (19% vs. 3%, respectively; P < 0.05).
Figure 5.
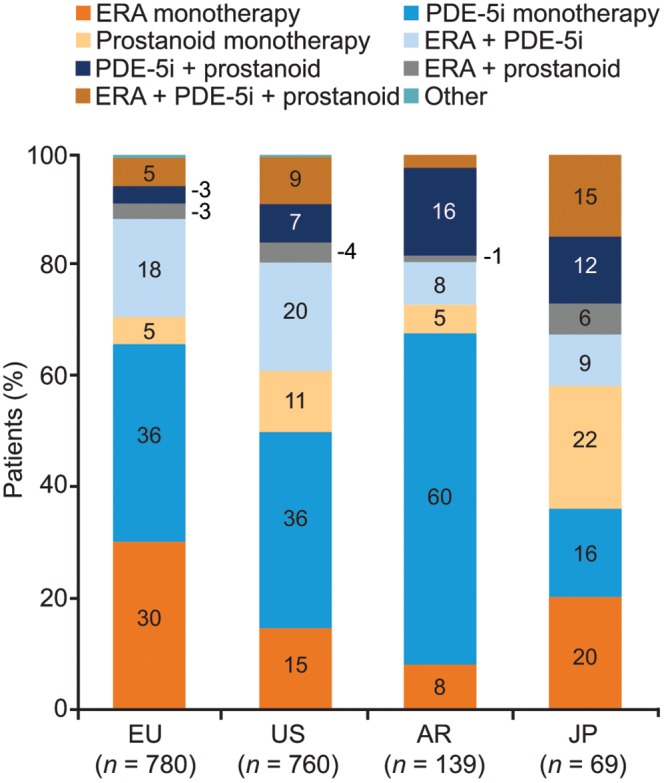
Usage of different PAH-specific drug regimens (patient record data). Response to the study question “Please select from the options listed which PAH-specific drugs this patient is currently receiving?” AR: Argentina; ERA: endothelin receptor antagonist; EU: Europe; JP: Japan; PAH: pulmonary arterial hypertension; PDE-5i: phosphodiesterase 5 inhibitor; US: United States.
In Europe and the United States, treatment choice was similar in patients who were eligible and those who were ineligible for PEA (no significant difference; data not shown). In patients with residual PH after surgery, the most common treatment choice in Europe was monotherapy with an ERA (36% of patients) or a PDE-5i (28% of patients; no significant difference); in the United States, the most common treatment choice in these patients was PDE-5i monotherapy (37% of patients) and ERA/PDE-5i dual therapy (27% of patients; no significant difference). In Japan, BPA was a fairly common treatment choice for patients whether they were ineligible or eligible for PEA (24% and 17%, respectively, of patients with CTEPH evaluated for PEA).
Results from physician questionnaire
Diagnosis of CTEPH
Physicians’ perceptions of the most commonly used techniques to diagnose CTEPH were broadly in line with results from their patients’ medical records, with echocardiography reported as the most commonly used test across all regions (data not shown).
Management of CTEPH
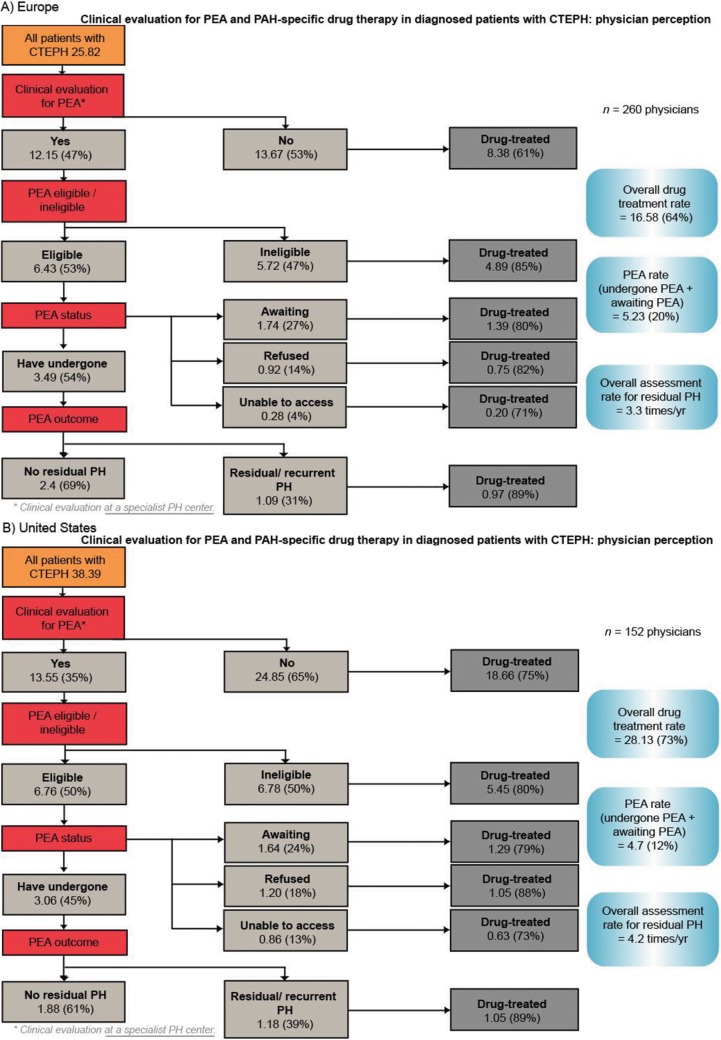
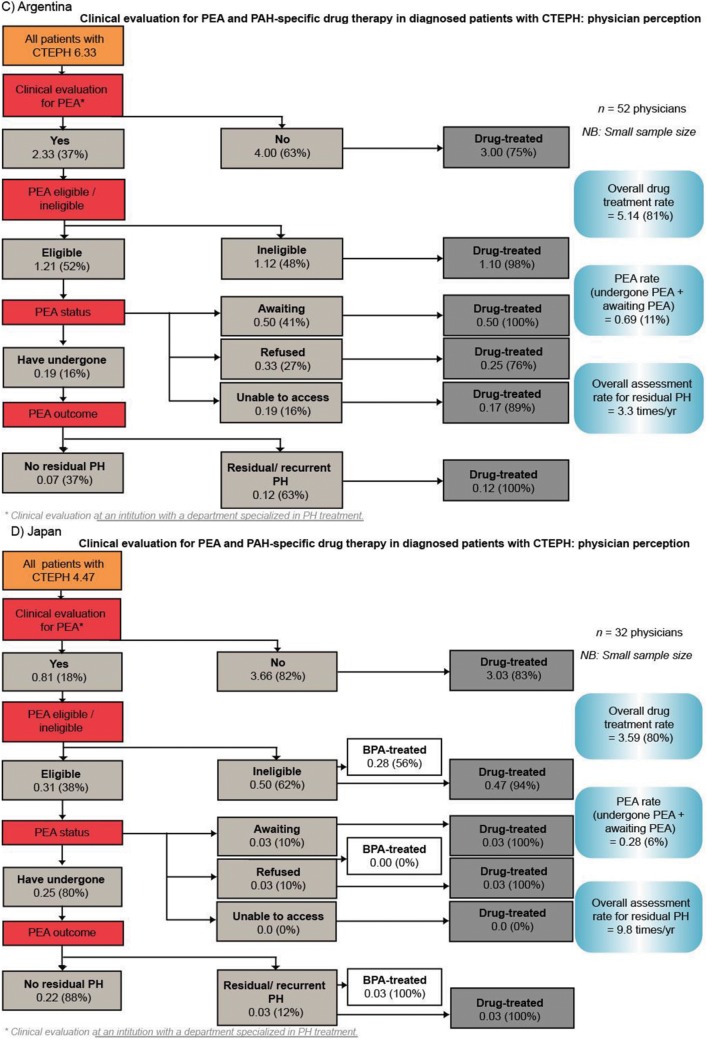
The rate of PEA evaluation reported by physicians across the four regions (Fig. S2) aligns with the rates calculated from patient records (Fig. 3). Of patients who were evaluated and considered eligible for PEA in Europe, the United States, Argentina, and Japan, 54%, 45%, 16%, and 80%, respectively, had undergone the procedure at the time of this survey. The rate of residual/recurrent PH after PEA was 31% in Europe, 39% in the United States, 63% in Argentina, and 12% in Japan (Fig. S2).
Physicians reported that the majority of their patients with CTEPH were receiving PAH-specific therapies (64% in Europe, 73% in the United States, 81% in Argentina, and 80% in Japan; Fig. S2). Within Europe, the treatment rate ranged from 56% in the United Kingdom to 81% in Spain (data not shown). Physicians from Europe, the United States, and Argentina reported that the most common reasons for not initiating PAH-specific drug therapy were that the patient was not suitable for drug therapy because of comorbidities or their general condition and patient refusal. In the United States, 35% of physicians reported lack of reimbursement for PAH-specific drug therapy as the reason for patients not being treated.
Discussion
To our knowledge, this physician-based patient record and perception study is the first to introduce a global aspect of CTEPH care and provides valuable insights into the management of CTEPH worldwide. A key feature was the inclusion of physicians from a wide range of clinical settings, including PEA centers, specialist PH centers, and nonspecialist centers. While most countries have designated specialist centers, CTEPH is also being managed in other settings, as evidenced by the 18%–81% of participating physicians who were not working in or affiliated with a specialist PH center.
Data from this study indicated gaps between the real-life practice of diagnosing and treating patients with CTEPH and the guidelines. Screening with V/Q scanning was extremely low worldwide (33%–54%), which is in accordance with low V/Q scanning rates in patients with PAH previously reported from US centers.8 Our finding suggests poor compliance with the 2009 guidelines that recommend V/Q scanning as the most appropriate test to screen for CTEPH in patients with PH, with subsequent CT angiography if the V/Q scan is indeterminate or reveals perfusion defects.1 The prevalence of V/Q scanning ranked seventh, after echocardiography, electrocardiography, RHC, CT angiography, lung function test, and chest X-ray. Echocardiography was the most commonly used test at both PH centers and nonspecialist centers; however, while it is an effective screening tool for the presence of PH, it is not an accurate diagnostic tool for CTEPH. Moreover, expertise in pulmonary vascular imaging tools is also required. These results therefore suggest that, on the basis of the guideline recommendations, a high proportion of patients in this study did not have a confirmed diagnosis of CTEPH, highlighting the difficulties in diagnosing CTEPH, even in specialist centers, and a potential need for increased education and/or resources. The low V/Q scanning rates found in this study may also indirectly support previous findings from PH registries indicating that knowledge gaps exist in correctly diagnosing PH,8 as it can be speculated that incorrect decisions made during PH diagnosis may subsequently affect the correct diagnostic workup for CTEPH, particularly the use of V/Q scanning.
In addition to our finding of low usage of V/Q scanning, a major discovery was the low rate of PEA evaluation worldwide, despite PEA being the standard of care and a potentially curative treatment for CTEPH. The main reasons reported for not referring a patient for PEA evaluation were that surgery was considered only if medical treatment was failing and patient refusal, which could reflect a patient’s preference regarding medical versus invasive surgery. Nevertheless, these results indicate a marked deviation from current treatment recommendations, which advise that PAH-specific therapy should be considered only in patients who are ineligible for PEA surgery or have residual PH after surgery.1 Because this survey was completed before the most recent CTEPH guidelines were published3,9 and in an era when medical therapy did not show any significant benefit, it is surprising that so few patients were referred for consideration of PEA surgery. This suggests that physicians may be unfamiliar with this surgical approach or that there may be misconceptions relating to the risks associated with the procedure, especially if physicians are unaware of the positive outcomes when PEA is carried out in expert centers with extensive experience in the surgery. Counterintuitively, this potential knowledge gap appeared to be present at specialist PH centers as well as nonspecialist centers, which is particularly concerning. The current 2013 and 2014 CTEPH guidelines are very clear and indicate that all patients with CTEPH should be referred to a dedicated multidisciplinary CTEPH team including an experienced PEA surgeon3,9 and that only the latter should diagnose a patient’s disease as inoperable.3
This study highlighted a number of regional variations in the use of PAH-specific therapies. In particular, more than half the patients in this survey were treated with PDE-5is, regardless of region. Among the other classes of PAH-specific therapies, prostanoids were used more frequently in Japan, perhaps because at the time of the study Japan was the only country where oral prostanoids were available (e.g., beraprost) and used as standard first-line therapy. Reasons of culture may also play a role. ERAs were used least in Argentina, probably for reasons of cost and reimbursement. Furthermore, some insurance plans (e.g., Medicaid in the United States) require that patients begin with sildenafil rather than an alternative treatment. Most patients in the survey received monotherapy, with triple or greater combination therapy relatively infrequent among all regions assessed, although it occurred more often in Japan and the United States, reflecting potential cultural differences, as well as drug availability (and possibly lack of surgical availability), in these regions. There were also regional differences in the choice of PAH-specific therapy in patients with residual/recurrent CTEPH after PEA—in Europe and the United States, patients most often received ERAs and/or PDE-5is, whereas in Japan, BPA was a common treatment choice because of the greater experience with this form of treatment and the cultural preference for BPA over PEA in Japan.
Limitations of this study include its retrospective nature—data were not collected in the course of a registry—and the potential bias in the physician sample, as practicing physicians were selected solely from the Medefield market research database. It should be noted that while a proportion of physicians in each country indicated that they practiced at “specialist PH centers” or “PH referral centers,” in the United States and Japan these were not verified and so may not equate to nationally recognized PH centers. In addition, there may be differences between respondents in the interpretation of survey questions. Finally, it should be noted that no follow-up questions were asked of physicians whose stated practices were not consistent with current guidelines. As well as addressing issues relating to noncompliance with guidelines, follow-up may also have highlighted areas where potential misunderstanding of questions may have occurred.
Overall, these findings indicate that while algorithms for the management of CTEPH exist,1,3 they are not closely followed and that there is a need for more education of physicians managing patients with possible CTEPH. The two most striking failures include low use of V/Q scanning for diagnosis of CTEPH and, importantly, poor referral rates for consideration of potentially curative PEA surgery. These findings suggest that resource utilization for the diagnosis and management of CTEPH is inadequate, further highlighting the importance of early referral to expert PEA centers for patients with this complex, but potentially curable, condition.
Appendix.
Figure S1.
Clinical evaluation for PEA in patients with CTEPH receiving PAH-specific drug therapy in PH centers and non-PH centers (patient record data for patients diagnosed with CTEPH and currently receiving PAH-specific drug therapy). BPA: balloon pulmonary angioplasty; CTEPH: chronic thromboembolic pulmonary hypertension; mPAP: mean pulmonary artery pressure; NYHA: New York Heart Association; PAH: pulmonary arterial hypertension; PEA: pulmonary endarterectomy; PH: pulmonary hypertension; pt: patient; PVR: pulmonary vascular resistance.
Figure S2.
Clinical evaluation for PEA and PAH-specific drug therapy in patients with CTEPH (physician perception data). Numbers are the mean number of patients per physician (percentage of patients as a proportion of each subgroup). BPA: balloon pulmonary angioplasty; CTEPH: chronic thromboembolic pulmonary hypertension; PAH: pulmonary arterial hypertension; PEA: pulmonary endarterectomy; PH: pulmonary hypertension.
Table S1.
Physician sample, continued
| UK | FR | DE | IT | ES | US | AR | JP | |
|---|---|---|---|---|---|---|---|---|
| No. of physicians | 50 | 52 | 51 | 50 | 57 | 152 | 52 | 32 |
| Patients with CTEPH currently managed, % | ||||||||
| 2–10 | 52 | 55.8 | 33.3 | 62 | 56.1 | 36.8 | 98.1 | 81.2 |
| 11–20 | 12 | 17.3 | 25.5 | 18 | 24.6 | 18.4 | 0 | 3.1 |
| 21–30 | 14 | 3.8 | 23.5 | 4 | 3.5 | 10.5 | 0 | 0 |
| 31 or more | 22 | 23.1 | 17.6 | 16 | 15.8 | 34.2 | 1.9 | 0 |
| Patients with CTEPH currently managed, no. | ||||||||
| Mean ± SEM | 27.6 ± 5.9 | 37.8 ± 11.2 | 25.9 ± 4.7 | 21.4 ± 4.9 | 17 ± 2.5 | 38.4 ± 5.1 | 6.3 ± 2.8 | 4.5 ± 0.7 |
| Median | 10 | 10 | 18 | 10 | 10 | 20 | 3 | 3 |
AR: Argentina; CTEPH: chronic thromboembolic pulmonary hypertension; DE: Germany; ES: Spain; FR: France; IT: Italy; JP: Japan; SEM: standard error of the mean; UK: United Kingdom; US: United States.
Source of Support: The study was carried out by Ipsos Healthcare (London), supported by Bayer Pharma (Berlin). Editorial assistance was provided by Adelphi Communications (Bollington, United Kingdom), supported by Bayer Pharma.
Conflict of Interest: HG reports personal fees for services rendered (includes honoraria, royalties, or fees for consultancy, lectures, speakers bureaus, or expert testimony) from Actelion, AstraZeneca, Bayer Healthcare Pharmaceuticals, GlaxoSmithKline, Janssen Cilag, Lilly, Pfizer, and United Therapeutics/OMT. IRP has received grants and personal fees from Bayer Healthcare Pharmaceuticals, Actelion, Gilead, and United Therapeutics. BH is a full-time employee of Bayer Pharma. SH declares no conflict of interest. DJ reports personal fees from Actelion, Bayer Healthcare Pharmaceuticals, GlaxoSmithKline, and Pfizer. NHK reports personal fees from Actelion; personal fees and nonfinancial support (includes drugs/equipment, travel, writing assistance, or administrative support) from Bayer Healthcare Pharmaceuticals; and grants from Gilead, Lung Biotechnology, and United Therapeutics. IL reports grants, personal fees, and nonfinancial support from Bayer Healthcare Pharmaceuticals, Actelion, and Pfizer; grants and personal fees from AOP Orphan and United Therapeutics; and personal fees and nonfinancial support from Servier, AstraZeneca, GlaxoSmithKline, and Medtronic.
Supplements
Appendix (16.9MB, pdf)
References
- 1.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiéry JL, Barbera JA, Beghetti M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30(20):2493–2537. [DOI] [PubMed]
- 2.D’Armini AM. Diagnostic advances and opportunities in chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2015;24(136):253–262. [DOI] [PMC free article] [PubMed]
- 3.Kim NH, Delcroix M, Jenkins DP, Channick R, Dartevelle P, Jansa P, Lang I, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl):D92–D99. [DOI] [PubMed]
- 4.Humbert M. Pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: pathophysiology. Eur Respir Rev 2010;19(115):59–63. [DOI] [PMC free article] [PubMed]
- 5.Freed DH, Thomson BM, Berman M, Tsui SS, Dunning J, Sheares KK, Pepke-Zaba J, Jenkins DP. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg 2011;141(2):383–387. [DOI] [PubMed]
- 6.Ryan JJ, Butrous G, Maron BA. The heterogeneity of clinical practice patterns among an international cohort of pulmonary arterial hypertension experts. Pulm Circ 2014;4(3):441–451. [DOI] [PMC free article] [PubMed]
- 7.Preston IR, Hinzmann B, Heinz S, Gall H, Jenkins D, Kim NH, Lang I. An international physician survey of pulmonary arterial hypertension management. Pulm Circ 2016;6(3):338–346. [DOI] [PMC free article] [PubMed]
- 8.McLaughlin VV, Langer A, Tan M, Clements PJ, Oudiz RJ, Tapson VF, Channick RN, Rubin LJ. Contemporary trends in the diagnosis and management of pulmonary arterial hypertension: an initiative to close the care gap. Chest 2013;143(2):324–332. [DOI] [PubMed]
- 9.Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC), endorsed by the European Respiratory Society (ERS). Eur Heart J 2014;35(43):3033–3069. [DOI] [PubMed]