Abstract Abstract
Portopulmonary hypertension (POPH) is a poorly understood complication of liver disease associated with significant morbidity and mortality. We sought to identify novel biomarkers of POPH disease presence and severity. We performed a prospective, multicenter, case-control study involving patients with liver disease undergoing right heart catheterization. POPH cases were defined as a mean pulmonary arterial pressure (mPAP) ≥25 mmHg and pulmonary vascular resistance (PVR) >240 dynes˙s˙cm−5. Plasma samples were collected from the systemic and pulmonary circulation, and antibody microarray was used to identify biomarkers. Characterization and validation of a candidate cytokine, macrophage migration inhibitory factor (MIF), was performed using enzyme-linked immunosorbent assay. Continuous variables were compared using a Mann-Whitney U test and correlated with disease severity using Spearman correlation. MIF levels were elevated in both the systemic and pulmonary circulation in patients with POPH compared with controls (median MIF level [interquartile range] in systemic circulation: 46.68 ng/mL [32.31–76.04] vs. 31.19 ng/mL [26.92–42.17], P = 0.009; in pulmonary circulation: 49.59 ng/mL [35.90–108.80] vs. 37.78 [21.78–45.53], P = 0.002). In patients with POPH, MIF levels were positively correlated with PVR (r = 0.58, P = 0.006) and inversely correlated with cardiac output (r = −0.57, P = 0.007). MIF >60 ng/mL or tricuspid regurgitation gradient >50 mmHg had a 92% sensitivity and specificity for the diagnosis of POPH, with a positive predictive value of 86% and a negative predictive value of 96%. MIF is a promising novel biomarker of POPH disease presence and severity in patients with liver disease and portal hypertension.
Keywords: portopulmonary hypertension, macrophage migration inhibitory factor, biomarker, transpulmonary, pulmonary arterial hypertension
Portopulmonary hypertension (POPH), a subtype of World Health Organization (WHO) group 1 pulmonary arterial hypertension (PAH) that develops in patients with liver disease and portal hypertension, is the third most common cause of associated PAH and affects 5%–6% of patients referred for liver transplantation.1-4 POPH is pathologically indistinct from idiopathic PAH, but it is associated with a significantly lower 5-year survival despite a higher cardiac index and lower right atrial pressure at diagnosis.5,6
POPH can complicate or preclude liver transplantation because of an elevated risk of perioperative death.7 Because of this increased risk, patients on the liver transplant list undergo yearly echocardiograms to screen for POPH, and patients with known POPH undergo right heart catheterizations every 3 months to ensure that their hemodynamic characteristics are acceptable for undergoing transplantation.8,9 As such, identification of noninvasive biomarkers of POPH disease presence, severity, and treatment response is needed to minimize repeated invasive procedures in this group of patients.
The pathogenesis of POPH, characterized by pulmonary vasoconstriction and vascular remodeling, is poorly understood. There is no known association between the presence of POPH and severity of liver disease or portal hypertension, but both female sex and autoimmune liver disease have been identified as clinical risk factors.4,10,11 An increased prevalence of spontaneous portosystemic shunts in patients with POPH as well as the development of POPH in patients with congenital portosystemic shunts with normal liver function suggests that vasoactive factors from the splanchnic circulation, rather than the presence of liver failure per se, contribute to disease development.12,13 These factors may represent novel therapeutic targets and may play an important role in the pathogenesis of other subtypes of PAH.
The purpose of our study was to identify biomarkers of POPH disease presence, severity, and treatment response. We also sought to compare circulating gradients of these biomarkers to determine whether they were derived from the splanchnic or pulmonary circulation. On the basis of the results of exploratory antibody microarray, we identified macrophage migration inhibitory factor (MIF) as a potential biomarker candidate. We chose to focus on MIF for the following reasons: (1) MIF is a pleiotropic proinflammatory cytokine expressed in the lung, liver, and spleen, key organs that are likely to be involved in the pathogenesis of POPH;14 (2) MIF is involved in inflammation and endothelial dysfunction in other subtypes of PAH, but its role in POPH has not been previously studied;15 and (3) high-quality immunoassays were available for measurement of circulating MIF.
Methods
Study design and subject selection
We performed a prospective multicenter case-control study. Patients with liver disease and suspected POPH (based on symptoms and/or echocardiogram) undergoing right heart catheterization as part of their clinical evaluation at 3 academic centers were enrolled between February 2014 and December 2015. Liver disease was defined as a clinical diagnosis of cirrhosis or portal hypertension with imaging or pathology consistent with cirrhosis or portal hypertension. Patients were divided into 2 groups, POPH case patients and liver disease controls, on the basis of pulmonary hemodynamic characteristics. POPH cases were defined as patients with an elevated mean pulmonary arterial pressure (mPAP) ≥25 mmHg and PVR >240 dynes˙s˙cm−5 with a normal pulmonary arterial wedge pressure (PAWP) ≤15 mmHg and a clinical diagnosis of POPH by an experienced PAH healthcare provider.8,16 We included patients with incident as well as prevalent disease with prior diagnosis of POPH who were receiving PAH therapy if they had previously met these hemodynamic criteria. Patients with hepatopulmonary syndrome were excluded from the control group. Clinical data, including demographic characteristics, liver disease etiology, liver disease severity, and transthoracic echocardiogram data, including tricuspid regurgitation (TR) gradient, derived from tricuspid regurgitation jet velocity, were collected from review of the medical record. TR gradient was used rather than right ventricular systolic pressure, because there was intercenter variation regarding estimation of right atrial pressure. The study was approved by the institutional review board (IRB; 2013-P-000416), and all patients provided written informed consent.
Sample collection and assays
At the time of catheterization, plasma samples were collected from the central vein (CV; internal jugular or femoral vein), the right atrium (RA), and the pulmonary arterial wedge (PAW) position. Before each sample collection, 2 mL of blood from the catheter was discarded to ensure that the sample was collected from the intended location. CV samples were collected from the catheter introducer, whereas RA and PAW samples were collected from the distal port of the Swan-Ganz catheter. All samples were collected in EDTA tubes, centrifuged at 3,000 g for 10 minutes, and stored at −80°C. An exploratory screen using a multiplexed antibody microarray was performed at R&D Systems (Human XL Cytokine Array). Circulating MIF levels were measured using commercial enzyme-linked immunosorbent assay using manufacturer’s instructions (R&D Systems). This assay had a sensitivity of 0.068 ng/mL with an intraplate and interplate coefficient of variation of 4.5%–6% and 8.4%–9.7%, respectively.
Validation and comparison with non-POPH WHO group 1 PAH
To validate our findings and compare MIF levels in patients with POPH with levels in patients with non-POPH WHO group 1 PAH, we also measured circulating MIF levels in a retrospective bank of available superior vena cava plasma samples from a validation cohort of patients with POPH and non-POPH group 1 PAH (idiopathic PAH, HIV-associated PAH, connective tissue disease–associated PAH, and drug/toxin-induced PAH).17
MIF expression in the lung
Archived lung pathology specimens from patients with WHO group 1 or 3 PH (documented mPAP ≥25 mmHg and a PAWP ≤15 mmHg) and controls with normal right heart size and function and an estimated right ventricular systolic pressure <35 mmHg determined by echocardiogram were identified from a search of the pathology laboratory system at a single center. Following hematoxylin and eosin staining, formalin-fixed, paraffin-embedded lung tissue blocks were examined by a thoracic surgical pathologist. Immunohistochemical staining for MIF (polyclonal goat immunoglobulin G [IgG] human MIF antibody, 1∶1,000 or 1∶1,500 dilution; R&D Systems) was performed using Vector ImmPRESS HRP Anti-Goat IgG Polymer Detection Kit in accordance with the manufacturer’s instructions (Burlingame, CA). The pathology study was approved by the IRB with waiver of informed consent.
Statistical analysis
Data are summarized as medians with interquartile ranges (IQRs) or absolute numbers and percentages. Case patients and controls were compared using a Mann-Whitney U test, χ2 test, or Fisher exact test, as appropriate. Matched plasma samples within individuals were compared using a Wilcoxon signed-rank test. MIF levels were correlated with continuous variables using Spearman rank correlation. To determine the value of MIF as a potential screening tool for POPH, we generated receiver operating characteristic (ROC) curves for CV MIF levels and TR gradient by echocardiogram and a combination of the two using a logistic model in controls and incident patients with POPH (i.e., treatment-naive patients with newly diagnosed POPH). We also calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of different MIF and TR gradient thresholds to identify cutoff values with optimal testing characteristics. We chose the CV as our primary site for analysis (rather than RA or PAW), because it would be most reproducibly obtained and similar to peripheral blood samples used for screening. As an exploratory analysis, we also compared patients with high (above the median) and low (below the median) CV and PAW MIF levels to determine whether MIF was predictive of mortality using Cox regression. Vital status was determined from the medical record as any death that occurred between the time of enrollment and the end of the follow-up period (May 20, 2016). Kaplan-Meier survival curves were generated. Patients who were lost to follow-up were censored at the time of their last clinic visit or hospitalization. All tests were 2-sided, and significance was defined as P < 0.05. Data analysis was performed in GraphPad Prism, version 6.0, and ROC curves were generated in R, version 3.2.2. Heat map was generated using GENE-E software (Broad Institute).
Results
Patient characteristics
Twenty-one patients with POPH and 31 controls with liver disease without pulmonary hypertension were included in the study (Fig. 1). Patients with POPH were not significantly different from controls with respect to age, sex, race/ethnicity, or liver disease etiology (Table 1). Compared with controls, patients with POPH had less severe liver disease as assessed by either Model for End-Stage Liver Disease (MELD) score (P = 0.002) or Child-Pugh class (P < 0.001). Patients with POPH had a median mPAP of 38 mmHg (IQR: 31–48 mmHg) and median PVR of 327.7 dynes˙s˙cm−5 (IQR: 267.0–534.9 dynes˙s˙cm−5), which were both significantly higher compared with the control group (P < 0.001). Patients with POPH also had a significantly lower cardiac output (CO) and PAWP compared with controls (Table 1).
Figure 1.
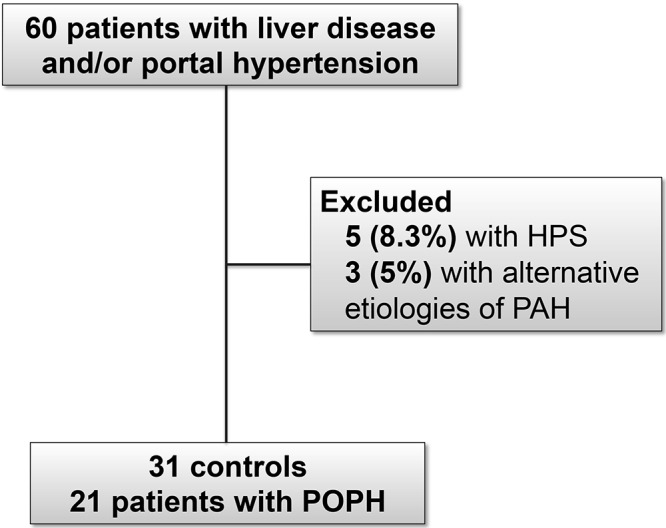
Flow chart of patients included in the study. A total of 8.3% of patients were excluded due to hepatopulmonary syndrome (HPS), and 5% of patients were excluded due to alternative etiologies of pulmonary arterial hypertension (PAH). Thirty-one controls and 21 case patients with portopulmonary hypertension (POPH) were included in the final cohort.
Table 1.
Patient characteristics
| Characteristic | Controls (n = 31) |
Portopulmonary hypertension (n = 21) |
P |
|---|---|---|---|
| Age, years | 58.0 (50.0–65.0) | 55.0 (48.0–63.0) | NS |
| Female sex | 9 (29.0) | 5 (23.8) | NS |
| Race/ethnicity | NS | ||
| White | 25 (80.6) | 15 (71.4) | |
| Hispanic/Latino | 4 (12.9) | 4 (19) | |
| Black | 2 (6.5) | 2 (9.5) | |
| Other | 0 | 2 (9.5) | |
| Comorbidities | NS | ||
| Hypertension | 17 (54.8) | 10 (47.6) | |
| Diabetes mellitus | 16 (51.6) | 10 (47.6) | |
| ESRD with hemodialysis | 3 (9.7) | 1 (4.8) | |
| HCC | 5 (16.1) | 4 (19.0) | |
| Systemic glucocorticoid use | 3 (9.7) | 1 (5.3) (n = 19) | |
| Etiology of liver disease | NS | ||
| Alcohol | 15 (48.4) | 11 (52.4) | |
| HCV infection | 9 (29.0) | 8 (38.1) | |
| HCV infection plus alcohol | 1 (3.2) | 4 (19.0) | |
| Nonalcoholic fatty liver disease | 4 (12.9) | 1 (4.8) | |
| Autoimmune | 0 | 1 (4.8) | |
| Other | 9 (29.0) | 4 (23.8) | |
| Liver disease severity | |||
| MELD | 17 (12–24) | 11 (9–14) | 0.002 |
| Child class | <0.001 | ||
| A | 2 (6.5) | 9 (42.9) | |
| B | 13 (41.9) | 11 (52.4) | |
| C | 16 (51.6) | 1 (4.8) | |
| Physical examination | |||
| Body mass index | 28.6 (25.6–32.3) (n = 27) | 29.1 (23.7–35.0) (n = 16) | NS |
| Oxygen saturation | 97 (95.5–99.0) (n = 29) | 96 (95–98) (n = 18) | NS |
| Tricuspid regurgitation gradient by echocardiogram, mmHg | 37 (35–45) (n = 26) | 53 (42–68) (n = 20) | 0.002 |
| Hemodynamic characteristics | |||
| mPAP, mmHg | 24 (19–30) | 38 (31–48) | <0.001 |
| PAWP, mmHg | 15.5 (11.5–20) | 10 (5.5–13.5) | <0.001 |
| PVR, dynes˙s˙cm−5 | 86.4 (50.4–119.0) | 327.7 (267.0–534.9) | <0.001 |
| CO, L/min | 7.7 (6.7–10.3) | 6.3 (5.3–7.6) | 0.001 |
Patients may have had more than one listed race/ethnicity, diagnosis, or comorbidity. Data are expressed as median (interquartile range) or number (%). CO: cardiac output; ESRD: end-stage renal disease; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; MELD: model for end-stage liver disease; mPAP: mean pulmonary arterial pressure; NS: not significant; PAWP: pulmonary arterial wedge pressure; PVR: pulmonary vascular resistance.
Antibody microarray screen
The relative expression of a subset of biomarkers from multiplexed antibody microarray in a limited sample set (3 controls and 3 case patients) is shown in Figure 2. One hundred and eight cytokines were analyzed. Relative cytokine expression was an average 1.18-fold higher in the 3 patients with POPH compared with controls. Nine cytokines were elevated in duplicate testing >1.5-fold in patients with POPH compared with controls (macrophage inflammatory protein-3 beta, hepatocyte growth factor, MIF, platelet-derived growth factor AA, interleukin-17A, monocyte chemoattractant protein-1, myeloperoxidase, leptin, and growth hormone) and no cytokines were reduced >0.5-fold in patients with POPH. For the 3 patients with POPH, compared with 3 controls, duplicate testing of MIF had the lowest P values without correction for multiple testing (0.01 and 0.009), and MIF was 1.6-fold higher in patients with POPH. The complete raw data set with duplicate values for all cytokines is included in Table S1. On the basis of microarray results, we focused our efforts on further characterizing MIF as a potential biomarker candidate.
Figure 2.
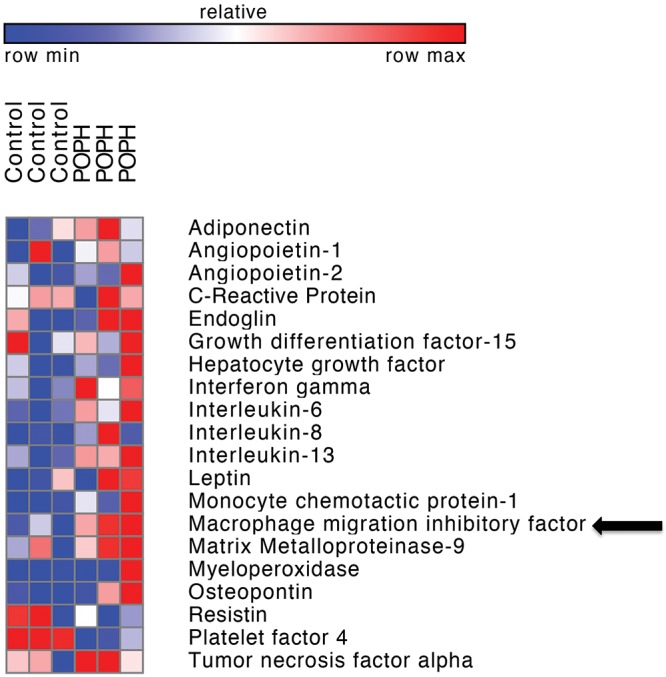
Antibody microarray. Heat map demonstrating relative expression of a subset of biomarkers from screening antibody microarray (Human XL Cytokine Array, R&D Systems) in patients with portopulmonary hypertension (POPH; n = 3) versus controls (n = 3). Macrophage migration inhibitory factor expression, indicated by an arrow, was 1.6-fold higher in patients with POPH.
Circulating MIF levels and correlation with disease severity
Both CV and PAW MIF levels were significantly higher in patients with POPH compared with controls (P = 0.009 and P = 0.002, respectively), and there was a trend toward higher RA MIF levels in patients with POPH (P = 0.07; Fig. 3). PAW MIF levels were significantly higher than RA MIF levels in patients with POPH (P = 0.01), whereas controls had similar PAW and RA MIF levels (P = 0.5). PAW MIF levels were not significantly different from CV MIF levels in patients with POPH (P = 0.27) or controls (P = 0.18). The absolute change in MIF across the pulmonary circulation (PAW-RA) was higher in patients with POPH compared with controls (median [IQR]: 9.04 ng/mL [1.22–48.40] vs. 2.15 ng/mL [−5.52 to 8.04]; P = 0.02). In a subgroup analysis, median (IQR) CV MIF in patients with incident POPH (65.30 ng/mL [35.68–80.26]; n = 13) was not significantly different from that in patients with prevalent POPH who were receiving PAH therapy (37.59 ng/mL [28.46–67.06]; n = 8; P = 0.3). There was no difference in CV MIF levels by sex (P = 0.35) or internal jugular versus femoral central venous site of access (P = 0.6).
Figure 3.
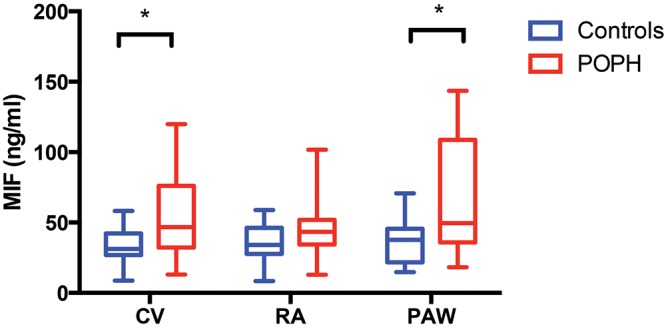
Circulating plasma macrophage migration inhibitory factor (MIF) levels. Median (interquartile range) central vein (CV), right atrium (RA), and pulmonary artery wedge (PAW) macrophage migration inhibitory factor (MIF) levels in patients with portopulmonary hypertension (POPH) versus controls. Both systemic (CV) and pulmonary (PAW) MIF levels were higher in patients with POPH than in controls (CV: 46.68 ng/mL [32.31–76.04] vs. 31.19 ng/mL [26.92–42.17], P = 0.009; PAW: 49.59 [35.90–108.80] vs. 37.78 [21.78–45.53], P = 0.002). There was also a trend toward higher RA MIF levels in patients with POPH (43.35 ng/mL [34.45–51.99] vs. 34.13 ng/mL [27.81–46.19], P = 0.07).
Circulating CV MIF was significantly correlated with PVR (r = 0.58, P = 0.006), inversely correlated with CO (r = −0.57, P = 0.007; Fig. 4A, 4B), and was not correlated with mPAP (r = 0.29, P = 0.2) or PAWP (r = 0.01, P = 0.95). CV MIF levels were also not correlated with liver disease severity as assessed by the MELD score (r = −.19, P = 0.4).
Figure 4.
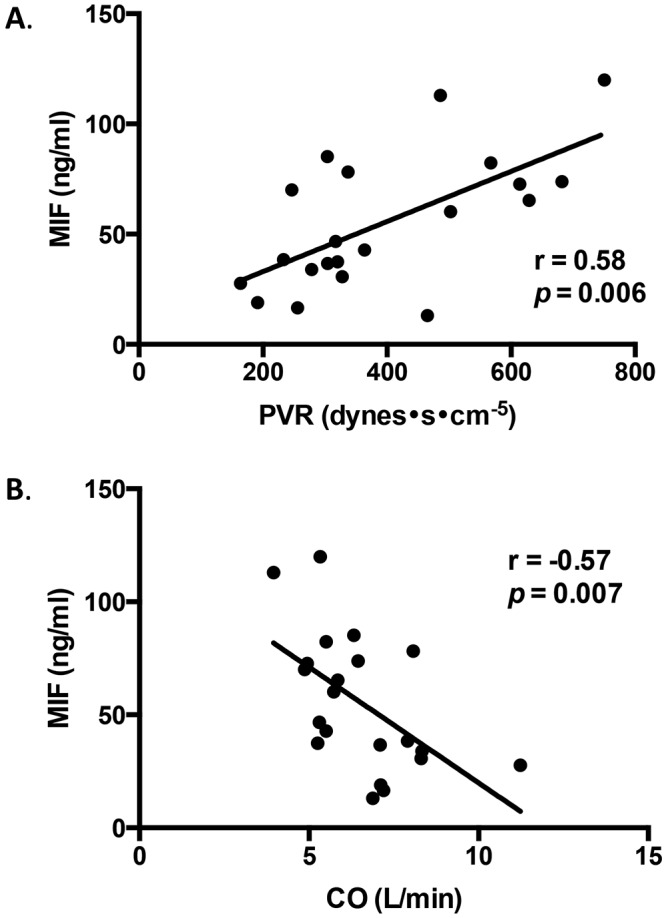
Correlation of macrophage migration inhibitory factor (MIF) levels with pulmonary hemodynamic characteristics. Central venous MIF is significantly correlated with pulmonary vascular resistance (r = 0.58, P = 0.006; A) and inversely correlated with cardiac output (r = −0.57, P = 0.007; B).
Using the same internal controls, we compared CV MIF levels to those in a retrospective cohort of patients with POPH (n = 27) and non-POPH WHO group 1 PAH (n = 21). Compared with control subjects, MIF was significantly higher in patients with POPH (median [IQR]: 38.85 ng/mL [32.90–65.97] vs. 31.19 ng/mL [26.92–42.17]; P = 0.007) and with non-POPH group 1 PAH (44.05 ng/mL [30.25–53.60]; P = 0.03). Patients with POPH and non-POPH group 1 PAH did not have significantly different CV MIF levels (P = 0.5; Fig. 5).
Figure 5.
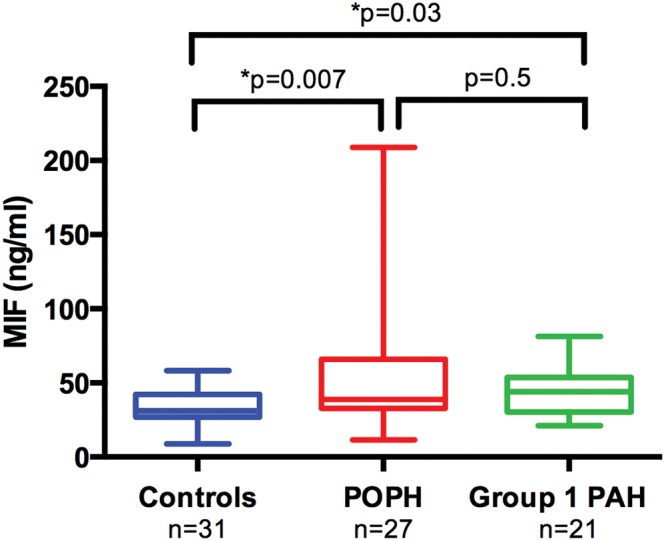
Median (interquartile range) macrophage migration inhibitory factor (MIF) levels in controls, patients with portopulmonary hypertension (POPH), and patients with non-POPH group 1 pulmonary arterial hypertension (PAH). Central vein (CV) MIF levels are higher in an external cohort of patients with POPH (38.85 ng/mL [32.9–66.0]; n = 27) compared with liver disease controls (31.2 ng/mL [26.9–42.2]; n = 31) but are not significantly different from patients with non-POPH group 1 PAH (44.1 ng/mL [30.3–53.6]; n = 21).
MIF as a screening test for POPH
We evaluated whether CV MIF levels could improve the screening for POPH among patients with liver disease. In controls (n = 26) and patients with incident POPH (n = 13) with adequate estimates of TR gradient by echocardiogram, CV MIF and TR gradient had similar testing characteristics (area under the curve [AUC]: 0.77 vs. 0.75). Combined, MIF and TR gradient had an AUC of 0.91 (P = 0.18, compared with TR gradient alone; Fig. 6). Additionally, the presence of either MIF >60 ng/mL or TR gradient >50 mmHg by echocardiogram had a 92% sensitivity and 92% specificity for the diagnosis of POPH with a positive predictive value of 86% and negative predictive value of 96%. With these cutoffs, a screening algorithm of MIF in conjunction with echocardiogram could have avoided 24 right heart catheterizations in the control group.
Figure 6.
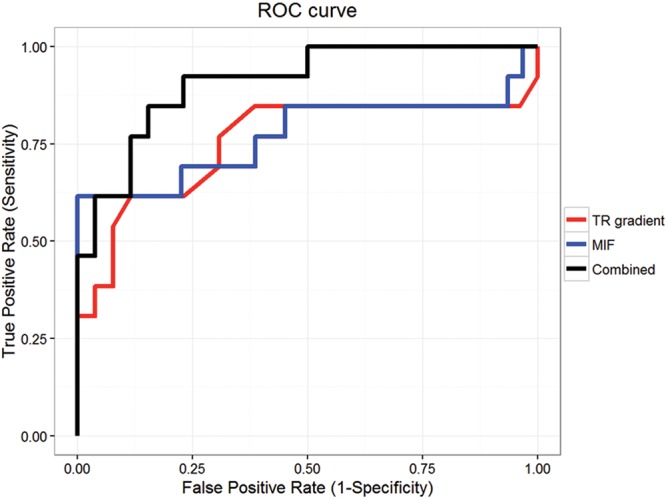
Screening test characteristics. Receiver operating characteristic curves for macrophage migration inhibitory factor (MIF; area under the receiver operating characteristic curve [AUC]: 0.77), tricuspid regurgitation (TR) gradient by transthoracic echocardiogram (AUC: 0.75), and combined MIF plus TR gradient (AUC: 0.91) are shown.
Treatment response and outcomes
Six patients with POPH underwent repeat catheterizations with sample collection before and after initiation of PAH therapy. The relationship between the absolute change in CV MIF and the absolute change in PVR after initiation of PAH therapy is depicted in Figure 7 (r = 0.71, P = 0.14). Pulmonary hemodynamic characteristics and CV MIF levels before and after initiation or uptitration of PAH therapy are detailed in Table 2.
Figure 7.
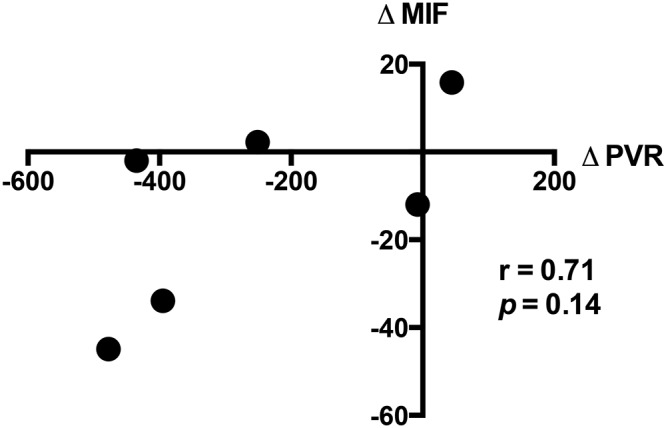
Treatment response, showing the correlation between absolute change in macrophage migration inhibitory factor (∆MIF; ng/mL) and absolute change in pulmonary vascular resistance (∆PVR; dynes˙s˙cm−5) in 6 patients who underwent cardiac catheterization with sample collection before and after initiation of pulmonary arterial hypertension therapy.
Table 2.
Treatment response
| Baseline | Posttreatment | ||||
|---|---|---|---|---|---|
| Patient | Hemodynamic characteristics | MIF (ng/mL) | PAH therapy | Hemodynamic characteristics | MIF (ng/mL) |
| Incident cases before and after initiation of PAH therapy | |||||
| 1 | mPAP: 55 mmHg; PVR: 750 dynes˙s˙cm−5; CO: 5.3 L/min | 120.00 | PDE5I and ERA | mPAP: 44 mmHg; PVR: 273 dynes˙s˙cm−5; CO: 7.0 L/min | 75.08 |
| 2 | mPAP: 51 mmHg; PVR: 629 dynes˙s˙cm−5; CO: 5.9 L/min | 65.30 | PDE5I and INH PGI2 | mPAP: 34 mmHg; PVR: 194 dynes˙s˙cm−5; CO: 8.2 L/min | 63.01 |
| 3 | mPAP: 40 mmHg; PVR: 364 dynes˙s˙cm−5; CO: 5.5 L/min | 42.80 | PDE5I (noncompliant) | mPAP: 42 mmHg; PVR: 408 dynes˙s˙cm−5; CO: 5.1 L/min | 58.60 |
| 4 | mPAP: 44 mmHg; PVR: 278 dynes˙s˙cm−5; CO: 8.3 L/min | 33.94 | PDE5I | mPAP: 43 mmHg; PVR: 270 dynes˙s˙cm−5; CO: 7.1 L/min | 21.92 |
| 5 | mPAP: 49 mmHg; PVR: 614 dynes˙s˙cm−5; CO: 5.0 L/min | 72.70 | PDE5I and INH PGI2 | mPAP: 32 mmHg; PVR: 219 dynes˙s˙cm−5; CO: 7.0 L/min | 38.84 |
| 6 | mPAP: 46 mmHg; PVR: 465 dynes˙s˙cm−5; CO: 6.9 L/min | 13.01 | ERA and PDE5I | mPAP: 30 mmHg; PVR: 214 dynes˙s˙cm−5; CO: 8.6 L/min | 15.21 |
| Prevalent cases before and after uptitration of PAH therapy | |||||
| 7 | mPAP: 31 mmHg; PVR: 317 dynes˙s˙cm−5; CO: 5.3 L/min on PDE5I and INH PGI2 | 46.68 | PDE5I, ERA, and INH PGI2 | mPAP: 34 mmHg; PVR: 171 dynes˙s˙cm−5; CO: 8.0 L/min | 30.46 |
| 8 | mPAP: 31 mmHg; PVR: 233 dynes˙s˙cm−5; CO: 7.9 L/min on PDE5I | 38.48 | PDE5I and ERA | mPAP: 31 mmHg; PVR: 159 dynes˙s˙cm−5; CO: 11.1 L/min | 56.81 |
Pulmonary hemodynamic characteristics and central venous macrophage migration inhibitory factor (MIF) levels before and after initiation or uptitration of pulmonary arterial hypertension therapy. CO: cardiac output; ERA: endothelin receptor antagonist; INH: inhaled; mPAP: mean pulmonary arterial pressure; PAH: pulmonary arterial hypertension; PDE5I: phosphodiesterase 5 inhibitor; PGI2: prostacyclin; PVR: pulmonary vascular resistance.
Three patients with POPH died during the study period, and one patient was lost to follow-up. The 3 deaths occurred at 20.1 months, 8.4 months, and 0.7 months after study enrollment and diagnostic right heart catheterization. Causes of death were liver failure, sepsis, and unknown. All 3 patients who died had PAW MIF levels >100 ng/mL. High CV MIF levels were not associated with mortality, but high PAW MIF levels were associated with an increased risk of death in patients with POPH (Fig. 8). Five patients have undergone liver transplant (8.9, 16.1, 18.6, 4.9, and 6.7 months after study enrollment), and all are alive at 12.5, 6.2, 1.2, 12.1, and 0.4 months after transplant.
Figure 8.
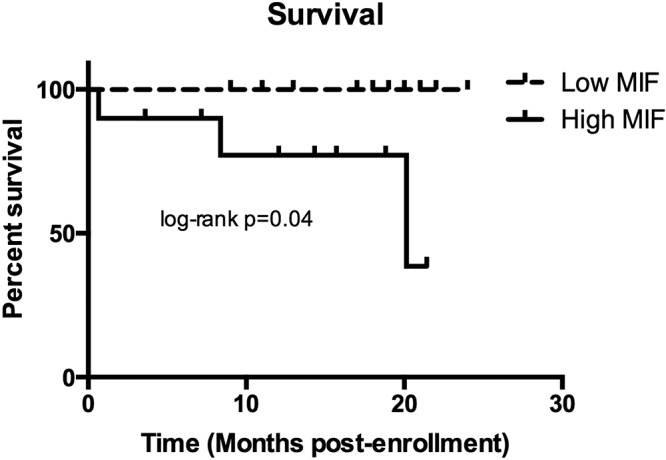
Kaplan-Meier survival curves for patients with POPH with high and low pulmonary macrophage migration inhibitory factor (MIF) levels above and below the median for the cohort (49.59 ng/mL) are depicted. Patients with high pulmonary MIF levels had poorer survival (log-rank P = 0.04).
Lung MIF expression
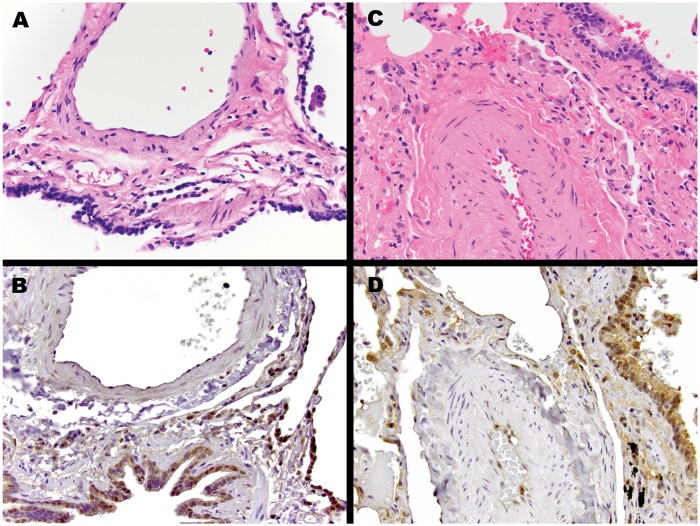
To evaluate MIF expression in the lungs, we performed immunohistochemical tests using archived lung biopsies. MIF was expressed predominantly in the endothelium and alveolar macrophages of both controls and patients with PH (Fig. 9). Because of a lack of available specimens, we could not evaluate MIF expression in subjects with POPH.
Figure 9.
Macrophage migration inhibitory factor (MIF) immunohistochemistry. Representative pulmonary parenchyma from control patient without pulmonary arterial hypertension (PAH) or lung disease (A and B) and patient with PAH (C and D). Compared with the control lung, the pulmonary artery from the patient with PAH demonstrates thickening and hypertrophy of the muscular medial layer. MIF immunostaining for both patients with PAH and control patients show expression in airway-lining ciliated bronchial epithelial cells, pulmonary macrophages, pneumocytes, and endothelial cells. Of note, the vascular smooth-muscle cells are negative. Hematoxylin and eosin stain (A, C) and MIF immunostain (B, D) images are presented at 400× original magnification.
Discussion
This study demonstrates that MIF is a promising novel biomarker of POPH disease presence and severity in patients with liver disease. We found that MIF was higher in both the systemic and pulmonary circulations of patients with POPH compared with controls and correlated with hemodynamic indices of disease severity. More importantly, the combination of circulating MIF >60 ng/mL or TR gradient >50 mmHg had an excellent negative predictive value of 96% to identify patients with liver disease who may not need a right heart catheterization to rule out POPH, and high pulmonary MIF levels were associated with an increased risk of death. Additionally, our results suggest that MIF production in the pulmonary circulation, rather than impaired hepatic clearance, may play a role in disease pathogenesis.
Earlier investigations of biomarkers in POPH have consisted of predominantly small studies that relied on echocardiogram rather than invasive hemodynamic testing for differentiation of case patients versus controls or did not correlate biomarker levels with disease severity or treatment response.18-20 In contrast to these studies, we enrolled patients with well-characterized liver disease and pulmonary hemodynamics and obtained samples at the same time as right heart catheterization, allowing biomarker levels to be temporally correlated with hemodynamics and disease severity. Interestingly, we found that MIF levels were strongly correlated with PVR and inversely correlated with CO but were not significantly correlated with mPAP. Of note, a diagnosis of POPH and elevated PVR, and not mPAP, are predictors of outcome in PAH,21 and MIF may play a pathogenic role in pulmonary vascular derangements described in POPH. We also found that circulating MIF levels in conjunction with TR gradient had a 96% NPV for the diagnosis of POPH. Given the current recommendation to screen all patients being considered for liver transplantation for POPH,8 this finding has important clinical implications. A screening algorithm combining MIF and echocardiogram with an excellent NPV could potentially decrease the number of right heart catheterizations in this group of patients, who often have an increased bleeding risk. Last, although the number of deaths was small, high pulmonary MIF levels were associated with an increased risk of death.
Despite significant advances in our knowledge regarding clinical and genetic risk factors for POPH,11,22 its pathogenesis remains poorly understood. Studies have identified decreased prostacyclin synthase expression,23 higher serum estradiol levels, and genetic variations in aromatase in patients with POPH,22 but the mechanistic link between these findings and the pathogenesis of POPH remains unknown. MIF, a critical upstream regulator of inflammation, inhibits prostacyclin synthase expression in animal models of hypoxia-induced pulmonary hypertension and is associated with pulmonary artery smooth-muscle cell proliferation.24,25 Studies involving other diseases have also shown that MIF can activate aromatase and increase estradiol levels.26 Additionally, MIF has been implicated in the pathogenesis of several autoimmune diseases, including autoimmune liver disease,27-30 which is a known risk factor for POPH.11 MIF levels in peripheral blood samples are elevated in patients with idiopathic PAH, where MIF contributes to inflammation and endothelial dysfunction,15 but this is the first study to identify higher circulating MIF levels in the pulmonary circulation of patients with PAH.
We also found that patients with POPH had an increased transpulmonary gradient of MIF compared with controls and similar RA MIF levels, suggestive of increased pulmonary production rather than decreased hepatic clearance. The source and potential stimuli for MIF production in POPH, however, remains to be determined. We found that MIF expression was prominent in alveolar macrophages, vascular endothelium, and lymphocytes in controls and patients with PAH, but we did not appreciate significant immunostaining in vascular smooth muscle cells, suggesting that smooth muscle is unlikely to be a source for higher circulating MIF levels in PAH.
Our study had several limitations. First, our sample size was small. Compared with similar studies of biomarkers in POPH, however, it is one of the largest studies of patients with POPH to date.18-20 In addition, although we found that CV MIF levels were similar to PAW MIF levels within individuals, potentially because of a prolonged half-life within the circulation, we did not specifically measure MIF levels in peripheral blood samples. We focused on assessing the importance of CV levels of MIF (compared with levels from other compartments) to describe a biomarker that may have a practical applicability in the future as a repetitive measure in clinical practice. We expect that CV MIF levels would be similar to MIF levels in peripheral blood samples, but this would need confirmation in order for MIF to be a practical screening test. MIF can also be upregulated in a variety of other conditions, including hypoxia and inflammatory and autoimmune diseases.31,32 Although we did not find significant differences in potential confounders, such as peripheral oxygen saturation, prevalence of hepatocellular carcinoma, or autoimmune liver disease between patients with POPH and controls (Table 1), it is possible that the presence of other conditions in which MIF levels are elevated could confound its utility as a screening test. Last, although we found that elevated pulmonary MIF levels were significantly associated with increased mortality, it should be noted that this was an exploratory post hoc analysis and not the primary outcome of our study. Because we did not routinely measure other prognostic PAH markers, such as 6-minute walk distance and brain natriuretic peptide, we were also unable to assess whether MIF levels correlated with these traditional markers of disease severity and prognosis.
Future studies to determine the correlation of MIF with hemodynamic response to PAH therapy and liver transplant are warranted, as is validation of MIF in conjunction with echocardiogram as a screening test for POPH in a larger, prospective cohort of patients with liver disease. Additional studies are also necessary to determine whether other differentially expressed biomarkers, such as hepatocyte growth factor and leptin, play a role in POPH disease pathogenesis or add synergistic value to the screening of patients with liver disease for POPH.
In conclusion, MIF is a novel biomarker of POPH disease presence and severity and may play an important role in disease pathogenesis. In addition, MIF is a promising screening tool for the presence of POPH in the high-risk population of patients with liver disease and portal hypertension and may also be an important prognostic indicator.
Appendix.
Table S1.
Raw data of pixel density for cytokines included in Proteome Profiler Human XL Cytokine antibody array kit (R&D Systems, Minneapolis, MN) performed at R&D Systems in duplicate for controls (n = 3) and patients with portopulmonary hypertension (POPH; n = 3)
| Control | POPH | |||||
|---|---|---|---|---|---|---|
| Biomarker | 1 | 2 | 3 | 1 | 2 | 3 |
| Adiponectin (a) | 49,475.05 | 51,813.08 | 53,522.44 | 55,451.35 | 57,121.03 | 51,293.78 |
| Adiponectin (b) | 48,409.73 | 49,735.91 | 53,539.46 | 53,638.29 | 56,941.99 | 53,726.59 |
| Aggrecan (a) | 42,337.03 | 33,407 | 39,124.06 | 37,635.05 | 43,924.06 | 43,649.43 |
| Aggrecan (b) | 43,803.35 | 34,796.37 | 39,260.97 | 37,627.76 | 44,340.47 | 44,042.34 |
| Angiogenin (a) | 48,575.81 | 47,943.91 | 51,092.87 | 50,782.59 | 53,224.32 | 54,202.95 |
| Angiogenin (b) | 47,950.39 | 49,051.35 | 48,820.47 | 49,131.56 | 50,935.71 | 53,827.05 |
| Angiopoietin-1 (a) | 4,577.84 | 7,119.2 | 4,712.32 | 5,570.24 | 6,515.66 | 5,567.81 |
| Angiopoietin-1 (b) | 4,270.8 | 6,897.23 | 4,054.49 | 5,721.73 | 5,888.62 | 5,311.81 |
| Angiopoietin-2 (a) | 7,388.97 | 4,646.7 | 5,370.14 | 6,734.39 | 6,149.48 | 11,491.46 |
| Angiopoietin-2 (b) | 6,974.19 | 4,525.99 | 5,071.2 | 6,541.58 | 5,816.52 | 10,307.86 |
| BAFF (a) | 36,824.92 | 12,810.34 | 14,595.86 | 31,761.63 | 12,899.46 | 29,878.09 |
| BAFF (b) | 35,090.44 | 12,770.65 | 14,148.67 | 30,428.97 | 12,428.77 | 27,502.8 |
| BDNF (a) | 4,868.67 | 4,154.14 | 4,848.42 | 5,141.68 | 5,105.23 | 6,755.46 |
| BDNF (b) | 4,503.3 | 4,230.29 | 4,483.05 | 4,983.71 | 4,812.77 | 6,235.35 |
| C-reactive protein (a) | 54,720.62 | 57,038.39 | 55,866.95 | 47,585.03 | 61,840.01 | 56,670.59 |
| C-reactive protein (b) | 52,023.71 | 56,764.57 | 56,988.16 | 41,436.97 | 63,026.04 | 56,401.63 |
| C5/C5a (a) | 26,880.62 | 26,495.81 | 28,324.27 | 22,486.49 | 34,547.66 | 28,819.25 |
| C5/C5a (b) | 27,324.57 | 26,209.84 | 28,707.46 | 22,821.89 | 34,077.78 | 29,083.35 |
| CD14 (a) | 21,054.19 | 14,609.63 | 18,332.97 | 18,935.71 | 26,904.11 | 25,932.77 |
| CD14 (b) | 19,336.72 | 15,288.52 | 19,893.28 | 19,371.56 | 26,252.77 | 27,424.22 |
| CD30 (a) | 4,303.2 | 4,010.75 | 6,633.13 | 4,270.8 | 4,289.43 | 4,581.08 |
| CD30 (b) | 4,634.54 | 4,183.3 | 7,536.42 | 4,628.06 | 4,748.77 | 4,483.86 |
| CD40 ligand (a) | 6,127.61 | 4,913.23 | 7,488.62 | 7,002.54 | 7,017.13 | 7,020.37 |
| CD40 ligand (b) | 7,797.28 | 5,099.56 | 7,313.63 | 7,405.18 | 7,789.99 | 7,788.37 |
| Chitinase 3-like 1 (a) | 57,829.89 | 59,574.9 | 59,099.35 | 56,523.96 | 60,417.43 | 57,890.65 |
| Chitinase 3-like 1 (b) | 58,441.53 | 59,017.53 | 56,608.22 | 55,395.46 | 58,857.13 | 58,610.04 |
| Complement factor D (a) | 45,576.72 | 45,046.09 | 46,562.65 | 45,570.24 | 49,713.23 | 48,519.91 |
| Complement factor D (b) | 43,177.94 | 43,029.68 | 45,726.59 | 44,027.76 | 48,474.54 | 48,539.35 |
| Cripto-1 (a) | 4,456.32 | 4,342.9 | 5,769.53 | 5,609.13 | 5,932.37 | 4,816.01 |
| Cripto-1 (b) | 4,308.87 | 4,070.7 | 5,148.16 | 5,344.22 | 5,042.04 | 4,285.38 |
| Cystatin C (a) | 42,534.7 | 38,213.48 | 40,565.28 | 37,703.1 | 41,587.66 | 47,457.03 |
| Cystatin C (b) | 42,581.68 | 39,012.27 | 40,379.76 | 37,946.95 | 42,130.44 | 46,264.52 |
| Dkk-1 (a) | 4,610.24 | 4,376.92 | 4,345.33 | 4,321.84 | 4,434.44 | 4,572.16 |
| Dkk-1 (b) | 4,322.65 | 4,222.19 | 4,004.27 | 4,322.65 | 4,123.35 | 4,406.9 |
| DPPIV (a) | 19,430.7 | 14,381.18 | 24,554.75 | 11,702.9 | 35,501.18 | 38,807.3 |
| DPPIV (b) | 19,316.47 | 14,224.82 | 23,883.96 | 11,749.89 | 35,797.68 | 39,267.46 |
| EGF (a) | 5,270.49 | 4,368.82 | 4,946.44 | 5,410.65 | 6,691.46 | 5,624.52 |
| EGF (b) | 4,930.24 | 4,103.1 | 4,515.46 | 5,453.58 | 6,423.3 | 5,690.14 |
| Emmprin (a) | 25,514.75 | 17,168.01 | 24,142.39 | 21,741.18 | 29,767.91 | 32,976.01 |
| Emmprin (b) | 26,339.46 | 17,531.76 | 24,390.29 | 21,983.41 | 31,657.13 | 32,724.06 |
| ENA-78/CXCL5 (a) | 5,102.8 | 4,091.76 | 4,436.06 | 5,670.7 | 5,567 | 5,251.86 |
| ENA-78/CXCL5 (b) | 5,165.18 | 4,103.1 | 4,530.04 | 6,239.41 | 5,370.14 | 5,690.14 |
| Endoglin (a) | 42,504.72 | 39,474.04 | 40,132.67 | 40,569.33 | 45,897.53 | 44,485.48 |
| Endoglin (b) | 44,210.85 | 40,539.35 | 40,144.82 | 41,313.84 | 44,061.78 | 45,533.78 |
| Fas ligand (a) | 8,272.82 | 8,490.75 | 6,702.8 | 7,451.35 | 7,640.11 | 9,931.15 |
| Fas ligand (b) | 6,987.15 | 7,363.86 | 5,654.49 | 6,684.97 | 6,038.49 | 7,862.9 |
| FGF basic (a) | 7,225.33 | 6,184.32 | 5,836.77 | 10,375.1 | 7,203.46 | 7,942.29 |
| FGF basic (b) | 8,634.95 | 7,249.63 | 8,084.87 | 10,597.89 | 8,258.24 | 9,260.37 |
| FGF-19 (a) | 9,596.57 | 6,616.92 | 7,992.52 | 9,123.46 | 10,684.57 | 14,792.72 |
| FGF-19 (b) | 9,436.97 | 6,801.63 | 8,316.57 | 9,861.48 | 10,581.68 | 17,103.2 |
| FGF-7 (a) | 5,376.62 | 4,853.28 | 5,535.41 | 4,922.14 | 6,125.99 | 5,657.73 |
| FGF-7 (b) | 4,381.78 | 3,890.04 | 4,160.62 | 4,368.01 | 4,909.18 | 4,123.35 |
| Flt-3 ligand (a) | 5,109.28 | 3,975.1 | 6,268.57 | 6,198.09 | 5,014.49 | 9,035.96 |
| Flt-3 ligand (b) | 4,754.44 | 4,058.54 | 5,848.11 | 5,780.06 | 5,106.85 | 8,103.51 |
| G-CSF (a) | 3,949.99 | 3,894.09 | 3,909.48 | 4,120.11 | 4,139.56 | 4,252.97 |
| G-CSF (b) | 3,949.18 | 3,941.89 | 3,938.65 | 4,036.67 | 4,004.27 | 4,570.54 |
| GDF-15 (a) | 40,438.09 | 20,039.91 | 29,624.52 | 31,984.42 | 27,638.09 | 39,824.01 |
| GDF-15 (b) | 40,287.41 | 20,970.75 | 29,311 | 34,463.41 | 27,331.05 | 40,420.27 |
| GM-CSF (a) | 5,122.24 | 4,570.54 | 6,665.53 | 9,153.43 | 6,621.78 | 5,607.51 |
| GM-CSF (b) | 5,494.9 | 4,820.06 | 6,822.7 | 9,681.63 | 6,907.76 | 5,792.22 |
| GRO alpha (a) | 4,065.03 | 3,673.73 | 4,045.58 | 4,060.16 | 4,146.04 | 4,265.94 |
| GRO alpha (b) | 3,981.58 | 3,882.75 | 4,074.75 | 4,783.61 | 4,103.91 | 4,368.82 |
| Growth hormone (a) | 23,878.29 | 3,857.63 | 5,267.25 | 39,828.06 | 4,882.44 | 28,370.44 |
| Growth hormone (b) | 23,081.94 | 3,859.25 | 4,863.81 | 39,254.49 | 4,692.87 | 28,107.15 |
| HGF (a) | 7,046.29 | 4,243.25 | 4,288.62 | 6,411.15 | 5,659.35 | 11,729.63 |
| HGF (b) | 7,796.47 | 4,201.13 | 4,855.71 | 7,336.32 | 6,422.49 | 12,474.95 |
| I-TAC (a) | 4,071.51 | 3,897.33 | 4,021.28 | 4,889.73 | 4,073.94 | 5,148.97 |
| I-TAC (b) | 4,522.75 | 4,417.43 | 4,267.56 | 5,093.08 | 3,928.92 | 5,351.51 |
| ICAM-1 (a) | 43,559.51 | 34,089.94 | 43,228.16 | 46,172.97 | 46,892.37 | 46,414.39 |
| ICAM-1 (b) | 42,049.43 | 34,022.7 | 42,674.04 | 45,490.04 | 47,115.96 | 47,282.04 |
| IFN-gamma (a) | 4,627.25 | 4,165.48 | 4,502.49 | 5,598.59 | 4,836.27 | 5,136.01 |
| IFN-gamma (b) | 4,730.95 | 4,048.01 | 4,551.1 | 5,623.71 | 4,893.78 | 5,567 |
| IGFBP-2 (a) | 37,399.3 | 31,482.14 | 34,622.19 | 36,414.19 | 37,768.72 | 40,511.81 |
| IGFBP-2 (b) | 37,499.76 | 32,535.3 | 33,633.84 | 36,483.86 | 37,968.82 | 38,718.19 |
| IGFBP-3 (a) | 35,184.42 | 31,469.99 | 30,911.81 | 29,920.22 | 31,862.09 | 38,716.57 |
| IGFBP-3 (b) | 34,237.38 | 31,311.2 | 30,785.43 | 30,343.91 | 32,413.78 | 39,155.66 |
| IL-1 alpha (a) | 4,764.16 | 4,152.52 | 4,513.03 | 6,364.97 | 4,868.67 | 6,346.34 |
| IL-1 alpha (b) | 4,628.06 | 4,023.71 | 4,467.66 | 6,127.61 | 4,598.9 | 6,053.89 |
| IL-1 beta (a) | 4,323.46 | 3,676.16 | 3,916.77 | 4,762.54 | 4,828.97 | 4,760.92 |
| IL-1 beta (b) | 4,369.63 | 3,816.32 | 4,036.67 | 4,402.04 | 4,634.54 | 4,336.42 |
| IL-10 (a) | 4,343.71 | 3,909.48 | 4,073.94 | 4,389.08 | 5,101.18 | 4,396.37 |
| IL-10 (b) | 4,235.96 | 3,786.34 | 3,970.24 | 4,251.35 | 4,815.2 | 4,805.48 |
| IL-11 (a) | 5,751.71 | 4,666.95 | 5,916.16 | 6,945.84 | 5,901.58 | 7,281.23 |
| IL-11 (b) | 5,652.87 | 4,923.76 | 5,936.42 | 6,459.76 | 6,138.14 | 7,252.87 |
| IL-12 p70 (a) | 4,473.33 | 4,060.16 | 4,129.84 | 5,054.19 | 4,887.3 | 4,671 |
| IL-12 p70 (b) | 4,534.9 | 4,231.91 | 4,187.35 | 4,864.62 | 5,398.49 | 4,789.28 |
| IL-13 (a) | 4,346.95 | 4,049.63 | 4,267.56 | 4,628.06 | 4,769.03 | 4,897.84 |
| IL-13 (b) | 4,101.48 | 3,834.95 | 3,949.18 | 4,441.73 | 4,237.58 | 4,675.86 |
| IL-15 (a) | 4,354.24 | 3,614.59 | 4,058.54 | 4,779.56 | 4,631.3 | 4,649.94 |
| IL-15 (b) | 4,327.51 | 4,022.09 | 3,994.54 | 4,165.48 | 4,329.13 | 4,331.56 |
| IL-16 (a) | 3,926.49 | 4,112.01 | 3,903 | 4,240.01 | 3,998.59 | 4,269.99 |
| IL-16 (b) | 3,854.39 | 3,736.92 | 4,071.51 | 3,832.52 | 3,790.39 | 4,427.96 |
| IL-17A (a) | 8,203.96 | 6,341.48 | 10,859.56 | 10,640.82 | 11,412.87 | 23,004.97 |
| IL-17A (b) | 9,284.67 | 7,615.81 | 11,457.43 | 11,877.08 | 10,068.06 | 25,284.67 |
| IL-18 Bpa (a) | 13,595.35 | 10,921.13 | 15,762.44 | 13,524.06 | 14,612.06 | 19,831.71 |
| IL-18 Bpa (b) | 14,433.03 | 10,981.89 | 16,776.72 | 13,121.43 | 16,122.14 | 20,157.38 |
| IL-19 (a) | 4,373.68 | 4,036.67 | 4,388.27 | 5,250.24 | 4,639.41 | 5,689.33 |
| IL-19 (b) | 4,413.38 | 4,129.84 | 4,287.81 | 5,062.29 | 4,589.99 | 5,710.39 |
| IL-1ra (a) | 4,214.09 | 4,204.37 | 4,892.16 | 4,325.89 | 4,434.44 | 4,159 |
| IL-1ra (b) | 3,961.33 | 3,846.29 | 5,038.8 | 4,127.41 | 4,516.27 | 4,441.73 |
| IL-2 (a) | 4,366.39 | 3,735.3 | 4,251.35 | 4,924.57 | 4,981.28 | 4,516.27 |
| IL-2 (b) | 4,633.73 | 4,133.89 | 4,110.39 | 5,141.68 | 5,083.35 | 4,303.2 |
| IL-22 (a) | 4,445.78 | 3,935.41 | 4,407.71 | 4,851.66 | 5,264.01 | 5,724.97 |
| IL-22 (b) | 4,603.76 | 4,005.89 | 4,335.61 | 4,799 | 5,001.53 | 5,396.87 |
| IL-23 (a) | 3,846.29 | 3,558.7 | 3,796.87 | 3,971.05 | 3,941.08 | 4,162.24 |
| IL-23 (b) | 4,069.89 | 3,806.59 | 3,885.18 | 3,886.8 | 3,973.48 | 4,344.52 |
| IL-24 (a) | 4,102.29 | 4,088.52 | 3,947.56 | 4,117.68 | 4,048.82 | 4,259.46 |
| IL-24 (b) | 4,041.53 | 3,965.38 | 3,919.2 | 4,386.65 | 4,013.18 | 4,218.95 |
| IL-27 (a) | 4,251.35 | 4,025.33 | 4,457.13 | 4,581.08 | 4,557.58 | 5,665.84 |
| IL-27 (b) | 4,255.41 | 4,412.57 | 4,573.78 | 4,509.78 | 4,658.85 | 5,732.27 |
| IL-3 (a) | 4,086.9 | 3,843.86 | 3,959.71 | 4,289.43 | 4,500.87 | 4,009.13 |
| IL-3 (b) | 3,940.27 | 3,741.78 | 3,863.3 | 4,096.62 | 4,577.03 | 4,127.41 |
| IL-31 (a) | 3,528.72 | 3,979.96 | 3,889.23 | 4,052.87 | 4,081.23 | 3,950.8 |
| IL-31 (b) | 3,932.16 | 3,999.41 | 4,106.34 | 4,044.77 | 4,089.33 | 4,005.89 |
| IL-32 alpha/beta/gamma (a) | 4,115.25 | 3,886.8 | 4,007.51 | 4,043.15 | 4,014.8 | 4,300.77 |
| IL-32 alpha/beta/gamma (b) | 4,059.35 | 3,859.25 | 3,970.24 | 4,235.15 | 4,052.06 | 4,145.23 |
| IL-33 (a) | 3,706.95 | 4,003.46 | 4,244.87 | 3,941.89 | 3,911.91 | 4,107.15 |
| IL-33 (b) | 3,757.18 | 3,985.63 | 4,131.46 | 3,962.95 | 3,880.32 | 4,042.34 |
| IL-34 (a) | 4,701.78 | 3,842.24 | 4,161.43 | 4,688.82 | 4,532.47 | 4,154.95 |
| IL-34 (b) | 4,230.29 | 3,659.96 | 4,168.72 | 4,468.47 | 3,971.05 | 4,130.65 |
| IL-4 (a) | 4,349.38 | 3,886.8 | 4,282.95 | 4,479.81 | 4,827.35 | 5,532.16 |
| IL-4 (b) | 4,699.35 | 4,118.49 | 4,379.35 | 4,692.87 | 4,556.77 | 4,837.89 |
| IL-5 (a) | 3,546.54 | 3,724.77 | 3,986.44 | 4,411.76 | 3,938.65 | 3,920.82 |
| IL-5 (b) | 3,728.82 | 3,836.57 | 4,068.27 | 4,496.01 | 3,728.82 | 4,085.28 |
| IL-6 (a) | 4,662.09 | 4,062.59 | 4,993.43 | 6,101.68 | 5,687.71 | 7,356.57 |
| IL-6 (b) | 4,914.04 | 4,336.42 | 4,918.09 | 6,555.35 | 5,499.76 | 7,184.01 |
| IL-8 (a) | 4,903.51 | 4,905.94 | 4,929.43 | 5,752.52 | 8,023.3 | 5,511.1 |
| IL-8 (b) | 4,858.95 | 5,515.15 | 5,102.8 | 6,408.72 | 9,343.81 | 5,345.03 |
| IP-10 (a) | 4,361.53 | 4,318.59 | 4,184.92 | 4,305.63 | 4,607.81 | 4,610.24 |
| IP-10 (b) | 4,343.71 | 4,265.94 | 4,283.76 | 4,282.95 | 4,440.92 | 4,892.97 |
| Kallikrein 3 (a) | 4,473.33 | 4,349.38 | 4,574.59 | 5,565.38 | 4,556.77 | 7,008.22 |
| Kallikrein 3 (b) | 4,972.37 | 4,354.24 | 4,743.1 | 6,244.27 | 4,866.24 | 7,281.23 |
| Leptin (a) | 4,009.94 | 6,708.47 | 26,090.75 | 4,730.14 | 40,552.32 | 36,914.04 |
| Leptin (b) | 4,167.91 | 6,688.22 | 27,488.22 | 4,575.41 | 41,138.04 | 37,633.43 |
| LIF (a) | 4,118.49 | 3,816.32 | 4,092.57 | 3,856.01 | 4,039.91 | 3,962.14 |
| LIF (b) | 3,633.23 | 3,843.86 | 4,031 | 3,995.35 | 3,875.46 | 4,271.61 |
| Lipocalin-2 (a) | 43,935.41 | 41,867.96 | 39,126.49 | 41,435.35 | 41,511.51 | 44,251.35 |
| Lipocalin-2 (b) | 42,157.18 | 40,065.43 | 39,346.85 | 41,650.85 | 41,312.22 | 43,188.47 |
| M-CSF (a) | 5,320.72 | 4,393.94 | 4,261.89 | 6,151.91 | 4,873.53 | 6,012.57 |
| M-CSF (b) | 5,098.75 | 4,561.63 | 4,749.58 | 6,142.19 | 4,753.63 | 6,559.41 |
| MCP-1 (a) | 5,313.43 | 5,227.56 | 5,993.13 | 9,079.71 | 6,985.53 | 14,634.75 |
| MCP-1 (b) | 4,936.72 | 5,204.06 | 5,654.49 | 8,602.54 | 6,074.14 | 11,991.3 |
| MCP-3 (a) | 3,806.59 | 3,890.04 | 3,933.78 | 4,022.09 | 3,935.41 | 4,043.15 |
| MCP-3 (b) | 3,792.01 | 3,919.2 | 3,957.28 | 4,224.62 | 3,920.01 | 4,031.81 |
| MIF (a) | 9,643.56 | 12,099.05 | 8,366.8 | 15,056.82 | 16,461.58 | 17,806.39 |
| MIF (b) | 9,532.57 | 12,142.8 | 8,414.59 | 14,255.61 | 17,626.54 | 17,547.96 |
| MIG (a) | 3,928.92 | 4,771.46 | 5,245.38 | 4,417.43 | 4,513.03 | 4,763.35 |
| MIG (b) | 4,235.15 | 4,636.16 | 5,233.23 | 4,153.33 | 4,291.05 | 4,708.27 |
| MIP-1 alpha/MIP-1 beta (a) | 3,694.8 | 4,078.8 | 3,980.77 | 3,967.81 | 3,962.14 | 4,036.67 |
| MIP-1 alpha/MIP-1 beta (b) | 3,646.19 | 3,876.27 | 3,900.57 | 3,821.18 | 3,834.14 | 3,997.78 |
| MIP-3 alpha (a) | 3,893.28 | 3,971.86 | 3,994.54 | 4,037.48 | 3,870.59 | 4,249.73 |
| MIP-3 alpha (b) | 3,866.54 | 3,984.82 | 3,834.95 | 3,826.85 | 3,540.87 | 4,304.82 |
| MIP-3 beta (a) | 4,088.52 | 4,418.24 | 5,205.68 | 4,895.41 | 4,685.58 | 11,016.72 |
| MIP-3 beta (b) | 4,434.44 | 4,816.82 | 5,439 | 5,373.38 | 5,195.15 | 12,375.3 |
| MMP-9 (a) | 23,242.34 | 34,550.9 | 15,947.96 | 29,369.33 | 37,898.34 | 40,125.38 |
| MMP-9 (b) | 25,308.16 | 34,780.16 | 16,929.84 | 31,836.97 | 38,814.59 | 39,637.68 |
| Myeloperoxidase (a) | 3,970.24 | 4,069.89 | 4,524.37 | 4,204.37 | 4,671.81 | 19,131.76 |
| Myeloperoxidase (b) | 4,359.91 | 4,394.75 | 5,002.34 | 4,585.94 | 5,274.54 | 19,384.52 |
| Negative control (a) | 3,448.52 | 3,720.72 | 3,973.48 | 3,852.77 | 3,876.27 | 3,941.08 |
| Negative control (b) | 3,488.22 | 3,738.54 | 3,813.89 | 3,635.66 | 3,503.61 | 3,740.97 |
| Osteopontin (OPN) (a) | 35,660.77 | 35,127.71 | 35,876.27 | 35,597.58 | 39,423 | 41,934.39 |
| Osteopontin (OPN) (b) | 36,302.39 | 35,356.16 | 34,507.15 | 35,870.59 | 40,054.09 | 41,600.62 |
| PDGF-AA (a) | 7,590.7 | 10,172.57 | 5,582.39 | 17,538.24 | 12,287 | 8,497.23 |
| PDGF-AA (b) | 7,337.13 | 9,839.61 | 5,358.8 | 16,857.73 | 12,153.33 | 8,374.09 |
| PDGF-AB/BB (a) | 4,656.42 | 9,930.34 | 4,157.38 | 7,457.84 | 6,683.35 | 5,321.53 |
| PDGF-AB/BB (b) | 4,247.3 | 9,611.96 | 4,457.13 | 8,148.87 | 6,633.13 | 5,191.1 |
| Pentraxin-3 (a) | 6,212.67 | 4,910.8 | 6,694.7 | 7,769.73 | 6,323.66 | 11,477.68 |
| Pentraxin-3 (b) | 6,906.14 | 5,627.76 | 7,960.11 | 8,374.9 | 6,168.92 | 12,158.19 |
| PF4/CXCL4 (a) | 57,154.24 | 54,213.48 | 54,479.2 | 40,127 | 41,249.84 | 45,182.19 |
| PF4/CXCL4 (b) | 52,260.27 | 54,831.61 | 53,351.51 | 38,979.86 | 41,018.95 | 45,058.24 |
| RAGE (a) | 6,634.75 | 4,277.28 | 4,565.68 | 4,534.09 | 5,084.16 | 5,582.39 |
| RAGE (b) | 7,128.11 | 4,494.39 | 4,644.27 | 4,949.68 | 5,119.81 | 5,895.1 |
| RANTES (a) | 39,853.99 | 41,006.8 | 23,384.11 | 45,642.34 | 41,897.13 | 35,163.35 |
| RANTES (b) | 40,141.58 | 38,945.84 | 24,694.9 | 45,287.51 | 42,260.87 | 36,268.37 |
| RBP-4 (a) | 59,311.61 | 59,420.97 | 57,823.41 | 61,807.61 | 61,830.29 | 61,105.23 |
| RBP-4 (b) | 60,585.94 | 58,495.81 | 56,527.2 | 61,684.47 | 62,564.27 | 62,118.7 |
| Relaxin-2 (a) | 3,940.27 | 4,339.66 | 4,516.27 | 4,402.85 | 5,694.19 | 5,046.09 |
| Relaxin-2 (b) | 3,683.46 | 4,056.11 | 3,951.61 | 4,247.3 | 4,157.38 | 4,338.04 |
| Resistin (a) | 22,460.57 | 23,546.14 | 13,109.28 | 18,477.18 | 12,946.44 | 16,427.56 |
| Resistin (b) | 25,428.87 | 26,247.91 | 14,527 | 20,014.8 | 14,277.48 | 17,829.89 |
| Reference spot (a) | 43,270.29 | 44,483.05 | 46,708.47 | 48,631.71 | 51,555.46 | 44,853.28 |
| Reference spot (b) | 43,865.73 | 47,107.86 | 47,260.16 | 51,318.9 | 54,133.28 | 44,536.52 |
| Reference spot (c) | 40,843.96 | 43,431.51 | 44,969.94 | 47,970.65 | 47,593.94 | 46,937.73 |
| Reference spot (d) | 40,363.56 | 41,449.13 | 46,931.25 | 45,922.65 | 46,817.84 | 45,675.56 |
| Reference spot (e) | 45,060.67 | 44,535.71 | 51,301.89 | 50,129.63 | 46,112.22 | 49,145.33 |
| Reference spot (f) | 45,392.82 | 43,874.65 | 50,541.99 | 50,559.81 | 47,414.09 | 46,031.2 |
| SDF-1 alpha (a) | 12,247.3 | 9,244.16 | 11,033.73 | 14,233.73 | 13,702.29 | 19,557.08 |
| SDF-1 alpha (b) | 12,442.54 | 9,387.56 | 11,042.65 | 14,194.85 | 15,915.56 | 20,809.53 |
| Serpin E1 (a) | 36,192.22 | 23,763.25 | 36,764.97 | 36,454.7 | 25,714.04 | 38,797.58 |
| Serpin E1 (b) | 36,995.86 | 24,715.96 | 37,941.28 | 33,866.34 | 25,931.15 | 38,634.75 |
| SHBG (a) | 35,062.9 | 32,288.22 | 34,419.66 | 38,209.43 | 40,391.91 | 40,647.91 |
| SHBG (b) | 35,193.33 | 32,938.75 | 34,617.33 | 37,376.62 | 39,661.99 | 40,765.38 |
| ST2 (a) | 34,708.06 | 15,909.08 | 22,115.46 | 21,848.92 | 21,605.89 | 38,766.8 |
| ST2 (b) | 35,595.96 | 15,280.42 | 19,803.35 | 21,790.59 | 21,298.85 | 40,306.04 |
| TARC (a) | 4,385.03 | 4,347.76 | 4,249.73 | 5,221.08 | 4,581.08 | 4,813.58 |
| TARC (b) | 4,238.39 | 4,371.25 | 4,268.37 | 4,600.52 | 4,139.56 | 4,319.41 |
| TFF-3 (a) | 37,903.2 | 32,803.46 | 36,916.47 | 33,532.57 | 33,399.71 | 42,076.16 |
| TFF-3 (b) | 38,931.25 | 34,200.92 | 36,656.42 | 32,316.57 | 32,713.53 | 42,152.32 |
| TfR (a) | 8,589.58 | 6,511.61 | 9,298.44 | 8,067.05 | 10,470.7 | 13,206.49 |
| TfR (b) | 8,161.84 | 7,329.03 | 9,261.18 | 8,732.97 | 10,000.82 | 13,151.41 |
| TGF-alpha (a) | 3,949.99 | 3,910.29 | 4,101.48 | 4,034.24 | 4,338.85 | 3,990.49 |
| TGF-alpha (b) | 3,864.92 | 3,996.97 | 3,745.84 | 3,865.73 | 3,974.29 | 3,968.62 |
| Thrombospondin-1 (a) | 4,435.25 | 5,100.37 | 4,409.33 | 6,684.97 | 5,485.18 | 5,276.16 |
| Thrombospondin-1 (b) | 4,881.63 | 5,009.63 | 4,615.91 | 6,491.35 | 5,996.37 | 5,273.73 |
| TNF alpha (a) | 5,760.62 | 5,607.51 | 4,503.3 | 6,394.14 | 5,771.15 | 4,923.76 |
| TNF alpha (b) | 6,222.39 | 6,630.7 | 4,880.82 | 7,233.43 | 7,845.89 | 6,816.22 |
| uPAR (a) | 19,002.95 | 8,170.75 | 10,615.71 | 11,935.41 | 11,684.27 | 26,396.97 |
| uPAR (b) | 18,230.09 | 7,806.19 | 9,293.58 | 12,140.37 | 9,696.22 | 25,731.86 |
| VEGF (a) | 4,252.97 | 4,357.48 | 4,556.77 | 4,261.89 | 4,148.47 | 5,153.03 |
| VEGF (b) | 4,269.18 | 4,482.24 | 4,497.63 | 4,239.2 | 3,846.29 | 5,170.85 |
| Vitamin D BP (a) | 50,776.92 | 48,411.35 | 51,728.82 | 51,765.28 | 33,650.85 | 53,288.32 |
| Vitamin D BP (b) | 49,500.16 | 47,619.05 | 51,398.29 | 51,286.49 | 32,974.39 | 55,137.03 |
BAFF: B cell activating factor; BDNF: brain-derived neurotrophic factor; BP: binding protein; EGF: epidermal growth factor; FGF: fibroblast growth factor; G-CSF: granulocyte-colony stimulating factor; GDF: growth differentiation factor; GM-CSF: granulocyte-macrophage colony-stimulating factor; GRO alpha: growth-regulated alpha protein; HGF: hepatocyte growth factor; ICAM-1: intercellular adhesion molecule 1; IFN: interferon; IGFBP: insulin-like growth factor-binding protein; IL: interleukin; I-TAC: interferon-inducible T cell alpha chemoattractant; LIF: leukemia inhibitory factor; MCP: monocyte chemotactic protein; M-CSF: macrophage colony-stimulating factor; MIF: macrophage migration inhibitory factor; MIP: macrophage inflammatory protein; MMP: matrix metalloproteinase; PDGF: platelet-derived growth factor; RAGE: receptor for advanced glycation endproducts; RANTES: regulated on activation, normal T cell expressed and secreted; RBP: retinol-binding protein; SDF: stromal cell-derived factor; SHBG: sex hormone–binding globulin; TARC: thymus and activation regulated chemokine; TFF: trefoil factor; TfR: transferrin receptor; TGF: transforming growth factor; TNF: tumor necrosis factor; uPAR: urokinase-type plasminogen activator receptor; VEGF: vascular endothelial growth factor.
Source of Support: HMD was supported by an institutional training grant (T32 5T32HL007893-17) from the National Institutes of Health. SAK was supported by funding from the Howard Hughes Medical Institute.
Conflict of Interest: ZKZ reports working part-time for Radikal Therapeutics. IRP reports receiving grants and personal fees from Actelion, Bayer, Gilead, and United Therapeutics. JMR-L reports receiving grants from Actelion and personal fees from Gilead. All other authors: none declared.
Supplements
Appendix (438.9KB, pdf)
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl):D34–D41. [DOI] [PubMed]
- 2.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest 2010;137(2):376–387. [DOI] [PubMed]
- 3.Krowka MJ, Swanson KL, Frantz RP, McGoon MD, Wiesner RH. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology 2006;44(6):1502–1510. [DOI] [PubMed]
- 4.Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology 2003;37(2):401–409. [DOI] [PubMed]
- 5.Krowka MJ, Edwards WD. A spectrum of pulmonary vascular pathology in portopulmonary hypertension. Liver Transpl 2000;6(2):241–242. [DOI] [PubMed]
- 6.Krowka MJ, Miller DP, Barst RJ, Taichman D, Dweik RA, Badesch DB, McGoon MD. Portopulmonary hypertension: a report from the US-based REVEAL registry. Chest 2012;141(4):906–915. [DOI] [PubMed]
- 7.Krowka MJ, Plevak DJ, Findlay JY, Rosen CB, Wiesner RH, Krom RA. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl 2000;6(4):443–450. [DOI] [PubMed]
- 8.Rodriguez-Roisin R, Krowka MJ, Herve P, Fallon MB; ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 2004;24(5):861–880. [DOI] [PubMed]
- 9.Krowka MJ, Wiesner RH, Heimbach JK. Pulmonary contraindications, indications and MELD exceptions for liver transplantation: a contemporary view and look forward. J Hepatol 2013;59(2):367–374. [DOI] [PubMed]
- 10.Hadengue A, Benhayoun MK, Lebrec D, Benhamou JP. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology 1991;100(2):520–528. [DOI] [PubMed]
- 11.Kawut SM, Krowka MJ, Trotter JF, Roberts KE, Benza RL, Badesch DB, Taichman DB, et al. Clinical risk factors for portopulmonary hypertension. Hepatology 2008;48(1):196–203. [DOI] [PMC free article] [PubMed]
- 12.Talwalkar JA, Swanson KL, Krowka MJ, Andrews JC, Kamath PS. Prevalence of spontaneous portosystemic shunts in patients with portopulmonary hypertension and effect on treatment. Gastroenterology 2011;141(5):1673–1679. [DOI] [PubMed]
- 13.Spruijt OA, Bogaard HJ, Vonk-Noordegraaf A. Pulmonary arterial hypertension combined with a high cardiac output state: three remarkable cases. Pulm Circ 2013;3(2):440–443. [DOI] [PMC free article] [PubMed]
- 14.Bacher M, Meinhardt A, Lan HY, et al. Migration inhibitory factor expression in experimentally induced endotoxemia. Am J Pathol 1997;150(1):235–246. [PMC free article] [PubMed]
- 15.Le Hiress M, Tu L, Ricard N, Phan C, Thuillet R, Fadel E, Dorfmüller P, et al. Pro-inflammatory signature of the dysfunctional endothelium in pulmonary hypertension: role of MIF/CD74 complex. Am J Resp Crit Care Med 2015;192(8):983–997. [DOI] [PubMed]
- 16.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl):D42–D50. [DOI] [PubMed]
- 17.Malhotra R, Paskin-Flerlage S, Zamanian RT, Zimmerman P, Schmidt JW, Deng DY, Southwood M, et al. Circulating angiogenic modulatory factors predict survival and functional class in pulmonary arterial hypertension. Pulm Circ 2013;3(2):369–380. [DOI] [PMC free article] [PubMed]
- 18.Benjaminov FS, Prentice M, Sniderman KW, Siu S, Liu P, Wong F. Portopulmonary hypertension in decompensated cirrhosis with refractory ascites. Gut 2003;52(9):1355–1362. [DOI] [PMC free article] [PubMed]
- 19.Pellicelli AM, Barbaro G, Puoti C, Guarascio P, Lusi EA, Bellis L, D’Ambrosio C, et al. Plasma cytokines and portopulmonary hypertension in patients with cirrhosis waiting for orthotopic liver transplantation. Angiology 2010;61(8):802–806. [DOI] [PubMed]
- 20.Peng T, Zamanian R, Krowka MJ, Benza RL, Roberts KE, Taichman DB, Rybak D, et al. Plasma levels of S100A4 in portopulmonary hypertension. Biomarkers 2009;14(3):156–160. [DOI] [PMC free article] [PubMed]
- 21.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010;122(2):164–172. [DOI] [PubMed]
- 22.Roberts KE, Fallon MB, Krowka MJ, et al. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Resp Crit Care Med 2009;179(9):835–842. [DOI] [PMC free article] [PubMed]
- 23.Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Resp Crit Care Med 1999;159(6):1925–1932. [DOI] [PubMed]
- 24.Itoh A, Nishihira J, Makita H, Miyamoto K, Yamaguchi E, Nishimura M. Effects of IL-1beta, TNF-alpha, and macrophage migration inhibitory factor on prostacyclin synthesis in rat pulmonary artery smooth muscle cells. Respirology 2003;8(4):467–472. [DOI] [PubMed]
- 25.Zhang B, Shen M, Xu M, Liu LL, Luo Y, Xu DQ, Wang YX, et al. Role of macrophage migration inhibitory factor in the proliferation of smooth muscle cell in pulmonary hypertension. Mediator Inflamm 2012;2012:840737. [DOI] [PMC free article] [PubMed]
- 26.Veillat V, Sengers V, Metz CN, Roger T, Leboeuf M, Mailloux J, Akoum A. Macrophage migration inhibitory factor is involved in a positive feedback loop increasing aromatase expression in endometriosis. Am J Pathol 2012;181(3):917–927. [DOI] [PubMed]
- 27.Becker H, Willeke P, Schotte H, Domschke W, Gaubitz M. Macrophage migration inhibitory factor may contribute to vasculopathy in systemic sclerosis. Clin Rheumatol 2008;27(10):1307–1311. [DOI] [PubMed]
- 28.Foote A, Briganti EM, Kipen Y, Santos L, Leech M, Morand EF. Macrophage migration inhibitory factor in systemic lupus erythematosus. J Rheumatol 2004;31(2):268–273. [PubMed]
- 29.Llamas-Covarrubias MA, Valle Y, Navarro-Hernandez RE, Guzmán-Guzmán IP, Ramírez-Dueñas MG, Rangel-Villalobos H, Estrada-Chávez C, Muñoz-Valle JF. Serum levels of macrophage migration inhibitory factor are associated with rheumatoid arthritis course. Rheumatol Int 2012;32(8):2307–2311. [DOI] [PubMed]
- 30.Assis DN, Leng L, Du X, Grieb G, Merk M, Garcia AB, McCrann C, et al. The role of macrophage migration inhibitory factor in autoimmune liver disease. Hepatology 2014;59(2):580–591. [DOI] [PMC free article] [PubMed]
- 31.Zhang Y, Talwar A, Tsang D, Bruchfeld A, Sadoughi A, Hu M, Omonuwa K, Cheng KF, Al-Abed Y, Miller EJ. Macrophage migration inhibitory factor mediates hypoxia-induced pulmonary hypertension. Mol Med 2012;18:215–223. [DOI] [PMC free article] [PubMed]
- 32.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol 2003;3(10):791–800. [DOI] [PMC free article] [PubMed]