Abstract Abstract
Despite new therapeutic options, pulmonary arterial hypertension (PAH) remains a progressive disease associated with substantial morbidity and mortality. As such, additional strategies for monitoring and adjunctive management of this disease are important. A 59-year-old woman with scleroderma-associated PAH received an implantable hemodynamic monitor (IHM) as part of a research protocol at our institution. Pulmonary artery pressures, heart rate, and cardiac output (sensor-based algorithm) were measured on a daily basis, and parameters of right ventricular (RV) performance and afterload were calculated. At the time of IHM implant, the patient had functional class III symptoms, was receiving triple-drug therapy, and had normal hemoglobin levels. Four months after implant, and with further optimization of prostacyclin therapy, she had improvement in her symptoms. However, shortly thereafter, while the patient was receiving stable drug therapy, her case regressed with worsening symptoms, and the patient received a new diagnosis of iron deficiency anemia. Oral iron supplementation resulted in normalization of hemoglobin levels and improvement in the patient’s iron profile. A gradual and sustained reduction in pulmonary pressures was noted after initiation of oral iron accompanied by increased RV performance and favorable reduction in RV afterload. The patient had significant symptomatic improvement. Iron deficiency is an underappreciated yet easily treatable risk factor in PAH. Use of IHM in this case longitudinally illustrates the optimization of pulmonary hemodynamics and RV afterload in tandem with clinical improvement achieved by a simple therapy.
Keywords: pulmonary arterial hypertension, implantable hemodynamic monitor, iron deficiency anemia
Despite new therapeutic options, pulmonary arterial hypertension (PAH) remains a progressive condition associated with substantial morbidity and mortality. As such, additional strategies for monitoring and adjunctive management are important.1 Iron deficiency is common in PAH and is associated with worsening hemodynamics, reduced exercise capacity, and increased mortality.2-6 Oral iron therapy for iron deficiency anemia in PAH therefore offers an attractive therapeutic target; however, the response to oral iron supplementation in the majority of patients with idiopathic PAH has not been robust.7
The feasibility and safety of using an implantable hemodynamic monitor (IHM; CardioMEMS HF System, St. Jude Medical, St. Paul, MN) in PAH has recently been demonstrated.8 Implantable hemodynamic monitoring in PAH offers a unique opportunity to learn about the hemodynamic effects of therapy on indexes of right ventricular (RV) performance and afterload in a longitudinal fashion. Herein we describes hemodynamic insights from IHM in a patient with scleroderma-related PAH who developed iron deficiency anemia and was treated with oral iron supplementation.
Case description
A 59-year-old woman with a history of scleroderma-associated PAH received an IHM as part of a research protocol at our institution. Pulmonary artery (PA) pressures, heart rate, and cardiac output (sensor-based algorithm) were measured on a daily basis. Parameters of RV performance, including RV power and stroke work as well as measures of RV afterload such as total pulmonary resistance (TPR) and pulmonary arterial capacitance, were calculated.
At implant, the patient had functional class III symptoms while receiving triple-drug therapy that included intravenous treprostinil, macitentan, and sildenafil, and hemoglobin (Hg) levels were normal (12.4 g/dL). Four months after receipt of the implant and after optimization of prostacyclin therapy, her condition improved to functional class I-II symptoms. Several months following this, while receiving stable therapy, the patient reported worsened functional class III symptoms, and she was found to have iron deficiency anemia (Hg: 8.3 g/dL; ferritin: 8 ng/mL; total iron-binding capacity [TIBC]: 490 μg/dL). RV function by echocardiography concomitantly decreased (tricuspid annular plane systolic excursion [TAPSE]: 14 mm; RV peak systolic velocity [RV S′]: 11 cm/s) compared with 6 months earlier (TAPSE: 18 mm; RV S′: 15 cm/s). The patient’s IHM revealed a subtle increase in her PA pressures, driven mainly by increase in TPR and heart rate. A diagnostic work up for anemia was recommended, and therapy with oral ferrous sulfate (325 mg three times a day) was initiated.
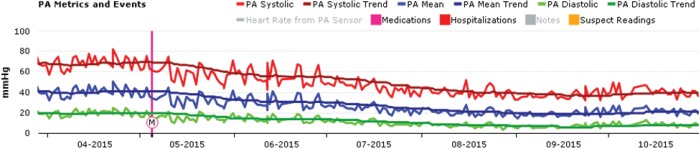
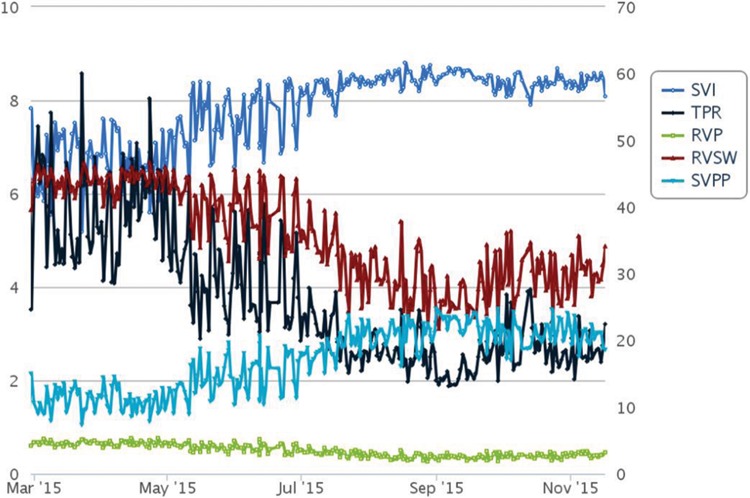
Figure 1 shows IHM trends for PA pressures with M representing the start of oral iron therapy. A gradual sustained reduction in PA pressure was observed after initiation of iron therapy. Shown in Figure 2 are the trends for cardiac output, stroke volume, and heart rate. Initiation of oral iron therapy was followed by a trend toward increase in cardiac output, driven by increase in stroke volume, while a reduction in heart rate was noted. RV afterload improved favorably with reduction in TPR and increase in PA capacitance (assessed by the ratio of stroke volume and pulse pressure; Fig. 3). A reduction in calculated RV stroke work and power was noted given optimized RV-PA coupling.
Figure 1.
Pulmonary artery pressure trends from an implantable hemodynamic monitor (IHM; CardioMEMS HF System, St. Jude Medical, St. Paul, MN). M: initiation of oral iron supplementation; PA: pulmonary artery.
Figure 2.
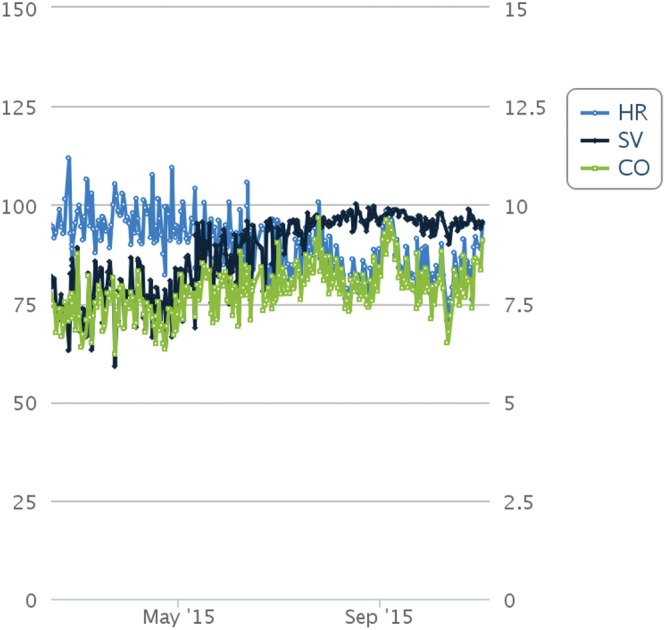
Heart rate (HR), stroke volume (SV), and cardiac output (CO) from implantable hemodynamic monitor (CardioMEMS HF System, St. Jude Medical, St. Paul, MN) obtained using proprietary analyses.
Figure 3.
Stroke volume index (SVI), total pulmonary resistance (TPR), right ventricular power (RVP), right ventricular stroke work (RVSW), and ratio of stoke volume to pulmonary pulse pressure (SVPP).
Four months later, the patient’s Hg levels normalized (12.8 g/dL), and she reported significantly improved functional class II symptoms. Six months after initiation of oral iron therapy, her iron profile improved (iron: 76 μg/dL; ferritin: 52 ng/mL; TIBC: 341 μg/dL), and cardiac magnetic resonance imaging demonstrated favorable RV function (TAPSE: 18 mm; RV ejection fraction: 53%).
Discussion
This case illustrates the optimization of pulmonary hemodynamics and RV function in tandem with clinical improvement achieved by simple therapy of oral iron supplementation, using novel longitudinal hemodynamic assessment with an IHM.
Iron deficiency is an underappreciated yet easily treatable risk factor in PAH. An iron-deficient state is akin to a pseudohypoxic environment and influences pulmonary vascular homeostasis. It does so by mimicking the pulmonary effects of hypoxia through stabilization of hypoxia-inducible factor, which is a transcription factor that regulates genes that provide compensation for hypoxia, including vascular endothelial growth factor.9,10 Iron chelation in humans mimics the increase in PA pressure with acute hypoxic exposure, suggesting a protective role for iron against pulmonary vasoconstriction.11,12 It has also been proposed that iron depletion inhibits the development of RV hypertrophy, which represents an important adaptive response for reducing RV wall stress in a pulmonary hypertension model.13 Additionally, there may be beneficial effects on myocardial function with an overall improvement in RV-vascular coupling. Iron-dependent alterations in myoglobin and muscle oxygen homeostasis may further affect functional status. However, the absorption of dietary iron may be repressed in patients with chronic illnesses because of chronically elevated levels of hepcidin, the master regulator of iron homeostasis. Some patients with IPAH exhibit increased circulating hepcidin levels despite iron deficiency, which likely accounts for the reduced response to oral iron supplementation in this cohort.3 Additional clinical study is needed to confirm the impact of iron supplementation in the treatment of pulmonary vascular disease.
The use of IHM in a select group of patients with PAH offers the ability to longitudinally follow the hemodynamic effects of therapies. This may also yield useful mechanistic insights. For example, we observed a relatively prompt initial decrease in PA pressures after initiation of oral therapy that was later sustained over several months, driven mainly by a decrease in TPR. It is plausible that iron replacement may have an early hemodynamic effect related to pulmonary vasorelaxation followed by a late effect related to vascular remodeling. In addition, combining IHM-derived parameters of RV performance and afterload paints an elegant and clinically meaningful picture of RV-PA coupling. As exemplified in this case, the reduction in pulmonary pressures combined with an increase in cardiac output was reassuring of improved RV-PA coupling rather than RV failure, which can also manifest by reduction in PA pressures. Similarly, an increasing stroke volume trend combined with reduction in heart rate was reassuring that the improvement in cardiac output was not merely driven by tachycardia, which can be seen in anemia.
Acknowledgments
This case was presented in a poster format at the 2016 International Society for Heart and Lung Transplantation Annual Meeting.
Source of Support: Funded by a National Heart, Lung, and Blood Institute grant awarded to RLB (HHSN268201400008C).
Conflict of Interest: None declared.
References
- 1.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012;142(2):448–456. [DOI] [PubMed]
- 2.van Empel VP, Lee J, Williams TJ, Kaye DM. Iron deficiency in patients with idiopathic pulmonary arterial hypertension. Heart Lung Circ 2014;23(3):287–292. [DOI] [PubMed]
- 3.Rhodes CJ, Howard LS, Busbridge M, Ashby D, Kondili E, Gibbs JS, Wharton J, Wilkins MR. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol 2011;58:300–309. [DOI] [PubMed]
- 4.Ruiter G, Lankhorst S, Boonstra A, Postmus PE, Zweegaman S, Westerhof N, van der Laarse WJ, Vonk-Noordegraaf A. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J 2011;37:1386–1391. [DOI] [PubMed]
- 5.Soon E, Treacy CM, Toshner MR, MacKenzie-Ross R, Manglam V, Busbridge M, Sinclair-McGarvie M, et al. Unexplained iron deficiency in idiopathic and heritable pulmonary arterial hypertension. Thorax 2011;66:326–332. [DOI] [PubMed]
- 6.Krasuski RA, Hart SA, Smith B, Wang A, Harrison JK, Bashore TM. Association of anaemia and long-term survival in patients with pulmonary hypertension. Int J Cardiol 2011;150:291–295. [DOI] [PubMed]
- 7.Ruiter G, Lankhorst S, Boonstra A, Postmus PE, Zweegman S, Westerhof N, van der Laarse WJ, Vonk-Noordegraaf A. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J 2011;37:1386–1391. [DOI] [PubMed]
- 8.Benza RL, Doyle M, Jaque PC, Cham M, White J, Raina A, Biederman R. A study to explore the safety and feasibility of using CardioMEMS HF system in PAH patients. Am J Respir Crit Care Med 2015;191:ASS29.
- 9.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFa targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001;292:464–468. [DOI] [PubMed]
- 10.Siddiq A, Aminova LR, Ratan RR. Hypoxia inducible factor prolyl 4-hydroxylase enzymes: center stage in the battle against hypoxia, metabolic compromise and oxidative stress. Neurochem Res 2007;32(4–5):931–946. [DOI] [PMC free article] [PubMed]
- 11.Smith TG, Balanos GM, Croft QP, Talbot NP, Dorrington KL, Ratcliffe PJ, Robbins PA. The increase in pulmonary arterial pressure caused by hypoxia depends on iron status. J Physiol 2008;586:5999–6005. [DOI] [PMC free article] [PubMed]
- 12.Smith TG, Talbot NP, Privat C, Rivera-Ch M, Nickol AH, Ratcliffe PJ, Dorrington KL, León-Velarde F, Robbins PA. Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials. JAMA 2009;302:1444–1450. [DOI] [PubMed]
- 13.Park AM, Wong CM, Jelinkova L, Liu L, Nagase H, Suzuki YJ. Pulmonary hypertension induced GATA4 activation in the right ventricle. Hypertension 2010;56:1145–1151. [DOI] [PMC free article] [PubMed]