Abstract Abstract
A loss of K+ efflux in pulmonary arterial smooth muscle cells (PASMCs) contributes to abnormal vasoconstriction and PASMC proliferation during pulmonary hypertension (PH). Activation of high-conductance Ca2+-activated (BK) channels represents a therapeutic strategy to restore K+ efflux to the affected PASMCs. However, the properties of BK channels in PASMCs—including sensitivity to BK channel openers (BKCOs)—are poorly defined. The goal of this study was to compare the properties of BK channels between PASMCs of normoxic (N) and chronic hypoxic (CH) rats and then explore key findings in human PASMCs. Polymerase chain reaction results revealed that 94.3% of transcripts encoding BKα pore proteins in PASMCs from N rats represent splice variants lacking the stress axis regulated exon insert, which confers oxygen sensitivity. Subsequent patch-clamp recordings from inside-out (I-O) patches confirmed a dense population of BK channels insensitive to hypoxia. The BK channels were highly activated by intracellular Ca2+ and the BKCO lithocholate; these responses require BKα-β1 subunit coupling. PASMCs of CH rats with established PH exhibited a profound overabundance of the dominant oxygen-insensitive BKα variant. Importantly, human BK (hBK) channels in PASMCs from human donor lungs also represented the oxygen-insensitive BKα variant activated by BKCOs. The hBK channels showed significantly enhanced Ca2+ sensitivity compared with rat BK channels. We conclude that rat BK and hBK channels in PASMCs are oxygen-insensitive BKα-β1 complexes highly sensitive to Ca2+ and the BKCO lithocholate. BK channels are overexpressed in PASMCs of a rat model of PH and may provide an abundant target for BKCOs designed to restore K+ efflux.
Keywords: pulmonary hypertension, stress axis regulated exon, oxygen sensitivity, lithocholate
Pulmonary hypertension (PH) is a severe, progressive disease in which small pulmonary arteries (PAs) show heightened vasoconstriction, proliferation of the pulmonary arterial smooth muscle cells (PASMCs), and vascular remodeling. Ultimately, the increased pulmonary vascular resistance results in right heart failure and death. One feature of PH shared between animal models and human forms of the disease is a loss of voltage-gated K+ (KV) channels in the PASMCs, which results in membrane depolarization, voltage-dependent Ca2+ influx, and vasoconstriction.1,2 The loss of K+ efflux also facilitates proliferation of PASMCs by inhibiting apoptosis.3 Thus, a recognized goal for the treatment of PH is to restore K+ efflux to the PASMCs. In proof-of-principle studies, Pozeg et al.4 achieved a lower pulmonary vascular resistance after using adenoviral gene therapy to transiently restore KV channel expression to PASMCs of rats with chronic hypoxia (CH)-induced PH. However, a more practical approach to restore K+ efflux to affected PASMCs may be to pharmacologically activate those K+ channels that show persistent and high expression levels in PASMCs during the development of PH rather than trying to restore depleted K+ channel types.5
Conceptually, the ideal K+ channel target in PASMCs for pharmacological activation would be (1) densely expressed in PASMCs during PH and capable of powerful hyperpolarization, (2) available for activation under conditions of high intracellular Ca2+ ([Ca2+]i) and depolarization that exist in PASMCs during PH, and (3) insensitive to inhibition by the hypoxic environment that may occur during PH. Considering that KV channels downregulate in PASMCs during PH and can be inactivated by hypoxia and [Ca2+]i,6,7 they may not be ideal pharmacological targets. In contrast, high-conductance Ca2+-activated K+ (BK) channels in PASMCs may represent suitable targets for K+ channel activators designed to ameliorate PH. BK channels exhibit a high single-channel conductance (150–250 picosiemens [pS]), which generates a strong hyperpolarizing K+ current. They are active under the conditions of high [Ca2+]i and depolarization, which are inherent to PASMCs during PH.1 Finally, several splice variants of BK channels are oxygen insensitive, and their open-state probability is unaffected by hypoxia.8 Unfortunately, the types of BK channel variants in PASMCs are unknown. Similarly, the biological and pharmacological properties of BK channels in PASMCs of preclinical models of PH are poorly defined, and reports disagree on whether the expression of BK channels in PASMCs increases or decreases during experimental PH.5,9 Finally, to our knowledge, the properties of BK channels in freshly isolated human PASMCs have not been explored.
Notably, the properties of BK channels in PASMCs from small PAs cannot be predicted using findings from other vascular beds; these properties are highly site specific largely because of the molecular diversity of channel composition.10 Although the BK channel pore-forming structure is a tetramer of α-subunits (BKα) encoded by a single gene, alternative splicing creates multiple BKα variants that can coassemble to form BK channels with variable Ca2+ sensitivity.11 Additionally, only some BKα splice variants contain the stress axis regulated exon (STREX), which confers oxygen sensitivity.8 The STREX insert was reported to be sparsely expressed in porcine PAs,12 but its prevalence in most arterial beds—including the rat and human pulmonary circulations—is unknown. Finally, small regulatory β1 subunits (BKβ1) can interact in 1∶1 stoichiometry with BKα subunits to enhance the Ca2+ sensitivity of BK channels.13,14 BKβ1 subunits also confer sensitivity to certain BK channel openers (BKCOs), including lithocholate (LC), which binds to BKβ1 subunits to activate BK channels.15 Thus, LC can be used as a pharmacologic tool to confirm the presence of functional BKβ1 subunits in BK channel complexes. Notably, BKβ1 subunits are reported to be functionally deficient in PASMCs, resulting in low Ca2+ sensitivity of pulmonary BK channels and presumed resistance to activation by BKβ1-dependent BKCOs.16
The goal of this study was to define the biological and pharmacological properties of BK channels related to their potential as pharmacological targets for PH in PASMCs freshly isolated from small rat and human PAs. Initially, we characterized BK channels in PASMCs of control rats and then determined whether these properties persisted in PASMCs from CH rats, which are a preclinical animal model used to develop new therapeutics for PH. Finally, we isolated PASMCs from small PAs of freshly obtained human lung samples to provide initial information on the properties of human BK (hBK) channels.
Methods
Animals
Procedures using animals were performed at the University of Arkansas for Medical Sciences, as approved by the Institutional Animal Care and Use Committee and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats 9–13 weeks of age anesthetized with isoflurane were euthanized by decapitation. Then the brain or lungs were removed and submerged in cold physiological saline solution (PSS) consisting of 119 mmol/L NaCl, 24 mmol/L NaHCO3, 5.5 mmol/L glucose, 4.7 mmol/L KCl, 1.6 mmol/L CaCl2, 1.2 mmol/L NaH2PO4, 1.2 mmol/L MgSO4, and 0.03 mmol/L ethylenediaminetetraacetic acid. Arteries were gently removed from adjacent tissue. Second- to fourth-order intralobar PAs with external diameters between 200 and 600 µm were used for patch-clamp, quantitative real-time polymerase chain reaction (qPCR), and Western blot experiments. Middle and posterior cerebral arteries, the circle of Willis, and the basilar artery were used to obtain cerebral arterial smooth muscle cells (CASMCs) for patch-clamp studies.
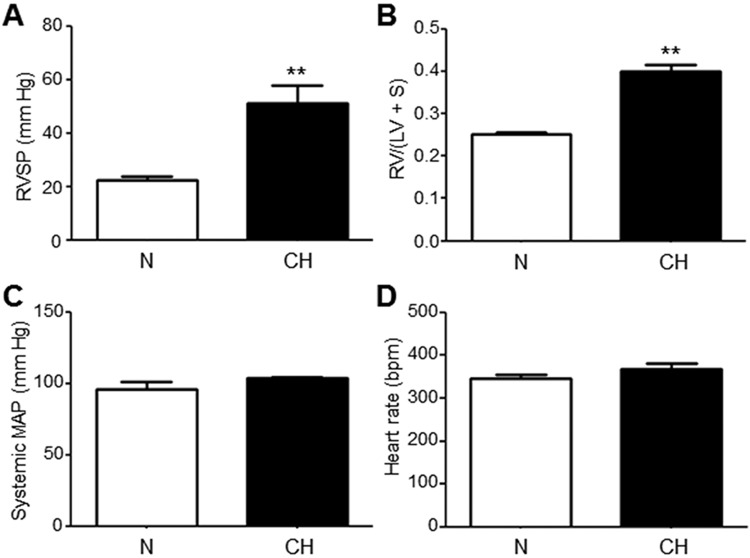
PH was induced by exposing 9-week-old male Sprague-Dawley rats to 3 weeks of normobaric CH in BioSpherix (Lacona, NY) environmental chambers. The chambers used a computer-regulated release of nitrogen into the chamber to maintain an inspired O2 level of 10%–11%. Immediately following the CH exposure, right ventricular systolic pressure (RVSP) was measured via catheter through the right external jugular vein under isoflurane anesthesia. Hearts were dissected to determine the ratio of the mass of the outer wall of the right ventricle (RV) to the mass of the left ventricle plus septum (RV/(LV + S)), a standard index of RV hypertrophy. The CH rats developed PH as indicated by an increase of RVSP and the presence of RV hypertrophy after 3 weeks of CH, without a rise in systemic mean arterial pressure or heart rate (Fig. S1). Age-matched normoxic (N) control rats were kept in normal room air (21% O2) for the same duration of time.
Human lung tissue
Transplant-quality human cadaver lungs were obtained from the National Disease Research Interchange or the Arkansas Regional Organ Recovery Agency. Lungs were removed by 30 minutes after cardiac death, placed in University of Wisconsin organ preservation solution, and then shipped to our laboratory on ice. The tissues were used for experiments within 3 days (or snap frozen in liquid nitrogen for later use in molecular analyses). The use of tissue from deceased organ donors was reviewed by the University of Arkansas for Medical Sciences Institutional Review Board and determined not to be human subjects research, as defined by 45 CFR 46.102(f). The lungs were not from donors with known PH. The following criteria were used in selecting samples: age 15–55 years; serologies suitable for transplant; no acute disease, bacterial infection, or cancer; <10 pack-years smoking history; and arterial oxygen tension (Pao2) > 100 mmHg on inhaled 100% O2. Asthma, diabetes, hypercholesterolemia, and systemic hypertension were not excluded.
After receipt of donor lungs in the laboratory, the airways were washed with phosphate-buffered saline (pH 7.4) and subsequently inflated with warm low-melting-point agarose to identify small airway versus vascular structures. After the agarose had cooled and solidified, small cubes (∼1 cm3) of lung tissue were cut and dissected in cold PSS to obtain small PAs (≤1-mm diameter). These arteries were used in Western blot, qPCR, and patch-clamp analyses.
Preparation of smooth muscle cells for patch-clamp experiments
All arterial smooth muscle cell (ASMC) isolations used enzymes from Worthington Biochemical (Lakewood, NJ) and protocols optimized for different preparations. To obtain ASMCs for patch-clamp experiments, dissected arteries were cut into 1-mm segments, incubated, and gently shaken in a 1.5-mL microcentrifuge tube filled with Ca2+-free Tyrode’s solution (CFT) at 37°C, which contained 143 mmol/L NaCl, 5.4 mmol/L KCl, 1.8 mmol/L CaCl2, 0.5 mmol/L MgCl2, 0.33 mmol/L NaH2PO4, 16.6 mmol/L glucose, and 5 mmol/L HEPES (pH adjusted to 7.4 with NaOH). The digestion sequence used to isolate PASMCs from control or N rats included 20 minutes in 1.8 mg/mL collagenase, 15 minutes in 0.5 mg/mL elastase, and another 10 minutes in 1.8 mg/mL collagenase. Isolation of PASMCs from CH rats required 20 minutes in 2 mg/mL collagenase, 20 minutes in 0.5 mg/mL collagenase, 15 minutes in 2 mg/mL elastase, and 10 minutes in 0.5 mg/mL collagenase. Finally, in enzyme-free CFT, the arterial segments were gently triturated with a fire-polished Pasteur pipette for 1–2 minutes to release PASMCs.
To obtain human PASMCs for patch-clamp experiments, human PAs (≤1-mm diameter) were cut into 1-mm segments and placed in warm (37°C) enzyme-containing CFT as follows: 20 minutes in 2 mg/mL collagenase, 20 minutes in 0.5 mg/mL elastase, 20 minutes in 2 mg/mL collagenase, and 15 minutes in 0.5 mg/mL collagenase. Then, in enzyme-free CFT, the arterial segments were gently triturated with a fire-polished Pasteur pipette for 1–2 minutes to release human PASMCs.
A subset of studies compared BK channel properties between rat PASMCs and CASMCs. To isolate CASMCs, rat cerebral arteries were cut into 1-mm segments and placed in warm (37°C) enzyme-containing CFT as follows: 20 minutes in 1 mg/mL papain and then 20 minutes in 0.9 mg/mL collagenase and 0.25 mg/mL elastase. Finally, in enzyme-free CFT, the arterial segments were gently triturated with a fire-polished Pasteur pipette for 1–2 minutes to release CASMCs. All ASMC suspensions were kept on ice and used in patch-clamp studies the same day.
Patch-clamp experiments
Patch-clamp experiments were performed on ASMCs obtained via enzymatic digestion of freshly dissected pulmonary or cerebral arteries as described above. The inside-out (I-O) patch-clamp configuration was used to record BK channel currents in I-O membrane patches at room temperature. These I-O membrane patches were obtained by sealing cells with a 10–20-MΩ pipette and then rapidly interfacing the pipette through the bath surface to remove the cell, followed by reintroducing the pipette into the bath. Cells were bathed in a solution that consisted of 145 mmol/L KCl, 10 mmol/L PIPES, 1 mmol/L EGTA, 1 mmol/L MgCl2, and variable CaCl2 (range: 10−8–10−4 mol/L) and was titrated to pH 7.4 using KOH. The amounts of Ca2+ required to achieve desired free Ca2+ concentrations were calculated using Maxchelator Ca-Mg-ATP-EGTA Calculator v1.0 with constants from National Institute of Standards and Technology database 46 v8 (http://www.stanford.edu/~cpatton/CaMgATPEGTA-NIST.htm). The pipette solution contained 145 mmol/L KCl, 5 mmol/L HEPES, 1.8 mmol/L CaCl2, and 1 mmol/L MgCl2 and was titrated to pH 7.4 using KOH. Different patch potentials are indicated in the figure legends. The cell-attached patch-clamp configuration also was used in one series of experiments. For this study, the composition of both the bath and pipette solutions was the same as the pipette solution used for I-O patches.
To achieve a hypoxic environment for patch-clamp studies, the bath solution was bubbled with nitrogen gas in a reservoir to remove dissolved O2. Then the hypoxic solution was drawn from the reservoir into a glass syringe before immediate, direct infusion into the patch-clamp chamber to superfuse the cells under study. An MI-730 O2 probe (Microelectrodes, Bedford, NH) was placed near the outflow port of the patch-clamp chamber to monitor the O2 concentration in the bath. The bath was reperfused with hypoxic solution as often as necessary to maintain a hypoxic environment (Pao2 = 26 ± 11 mm Hg; O2 = 3.4% ± 1.5%), which was shown earlier to inhibit KV channels.17,18
Patch-clamp studies were performed on an Olympus IMT-2 inverted microscope (Tokyo), using an L/M-EPC7 amplifier (List Medical, Darmstadt) and a TL-1 DMA interface (Axon Instruments/Molecular Devices, Sunnyvale, CA). Data were filtered at 1 kHz using a Frequency Devices 902 low-pass filter (Ottawa, IL) before digitization. Traces were recorded using Fetchex (ver. 6). Analysis of single-channel data was performed using Clampfit 10.3.1.5 (Molecular Devices).
Western blotting
Protein lysates were prepared by homogenizing tissues in Thermo Scientific radioimmunoprecipitation assay buffer (Waltham, MA), using stainless steel beads in a Next Advance BBX24B-CE bullet blender (Averill Park, NY). Protein lysates were size separated on an Invitrogen (Grand Island, NY) 3%–8% gradient bis-tris polyacrylamide minigel and transferred to a polyvinylidene fluoride membrane for blotting for 2 hours on ice. The membrane was blocked using 10% dry milk in Tris-buffered saline containing 0.1% Tween-20. A monoclonal anti-BKα antibody (75-022; Neuromab, Davis, CA) was used at a dilution of 1∶400, and a polyclonal anti-BKβ1 antibody (APC-036; Alomone Labs, Cambridge) was used at a dilution of 1∶400. Beta-actin was detected with a monoclonal antibody from Sigma-Aldrich (A5441; St. Louis, MO) at a dilution of 1∶5,000. Horseradish peroxidase–conjugated secondary antibodies provided chemiluminescent signals, which were detected using X-ray film. After the blots were scanned, densitometry was performed on the images, using ImageJ (http://imagej.nih.gov/ij/).
Expression of hBK channels in HEK293 cells
A plasmid bicistronically expressing a Flag tag (DYKDDDDK) fused to an isoform of human BKα subunit (GenBank U11058.2) and mCherry was a generous gift from S. England (Washington University).19 A plasmid expressing human BKβ1 subunit (GenBank NM_004137.3; University of Texas Health Science Center, San Antonio) was provided by J. Denton (Vanderbilt University).13 Human embryonic kidney (HEK) 293 cells (ATCC, Manassas, VA) were transfected using the Ca2+ phosphate method with 0.2 μg of hBKα plasmid and 0.8 μg of hBKβ1 plasmid (α + β1) or with 0.8 μg of negative control plasmid without the hBKβ1 gene (BKα only). Briefly, HEK293 cells were plated on 35-mm culture dishes in 10% fetal bovine serum/Dulbecco’s modified Eagle medium 1 day before transfection. A total of 1 μg of DNA plasmid was incubated for 1 min with 100 μL of CaCl2 and 100 μL of 2× HEPES-buffered solution before the solution was applied to ∼50% confluent dishes. Patch-clamp experiments were carried out 24–48 hours after transfection. We routinely obtained >80% positive transfection confirmed by red fluorescence.
Quantitative polymerase chain reaction (qPCR)
Rat or human PAs were snap frozen in liquid nitrogen and pulverized for RNA isolation. Total RNA was isolated using the Qiagen RNeasy Mini Kit (Venlo) according to the manufacturer’s instructions. Total RNA was treated with RNase-Free DNase (Qiagen) to remove contaminating DNA. To generate complementary DNA (cDNA), 500 ng of total RNA was reverse transcribed using the Bio-Rad iScript cDNA synthesis kit (Hercules, CA). Separate primer sets for rat (forward: GAGTCAACATTCCCATCATC; reverse: TGTGTCAGGGTCATCATCAT) and human (forward: GTACGCCATTAAGTCGGGCT; reverse: TGCAAGACTCCGATGCTGTC) samples were designed against cDNA sequences corresponding to regions common to all recognized rat or human BKα splice variants, respectively, to detect total BKα transcript. Two additional primer sets—again separate for rat (forward: TCCATCTACAAGAGAATGAGCCGAGC; reverse: CACGGAAACTGGTGGAGCAATCAT) and human (forward: ACGTGGACACCCTTGAGAGA; reverse: TAACAAGGGGTCATGCCTCATC) samples—were designed against a region of the cDNA sequence of the rat or human STREX, respectively, to detect the STREX-containing BKα splice variant. Amplification was accomplished using qPCR with iQ SYBR Green Supermix (Bio-Rad) using a CFX96 Touch qPCR detection system (Bio-Rad). Primer sets for rat β-actin (forward: ATCCTGTGGCATTCCATGAAACTAC; reverse: AGGAGCCAGGGCAGTAATCTC) and human glyceraldehyde 3-phosphate dehydrogenase (GAPDH; forward: AGGGCTGCTTTTAACTCTGGT; reverse: CCCCACTTGATTTTGGAGGGA) were used as amplification controls for rat and human samples, respectively. Amplification reactions containing all components except cDNA template were used as negative controls. The relative abundances of human total BKα and human STREX-containing BKα transcripts were estimated by the ΔΔCt method and reported as percentage of total BKα expression. For rat samples, commercially synthesized DNA fragments (GenScript, Piscataway, NJ) containing either rat STREX-containing BKα or rat STREX-lacking BKα were amplified in parallel to quantify absolute transcript copy numbers.
Statistical analysis
Results are presented as means ± SE. Comparisons between 2 groups were made using unpaired Student t tests unless noted otherwise. Experiments with more than 2 groups were analyzed using ANOVA followed by a post hoc Tukey test; P < 0.05 was regarded as significant. Two levels of statistical significance were used. In the figures, one asterisk indicates that P ≤ 0.05, and 2 asterisks indicate that P ≤ 0.01. Data from patch-clamp studies in which the open-state probability (NPO) of BK channels is plotted as a function of LC concentration exhibited a log-normal distribution; therefore, statistics were performed on the base 10 logarithms of the values, and data are presented on a logarithmic scale. Data generated from patch-clamp studies in which the NPO of BK channels was plotted as a function of Ca2+ concentration were fitted with variable slope and 4-parameter sigmoids, using GraphPad Prism (GraphPad, La Jolla, CA).
Results
High Ca2+ sensitivity of BK channels in rat PASMCs infers functional BKα-β1 complexes
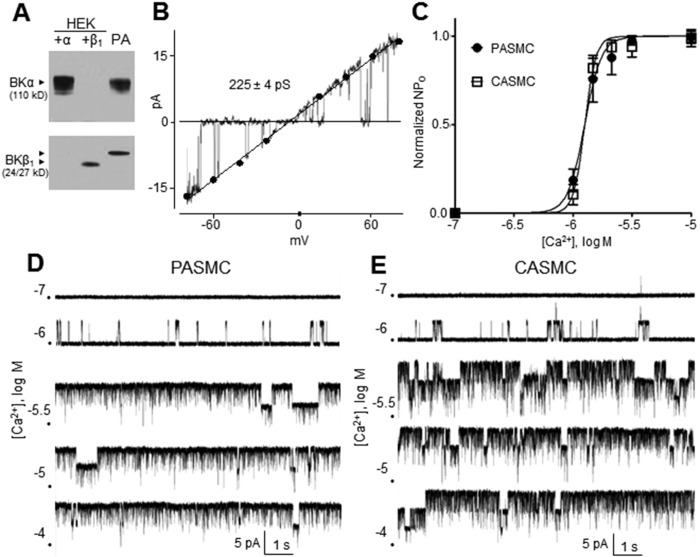
The ability of BK channels to generate a strong hyperpolarizing current depends on their abundance and the degree of functional coupling between BKα and BKβ1 subunits, since BKβ1 increases the apparent voltage and Ca2+ sensitivity of the channel. In protein lysates from third- and fourth-order rat PAs, immunoreactive bands corresponding to the BKα and β1 subunits were detected using monoclonal anti-BKα and polyclonal anti-BKβ1 antibodies, respectively (Fig. 1A, right lane). The antibodies were verified for target fidelity using protein lysates from HEK293 cells expressing either the human BKα or the BKβ1 subunit (Fig. 1A, left lanes). Notably, rat and human BK channels share the anti-BKα and anti-BKβ1 epitopes. The apparent size of the BKβ1 subunit in HEK293 cells is slightly smaller (24 kD) than that in rat PA (≥27 kD), which may reflect differences in posttranslational modification of BKβ1. Meera et al.20 have reported N-glycosylation of the BKβ1 subunit in Xenopus laevis oocytes, which results in an apparent 4-kD increase in molecular mass on polyacrylamide gel electrophoresis. Notably, in I-O patches of rat PASMCs, BK channels exhibited a single-channel conductance of 225 ± 4 pS, in agreement with earlier reports.21,22 This value was determined by fitting a linear trend line to unitary BK current amplitudes obtained at patch potentials between −80 and +80 mV (Fig. 1B; n = 4–13).
Figure 1.
A, Western blot of pulmonary artery (PA) lysate (third lane) from a normoxic rat reveals BKα and BKβ1 subunits. To confirm the specificity of antibodies, the first and second lanes contain lysates from human embryonic kidney (HEK) cells expressing either the BKα or BKβ1 subunit, respectively. B, BK channel current in an inside-out (I-O) patch of a PA smooth muscle cell (PASMC) from a normoxic rat during an 800-ms voltage ramp from −80 to +80 mV reveals a unitary conductance of 225 ± 4 pS (n = 5–11 per point). C, Exposure to a range of Ca2+ concentrations reveals similar Ca2+ sensitivities between BK channels in I-O patches from PASMCs and cerebral arterial smooth muscle cells (CASMCs) clamped at +20 mV (n = 4–7 per point). D, E, Representative K+ current recordings reveal increasing BK channel opening in an I-O patches of rat PASMCs (D) or CASMCs (E) exposed to elevations of [Ca2+]i at a patch potential of +20 mV. BK channel: high-conductance Ca2+-activated channel; NPO: open-state probability.
The Ca2+ sensitivity of BK channels in different vascular beds is highly variable and positively correlates to the ratio of BKβ1 to BKα subunits.21 Importantly, BK channels in rat PASMCs were reported to exhibit low Ca2+ sensitivity possibly related to deficient BKβ1.16 However, we observed BK channels highly sensitive to [Ca2+]i only in I-O patches of rat PASMCs. BK channel NPO sharply increased when [Ca2+]i was elevated from 10−6 to 10−5.5 mol/L (Fig. 1D), resulting in a steep Ca2+ activation curve (Fig. 1C, circles). This curve is nearly identical to that reported for cloned BKα-β1 channels.23 In contrast, the Ca2+ activation curve for BK channels devoid of BKβ1 exhibits a markedly diminished slope and rightward shift.23 Furthermore, we also compared Ca2+ activation curves between BK channels from rat PASMCs and rat CASMCs. The latter BK channels of rat cerebral arteries are known to exhibit high Ca2+ sensitivity,21 a finding we verified in CASMCs by observing their marked activation by stepwise [Ca2+]i elevations from 10−6 to 10−5.5 mol/L (Fig. 1E). The resulting steep Ca2+ activation curve of CASMC BK channels was nearly identical to that of BK channels in PASMCs (Fig. 1C; n = 4–8). Half-maximal Ca2+ activation values were −5.90 ± 0.02 log mol/L (PASMCs) and −5.90 ± 0.01 log mol/L (CASMCs), suggesting similarly high Ca2+ sensitivities of BKα-β1 channel complexes in both vascular beds.
Rat PASMCs primarily express oxygen-insensitive STREX− BKα variants
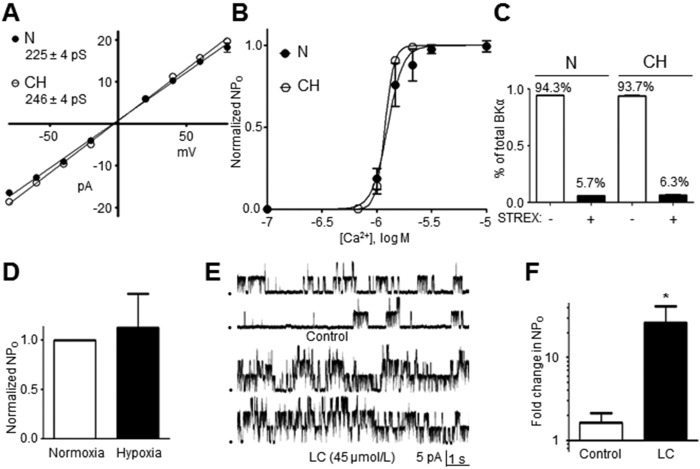
It is unclear whether BK channels in PASMCs are inactivated by hypoxia, which potentially would render them unavailable to BKCO therapy in some forms of PH characterized by hypoxemia. BKα splice variants that include a 59–amino acid STREX insert in the intracellular carboxy terminus (Fig. 2A) are inhibited by hypoxia, as originally described in mouse anterior pituitary cells.8 We evaluated the relative percent of BKα variants containing (STREX+) or lacking (STREX−) the STREX insert in cDNA prepared from rat third- to fifth-order PAs. Using primers designed to amplify the STREX insert, real-time PCR revealed that only 5.7% ± 0.1% of BKα transcripts are STREX+ in rat PAs, whereas 94.3% ± 0.1% are the STREX− hypoxia-insensitive variant (Fig. 2B; n = 7). The functional correlate of this observation was confirmed directly in patch-clamp studies by recording BK channel currents in I-O patches of PASMC in room air (21% O2) and then during hypoxia (3.4% ± 1.5% O2), which was achieved by perfusing the patch-clamp chamber with recording solution heavily bubbled with nitrogen gas. The NPO of BK channels was not significantly different between normoxia and hypoxia, confirming the predominance of a STREX− BK channel variant insensitive to acute hypoxia in rat PASMCs (Fig. 2C, 2D; n = 7). We also exposed BK channels in cell-attached patches of rat PASMCs to acute hypoxia in a context in which the cytosolic milieu is preserved. Hypoxia still had no effect on BK channel NPO (n = 3; Fig. S2).
Figure 2.
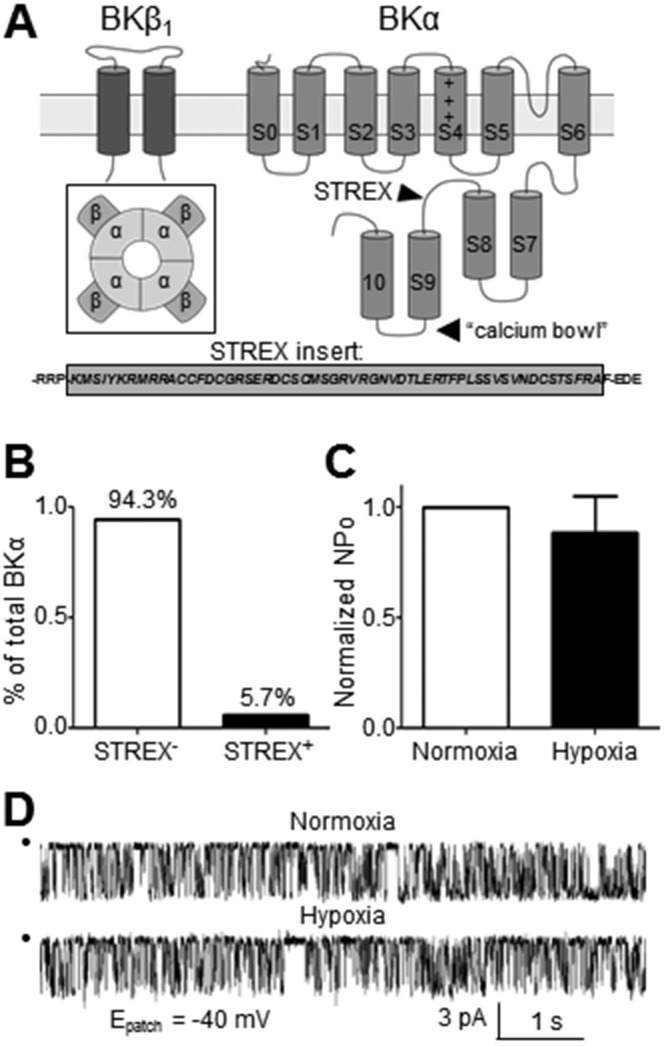
The alternatively spliced stress axis regulated exon (STREX) sequence located between the S8 and S9 regions of the BKα subunit confers intrinsic oxygen sensitivity. A, Inset depicts the assembled high-conductance Ca2+-activated (BK) channel complex. B, Relative abundance of BKα transcript containing the STREX sequence in rat pulmonary artery was 5.7% ± 0.1%, as determined by quantitative real-time polymerase chain reaction (n = 7). C, Open-state probability (NPO) of BK channels in inside-out patches of pulmonary arterial smooth muscle cells from normoxic rats failed to respond to hypoxic bath solution (n = 7). D, Sample recording demonstrates the lack of effect of hypoxic bath solution on BK channel current (patch potential [Epatch] = −40 mV; [Ca2+]i = 10−6 mol/L).
BK channels in rat PASMCs are activated by LC
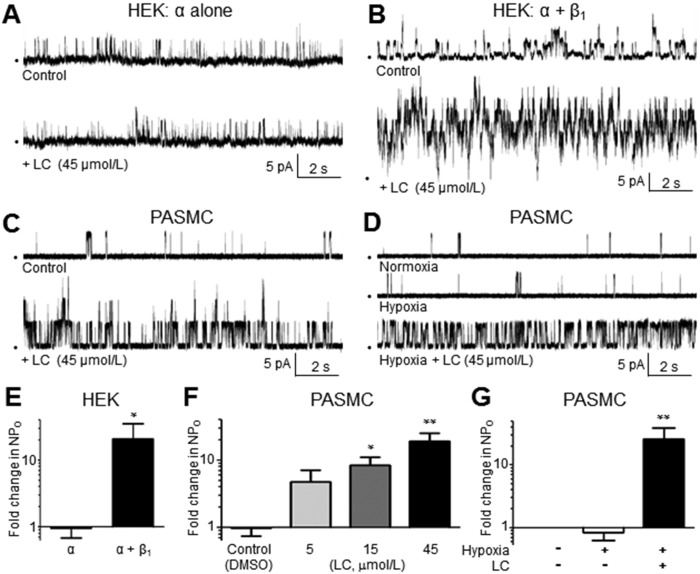
The high Ca2+ sensitivity of BK channels in rat PASMCs (Fig. 1C) suggests a functionally coupled BKα-β1 complex. Importantly, this functional coupling is required for channel activation by several classes of BKCOs, including LC, an activator of BK channels that elicits activation via a known binding site on the second transmembrane domain of the BKβ1 subunit.15 LC has been shown to selectively activate β1-coupled BK channels at concentrations of 15–300 μmol/L.24 Here, we used HEK293 cells to express a STREX− BKα variant alone or with BKβ1 in order to verify that LC activation of BK channels requires both subunits. HEK293 cells coexpressing cloned human BKα and β1 subunits exhibited a 20.7-fold increase in NPO in response to 45 μmol/L LC, whereas cells lacking BKβ1 subunits showed no change in NPO (Fig. 3A, 3B, 3E). This finding confirmed earlier reports that cloned BKβ1 subunits are required for LC-induced activation of the BK channel.15,25 Notably, our patch-clamp studies in HEK293 cells used a lower Ca2+ concentration in the bath (10−6.17 mol/L) to reduce the high number of cloned BK channels in I-O membrane patches. Thus, the NPO response to LC cannot be compared quantitatively between BK channels in HEK293 cells and PASMCs.
Figure 3.
A, B, Exposure to lithocholate (LC) fails to increase high-conductance Ca2+-activated (BK) channel opening in inside-out (I-O) patches of human embryonic kidney (HEK) 293 cells expressing cloned BKα subunits only (A), but LC activates BK channels in HEK293 cells coexpressing cloned BKα and BKβ1 subunits (B). C, D, LC (45 µmol/L) activates BK channels in I-O patches of rat pulmonary arterial smooth muscle cells (PASMCs) exposed to normoxic (C) or hypoxic (D) bath solutions, confirming the presence of a functionally coupled BKβ1 subunit (n = 9–12; patch potential = +20 mV; [Ca2+]i = 10−6 mol/L). E, Average change in open-state probability (NPO) of cloned BKα or BKα-β channels exposed to LC in I-O patches of HEK293 cells. F, Average increase in NPO of BK channels in I-O patches of PASMCs in response to 3 concentrations of LC (n = 4–5; patch potential = +20 mV; [Ca2+]i = 10−6.17 mol/L). G, LC activates BK channels in I-O patches of rat PASMCs in the presence of hypoxia. DMSO: dimethyl sulfoxide.
To confirm the sensitivity of pulmonary BK channels to LC, I-O patches from rat PASMCs were exposed to LC in bath solution containing 10−6 mol/L bath Ca2+. Addition of 45 μmol/L LC elicited a 19 ± 6-fold increase in NPO (Fig. 3C, 3F; n = 11), confirming a functionally coupled BKβ1 subunit. Lower LC concentrations of 5 and 15 μmol/L resulted in smaller 5 ± 2- and 8 ± 3-fold increases in NPO, respectively (Fig. 3F; n = 7, 9). The use of higher concentrations (>45 μmol/L) of LC was limited by low drug solubility. The solvent for LC (0.1% dimethyl sulfoxide [control]) did not significantly change the NPO of BK channels in PASMCs (Fig. 3F; n = 9), and LC did not alter the pH of the bath solution (data not shown).
We also verified that hypoxia does not prevent LC-induced activation of BK channels in rat PASMCs. Although the vast majority of BK channels in rat PASMCs appear to be STREX− oxygen-insensitive splice variants (Fig. 2), we considered the possibility that hypoxia could disrupt the binding of LC to the BKβ1 subunit or, alternatively, disrupt distal signaling pathways required for BK channel activation. However, the addition of LC (45 µmol/L) to the bath solution caused a pronounced 25 ± 13-fold increase in NPO, despite the presence of low Pao2 (Fig. 3D, 3G; n = 9), showing persistent sensitivity of BK channels to LC under hypoxic conditions. Collectively, these data suggest that BKβ1-specific pharmacological activators offer the potential to activate the BK channel variant in PASMCs, regardless of the presence of hypoxia.
PASMCs of CH rats express high numbers of BK channels exhibiting normal properties
The ability of BKCOs to restore K+ efflux to PASMCs during PH will rely on the persistent expression of BK channels as the therapeutic target. Thus, our next series of studies evaluated whether BK channels in PASMCs retain normal expression and properties during the development of PH in rats exposed to CH for 3 weeks. Age-matched rats exposed to a similar duration of normoxia (N) were used as control (separate from the untreated rats used in Figs. 1–3). After introduction to N or CH for 3 weeks, RVSPs were 22 ± 1 mmHg (n = 7) and 51 ± 7 mmHg (n = 4), respectively, similar to findings of other studies (Fig. S1A).26 RV hypertrophy assessed as the ratio RV/(LV + S) was evident in CH rats (0.400 ± 0.071, n = 21) compared with N rats (0.252 ± 0.005, n = 18; Fig. S1B). Systemic mean arterial pressure and heart rate were similar between rat groups (Fig. S1C, S1D).
After animals were maintained in N or CH for 3 weeks, intralobular PAs (second to fourth order, 200–600-μm diameter) were dissected from isolated lungs, and the expression level of the BKα subunit was analyzed by Western blot (Fig. 4A). Each lane was loaded with protein lysate from PAs of a single N or CH rat. This analysis revealed that the abundance of BKα subunits was profoundly increased in PAs of CH compared with N rats, corresponding to a 3.2 ± 0.5-fold increase in immunoreactivity (Fig. 4B; n = 5). Real-time PCR using cDNA prepared from rat intralobar PAs—and using primers recognizing rat cDNA sequence shared by BKα splice variants—disclosed a corresponding 1.8 ± 0.2-fold increase in total BKα transcript in PAs of CH compared with N rats (Fig. 4C; n = 6–7). Interestingly, only PAs of CH rats showed an increased expression of BKα protein; mesenteric and femoral arteries from the same animals failed to show this abnormality in protein lysates pooled from 3 N or CH rats (Fig. 4D). Western blots were also performed to compare expression of the BKβ1 subunit using the same pooled PA lysate. Immunoreactivity corresponding to anti-BKβ1 was not significantly different between PAs of N and CH rats (Fig. 4E; n = 6), despite a marked increase in anti-BKα immunosignal (Fig. 4A), suggesting an increased abundance of BKα pore proteins in PAs of CH rats compared with accessory BKβ1 proteins.
Figure 4.
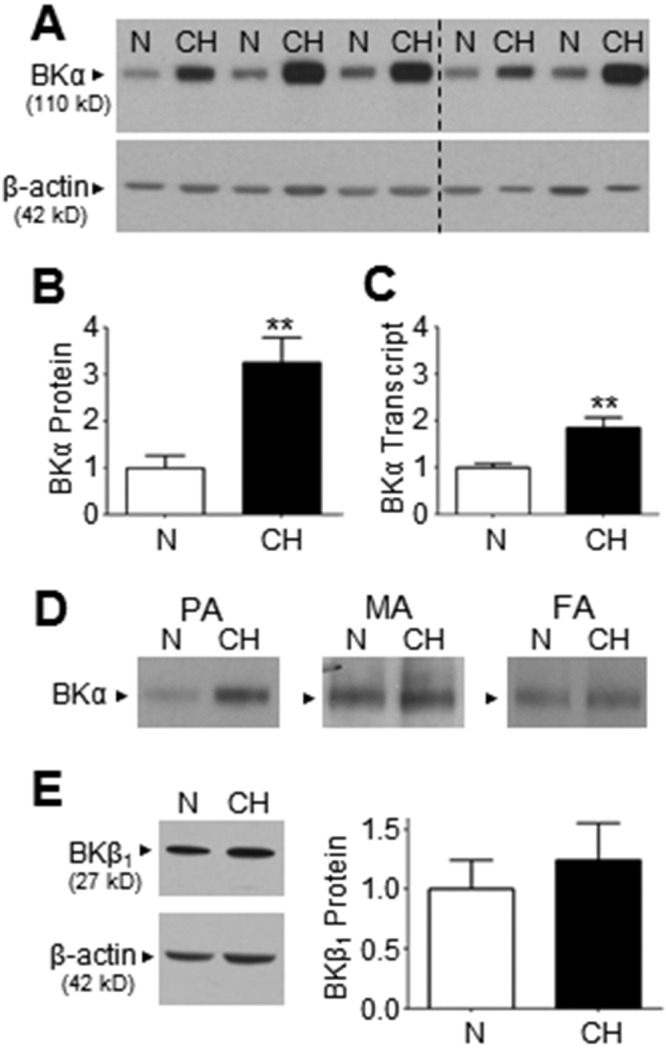
A, Western blot reveals accentuated immunoreactive bands corresponding to the BKα pore protein in pulmonary artery (PA) lysate from chronic hypoxic (CH) compared with normoxic (N) rats (n = 5 each). Dashed line indicates the location of 2 deleted lanes that were determined to be statistical outliers by the Grubbs outlier test (P < 0.05). B, Bar graph derived from data in A depicts the average 3.2 ± 0.5-fold increase in BKα protein expression in PA lysate of CH compared with N rats. C, Real-time reverse transcription polymerase chain reaction reveals a 1.8 ± 0.2-fold increase in BKα transcript expression in PA from CH compared with N rats (n = 6–7). D, Western blots using protein lysate from pulmonary, mesenteric (MA), or femoral (FA) arteries of N and CH rats indicate that only PAs respond to chronic hypoxia by increasing BKα abundance (each lane was loaded with lysate pooled from 3 rats). E, Western blot suggests a similar expression level of BKβ1 subunit protein between PA lysate from CH and N rats, as confirmed by averaged values from 6 experiments using different lysate preparations. **P ≤ 0.01.
To resolve the question of whether BK channels in CH rats retain normal properties after the development of PH, we compared unitary conductance, hypoxia, Ca2+, and LC sensitivity of BK channels between I-O patches from PASMCs of N and CH rats. Briefly, we detected BK channels of apparently similar phenotype in PAs of N and CH rats, and the properties of high Ca2+ sensitivity, insensitivity to hypoxia, and activation by LC were indistinguishable (Fig. 5). For example, the unitary conductance of BK channels in PASMCs of CH rats was determined to be 246 ± 4 pS, which was not significantly different from the value of 225 ± 4 pS in N rats (Fig. 5A; n = 6–12; data for N rats plotted from Fig. 1B). Although BKα subunits were more abundant in PAs of CH rats (Fig. 4A, 4B), the hypoxia-insensitive STREX− variant persisted as the predominant isoform, representing 93.7% ± 0.4% (n = 6) of total BKα transcripts in PAs of CH rats compared with 94.3% ± 0.1% in N rats (Fig. 5C; n = 6; data for N rats plotted from Fig. 2B). Accordingly, BK channels in I-O patches from PASMCs of CH rats were insensitive to acute hypoxia and failed to alter NPO in response to depletion of bath Pao2 (Fig. 5D; n = 11). Surprisingly, considering that expression of BKα proteins increased in PAs of CH rats independently of the BKβ1 protein that confers channel sensitivity to [Ca2+]i, Ca2+ activation curves revealed a Ca2+ half-maximal effective concentration (EC50) value of −5.93 ± 0.01 log mol/L for BK channels in PASMCs from CH rats, which was nearly identical to the value of −5.90 ± 0.02 log mol/L obtained in PASMCs of N rats (Fig. 5B; n = 5–12 per group; N rat data plotted from Fig. 1C). Similarly, the NPO of BK channels in PASMCs of CH rats markedly increased by 26 ± 16-fold in response to 45 μmol/L LC, the BKβ1-dependent BKCO (Fig. 5E, 5F; n = 9). Collectively, these data imply that BK channels are highly upregulated in PASMCs from a CH rat model of PH and that these channels retain normal properties, including sensitivity to BKβ1-dependent BKCOs.
Figure 5.
A, Summarized data from inside-out (I-O) patch recordings of pulmonary arterial (PA) smooth muscle cells (PASMCs) reveal a unitary high-conductance Ca2+-activated (BK) channel conductance of 246 ± 4 pS in PASMCs of chronic hypoxic (CH) rats (n = 5–12 per point), which was nearly identical to the conductance of 225 ± 4 pS measured in PASMCs of normal (N) rats. B, BK channels in I-O patches of PASMCs exhibit similar Ca2+ sensitivity between CH and N rats (n = 4–7 per point). C, Quantitative real-time polymerase chain reaction reveals that the abundance of stress axis regulated exon (STREX)-containing BKα transcript remains low (6.3% ± 0.4%) in PAs of CH rats (n = 6). D, Exposure to acute hypoxia (3.4% ± 1.5% O2) of BK channels in I-O patches of PASMCs from CH rats does not significantly alter open-state probability (NPO; n = 11). E, F, BK channels in I-O patches of PASMCs from CH rats are activated by lithocholate (LC; 45 µmol/L), as confirmed by bar graph depicting averaged values (n = 9; F).
hBK channels in human PASMCs show higher Ca2+ sensitivity and are activated by BKCOs
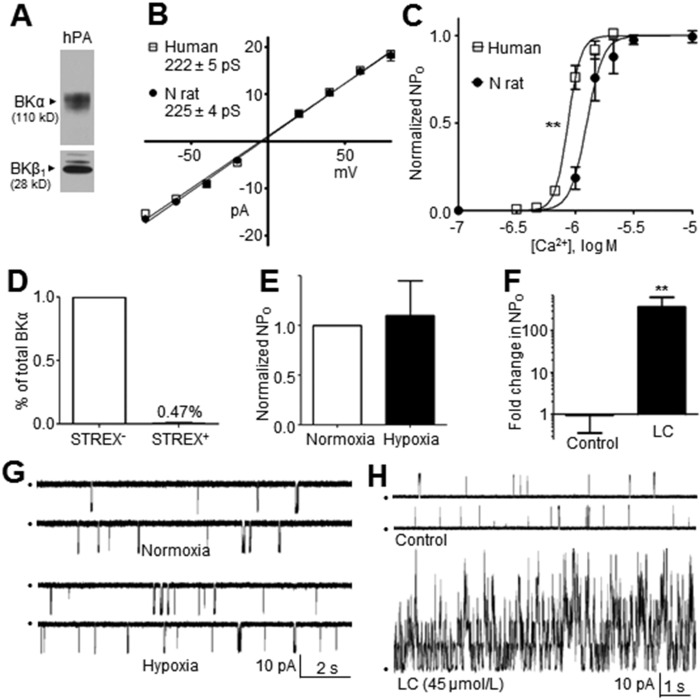
In a final series of studies designed to ensure the relevance of our findings in rat PASMCs to hBK channels in human PASMCs, we performed key experiments using 500–1000-μm-diameter PAs from 12 human lung samples. Western blots detected the BKα and BKβ1 subunits in protein lysates from human PAs (Fig. 6A). The multiple bands in the anti-BKβ1 blot may represent multiple posttranslational modifications of the BKβ1 subunit or nonspecific immunoreactivity, although these bands were not observed in Western blots of cloned human BKα subunits or rat BKα subunits (Figs. 1A, 4E, respectively). Patch-clamp analysis of hBK channel currents in I-O patches from human PASMCs revealed a standard unitary conductance of 222 ± 5 pS (Fig. 6B; n = 3–4). PCR analysis revealed that the hypoxia-insensitive STREX− variant accounted for 99.5% ± 0.1% of BKα transcripts expressed in human PAs (Fig. 6D; n = 3), implying a homogenous population of STREX− BK channels resistant to hypoxia. This finding concurred with our next observation that BK channel NPO recorded in I-O patches from freshly isolated human PASMCs was not significantly altered when patches were exposed to hypoxic bath solutions (Fig. 6E, 6G; n = 5). However, the Ca2+ activation curve for hBK channels exhibited a consistent leftward shift compared with that of BK channels from rat PASMCs, resulting in an EC50 value of −6.06 ± 0.01 log mol/L (n = 2–5 per point) compared with BK channels in PASMCs from N rats, which exhibited an EC50 value of −5.90 ± 0.02 log mol/L (Fig. 6C; N rat data plotted from Fig. 1C). Finally, hBK channels also exhibited high sensitivity to LC, showing a 141 ± 31-fold increase in NPO in response to 45 μmol/L LC (Fig. 6F, 6H; n = 4, 8). Because of the elevated Ca2+ sensitivity of hBK channels, this experiment was performed under conditions designed to reduce basal channel activity in order to accurately quantitate LC-induced increases in NPO and identify multiple stacked openings. Accordingly, patch potential was held at +40 mV to increase single-channel amplitude, but bath [Ca2+] was reduced 100-fold to 10−8 mol/L (instead of 10−6 mol/L used for rat PASMCs). Thus, the magnitude of NPO increase in response to LC cannot be directly compared with that of N or CH rats.
Figure 6.
A, Western blot reveals BKα and BKβ1 subunits in protein lysates from human pulmonary arteries (PAs). B, Unitary human high-conductance Ca2+-activated (hBK) channel conductance in human PA smooth muscle cells (PASMCs) was 222 ± 5 pS, a value nearly identical to that in PASMCs of normal (N) rats. C, Ca2+ sensitivity of hBK channels in PASMCs is higher than that of BK channels in PASMCs of N rats (n = 2–5 per point). Asterisks indicate a statistical difference between half-maximal effective concentration values, using a nonlinear fit comparison (P ≤ 0.01). D, Quantitative real-time polymerase chain reaction using human PA reveals that only rare (0.47% ± 0.08%) BKα transcripts contain the stress axis regulated exon (STREX) insert that confers oxygen sensitivity (n = 3). E, Averaged values confirm that acute hypoxia does not significantly affect the open-state probability (NPO) of hBK channels in PASMCs freshly isolated from lungs (n = 4). F, Summarized data from hBK channels in inside-out (I-O) patches of PASMCs verify that LC (45 µmol/L) profoundly increases NPO (n = 4, 8). G, Exposure of an I-O patch from a human PASMC to hypoxic bath solution fails to affect hBK current (patch potential = −40 mV; [Ca2+]i = 10−6.17 mol/L). H, Representative recording of hBK channel current in an I-O patch from human PASMCs reveals that LC (45 µmol/L) profoundly activates the channel (patch potential = +40 mV; [Ca2+]i = 10−8 mol/L. LC: lithocholate.
Discussion
The biological and pharmacological properties of BK channels vary between smooth muscle cells of different vascular beds, and BK channels in small PAs are understudied. Here, we provide new findings to demonstrate that (1) the BK channel population in rat PASMCs is composed primarily of hypoxia-insensitive STREX− splice variants; (2) BKα subunits in rat PASMCs are functionally coupled to BKβ1 subunits to form channel complexes that are highly Ca2+ sensitive and activated by LC, a BKβ1-dependent BKCO; (3) PAs of CH rats with established PH show an increased abundance of BKα but not BKβ1 subunits, yet these channels appear phenotypically normal; and (4) BKα subunits in human PASMCs are also STREX− splice variants highly coupled to BKβ1, and these hBK channels show higher Ca2+ sensitivity than rat BK channels and are activated by LC. Collectively, these results imply that the properties of BK channels in PASMCs are consistent with those desired for a pharmacological target to increase K+ efflux in PASMCs, an ionic flux that is compromised during the development of PH.
The oxygen sensitivity of BK channels varies between cell types and serves a variety of important functions. For example, BK channels of carotid body chemoreceptor cells, which are essential for the maintenance of systemic O2 homeostasis, are highly oxygen sensitive.27 A splice insert, STREX, confers intrinsic oxygen sensitivity to the pore-forming BKα subunit independently of auxiliary channel subunits or cytosolic factors.8 A recent study detected STREX+ BKα transcripts in porcine PAs, but their abundance was not defined.12 Our findings suggest that STREX+ BKα variants are rare in rat and human PASMCs and that the vast majority of pulmonary BKα transcripts represent oxygen-insensitive STREX− variants. Accordingly, the NPO of BK channels in isolated patches of rat and human PASMCs was unaffected by acute hypoxia, a property that may confer an important advantage for therapeutic strategies relying on the pharmacological opening of BK channels for the treatment of PH.
Our finding that BKα subunits in rat PASMCs are functionally coupled to BKβ1 subunits to form highly Ca2+-sensitive BK channels contrasts with results of an earlier report.16 These authors suggested that BKα-BKβ1 coupling and Ca2+ sensitivity are lower in rat PASMCs compared with CASMCs. However, their analysis only compared channel NPO at 3 values of [Ca2+]i without establishing a concentration-response curve to calculate EC50 values; additionally, BKα-BKβ1 coupling was not evaluated using BKβ1-selective openers. In contrast, we observed that the high Ca2+ sensitivity of BK channels in rat PASMCs is nearly identical to that of BK channels formed by cloned BKα and BKβ1 proteins in Xenopus oocytes.23 Notably, the Ca2+ sensitivity of BK channels varies between different circulatory beds. It is low in BK channels of hamster and rat skeletal muscle arterioles, rendering the channels inactive under resting conditions.21,22 In contrast, BK channels in rat CASMCs exhibit high Ca2+ sensitivity, which is linked to higher expression of the BKβ1 subunit.21 Thus, our finding of high Ca2+ sensitivity of BK channels in rat PASMCs argues for functionally coupled BKα-BKβ1 complexes.
We also observed that BK channels in rat PASMCs are activated by LC, a BKβ1-dependent BKCO. Dopico et al.28 initially reported LC-elicited activation of BK channels in rabbit PASMCs in a panel of preparations. However, although LC is useful as a pharmacological tool to identify the presence of functional BKβ1 subunits, it is not suitable for in vivo use. Off-target effects include the release of intracellular Ca2+ and hepatotoxicity.29,30 The Ca2+-releasing action of LC may confound vascular reactivity assays designed to evaluate its vasodilator effect.31 Tamoxifen, another compound with BKCO properties, initially was thought to activate BK channels by binding to BKβ1 subunits;32 however, later findings suggest a more complex interaction.33 To our knowledge, there are no BKβ1-selective BKCOs suitable for in vivo use. However, the availability of other BKβ1-dependent BKCOs, including dehydrosoyasaponin-1 and β-estradiol, suggests the feasibility of targeting BKβ1 subunits to activate vascular BK channels.32,34 This strategy may offer a distinct advantage because of the tissue-specific expression of BKβ1 in smooth muscle cells. Accordingly, LC activates BK channels by binding to BKβ1 subunits in smooth muscle cells but does not bind to other BKβ subunits (i.e., β2, β3, β4) that compose BK channels in other tissues, potentially minimizing side effects.25
Our studies also address the uncertain fate of BK channels in PASMCs during CH-induced PH. Resnik et al.5 reported an increased expression of BKα subunits in whole-lung protein lysates of Sprague-Dawley rats exposed to hypobaric chronic hypoxia for 3 weeks. However, Bonnet et al.9 subsequently reported reduced BKα expression in intralobar arteries of Wistar rats exposed to hypobaric hypoxic conditions. Our results clearly indicate that BKα transcript and protein markedly increase in intralobar arteries of Sprague-Dawley rats exposed to CH for 3 weeks. The reason for the discrepancy in findings between laboratories is not readily apparent but may relate to different rats strains, differential effects of normobaric hypoxia (our study) versus hypobaric hypoxia,9 or other unrecognized factors. Notably, in our studies, BKβ1 expression in PAs of CH rats was normal, despite a concomitant increase in BKα expression, a finding that implies a relative deficit of BKβ1 subunits. Thus, we anticipated reduced BK channel sensitivity to [Ca2+]i and LC in PASMCs from CH rats. Instead, BK channel properties in PASMCs of N and CH rats were indistinguishable, suggesting that BKβ1 subunits may be expressed in excess in PASMCs of N rats. Collectively, our data provide initial evidence that PAs of CH rats express an overabundance of apparently normal BK channels that are sensitive to BKCOs, suggesting that they represent credible pharmacological targets to restore K+ efflux to PASMCs during PH.
Earlier studies of hBK channels have been limited to cultured human PASMCs,35 whereas our final experiments explored BK channel properties in freshly isolated human PASMCs. Importantly, hBK channels appear to represent STREX− splice variants insensitive to acute hypoxia, implying that they would be available for activation during hypoxemia, a feature of some forms of PH. Similar to those in rat, hBK channels also appear to be functionally coupled BKα-BKβ1 complexes sensitive to the BKCO LC. However, hBK channels exhibit higher Ca2+ sensitivity than their rat counterparts. This finding raises the possibilities that the hBKα isoform may be distinct from its rat equivalent or, alternatively, regulated differently by Ca2+-sensitizing mechanisms, concepts that deserve further attention.
Our study has several limitations. First, we could not use the whole-cell patch-clamp technique to confirm that the upregulation of BK channels in PAs of CH rats was associated with increased BK current density at the whole-cell level, although we attempted these studies. In the whole-cell configuration, we observed overwhelming KV channel current in PASMCs even under conditions that elicit abundant BK channel current in other cells, a previously reported phenomenon.36 For this reason, we focused on single-channel recordings to define BK channel properties without interference from other K+ channels. Another potential limitation involved the use of human donor tissue lacking detailed patient health histories, which implies that patient age, sex, genetic background, and disease processes may have influenced our results. Nevertheless, hBK channel properties, including Ca2+ sensitivity, were consistent between PASMCs from different lung samples.
Human forms of PH show diverse clinical presentations with multiple genetic and environmental factors influencing the disease process.37 Medications for PH have been introduced and refined to optimize patient outcomes, but resistance to vasodilator drugs remains a key challenge, largely related to structural remodeling of the pulmonary vasculature.38,39 A BKCO (NS1619) was recently reported to ameliorate monocrotaline-induced PH in rats,40 which is a promising result, but unfortunately NS1619 also exhibits several off-target effects, including inhibition of L-type voltage-gated Ca2+ channels,41 making it difficult to credit the BK channel alone for the therapeutic effect. Earlier investigations have demonstrated that sarcolemmal K+ efflux in PASMCs establishes a negative membrane potential, which inactivates voltage-dependent Ca2+ channels to lower pulmonary vascular tone.42 Sarcolemmal K+ efflux also inhibits proliferation of PASMCs by inducing apoptotic signaling pathways.3 Our new findings show that human PASMCs express hypoxia-insensitive hBK channels readily activated by BKβ1 subunit-dependent BKCOs, implicating hBK channels as receptive targets for increasing K+ efflux in human PASMCs. Collectively, our findings—combined with earlier discoveries by other laboratories—may encourage the development of new BKCOs with favorable solubility and pharmacokinetic and hemodynamic profiles optimized for the treatment of PH.
Acknowledgments
We thank Anna N. Bukiya and Alex M. Dopica (University of Tennessee Health Science Center) for advice in preparing and using lithocholate in patch-clamp studies. Sarah England (Washington University) and Jerod Denton (Vanderbilt University) kindly provided the BKα and BKβ cDNAs, respectively. Finally, we thank Philip Palade (University of Arkansas for Medical Sciences) for providing a helpful review of the manuscript.
Appendix.
Figure S1.
After exposure to 3 weeks of chronic normobaric normoxia (N) or hypoxia (CH), rats were anesthetized with isoflurane and cardiovascular parameters were measured. Right ventricular systolic pressure (RVSP) was significantly higher in CH compared with N rats (A), a finding that corresponded to an increased right ventricle/(left ventricle + septum) ratio (RV/(LV + S)) in the CH animals (B). Systemic mean arterial pressure (MAP; C) and heart rate (D) were not significantly different between N and CH rats. **P ≤ 0.01.
Figure S2.
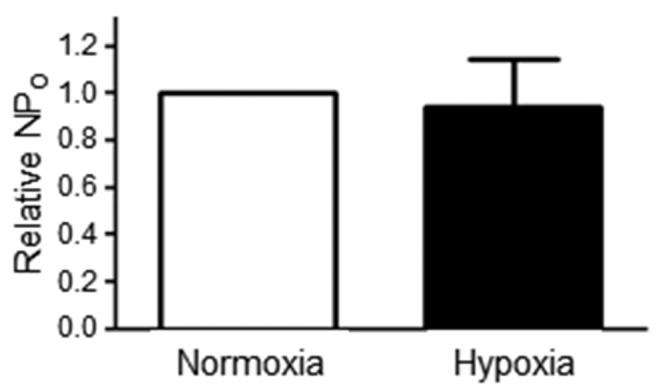
To rule out the possibility that the loss of soluble cytosolic factors in inside-out patches accounted for the failure of hypoxia to affect high-conductance Ca2+-activated (BK) channel activity, we exposed BK channels in cell-attached patches of rat pulmonary arterial smooth muscle cells to hypoxic bath solution. Although the cytosolic milieu is preserved in cell-attached patches, hypoxia did not significantly affect the open-state probability (NPO) of BK channels (n = 3).
Source of Support: This research was supported by National Heart, Lung, and Blood Institute grant R01-HL-083013 (NJR), American Heart Association National Scientist Development grant 0830060N (SWR), a training stipend from Clinical and Translational Science Award UL1RR029884 (NDD), and American Heart Association Southwest Affiliate predoctoral grant PRE17240055 (NDD). A gift from the University of Arkansas for Medical Sciences College of Medicine Dean’s Society provided funds for biotelemetry equipment.
Conflict of Interest: None declared.
Supplements
Appendix (562KB, pdf)
References
- 1.Yuan JX-J, Aldinger AM, Juhaszova M, Wang J, Conte JV, Gaine SP, Orens JB, Rubin LJ. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation 1998;98(14):1400–1406. [DOI] [PubMed]
- 2.Wang J, Weigand L, Wang W, Sylvester JT, Shimoda LA. Chronic hypoxia inhibits KV channel gene expression in rat distal pulmonary artery. Am J Physiol 2005;288(6):L1049–L1058. [DOI] [PubMed]
- 3.Burg ED, Remillard CV, Yuan JX-J. Potassium channels in the regulation of pulmonary artery smooth muscle cell proliferation and apoptosis: pharmacotherapeutic implications. Br J Pharmacol 2008;153:S99–S111. [DOI] [PMC free article] [PubMed]
- 4.Pozeg ZI, Michelakis ED, McMurtry MS, Thébaud B, Wu X-C, Dyck JRB, Hashimoto K, et al. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation 2003;107(15):2037–2044. [DOI] [PubMed]
- 5.Resnik E, Herron J, Fu R, Ivy D, Cornfield DN. Oxygen tension modulates the expression of pulmonary vascular BKCa channel α- and β-subunits. Am J Physiol 2005;290(4):L761–L768. [DOI] [PubMed]
- 6.Platoshyn O, Brevnova EE, Burg ED, Yu Y, Remillard CV, Yuan JX-J. Acute hypoxia selectively inhibits KCNA5 channels in pulmonary artery smooth muscle cells. Am J Physiol 2006;290(3):C907–C916. [DOI] [PMC free article] [PubMed]
- 7.Cox RH, Petrou S. Ca++ influx inhibits voltage-dependent and augments calcium-dependent K+ currents in arterial myocytes. Am J Physiol 1999;277(1):C51–C63. [DOI] [PubMed]
- 8.McCartney CE, McClafferty H, Huibant J-M, Rowan EG, Shipston MJ, Rowe ICM. A cysteine-rich motif confers hypoxia sensitivity to mammalian large conductance voltage- and Ca-activated K (BK) channel α-subunits. Proc Natl Acad Sci USA 2005;102:17870–17876. [DOI] [PMC free article] [PubMed]
- 9.Bonnet S, Dumas-de-La-Roque E, Bégueret H, Marthan R, Fayon M, Dos Santos P, Savineau J-P, Baulieu E-E. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA 2003;100:9488–9493. [DOI] [PMC free article] [PubMed]
- 10.Bentzen BH, Olesen S, Rønn LCB, Grunnet M. BK channel activators and their therapeutic perspectives. Front Physiol 2014;5:389. [DOI] [PMC free article] [PubMed]
- 11.Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron 1994;13(6):1315–1330. [DOI] [PubMed]
- 12.Dospinescu C, Widmer H, Rowe I, Wainwright C, Cruickshank SF. Hypoxia sensitivity of a voltage-gated potassium current in porcine intrapulmonary vein smooth muscle cells. Am J Physiol 2012;303(5):L476–L486. [DOI] [PubMed]
- 13.Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature 2000;407(6806):870–876. [DOI] [PubMed]
- 14.Jiang Z, Wallner M, Meera P, Toro L. Human and rodent MaxiK channel β-subunit genes: cloning and characterization. Genomics 1999;55:57–67. [DOI] [PubMed]
- 15.Bukiya AN, Vaithianathan T, Toro L, Dopico AM. The second transmembrane domain of the large conductance, voltage- and calcium-gated potassium channel β1 subunit is a lithocholate sensor. FEBS Lett 2008;582(5):673–678. [DOI] [PMC free article] [PubMed]
- 16.Zheng Y-M, Park SW, Stokes L, Tang Q, Xiao J-H, Wang Y-X. Distinct activity of BK channel β1-subunit in cerebral and pulmonary artery smooth muscle cells. Am J Physiol 2013;304(8):C780–C789. [DOI] [PMC free article] [PubMed]
- 17.Post JM, Hume JR, Archer SL, Weir EK. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol 1992;262:C882–C890. [DOI] [PubMed]
- 18.Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol 1993;264:L116–L123. [DOI] [PubMed]
- 19.Lorca RA, Prabagaran M, England SK. Functional insights into modulation of BKCa channel activity to alter myometrial contractility. Front Physiol 2014;5:289. [DOI] [PMC free article] [PubMed]
- 20.Meera P, Wallner M, Toro L. A neuronal β subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA 2000;97(10):5562–5567. [DOI] [PMC free article] [PubMed]
- 21.Yang Y, Sohma Y, Nourian Z, Ella SR, Li M, Stupica A, Korthuis RJ, Davis MJ, Braun AP, Hill MA. Mechanisms underlying regional differences in the Ca2+ sensitivity of BKCa current in arteriolar smooth muscle. J Physiol 2013;591(5):1277–1293. [DOI] [PMC free article] [PubMed]
- 22.Jackson WF, Blair KL. Characterization and function of Ca2+-activated K+ channels in arteriolar muscle cells. Am J Physiol 1998;274:H27–H34. [DOI] [PubMed]
- 23.Meera P, Wallner M, Jiang Z, Toro L. A calcium switch for the functional coupling between α (hslo) and β subunits (KV,Caβ) of maxi K channels. FEBS Lett 1996;382(1/2):84–88. [DOI] [PubMed]
- 24.Bukiya AN, Liu J, Toro L, Dopico AM. β1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-sized arteries. Mol Pharmacol 2007;72(2):359–369. [DOI] [PubMed]
- 25.Bukiya AN, Vaithianathan T, Toro L, Dopico AM. Channel β2-4 subunits fail to substitute for β1 in sensitizing BK channels to lithocholate. Biochem Biophys Res Commun 2009;390(3):995–1000. [DOI] [PubMed]
- 26.Li W-Q, Li X-H, Du J, Zhang W, Li D, Xiong X-M, Li Y-J. Rutaecarpine attenuates hypoxia-induced right ventricular remodeling in rats. Arch Pharmacol 2016;389(7):757–767. [DOI] [PubMed]
- 27.Riesco-Fagundo AM, Perez-Garcia MT, Gonzalez C, Lopez-Lopez JR. O2 modulates large-conductance Ca2+-dependent K+ channels of rat chemoreceptor cells by a membrane-restricted and CO-sensitive mechanism. Circ Res 2001;89:430–436. [DOI] [PubMed]
- 28.Dopico AM, Walsh JV, Singer JJ. Natural bile acids and synthetic analogues modulate large conductance Ca2+-activated K+ (BKCa) channel activity in smooth muscle cells. J Gen Physiol 2002;119(3):251–273. [DOI] [PMC free article] [PubMed]
- 29.Fernández-Mariño AI, Porras-Gonzalez C, Gonzalez-Rodriguez P, Selent J, Pastor M, Urena J, Castellano A, Valverde MA, Fernandez-Fernandez JM. Tungstate activates BK channels in a β-subunit- and Mg2+-dependent manner: relevance for arterial vasodilation. Cardiovasc Res 2012;95:29–38. [DOI] [PubMed]
- 30.Hofmann AF. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metab Rev 2004;36:703–722. [DOI] [PubMed]
- 31.Bukiya AN, McMillan JE, Fedinec AL, Patil SA, Miller DD, Leffler CW, Parrill AL, Dopico AM. Cerebrovascular dilation via selective targeting of the cholane steroid-recognition site in the BK channel β1-subunit by a novel nonsteroidal agent. Mol Pharmacol 2013;83(5):1030–1044. [DOI] [PMC free article] [PubMed]
- 32.Dick GM, Sanders, KM. (Xeno)estrogen sensitivity of smooth muscle BK channels conferred by the regulatory β1 subunit: a study of β1 knockout mice. J Biol Chem 2001;276(37):34594–34599. [DOI] [PubMed]
- 33.Pérez G. Dual effect of tamoxifen on arterial KCa channels does not depend on the presence of the β1 subunit. J Biol Chem 2005;280(23):21739–21747. [DOI] [PubMed]
- 34.McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the β subunit of high conductance calcium-activated potassium channels. Neuron 1995;14(3):645–650. [DOI] [PubMed]
- 35.Peng W, Hoidal JR, Karwande SV, Farrukh IS. Effect of chronic hypoxia on K+ channels: regulation in human pulmonary vascular smooth muscle cells. Am J Physiol 1997;272:C1271–C1278. [DOI] [PubMed]
- 36.Dubuis E, Potier M, Wang R, Vandier C. Continuous inhalation of carbon monoxide attenuates hypoxic pulmonary hypertension development presumably through activation of BKCa channels. Cardiovasc Res 2005;65:751–761. [DOI] [PubMed]
- 37.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Sanchez MAG, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl):D34–D41. [DOI] [PubMed]
- 38.Galiè N, Ghofrani AH. New horizons in pulmonary arterial hypertension therapies. Eur Respir Rev 2013;22(130):503–514. [DOI] [PMC free article] [PubMed]
- 39.Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J 2012;40(6):1555–1565. [DOI] [PMC free article] [PubMed]
- 40.Revermann M, Neofitidou S, Kirschning T, Schloss M, Brandes RP, Hofstetter C. Inhalation of the BKCa-opener NS1619 attenuates right ventricular pressure and improves oxygenation in the rat monocrotaline model of pulmonary hypertension. PLoS ONE 2014;9(1):e86636. [DOI] [PMC free article] [PubMed]
- 41.Edwards G, Niederste-Hollenberg A, Schneider J, Noack T, Weston AH. Ion channel modulation by NS 1619, the putative BKCa channel opener, in vascular smooth muscle. Br J Pharmacol 1994;113(4):1538–1547. [DOI] [PMC free article] [PubMed]
- 42.Gurney AM, Osipenko ON, MacMillan D, Kempsill FE. Potassium channels underlying the resting potential of pulmonary artery smooth muscle cells. Clin Exp Pharmacol Physiol 2002;29(4):330–333. [DOI] [PubMed]