Abstract Abstract
Sarcoidosis-associated pulmonary hypertension (SAPH) is estimated to occur in at least 5% or more of sarcoidosis patients, and it contributes to significant morbidity and mortality. Optimal therapy for SAPH is not well established. We performed a 24-week open-label trial of tadalafil for SAPH at 2 academic medical centers. Subjects were required to have confirmed sarcoidosis plus a right heart catheterization within 12 months of enrollment showing a mean pulmonary artery pressure ≥ 25 mmHg, a pulmonary artery wedge pressure ≤ 15 mmHg, and a calculated pulmonary vascular resistance ≥ 3 Wood units. Subjects received 20 mg/day of tadalafil for the first 4 weeks and then 40 mg/day for the subsequent 20 weeks. Sixteen patients were screened, 12 of whom met criteria for enrollment. At 24 weeks, there was no overall improvement in 6-minute walk distance (6MWD). Five of the 12 subjects dropped out of the study early (2 for social reasons, 3 for medical reasons). There was no significant change in short form 36, St. George’s respiratory questionnaire, or maximum Borg dyspnea scores over the 24 weeks. There were no significant adverse events or laboratory abnormalities clearly related to tadalafil in the cohort. The study did not meet the primary end point of change in 6MWD because of the small sample size. Tadalafil was generally safely administered in this cohort of SAPH patients. There was a relatively high dropout rate but no major adverse events and no clinical worsening. Larger studies are needed to explore this question further. (Trial registration: ClinicalTrials.gov identifier: NCT01324999)
Keywords: sarcoidosis, pulmonary hypertension, tadalafil
Sarcoidosis is a multiorgan system disease of unknown etiology, characterized by noncaseating granulomatous tissue infiltration and inflammation. The most commonly affected organ system is the lung, most often in the form of alveolitis and/or parenchymal fibrosis and associated sequelae (such as bronchiectasis and cavity formation). Sarcoidosis-associated pulmonary hypertension (SAPH) is seen in at least 5% of patients with sarcoidosis1 and more often in patients with advanced, end-stage disease. This higher frequency of SAPH is noted particularly in patients who are being evaluated for or awaiting lung transplantation, and increased mortality is noted in this population as well.2-5 The optimal treatment approach to SAPH remains unclear. Conventional corticosteroid and immunomodulator therapies (such as methotrexate) utilized to treat sarcoidosis do not clearly improve SAPH. Furthermore, there is a relative paucity of data with regard to the utility of treating SAPH with pulmonary arterial hypertension (PAH)-specific therapies. We report the results of a prospective, open-label trial of tadalafil therapy in a defined cohort of SAPH patients. The study was conducted at 2 academic medical centers in the United States.
Methods
This was an investigator-initiated study, approved by the institutional review boards of the University of Cincinnati and the University of North Carolina. Informed consent was obtained from each subject before study entry.
Enrollment criteria
Subjects were recruited at the outpatient pulmonary clinics at the University of Cincinnati and the University of North Carolina if they met all of the following inclusion criteria: (1) confirmed sarcoidosis; (2) right heart catheterization within 12 months of study enrollment, with the following findings: mean pulmonary artery pressure (MPAP) ≥ 25 mmHg, pulmonary artery wedge pressure ≤ 15 mmHg, and pulmonary vascular resistance (PVR) ≥ 3 Wood units; (3) forced vital capacity > 40% of predicted; (4) World Health Organization (WHO) functional class (FC) 2 or 3; and (5) receiving stable sarcoidosis therapy (e.g., corticosteroids) for at least 3 months before study entry. Subjects were excluded from participation if they met any of the following criteria: (1) use of any agent for treatment of PAH within 1 month of study entry; (2) uncontrolled systemic hypertension; (3) pregnancy; (4) exercise limitation from a cause thought other than a cardiopulmonary cause (e.g., arthritis); (5) pulmonary hypertension (PH) thought not to be related to sarcoidosis; (6) WHO FC 4; (7) significant left ventricular dysfunction; (8) hepatic dysfunction thought not to be related to sarcoidosis liver involvement; (9) presence of a comorbid illness thought to affect survival during the course of the study.
Drug administration
Subjects were screened for study eligibility within 30 days of enrollment. Enrolled subjects received 20 mg daily of oral tadalafil starting at enrollment (week 0) for 4 weeks. They then received 40 mg daily of tadalafil until week 24 after enrollment. The drug was given in an open-label fashion, with no control/placebo group. Reduction in the dose of tadalafil back to 20 mg/day was allowed at the discretion of study investigators if thought necessary.
Study end points
The primary efficacy end point was the change in the 6-minute walk distance (6MWD) between week 0 and week 24. Secondary end points included the change in the following parameters over the 24-week study period: (1) WHO FC; (2) maximum modified Borg dyspnea scale6 during the 6-minute walk test (6MWT); (3) St. George’s respiratory questionnaire (SGRQ) score;7 (4) short form 36 (SF-36) score; (5) serum pro-brain natriuretic peptide (BNP) level; and (6) oxygen saturation nadir at rest and during 6MWT.
Data collection and safety monitoring
Subjects were interviewed and physical examination was performed at study visits every 4 weeks until study completion (week 24). Study subjects were asked about their level of dyspnea; presence of peripheral edema, wheezing, cough, or chest pain; and symptoms of liver disease and right heart failure (e.g., syncope, near-syncope, abdominal distension). At each visit, the subjects underwent the following: physical examination, including vital signs and pulse oximetry, and a formal examination for the degree of peripheral edema; 6MWT, where 6MWD with maximum Borg scale assessment, heart rate, oximetry, and blood pressure were recorded; WHO FC determination; serum BNP testing; and a pregnancy test for all female subjects with childbearing potential. Spirometry, diffusing capacity, complete blood counts, comprehensive metabolic panel, SGRQ scores, and SF-36 scores were measured at weeks 0, 8, 16, and 24. An assessment of compliance with the study drug and of possible study drug toxicity was made at the week 4 study visit and all subsequent visits. Subjects were allowed to receive diuretics to control peripheral edema as well as treatment of any other complications or acute illnesses that occurred during the course of the study.
Statistical analysis
The study design was open label, proof of concept. Baseline demographics were collected at the baseline or screening visit. Spirometry and single-breath diffusing capacity for carbon monoxide (DLco) parameters were documented as percent predicted of normal. SF-36 and SGRQ data were scored using algorithms and calculators published and provided by Hays8 and the SGRQ score calculator.9
All study outcome variables were compared with baseline values at weeks 8, 16, and 24. Student t tests were utilized to determine significance of the changes observed between baseline and week 24 data. When data were missing because of patient dropout or a missed study visit, the last observation carried forward was used for interim and final analyses.
Results
Twelve subjects were enrolled in this study over a period of 29 months. Table 1 displays the demographics and baseline characteristics of the study cohort. The patients were predominantly African American women. Most had mild to moderate PH (MPAP = 34.8 ± 7.1 mmHg), which is consistent with previous reports of SAPH.10 The majority of the subjects (7/12 [58%]) had WHO FC 2 status at the time of enrollment.
Table 1.
Baseline demographics and clinical characteristics (intent to treat population; n = 12 unless otherwise noted)
| Baseline characteristic | Value |
|---|---|
| Age, years | 54.5 ± 9.48 |
| Sex, no. (%) | |
| Male | 4 (33) |
| Female | 8 (67) |
| Race, no. (%) | |
| Black | 9 (75) |
| White | 3 (25) |
| Sarcoidosis therapy, no. (%) | |
| Corticosteroid | 8 (67) |
| Methotrexate | 5 (41.6) |
| Hydroxycholoroquine | 1 (8.3) |
| None | 3 (25) |
| Prednisone (or equivalents) daily dose, mg | 20.9 ± 12.7 |
| Chest X-ray stage (n = 12) | |
| 0 | 1 |
| 1 | 0 |
| 2 | 1 |
| 3 | 6 |
| 4 | 4 |
| Spirometry, percent predicted | |
| FVC | 65.9 ± 13.4 |
| FEV1 | 58.0 ± 14.7 |
| DLco (n = 8) | 60.3 ± 25.0 |
| Severe airflow obstruction (FEV1 < 50% predicted), no. (%)a | 2 (16.7) |
| Isolated DLco reduction, no. (%) | 1 (8.3) |
| Principally restrictive disease suggested by spirometry, no. (%) | 4 (33) |
| Quality of life metrics | |
| SF-36 global score | 38.2 ± 5.1 |
| SGRQ total score | 62.5 ± 14.2 |
| Hemodynamic data | |
| Right atrial pressure, mmHg | 6.2 ± 4.0 |
| MPAP, mmHg | 34.8 ± 7.1 |
| PVR, Wood units (thermodilution) | 6.2 ± 3.6 |
| PAWP, mmHg | 10.3 ± 3.6 |
| Cardiac index, L/min/m2 (thermodilution) | 2.4 ± 0.6 |
| Oxygen and functional assessments | |
| Requires supplemental oxygen at rest, no. (%) | 5 (41.6) |
| 6-minute walk distance, m | 360.0 ± 163.3 |
| Maximum Borg score during 6MWT | 5.0 ± 2.3 |
| WHO functional class, no. (%) | |
| 2 | 7 (58.3) |
| 3 | 5 (41.6) |
| Serum pro-BNP, pg/mL (n = 8) | 77.6 ± 85.2 |
Except where otherwise indicated, data are means ± SD. 6MWT: 6-minute walk test; BNP: brain natriuretic peptide; DLco: single-breath diffusing capacity for carbon monoxide; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; MPAP: mean pulmonary artery pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; SF-36: short form 36; SGRQ: St. George’s respiratory questionnaire; WHO: World Health Organization.
Without proportionate decrease in FVC.
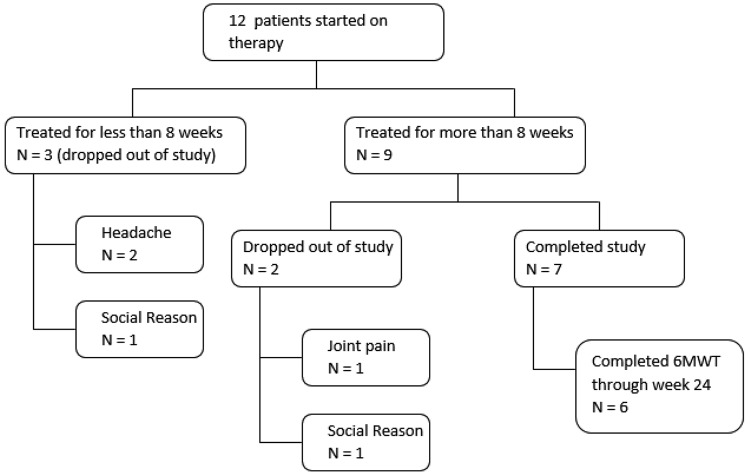
With regard to the disposition of the subjects (Fig. 1), 58% (7/12) of subjects completed this 24-week trial. Of the 5 subjects who discontinued this study, 3 (60%) discontinued for adverse effects (headache [n = 2] or joint pain [n = 1]), while 2 (40%) discontinued for social reasons (lack of continued interest or inability to attend further study visits). In terms of timing of study discontinuation, 3 (60%) of those who discontinued did so within 8 weeks of enrollment, and the remaining 2 (40%) discontinued after week 8.
Figure 1.
Disposition of study subjects with duration of treatment and reasons for study drop-out. 6MWT: 6-minute walk test.
Subjects who discontinued tadalafil and dropped out of the study for any reason could not be predicted by development of edema, 6MWD, Borg score, baseline supplemental oxygen requirement, WHO FC, SF-36 score, or SGRQ score (data not shown). Of those who received at least 1 dose of study drug, none developed hepatic or renal impairment during this trial, on the basis of interval assessments of liver transaminases, bilirubin, and serum creatinine. Furthermore, no subjects developed significantly increased peripheral edema during the course of the study, on the basis of investigator physical examination assessment at each study visit. One subject changed the dose of immunomodulatory medications during the trial (reduction of prednisone from 40 to 30 mg/day at week 12). In addition, there was one subject who reduced the dose of tadalafil back to 20 mg daily after week 4 because of joint pain (resolved after dose decrease, and the patient remained in the trial for the full duration).
In addition to the aforementioned joint pain and headache, 2 other patients experienced adverse effects of herpes zoster and influenza complicated by pneumonia. Both were thought to be unrelated to the study drug and were resolved with appropriate therapy. All of the adverse events noted in the study population are reported in Table 2.
Table 2.
Adverse events experienced in patients who received at least 1 dose of study drug, with reported severity, outcome, and relationship to study drug
| Adverse event | No. (%) | Severity | Relationship to study drug | Outcome |
|---|---|---|---|---|
| Headache | 2 (17) | Adverse event | Probably related | Resolved with cessation of study drug (patient dropped out) |
| Joint pain | 1 (8) | Adverse event | Probably related | Resolved with cessation of study drug (patient dropped out) |
| Herpes zoster | 1 (8) | Adverse event | Unrelated | Resolved with treatment |
| Influenza and pneumonia | 1 (8) | Adverse event | Unrelated | Resolved with treatment |
The study’s primary end point—a significant change in 6MWD between week 0 and week 24—was not met in the 6 of the 7 study completers who performed the 6MWT through week 24 (Fig. 2; Table 3). The following secondary end points also did not significantly change after 24 weeks of tadalafil therapy: maximum modified Borg dyspnea scale during the 6MWT, SF-36 and SGRQ scores, and serum BNP level (Table 3). One patient did change their WHO FC from baseline to week 24, improving from FC 2 to class 1. Oxygen requirement at rest and nadir during 6MWT did not change significantly during the course of the trial in this cohort.
Figure 2.

Six-minute walk distance of the 6 patients who completed the 6-minute walk test from baseline through week 24, with each distance shown at every 4 weeks.
Table 3.
Key study outcomes at each 8-week interval as compared with baseline
| Characteristic | Week 0 | Week 8 | Week 16 | Week 24 |
|---|---|---|---|---|
| 6-minute walk distance, m (n = 6) | 419.1 ± 170.1 | 418.1 ± 143.5 | 436.5 ± 178.1 | 377.1 ± 140.9 |
| Oxygen saturation at rest, % (n = 6) | 96.5 ± 1.8 | 95.2 ± 2.6 | ||
| Oxygen desaturation (at nadir) during 6MWT, % (n = 6) | 9 ± 6 | 9 ± 9 | ||
| Maximum Borg score during 6MWT (n = 6) | 4.8 ± 1.2 | 5.0 ± 2.4 | 5.3 ± 2.1 | 6.3 ± 2.3 |
| Brain natriuretic peptide, pg/mL | 58.8 ± 89.6 | 32.3 ± 46.6 | 37.5 ± 51.4 | 42.6 ± 49.7 |
| SF-36 global score | 38.5 ± 5.3 | 37.7 ± 6.7 | 39.2 ± 6.5 | 38.9 ± 8.5 |
| SGRQ total score | 64.5 ± 15.5 | 65.1 ± 18.9 | 64.3 ± 15.2 | 71.1 ± 15.2 |
| WHO functional class, no. | ||||
| 1 | 0 | 1 | 1 | 1 |
| 2 | 3 | 2 | 2 | 2 |
| 3 | 4 | 4 | 4 | 4 |
| 4 | 0 | 0 | 0 | 0 |
Outcomes determined on the basis of 7 patients who completed the study, unless noted otherwise. Except where otherwise indicated, data are means ± SD. 6MWT: 6-minute walk test; SF-36: short form 36; SGRQ: St. George’s respiratory questionnaire; WHO: World Health Organization.
Discussion
This proof-of-concept trial did not show a significant difference in the primary efficacy end point of 6MWD at 24 weeks in SAPH patients treated with tadalafil. This was largely due to small sample size and compounded by patient dropouts. This is consistent with what was observed by Judson et al.11 in a similarly sized open-label trial of ambrisentan for SAPH that enrolled slightly more patients (n = 21). Our sample size was further reduced by study dropouts, making it even less likely to detect a trend toward statistical significance with any of our measures. Interestingly, one patient who completed the study did demonstrate a 46-m improvement in 6MWD (Fig. 1), far above the minimal important difference of 33 m established for PAH patients.12 This particular patient had mild PH (MPAP = 25 mmHg) and severe airway obstruction (forced expiratory volume in 1 second = 46% predicted).
A significant number of patients dropped out of the study early as a result of known adverse effects of tadalafil (headache and joint pain). Three of the patients who remained in the trial for the full 24 weeks actually experienced a decline in their 6MWD. The reason for this is unclear, but one possible explanation is worsening of ventilation/perfusion (V/Q) mismatch in patients with parenchymal lung disease due to administration of a pulmonary vasodilator agent (tadalafil). All three of these patients had stage 3 radiographic disease. However, none of the patients in the trial clearly demonstrated worsening of oxygen saturation while receiving tadalafil therapy, which would be an expected consequence of increased V/Q mismatch.
We also sought to evaluate safety and tolerability in this cohort of SAPH patients exposed to tadalafil. As stated above, the number of adverse events was small. Joint pain/arthralgia and headache are known adverse effects of tadalafil. The two other patients who developed infectious complications (herpes zoster and influenza with pneumonia) were both on significant immunosuppressant regimens consisting of prednisone and methotrexate as therapy directed at sarcoidosis.
With regard to SF-36 and SGRQ scores, there was no significant improvement over the 24-week trial. Similarly, there was no improvement noted in WHO FC, as assessed by the investigators. Thus, there seems to be general agreement between investigator and patient self-assessment of functional capacity.
There was no significant difference noted in pro-BNP levels from baseline to week 24. However, the baseline levels were within normal range before treatment, suggesting that most of the patients did not have significant right ventricular strain due to PH at the outset of the study. Indeed, the average baseline cardiac index was within normal range. The right atrial pressures were not elevated, indicating that the patients were relatively euvolemic (Table 1) without significant right heart strain.
Aside from small sample size, the multifactorial nature of SAPH has made it very difficult to identify any signal of efficacy from PAH therapies in general. Isolated, intrinsic pulmonary arteriopathy (possibly in the form of granulomatous vascular disease) may be seen in a small number of SAPH patients. However, the majority of SAPH patients have left heart dysfunction (WHO group 2 PH) and/or restrictive lung disease related to parenchymal fibrosis (WHO group 3 PH). Most trials of PAH therapy for SAPH have had small sample sizes and were not placebo controlled. Furthermore, many have been retrospective and/or observational studies.13-15 Overall, the body of literature looking at PAH therapies to treat SAPH has not shown a benefit in traditional PAH trial end points. One exception to this is a recent study by Baughman et al.16 looking at the efficacy of bosentan for SAPH in a 16-week, double-blind, placebo-controlled trial. In that study, no difference was observed in 6MWD, but significant improvements were seen in MPAP and PVR at week 16. Our study did not look at hemodynamics at week 24, but it is possible that they may have improved. Milman et al.17 found that treating SAPH with sildenafil was associated with improvement in hemodynamics but not 6MWD. Changes in pulmonary hemodynamics may not result in functional or 6MWT improvement within the 24 weeks of this study and thus may require a more prolonged course of treatment to improve 6MWD and functional capacity.13
There are limitations to this study, the most significant being the unblinded, open-label study design with no placebo control group. As stated above, the small sample size also limits the ability to detect significant differences in all of the study end points. In addition to these design and sample size issues, it is possible that the exclusion criteria for forced vital capacity (≥40% predicted) was too liberal. This could have allowed for patients who were less likely to have intrinsic pulmonary arteriopathy and more likely to have SAPH driven largely by parenchymal fibrosis and destruction/distortion. One could argue that a PAH-specific therapy is not as likely to improve outcomes in this context. Another possibility is that the dosing schedule employed in the study was not optimal for this population. In the Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) trial of tadalafil for PAH, it was noted that 20 mg daily of tadalafil improved 6MWD almost as much as 40 mg daily and that the increase was significant compared with placebo.18 Given the aforementioned hypothesis that some SAPH may actually develop worsening V/Q mismatch with PAH therapy, it is plausible that a lower dose of tadalafil for the duration of this trial may have led to beneficial functional and hemodynamic outcomes while mitigating some of the potential V/Q mismatch.
In summary, though the primary and secondary efficacy end points were not met, we were able to glean some preliminary safety and tolerability data with respect to the use of tadalafil in SAPH. Seven out of 12 patients were able to continue tadalafil therapy and complete the study. Those who withdrew because of medical reasons experienced known side effects of tadalafil. There was no clear clinical worsening on the basis of the end points assessed over the 24-week duration of the study. None of the patients required additional therapy or hospitalization. There was no indication of harm in terms of hepatic, renal, or other organ system dysfunction. The exact role tadalafil may play in this population remains unclear. A more rigorous, larger, and/or longer controlled trial with stricter inclusion/exclusion criteria might identify a cohort of SAPH patients who would benefit from tadalafil therapy.
Source of Support: This research was funded by United Therapeutics.
Conflict of Interest: None declared.
References
- 1.Bourbonnais JM, Samavati L. Clinical predictors of pulmonary hypertension in sarcoidosis. Eur Respir J 2008;32(2):296–302. [DOI] [PubMed]
- 2.Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis-associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest 2010;138:1078–1085. [DOI] [PubMed]
- 3.Arcasoy SM, Christie JD, Pochettino A, Rosengard BR, Blumenthal NP, Bavaria JE, Kotloff RM. Characteristics and outcomes of patients with sarcoidosis listed for lung transplantation. Chest 2001;120:873–880. [DOI] [PubMed]
- 4.Handa T, Nagai S, Miki S, Fushimi Y, Ohta K, Mishima M, Izumi T. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest 2006;129:1246–1252. [DOI] [PubMed]
- 5.Sulica R, Teirstein AS, Kakarla S, Nemani N, Behnegar A, Padilla ML. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest 2005;128:1483–1489. [DOI] [PubMed]
- 6.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–381. [PubMed]
- 7.Jones PW, Quirk FH, Baveystock CM. The St George’s respiratory questionnaire. Respir Med 1991;85(suppl B):25–31; discussion 33–37. [DOI] [PubMed]
- 8.Hays RD, ed. Rand-36 health status inventory. San Antonio, TX: Psychological Corporation, 1998.
- 9.Jones PW. St. George’s respiratory questionnaire manual. London: St. George’s University of London, 2008.
- 10.Nunes H, Humbert M, Capron F, Brauner M, Sitbon O, Battesti JP, Simonneau G, Valeyre D. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax 2006;61(1):68–74. [DOI] [PMC free article] [PubMed]
- 11.Judson MA, Highland KB, Kwon S, Donohue JF, Aris R, Craft N, Burt S, Ford HJ. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis 2011;28(2):139–145. [PubMed]
- 12.Mathai SC, Puhan MA, Lam D, Wise RA. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2012;186(5):428–433. [DOI] [PMC free article] [PubMed]
- 13.Barnett CF, Bonura EJ, Nathan SD, Ahmad S, Shlobin OA, Osei K, Zaiman AL, et al. Treatment of sarcoidosis-associated pulmonary hypertension: a two-center experience. Chest 2009;135(6):1455–1461. [DOI] [PMC free article] [PubMed]
- 14.Foley RJ, Metersky ML. Successful treatment of sarcoidosis-associated pulmonary hypertension with bosentan. Respiration 2008;75(2):211–214. [DOI] [PubMed]
- 15.Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest 2006;130(5):1481–1488. [DOI] [PubMed]
- 16.Baughman RP, Culver DA, Cordova FC, Padilla M, Gibson KF, Lower EE, Engel PJ. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest 2014;145:810–817. [DOI] [PubMed]
- 17.Milman N, Burton CM, Iversen M, Videbaek R, Jensen CV, Carlsen J. Pulmonary hypertension in end-stage pulmonary sarcoidosis: therapeutic effect of sildenafil? J Heart Lung Transplant 2008;27(3):329–334. [DOI] [PubMed]
- 18.Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009;119(22):2894–2903. [DOI] [PubMed]